Abstract
Striatal neurons are known to express GABAA receptor subunits that underlie both phasic and tonic inhibition. Striatal projection neurons, or medium spiny neurons (MSNs), are divided into two classes: MSNs containing the dopamine D1 receptor (D1-MSNs) form the direct pathway to the substantia nigra and facilitate movement while MSNs expressing the dopamine D2 receptor (D2-MSNs) form the pallidal pathway that inhibits movement. Consequently, modulating inhibition in distinct classes of MSNs will differentially impact downstream network activity and motor behavior. Given the powerful role of extrasynaptic inhibition in controlling neuronal excitability, we examined the nature of striatal tonic inhibition and its potential role in preventing excitotoxicity. Consistent with earlier studies in young (P16–P25) mice, tonic GABA currents in D2-MSNs were larger than in D1-MSNs. However, with age (>P30 mice) the tonic GABA currents increased in D1-MSNs but decreased in D2-MSNs. These data demonstrate a developmental switch in the MSN subtype expressing larger tonic GABA currents. Compared to wild-type, MSNs from adult mice lacking the GABAAR δ subunit (Gabrd−/− mice) had both decreased tonic GABA currents and reduced survival following an in vitro excitotoxic challenge with quinolinic acid. Furthermore, muscimol-induced tonic GABA currents were accompanied by reduced acute swelling of striatal neurons after exposure to NMDA in WT mice but not in Gabrd−/− mice. Our data are consistent with a role for tonic inhibition mediated by GABAAR δ subunits in neuroprotection against excitotoxic insults in the adult striatum.
Keywords: medium spiny neuron, excitotoxicity, Huntington’s disease, cell swelling, delta subunit
The striatum, a major input nucleus of the basal ganglia, is crucial for extrapyramidal motor processing and psychomotor behaviors (Nakano et al., 2000; Schultz, 2006; Balleine et al., 2007). Both the local-circuit and projection neurons of the striatum are inhibitory (Tepper et al., 2004), with cortical and thalamic afferents constituting the primary source of excitation. Over 95% of the striatal neurons are medium spiny neurons (MSNs), which are GABAergic projection neurons. MSNs are divided into two classes based on their axonal projection and differential expression of dopamine receptor subtypes (Gerfen et al., 1990). MSNs that contain the dopamine D1 receptor (D1-MSNs) give rise to the direct pathway projections to the basal ganglia output nuclei, which includes the internal segment of the globus pallidus (GPi) and the substantia nigra. Activation of the direct pathway disinhibits the thalamus and facilitates movement (Le et al., 1991; Wilson, 2007). The indirect, pallidal projecting, MSNs express the dopamine D2 receptor (D2-MSNs) and result in inhibition of movement (Gerfen and Young, 1988; Surmeier and Kitai, 1994). Given its pivotal role in feedback regulation of movement, it is not surprising that degenerative diseases affecting striatal circuits, such as Huntington’s and Parkinson’s disease, are associated with severe motor deficits.
Medium spiny neurons receive afferent excitation and local-circuit inhibition and are also synaptically interconnected through GABAergic collaterals (Wilson and Groves, 1980; Koos et al., 2004; Taverna et al., 2008). Given the preponderance of inhibitory connections, striatal neurons express an abundance of GABAA receptor (GABAAR) subunits including α1–5, β1–3, γ1–3 and δ (Fujiyama et al., 2000; Schwarzer et al., 2001). The subunit composition of the pentameric GABAARs varies depending on their anatomical location (Pirker et al., 2000), and developmental stage, and determines the physiological and pharmacological properties of GABA currents (Hevers and Luddens, 1998; Mody and Pearce, 2004). Generally, GABAARs containing a γ subunit in combination with α and β subunits are located at the synapse and mediate fast phasic transmission. Receptors containing δ in combination with α4 or α5 subunits form high affinity extrasynaptic receptors that open tonically in low ambient GABA levels and give rise to a steady-state GABA conductance. Interestingly, recent studies in young (P16–25) mice have indicated that a higher expression of GABAAR containing α5 subunits in D2-MSNs, as compared to D1-MSNs, may contribute to the larger tonic GABA currents of D2-MSNs in young mice (Ade et al., 2008; Janssen et al., 2009). However, GABAAR subunits are developmentally regulated, with a progressive decline in α5 subunits and an increase in α4 and δ subunits in the adult striatum (Laurie et al., 1992). Previous studies have also demonstrated an increase in tonic inhibition in striatal MSNs during development (Kirmse et al., 2008). Therefore, it is possible that the magnitude of, and the GABAAR subunit contribution to, tonic GABA currents in adult striatal neurons may be different from those in the developing striatum.
Inhibitory regulation of the adult striatum is of considerable interest due to the vulnerability of striatal MSNs to excitotoxic damage which has been suggested to contribute to neurodegenerative diseases, such as Huntington’s disease (Graveland et al., 1985; Vonsattel et al., 1985). Tonic inhibition can greatly decrease cellular excitability (Farrant and Nusser, 2005), suggesting that extrasynaptic GABAergic inhibition could also reduce vulnerability to excitotoxic injury. The selective loss of projections from presumed D2-MSNs in Huntington’s disease (Reiner et al., 1988) is consistent with a differential MSNs vulnerability in neurodegenerative disease. Therefore, we examined whether differences in the amplitude of tonic GABA currents between adult D1- and D2-MSNs may contribute to non-uniform MSN loss during excitotoxic insults, and whether augmenting tonic inhibition could protect against excitotoxic injury. Parts of this study have been previously presented as an abstract at the Society for Neuroscience (Santhakumar and Mody, 2008).
EXPERIMENTAL PROCEDURES
Animals
Young (16–25 day old) and adult (>P30) male Drd2-EGFP (D2-GFP) and Drd1a-EGFP (D1-GFP) mice (Gong et al., 2003; generously provided by Dr. X William Yang at the University of California, Los Angeles) back-crossed for >10 generations with C57BL/6 mice were used in experiments distinguishing between MSNs expressing D1 and D2 subtype of dopamine receptors. Adult C57BL/6 and Gabrd−/− on the same genetic background (Jackson Laboratories, Bar Harbor, ME, USA) were used in experiments characterizing the role of the GABAAR δ subunit in striatal inhibition. Since previous studies have shown that striatal MSNs express either D1 or D2 dopamine receptors (Gerfen et al., 1990; Day et al., 2008), D2-GFP mice were used in a majority of the experiments and GFP-negative MSNs were presumed to express the D1 dopamine receptor (Kreitzer and Malenka, 2007; Gertler et al., 2008; Ade et al., 2008). While recording from GFP-negative MSNs in D2-GFP mice, care was taken to record from cells located at the same depth where GFP-positive cells were also visible. Additionally, we determined the responses of GFP-negative striatal neurons to positive current injections and excluded those with firing characteristics of interneurons from further analysis. Moreover, data from confirmatory experiments performed in D1-GFP mice were similar to those using D2-GFP mice and the results from the two strains were pooled.
Slice preparation
Mice were anesthetized with halothane (Halocarbon laboratories, River Edge, NJ, USA) and decapitated according to a protocol approved by the UCLA Chancellor’s Animal Research Committee. All efforts were made to minimize the number of animals and to reduce their suffering. Coronal brain slices (350 μm) were cut on a Leica VT1000S (Wetzlar, Germany) or a Microm HM 650 V (Thermo Scientific, Walldorf, Germany) in ice-cold sucrose artificial CSF (sucrose-aCSF) containing (in mM) 85 NaCl, 75 sucrose, 24 NaHCO3, 25 D-glucose, 4 MgCl2, 2.5 KCl, 1.25 NaH2PO4, and 0.5 CaCl2. A sodium-free slicing solution containing (in mM) 135 N-methyl-D-glucamine, 130 HCl, 20 Choline-HCO3, 10 D-glucose, 1.5 MgCl2, 1.2 KH2PO4, 1.0 KCl and 0.5 CaCl2 which was recently shown to promote interneuronal viability (Tanaka et al., 2008) was used to prepare slices for experiments measuring NMDA currents (Fig. 4D, E). Slices were incubated at 32±1 °C for 30 min in a submerged holding chamber containing an equal volume of sucrose-aCSF and recording aCSF and subsequently held at room temperature. The recording aCSF contained (in mM) 126 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 D-glucose, 1 glutamine and 1.5 Na-Pyruvate. All solutions were saturated with 95% O2 and 5% CO2 and maintained at a pH of 7.4.
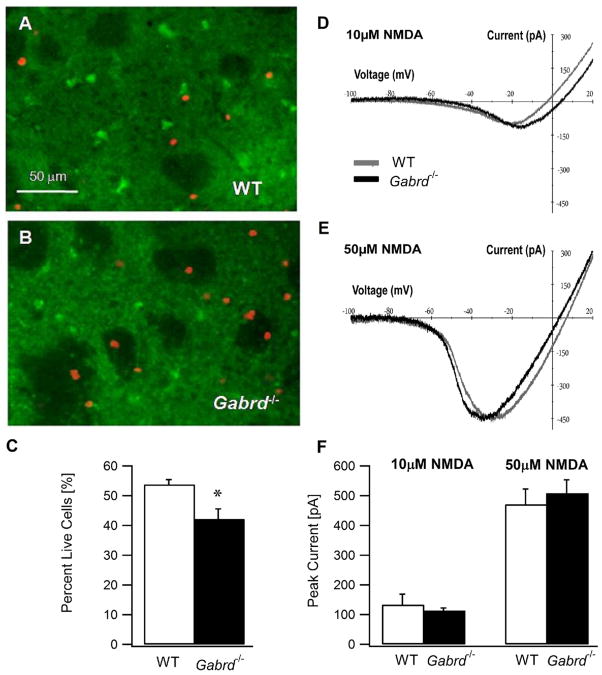
Fig. 4.
Enhanced susceptibility of striatal MSNs in Gabrd−/− mice to excitotoxic cell death. (A, B) Confocal photomicrograph of cortico–striatal slices from a wild-type mouse (A) and a Gabrd−/− mouse (B) showing the assay for live and dead cells following in vitro exposure to quinolinic acid (0.5 mM, 1 h). The live cells are stained with Calcein (in green) and dead cells incorporate the nuclear stain Ethidium Homodimer-1 (in red). Both Calcein and Ethidium Homodimer-1 images of a given field of view were obtained at 10× using appropriate filters and overlaid for illustration. (C) Summary data show the percent of MSNs that survive an hour long in vitro exposure to the excitotoxin quinolinic acid (0.5 mM) in wild-type and Gabrd−/− mice. (D, E) Representative NMDA current–voltage plots obtained in MSNs from adult wild-type and Gabrd−/− mice illustrate comparable peak NMDA currents and voltage-dependence in the presence 10 μM (D) and 50 μM (E) NMDA. (F) Summary histogram shows that the peak NMDA currents are similar in MSNs of wild type and Gabrd−/− mice. Asterisk denote a statistically significant (P<0.05) difference.
Electrophysiology
Slices were transferred to a submerged recording chamber and perfused with oxygenated aCSF at 34±1 °C. The perfusing aCSF contained the glutamate receptor antagonists D-2-amino-5-phosphonovalerate (10 μM D-AP-5, Tocris, Ellisville, MO, USA), 6,7-Dinitroquinoxaline-2,3-dione (25 μM DNQX, Tocris, Ellisville, MO, USA) and 5 μM GABA (Sigma-Aldrich, St. Louis, MO, USA). Green fluorescent protein (GFP) expression of the neurons was determined under epi-fluorescence microscopy (Fig. 1B) and striatal medium spiny neurons were recorded under IR-DIC video-microscopy. Whole-cell voltage- and current-clamp recordings from dorsal striatal MSNs were obtained at a holding potential of −70 mV using Axon Instruments MultiClamp 700A (Molecular Devices, Sunnyvale, CA, USA). Recordings were low-pass filtered at 3 kHz and acquired with custom written LabView-based software (EVAN) at 10-kHz. The recording microelectrodes (5–7 MΩ) contained (in mM): 125 KCl, 10 K-Gluconate, 2 MgCl2, 10 HEPES, 0.2 EGTA, 2 MgATP, 0.5 NaGTP, and 10 phospho-creatine at a pH of 7.26. Only cells showing the firing properties characteristic of MSNs (Kreitzer and Malenka, 2007; Ade et al., 2008) in response to depolarizing current steps were included in the analysis. Recordings were discontinued if series resistance increased by >25%. In some experiments, the GABA uptake inhibitor 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyrid-inecarboxylic acid hydrochloride (10 μM NO-711, Sigma-Aldrich, St. Louis, MO, USA) and an inverse agonist selective for GABAARs containing the α5 subunit (100 nM L655,708, Tocris, Ellisville, MO, USA) were included in the external solution. Tonic GABA current, the steady-state current blocked by the GABAAR antagonist bicuculline methiodide (100 μM BMI, Sigma-Aldrich, St. Louis, MO, USA) was measured using custom macros in IgorPro6.0 software (WaveMetrics, Lake Oswego, OR, USA) as described previously (Glykys and Mody, 2007). All salts were purchased from Sigma–Aldrich (St. Louis, MO, USA).
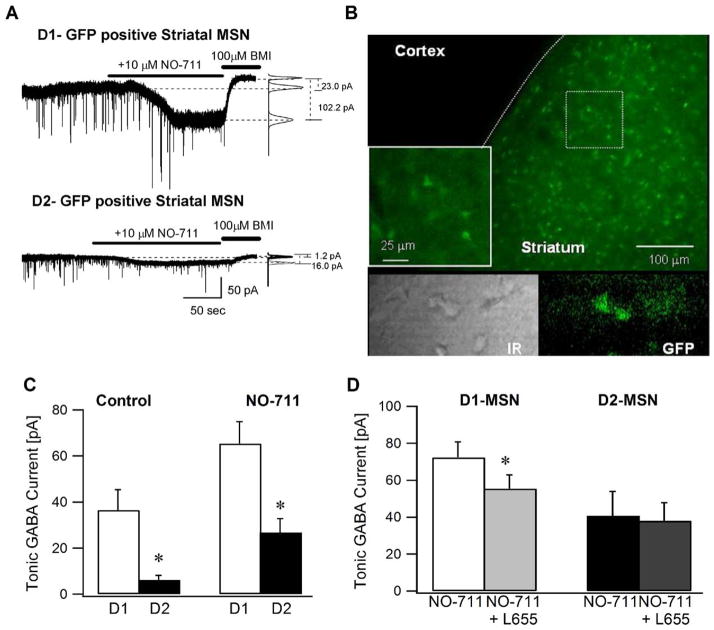
Fig. 1.
Adult striatal medium spiny neurons expressing D1 receptors have larger tonic GABA currents. (A) Representative voltage-clamp recordings (Vh=− 70 mV) from adult D1-MSNs (above) and D2-MSNs (below) illustrate the magnitude of tonic GABA current blocked by a saturating concentration of BMI (100 μM). Panels to the right show Gaussian fits to all-points histograms derived from 30 s recording periods in control conditions and in the presence of the GABA transporter-1 blocker NO-711 (10 μM) and a 15 s recording period during the perfusion of BMI used to determine the tonic current. The dashed lines indicate the Gaussian means and the difference currents are noted. (B) Confocal image of a cortico–striatal slice from a D2-GFP mouse shows that the GFP expression is restricted to the striatum. Inset illustrates a magnified image of the boxed area. The lower panel shows an IR-DIC (left) and fluorescence image (right) of a representative cortico-striatal slice from a D2-GFP mouse. (C) Summary histogram of the tonic GABA currents in D1- and D2-MSNs under control conditions and in the presence of the GABA transporter antagonist NO-711. (D) Histogram shows the effect of L655,708 (100 nM), an inverse benzodiazepine site agonist selective for GABAAR containing the α5 subunit on tonic inhibition in adult D1- and D2-MSNs in the presence of 10 μM NO-711. Tonic GABA currents were recorded in the presence of glutamate receptor blockers and 5 μM GABA. Asterisk denotes a statistically significant (P<0.05) difference in mean values using Student t-test.
Experiments examining NMDA current–voltage relationships were performed in the presence of the GABAA antagonist, picrotoxin (100 μM) and the non-NMDA glutamate receptor antagonist 6,7-Dinitroquinoxaline-2,3-dione (DNQX, 25 μM, Tocris, Ellisville, MO, USA or Sigma, St. Louis, MO, USA). Whole-cell voltage-clamp recordings were obtained using Axopatch 200A (Molecular Devices, Sunnyvale, CA, USA) with a cesium based internal solution containing (in mM): 140 CsCl, 4 NaCl, 1 MgCl2, 10 HEPES, 0.1 EGTA, 2 MgATP, 5 QX-314 at a pH of 7.26. MSNs were voltage clamped at −65 mV for 3–5 min before application of the voltage ramp protocol. The voltage was ramped from −65 to +40 mV for 1 s, followed by a 0.5 s holding period at +40 mV and a second ramp from +40 to −100 mV for 1 s and a return to −65 mV over 0.5 s. The ramps were repeated five times, with 15 s between each ramp. The averages of the five current traces obtained in the absence of NMDA were subtracted from those obtained after a 1–3 min perfusion of 10 or 50 μM NMDA to construct the NMDA current–voltage curves for each cell.
Live-dead assay
Striatal slices from age-matched adult C57/BL6 and Gabrd−/− mice were prepared on the same day and incubated for 1 h at room temperature in quinolinic acid (0.5 mM QA, Tocris, Ellisville, MO, USA). Following incubation in quinolinic acid, the slices were incubated in 4 μM of ethidium homodimer-1 (to highlight the dead cells in red) and 2 μM of calcein acetoxymethyl ester (to highlight the live cells in green) in the dark for 30 min (LIVE/DEAD stain from Molecular Probes, Carlsbad, CA, USA). The slices were then washed in control aCSF at room temperature for 15 min (Monette et al., 1998). Care was taken to incubate pairs of slices from C57/BL6 and Gabrd−/− mice simultaneously in the various solutions. The number of live and dead cells in a given field of view was quantified at a depth of 30–70 μm below the surface of the slice using a 40× objective and epi-fluorescence microscopy with the appropriate filters (Ratzliff et al., 2004). Specifically, in each genotype a primary observer visualized and counted the green cells with calcein at a depth of 30 μm (where live cells were seen below slicing induced cell damage) and the number of live cells with calcein was determined by focusing down to a depth of 70 μm. Only cells showing localized somatic fluorescence were counted. Then the filters were changed and the observer counted the number of dead cells incorporating ethidium in the same field. Only cells with the strong nuclear staining within the observed depth and field were counted and care was taken to exclude the profiles with bleed through fluorescence from deeper cells. The correspondence between the depth at which the calcein and ethidium profiles were visualized was verified by switching filters to ascertain that the counting was done at identical levels. A second investigator blind to the genotype obtained confocal image stacks from a depth of 30–70 μm in two slices each from a WT and Gabrd−/− mouse. The percent cell loss quantified using the confocal image stacks was not statistically different from those reported by the primary observer and the data from the two observations were pooled. Images for illustrative purposes were obtained using a confocal microscope with a 10× objective.
Cell swelling measurement
To assess NMDA induced swelling, slices were transferred to the submerged recording chamber and perfused for 6–8 min with control aCSF followed by 10 min perfusion of 50 μM NMDA (Sigma-Aldrich, St. Louis, MO, USA). The effect of muscimol (Tocris, Ellisville, MO, USA) on NMDA induced swelling was examined by perfusing the slices with muscimol (50 nM) first for 4–6 min followed by aCSF containing muscimol and NMDA (50 μM). Experiments were performed at room temperature and images at 40× magnification were acquired at 2 min intervals using a WAT-992H3 camera coupled to a Snappy image capture system. Cell outlines were determined using the “Cell Outliner” plugin or by manual selection of ROI in ImageJ Software (National Institutes of Health, Bethesda, MD, USA). Cell area and perimeter were calculated in ImageJ based on the outline. Data are presented as the percent increase in area or perimeter of a given cell 8–10 min after NMDA perfusion relative to the measurements obtained in the control period. No visible swelling was observed during a 15 min perfusion of aCSF or muscimol (50 nM, n=2 slices).
Statistics
All data are shown as mean±SEM. Statistical analysis was performed by paired and unpaired Student’s t-test (Microsoft Excel) and two-way ANOVA with post hoc comparisons performed by Bonferroni’s post-test (GraphPad Prism software, La Jolla CA, USA). Significance was set to P≤0.05.
RESULTS
Adult striatal D1-MSNs express larger tonic GABA currents
Using coronal slices from adult (>P30) male Drd2-EGFP and Drd1a-EGFP mice (Gong et al., 2003) to distinguish between neurons expressing D1 and D2 subtype of dopamine receptors (Fig. 1B), we examined the magnitude of tonic GABA currents in the two types of striatal MSNs. Since previous studies have shown that MSNs express either D1 or D2 dopamine receptors (Gerfen et al., 1990), Drd2-EGFP mice were used in a majority of the experiments and GFP-negative MSNs were presumed to express the D1 dopamine receptor. Additionally, we examined the firing characteristics of all recorded neurons to distinguish between MSNs and striatal interneurons (Kawaguchi, 1993, 1995; Taverna et al., 2007).
The amplitude of tonic GABA currents in medium spiny neurons from adult mice was significantly larger in D1-MSN (36.5±8.9 pA, in n=6 cells from four mice, of which two were D1-GFP cells) compared to D2-MSN (6.2±2.0 pA in n=9 cells from five mice P<0.05) (Fig. 1A, C). The input resistance (Rin), measured as the voltage deflection elicited by a 400 pA hyperpolarizing current step from a membrane potential of −70 mV, was not significantly different between D1-MSN (126.17±9.65 MΩ) and D2-MSN (149.14±21.9 MΩ). Rin was measured in glutamate and GABAA receptor antagonists. Similarly, in the presence of glutamate and GABAA receptor antagonists, the difference in the current needed to hold D1- and D2-MSN at −70 mV did not reach statistical significance (D1-MSN: (Ihold) 25.8±26.4 pA and D2-MSN: 7.54±28.93 pA). The GABA transporter-1 (GAT-1) antagonist NO-711 (10 μM), added after recording the baseline currents in aCSF, enhanced tonic GABA currents in five of the six D1-MSN (63.8±8.5 pA, average of all six cells) and all D2-MSN (25.4±6.8 pA). In additional experiments performed in the presence of NO-711, tonic GABA currents were significantly larger in D1-MSN (D1-MSN: 65.4±9.5 pA in n=13 cells from nine mice; D2-MSN: 26.8±6.1 pA in n=12 cells from seven mice, P<0.05; Fig. 1C). In contrast to previous reports suggesting that adult MSN lack tonic GABA currents (Gertler et al., 2008), and in agreement with Janssen et al. (2009) we find that tonic GABA currents are expressed in adult MSNs. Our results demonstrate that in adult mice, D1-MSNs, which contribute to the direct pathway, have larger tonic GABA currents than D2-MSN that give rise to the indirect pathway.
GABAA receptors containing δ subunits mediate tonic GABA currents in adult striatal MSNs
GABAAR α5 and δ subunits are expressed in the striatum (Laurie et al., 1992; Schwarzer et al., 2001), both subunits are known to mediate extrasynaptic inhibition. Since GABAAR subunit expression is developmentally regulated (Laurie et al., 1992), we examined which GABAAR subunits underlie tonic GABA currents in adult MSNs. In the presence of NO-711 (10 μM), to augment extrasynaptic GABA levels, perfusion of the α5 selective GABAAR inverse agonist L655,708 (100 nM) caused a small decrease (22.7±6.7%) in tonic GABA currents in D1-MSNs (Fig. 1D; tonic GABA currents: 72.3±8.5 pA in NO-711 and 55.4± 7.5 pA in L655,708 in n=17 cells from eight mice, P<0.05). Conversely, L655,708 failed to decrease tonic GABA currents in D2-MSN (Fig. 1D; tonic GABA currents: 40.7±13.2 pA in NO-711 and 38.0±7.3 pA in L655, 708; n=9 cells from five mice, P>0.05) indicating minimal contribution from receptors with α5 subunits.
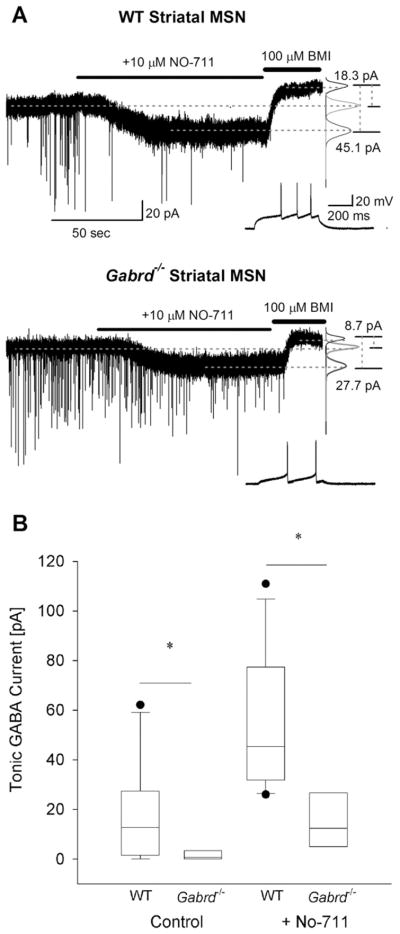
Next, we examined the contribution of GABAAR δ subunit to striatal extrasynaptic GABA currents using mice lacking the δ subunit (Gabrd−/− mice). Only cells that showed the delayed, repetitive non-adapting firing pattern characteristic of MSNs (Kawaguchi et al., 1989; Ade et al., 2008) were included in the analysis. Compared to wild-type, the magnitude of tonic GABA currents were significantly decreased in MSNs from Gabrd−/− mice (WT: 17.3± 6.1 pA in n=10 cells from six mice; Gabrd−/−: 1.8±1.1 pA in n=8 cells from four mice, P<0.05) (Fig. 2A, B). Similarly, tonic GABA currents in the presence of NO-711(10 μM) were also larger in the wild-type mice (56.2±8.1 pA in n=9 cells from six mice) compared to Gabrd−/− mice (15.8±1.1 pA in n=8 cells from four mice, P<0.05). The presence of the long whiskers, indicating the spread of data, in the box plot from wild-type mice (Fig. 2B) demonstrate the high degree of variability in the amplitude of tonic GABA currents (0 to 62.1 pA in aCSF and 26.0 to 110.9 pA in 10 μM NO-711) in MSNs from wild-type mice. This wide range is consistent with the inclusion of currents from both D1- and D2-MSNs among the recorded neurons. In contrast, tonic GABA currents in MSNs from Gabrd−/− mice were confined to a narrow range of 0 to 8.7 pA under control conditions and 5.0 to 35.2 pA in NO-711 (10 μM), suggesting that the amplitude of tonic GABA currents in D1- and D2-MSNs was not different in Gabrd−/− mice (Fig. 2A, B). In four of four cells recorded in NO-711, the α5 selective inverse agonist L655,708 decreased tonic GABA currents in MSNs from wild-type mice (77.4 ±13.4 pA in NO-711 and 56.2 ±8.5 pA in L655,708 in n =4 cells from two mice, P<0.05). However, in MSNs from Gabrd−/− mice, the effect of L655,708 on tonic GABA currents was variable with a decrease observed in nine out of 15 cells tested (53.6 ±5.0 pA in NO-711 and 31.9 ±9.7 pA in L655,708, n =9 cells from five mice, P<0.05) and an increase in six out of 15 cells which did not reach statistical significance (37.3 ±11.1 pA in NO-711 and 42.0 ±10.2 pA in L655,708, n =6 cells from five mice, P>0.05). Interestingly, the Rin of WT MSNs in the presence of glutamate and GABAA receptor antagonists was significantly greater than in MSN from Gabrd−/− mice (WT: 167.04 ±17.33 MΩ, Gabrd−/−: 118.52 ±7.22 MΩ; P<0.05) suggesting the presence of compensatory changes to account for decreases in GABAA conductance in Gabrd−/− mice.
Fig. 2.
Tonic GABA currents are decreased in Gabrd−/− mice lacking GABAA receptor delta subunits. (A) Example voltage-clamp traces from striatal MSNs in adult wild-type (above) and Gabrd−/− mice (below) show the difference in tonic GABA currents in control conditions and in the presence of NO-711 (10 μM) between the two genotypes. Recordings were obtained at a holding potential of −70 mV. To the right are panels showing Gaussian fits to all-points histograms derived from 30 s recording periods in control conditions and in the presence of NO-711 (10 μM) and a 15 s recording period during the perfusion of BMI used to determine the tonic current. The difference currents are noted to the right were calculated from the Gaussian means indicated by the dashed lines. Inset shows the characteristic firing pattern of the MSNs recorded in response to a depolarizing current injection (+80 pA in the WT in the upper panel and +20 pA in Gabrd−/− in the lower panel) from a holding potential of −70 mV. (B) Summary box-plot shows the decrease in tonic GABA currents in MSNs from Gabrd−/− mice in the absence and presence of NO-711. The upper and lower sides of the box represent the upper/lower quartiles and the median value is represented as a line. The maximum and minimum values are represented by the extent of the whiskers with outliers shown as black dots. Asterisk denotes a statistically significant (P<0.05) difference in mean values using Student’s t-test. Recordings were obtained in the presence of glutamate receptor blockers and 5 μM GABA.
Taken together, these data demonstrate that in contrast to MSNs from young mice (Janssen et al., 2009), δ subunit-containing GABAARs underlie the differential expression of tonic GABA currents between D1 and D2-MSNs in the adult striatum. The results also suggest that both GABAAR α5 and δ subunits are expressed in adult D1-MSNs with the δ subunits contributing a major fraction of extrasynaptic GABA currents in adult MSN.
Developmental changes in tonic GABA currents in striatal MSNs
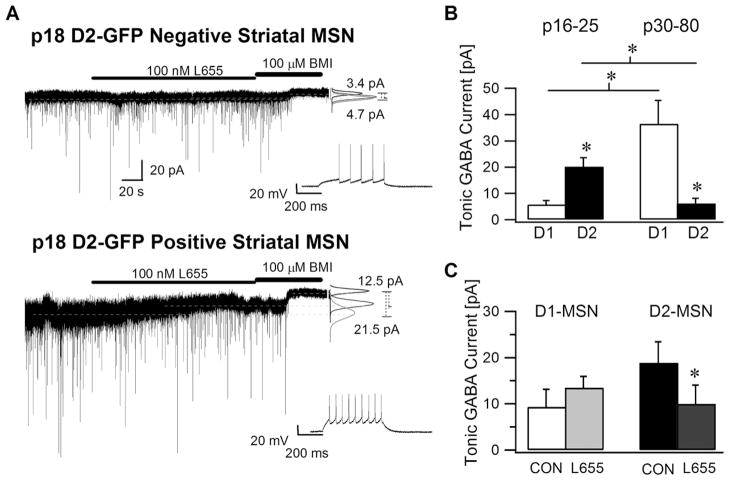
Contrary to our findings in adult MSNs, recent studies have shown that the magnitude of tonic GABA currents is larger in D2-MSNs than in D1-MSNs in young mice (Ade et al., 2008; Janssen et al., 2009). To determine whether these conflicting results were due to developmental differences, we examined the magnitude and cell type specificity of tonic GABA currents in young animals. Consistent with the earlier study (Ade et al., 2008) and in contrast to adult MSNs, we found that the amplitude of tonic GABA currents in P16–25 mice was significantly lower in the D1-MSNs (5.8±1.5 pA in n=13 cells from seven mice) than in D2-MSNs (20.2±.3.4 pA in n=16 cells from eight mice, P<0.05) (Fig. 3A, B). Notably, while there is an increase in tonic current in D1-MSNs during development (Young: 5.8±1.5 pA; Adult; 36.5±8.9 pA, P<0.01), there appears to be a developmental decrease in extrasynaptic GABA currents in D2-MSNs (Young: 20.2±.3.4 pA; Adult; 6.2± 2.0 pA, P<0.01) (Fig. 3B). The Rin and Ihold of young D1-and D2-MSNs measured in the presence of glutamate and GABAA receptor antagonists were not statistically different (D1-MSN: Rin= 147.32±6.25 MΩ and Ihold @ −70 mV = −34.23±20.04 pA and D2-MSN: Rin=186.83±18.93 MΩ and Ihold @ −70 mV=−64.2±64.9 pA). Although the developmental changes in Rin and Ihold did not reach statistical significance, the observed trend is consistent with previous studies showing that MSNs in young mice have more depolarized resting membrane potentials and higher input resistances compared to MSNs from adult mice (Kirmse et al., 2008).
Fig. 3.
Striatal medium spiny neurons expressing D2 receptors have larger tonic GABA currents in young mice. (A) Recordings from a representative D1-MSN (above) and D2-MSN (below) from juvenile (P18) D2-GFP mice show the tonic GABA current blocked by a saturating concentration of BMI (100 μM). The effect of the α5 selective GABAA receptor inverse agonist, L655,708 (100 nM) on tonic GABA currents in D1 and D2-MSN is also illustrated. As before, the graphs to the right show Gaussian fits to all-points histograms derived from 30 s periods in control and L655,708 and a 15 s recording in BMI with dashed lines indicating the means used to calculate the tonic current. Inset shows the characteristic firing pattern of the MSN recorded in response to a depolarizing current injection (+120 pA) from a holding potential of −70 mV. (B) Summary data compare the tonic GABA currents in juvenile D1- and D2-MSNs with the tonic GABA currents from adult D1 and D2-MSNs (same as control data as in Fig. 1C) under control conditions in the presence of glutamate receptor blockers and 5 μM GABA. (C) Histogram shows the effect of L655,708 (100 nM) on tonic GABA currents in juvenile D1- and D2-MSNs. All recordings were performed at a holding potential at −70 mV. Asterisk denotes a statistically significant (P<0.05) difference in mean values.
Interestingly, the contribution of GABAAR containing the α5 subunit to tonic GABA currents is also altered during development. In contrast to its effect in adult D1-MSN, where L655,708 (100 nM) decreased tonic GABA currents (Fig. 1D), L655,708 (100 nM) failed to block tonic GABA currents in D1-MSNs from young mice (9.3±3.8 pA in control and 13.4±2.5 pA in L655,708, n=5 cells from three mice, P>0.05) (Fig. 3C). Moreover, although L655,708 (100 nM) had no effect on tonic GABA currents in adult D2-MSNs (Fig. 1D), it significantly reduced tonic GABA currents in D2-MSNs from young mice (18.8±4.6 pA in control and 10.0±4.1 pA in L655,708, n=7 cells from three mice, P<0.05) (Fig. 3C). These findings reconcile our data with earlier reports in young mice (Ade et al., 2008) and demonstrate that developmental regulation of the GABAAR subunits contributing to tonic GABA currents differs between D1- and D2-MSNs.
Tonic GABA currents protect against excitotoxic neuronal injury
Given the correlation between our findings of enhanced tonic GABA currents in adult D1-MSNs and the relative sparing of the direct pathway in early neurodegenerative disease (Reiner et al., 1988; Albin et al., 1992; Deng et al., 2004), we examined whether extrasynaptic inhibition can decrease excitotoxic damage. We subjected cortico–striatal slices from adult mice to excitotoxic damage by incubating slices from wild-type and Gabrd−/− mice in a selective NMDA receptor agonist, quinolinic acid (0.5 mM) for 1 h. Following the quinolinic acid treatment the cells were incubated in Calcein (2 μM) and Ethidium Homodimer-1 (4 μM) to identify the live (green calcein stained) and dead (red ethidium stained) cells. Exposure to quinolinic acid resulted in a greater cell loss in slices from Gabrd−/− mice (Fig. 4A, B). Summary data in Fig. 4C, show that following quinolinic acid exposure, 54.5±1.8% (n=259/478 cells from 10 slices in three mice) of the wild-type cells survived. However, a significantly lower proportion of cells from Gabrd−/− mice (43.1±2.2%; n=201/475 cells from nine slices in three mice, P<0.05) survived the 1 h incubation in quinolinic acid (Fig. 4C). Additional experiments were performed to examine whether differences in NMDA currents between MSNs from wild-type and Gabrd−/− mice could contribute to the enhanced toxicity of quinolinic acid in Gabrd−/− mice. The peak currents induced by 10 and 50 μM NMDA during a voltage ramp protocol (see Experimental Procedures) and their voltage-dependence were not different between wild-type and Gabrd−/− mice (Fig. 3D–F; average peak currents in 10 μM NMDA: WT: 133.6±34.9 pA, n=4 cells and Gabrd−/−: 113.6±8.5 pA, n=3 cells, P>0.1, unpaired t-test; in 50 μM NMDA: WT: 470.9±51.7 pA and Gabrd−/−: 508.4±45.2 pA, P>0.1, unpaired t-test). These findings indicate that deletion of the GABAAR δ subunit does not enhance NMDA currents in MSNs. Since GABAAR δ subunit is the major determinant of tonic inhibition in the adult striatum, we reason that the increased cell vulnerability in Gabrd−/− mice indicates that tonic GABA conductance decreases excitotoxic cell death in adult MSN.
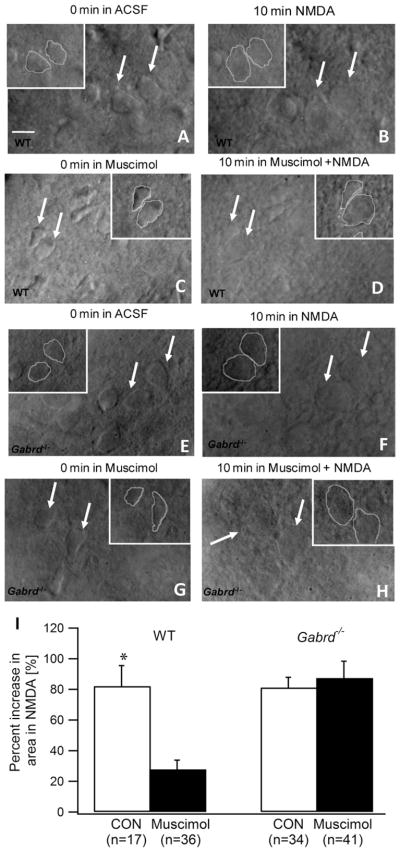
Finally, we investigated whether selectively enhancing tonic GABA currents could decrease the swelling and striatal cell death induced by exposure to NMDA. This in vitro model has been previously used to simulate excitotoxic cell death in Huntington’s disease, making it an ideal assay to test our hypothesis (McGeer and McGeer, 1976; Schwarcz and Coyle, 1977; Colwell and Levine, 1996). Under IR-DIC imaging, we exposed striatal slices to NMDA (50 μM) for 10 min either in control aCSF or in the presence of muscimol (50 nM), a δ subunit selective GABAAR agonist (Shivers et al., 1989; Mihalek et al., 1999) which preferentially enhances tonic inhibition at the low concentrations used in this study (Glykys and Mody, 2006). As expected, exposing wild-type slices in control aCSF to NMDA for 10 min resulted in swelling of the striatal cells (Fig. 5A, B), with an increase in both the cell area (82.1 ±13.4% increase) and cell perimeter (30.1 ±7.1% increase, in n =17 cells from six slices in three mice, P<0.01). Muscimol (50 nM) decreased the magnitude of cell swelling induced by NMDA in slices from wild-type mice (Fig. 5C, D). In muscimol, NMDA increased cell area by 27.8 ±6.0% (P<0.01) and failed to increase the cell perimeter (8.4 ±2.8% increase, P>0.05 in n =36 cells from 10 slices in four mice). The effect of muscimol in reducing the excitotoxic increase in cell area (Fig. 5I) and perimeter was statistically significant (P<0.05). To determine whether GABA currents through receptors containing δ subunits underlie the neuroprotection by muscimol, we tested the ability of muscimol to decreases excitotoxic cell swelling in Gabrd−/− mice. As with the wild-type, NMDA perfusion caused swelling of striatal neurons in slices from Gabrd−/− mice incubated in aCSF (81.2 ±6.6% increase in cell area, P<0.01 and 24.6 ±2.9% increase in cell perimeter, P<0.05 in n =34 cells from nine slices in three mice) (Fig. 5E, F). However, in contrast to the results from the wild-type, muscimol (50 nM) did not decrease NMDA induced cell swelling in Gabrd−/− mice (87.4 ±10.9% increase in cell area, P<0.01 and 28.9 ±3.9% increase in cell perimeter, P<0.05 in n =41 cells from 10 slices in three mice) (Fig. 5G, H). Summary data (Fig. 5I) show the genotype specific difference in the effect of muscimol treatment which was confirmed by two way ANOVA (interaction between genotype and muscimol treatment: F(1,125) =10.05; P<0.01; effect of treatment with muscimol: F(1,125) =6.38; P<0.05; and effect of genotype: F(1,125) =9.45; P<0.01). These results are consistent with a role for GABAA receptors containing δ subunits in mediating the neuroprotective action of muscimol. Taken together, these data demonstrate that tonic inhibition mediated by GABAAR containing δ subunits protects striatal neurons from excitotoxic cell injury.
Fig. 5.
Enhancement of tonic currents mediated by δ subunit-containing GABAARs decreases excitotoxic cell swelling of MSNs. (A–D). Illustrative IR-DIC images of wild-type striatal MSN obtained using a 40× objective show the MSNs before (A) and 10 min after exposure 50 μM NMDA (B) show the swelling and indistinct outlines after NMDA exposure in aCSF (n =17 cells from six slices in three mice). Images from slices in which tonic inhibition was selectively enhanced by a 5 min perfusion of 50 nM muscimol before (C) and during the 10 min exposure to 50 μM NMDA (D) show that muscimol decreases NMDA induced swelling in MSNs (n =36 cells from 10 slices in four mice). Insets show outlines used to measure the area and perimeter of the cells indicated by arrows. (E–H). Images of striatal MSNs from Gabrd−/− mice before (E) and 10 min after exposure 50 μM NMDA (F) show the NMDA induced swelling (n =34 cells from nine slices in three mice). Images obtained following enhancement of tonic inhibition by muscimol before (G) and during the 10 min exposure to NMDA (H) show that muscimol does not protect against NMDA induced swelling of MSNs in Gabrd−/− mice (n =41 cells from 10 slices in three mice). Insets show outlines used to measure the area and perimeter of the cells indicated by the arrows. Scale bar in (A) represents 10 μm. (I) Summary histogram shows the degree of NMDA induced increase in cell area in wild-type and Gabrd−/− mice in the absence and presence of muscimol. Asterisk denotes a statistically significant (P<0.05) difference.
DISCUSSION
Although it has been known that the striatal medium spiny neurons expressing D1- and D2-dopamine receptors are two distinct neuronal classes (Gerfen and Young, 1988), it has been difficult to characterize the physiological differences between the two MSNs subtypes. Recent experiments using D1- and D2-BAC transgenic mice (Gong et al., 2003; Heintz, 2004) have revealed differences in the synaptic and intrinsic properties of the two types of MSNs (Kreitzer and Malenka, 2007; Day et al., 2008; Gertler et al., 2008; Ade et al., 2008; Cepeda et al., 2008; Valjent et al., 2009). Our results demonstrate differences in the GABAAR subunits underlying tonic GABA currents between adult D1- and D2-MSNs. Our data indicate that a decrease in the contribution of the GABAAR α5 subunit in D2-MSNs and an increase in the tonic GABA currents mediated by GABAAR containing the δ subunit in adult D1-MSNs might underlie the developmental reversal in the tonic GABA current profile of MSNs. Unlike certain active and passive neuronal properties that distinguish D1- and D2-MSNs, which are not different between young and adult mice (Gertler et al., 2008), tonic GABA currents are larger in D2-MSNs in young (P16–25) mice and in D1-MSNs in the adult (>P30) mice. We demonstrate that striatal neurons from adult mice lacking the GABAAR δ-subunit are more vulnerable to excitotoxic cell death and that pharmacologically-enhancing tonic inhibition decreases the excitotoxic cell swelling. Overall, our results are consistent with a neuroprotective role of tonic inhibition in striatal excitotoxic injury, which has been proposed to contribute to neurodegenerative diseases.
Developmental regulation of striatal tonic inhibition
Earlier studies have identified the developmental regulation of GABA receptor expression in the striatum (Laurie et al., 1992) and a progressive developmental enhancement of tonic inhibition in MSNs (Kirmse et al., 2008). Therefore, we expected an increase in the magnitude of tonic inhibition in striatal neurons during development. However, in agreement with Janssen et al. (2009), we found an alteration in the subset of striatal MSN that demonstrate larger tonic GABA currents with maturation of the circuit. What are the changes that account for this developmental switch in tonic inhibition? In young (P16–25) mice, an inverse agonist specific for the GABAA receptor α5 subunit appears to preferentially decrease tonic GABA currents in D2-MSNs and abolish the differences in extrasynaptic inhibition between the D1- and D2-MSNs (Fig. 3D and Ade et al., 2008). Thus, in young mice, the expression of GABAAR α5 subunits in D2-MSNs accounts for the enhanced tonic GABA currents. The GABAAR α5 subunit selective inverse agonist L655,708 did not decrease the small tonic GABA currents observed in D1-MSNs suggesting that GABAARs with other subunits might underlie these currents. THIP, a preferential agonist of GABA receptors containing δ subunits (Brown et al., 2002), augments tonic GABA currents in both D1- and D2-MSNs alike (Ade et al., 2008). Therefore, it is likely that GABAAR δ subunits contribute to part of the extrasynaptic inhibition in young mice. However, the absence of changes in the distribution of tonic GABA currents in MSNs from young Gabrd−/− mice (Janssen et al., 2009) indicates that the contribution of GABAAR δ subunits to tonic GABA currents in young MSNs is minimal. In the adult striatum, we find a marked increase in tonic GABA currents in D1-MSNs. The dramatic decrease in extrasynaptic GABA currents in MSN from Gabrd−/− mice (Fig. 3) suggests that increased expression of GABAAR δ subunits underlies the enhanced tonic inhibition in adult D1-MSNs. Furthermore, L655,708, which had no effect on D1-MSNs in young mice, decreases tonic GABA currents in adult D1-MSNs (Fig. 1D) indicating that in addition to δ subunits, the contribution of GABAAR α5 subunits to tonic GABA currents is also increased in adult D1-MSNs. In contrast, the amplitude of tonic GABA currents undergoes a statistically significant developmental decrease in D2-MSNs. Moreover, tonic currents in adult D2-MSNs are not blocked by L655,708 which indicates a developmental decrease in the contribution of GABAAR α5 subunits to tonic GABA currents in these cells. The results of our physiological studies are in agreement with earlier anatomical studies showing a decrease in the expression of α5 subunit and an increase in expression of α4 and δ subunit with striatal development (Laurie et al., 1992). This developmental switch in tonic inhibitory control of the striatal output neurons from the indirect pathway to those of the direct pathway is likely to alter the input processing in the striatal circuit.
Role in shaping membrane properties
There are several differences between the physiological properties of D1- and D2-MSNs. D1-MSNs rest at a more hyperpolarized membrane potential, have a lower input resistance (Gertler et al., 2008) and are less excitable than D2-MSNs. A recent study examined the mechanisms underlying the difference in passive membrane properties of adult D1 and D2-MSNs but failed to find differences in extrasynaptic inhibition between adult D1 and D2-MSNs (Gertler et al., 2008). Our findings that tonic GABA currents in adult D1-MSNs is greater than in D2-MSNs are consistent with a recent report (Janssen et al., 2009) and indicate a role for extrasynaptic inhibition in shaping the passive membrane properties of adult D1-MSNs. Since MSNs rest at hyperpolarized membrane potentials (Gertler et al., 2008) likely to be more negative than the reversal potential of GABA currents, it is not clear if tonic GABA currents would be depolarizing or hyperpolarizing. However, the shunting conductance provided by tonically open extrasynaptic GABA receptors is likely to contribute to the lower input resistance (Gertler et al., 2008) and decreased excitability (Kreitzer and Malenka, 2007) of adult D1-MSNs. Indeed, the oscillation of MSN membrane potential between up and down states close to the GABA reversal potential can render GABA currents either depolarizing or hyperpolarizing (Bracci and Panzeri, 2006). However, the membrane shunting effects of tonic GABAergic conductance should decrease the excitability of MSN with tonic GABA currents and reduce excitotoxic damage. It is intriguing to speculate that a shunting tonic inhibition in the adult striatum may selectively decrease the excitability of the direct pathway (D1-MSN), allowing for selective facilitation of behaviorally relevant inputs, while the indirect pathway (D2-MSN), with a much lower level of tonic inhibition, can be more readily activated to suppress unintended movement.
Protection against excitotoxic cell damage
Loss of striatal medium spiny neurons is the pathological hallmark of Huntington’s disease, an autosomal dominant neurodegenerative movement disorder (Huang et al., 1995; Estrada Sanchez et al., 2008). Early experiments have shown that in vivo injection of the NMDA agonist, quinolinic acid, into the striatum can reproduce the cell loss and behavioral alterations in Huntington’s disease. Studies on striatal tissue from patients with Huntington’s disease have demonstrated that fibers containing enkephalin, presumed to arise from D2-MSNs, are selectively lost relatively early in the disease process while the striato–nigral fibers enriched in Substance P are relatively intact (Reiner et al., 1988). Interestingly, D1 but not D2 dopamine receptors have been shown to enhance potentially excitotoxic NMDA currents in MSNs (Surmeier et al., 2007), suggesting that the susceptibility to glutamate alone may not account for the differential cell loss. Our findings that tonic GABA currents are enhanced in adult D1-MSN which appear less vulnerable to degeneration are consistent with a potential neuroprotective role for tonic inhibition. Our results demonstrating increased excitotoxic striatal cell loss without changes in NMDA currents in Gabrd−/− mice, in conjunction with larger δ subunit mediated tonic GABA currents in adult MSNs, support the role for tonic inhibition in protecting against excitotoxic cell death in D1-MSN.
Huntington’s disease is characterized by progressive and differential neuronal loss with early degeneration of the the striatal projections from presumed D2-MSNs to the external segment of the Globus Pallidus. Since glutamate excitotoxicity is known to contribute to striatal cell loss, several clinical trials have examined the effects of various NMDA receptor antagonists to prevent glutamate toxicity in neurodegenerative diseases (Murman et al., 1997; Kremer et al., 1999; Lucetti et al., 2002; Beister et al., 2004). However, the outcome of these studies have been variable (Estrada Sanchez et al., 2008). Our results show that enhancing tonic inhibition decreases NMDA-induced cell swelling in MSN and suggest that selective enhancement of extrasynaptic GABAergic inhibition could reduce excitotoxic injury to the striatum.
CONCLUSION
We have demonstrated a developmental switch in the MSN subtype expressing tonic GABA currents, and using pharmacological and genetic tools we have identified the respective GABAA receptor subunits involved. We further show that tonically active GABAA receptors can protect against excitotoxic damage of striatal neurons. Therefore, the differential expression of extrasynaptic GABAA receptors can influence developmental changes in striatal information processing and may thwart striatal neuronal vulnerability in neurological diseases.
Acknowledgments
We thank Dr. X William Yang for providing us the Drd2-EGFP and Drd1a-EGFP mice and Dr. Yijun Cui for help with genotyping the mice, and Dr. Seema Tiwari-Woodruff for help with confocal imaging. We also thank Mahsan Rafizadeh and Reyes Main Lazaro for their help with maintaining the animal colonies and genotyping. This work was supported by NIH Grant NS30549 and the Coelho Endowment to I.M.
Abbreviations
- aCAF
artificial cerebro spinal fluid
- AP-5
D-2-amino-5-phosphonovalerate
- BMI
bicuculline methiodide
- DNQX
6,7-Dinitroquinoxaline-2,3-dione
- D1
dopamine receptor type 1
- D2
dopamine receptor type 2
- GABA
γ-aminobutyric acid
- GABAAR
GABAA receptor
- GFP
green fluorescent protein
- IR-DIC
infrared-differential interference contrast
- L655, 708
11,12,13,13a-tetrahydro-7-methoxy-9-oxo-9H-imidazo[1, 5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylic acid, ethyl ester
- MSN
medium spiny neuron
- NMDA
N-Methyl-D-aspartic acid
- NO-711
1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridine carboxylic acid hydrochloride
References
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Reiner A, Anderson KD, Dure LS, Handelin B, Balfour R, Whetsell WO, Jr, Penney JB, Young AB. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington’s disease. Ann Neurol. 1992;31:425–430. doi: 10.1002/ana.410310412. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beister A, Kraus P, Kuhn W, Dose M, Weindl A, Gerlach M. The N-methyl-D-aspartate antagonist memantine retards progression of Huntington’s disease. J Neural Transm Suppl. 2004;68:117–122. doi: 10.1007/978-3-7091-0579-5_14. [DOI] [PubMed] [Google Scholar]
- Bracci E, Panzeri S. Excitatory GABAergic effects in striatal projection neurons. J Neurophysiol. 2006;95:1285–1290. doi: 10.1152/jn.00598.2005. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Andre VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Levine MS. Glutamate receptor-induced toxicity in neostriatal cells. Brain Res. 1996;724:205–212. doi: 10.1016/0006-8993(96)00323-x. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YP, Albin RL, Penney JB, Young AB, Anderson KD, Reiner A. Differential loss of striatal projection systems in Huntington’s disease: a quantitative immunohistochemical study. J Chem Neuroanat. 2004;27:143–164. doi: 10.1016/j.jchemneu.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Estrada Sanchez AM, Mejia-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39:265–276. doi: 10.1016/j.arcmed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABA(A) receptor subunits in the striatum of the rat. J Comp Neurol. 2000;416:158–172. [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS., III Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 1988;460:161–167. doi: 10.1016/0006-8993(88)91217-6. [DOI] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, Surmeier DJ. Dichotomous anatomical properties of adult striatal medium spiny neurons. J Neurosci. 2008;28:10814–10824. doi: 10.1523/JNEUROSCI.2660-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABA A receptor alpha5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graveland GA, Williams RS, DiFiglia M. Evidence for degenerative and regenerative changes in neostriatal spiny neurons in Huntington’s disease. Science. 1985;227:770–773. doi: 10.1126/science.3155875. [DOI] [PubMed] [Google Scholar]
- Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhou D, Sapp E, Aizawa H, Ge P, Bird ED, Vonsattel JP, DiFiglia M. Quinolinic acid-induced increases in calbindin D28k immunoreactivity in rat striatal neurons in vivo and in vitro mimic the pattern seen in Huntington’s disease. Neuroscience. 1995;65:397–407. doi: 10.1016/0306-4522(94)00494-p. [DOI] [PubMed] [Google Scholar]
- Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci. 2009;29:5116–5126. doi: 10.1523/JNEUROSCI.4737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci. 1995;18:527–535. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol. 1989;62:1052–1068. doi: 10.1152/jn.1989.62.5.1052. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Dvorzhak A, Kirischuk S, Grantyn R. GABA transporter 1 tunes GABAergic synaptic transmission at output neurons of the mouse neostriatum. J Physiol. 2008;586:5665–5678. doi: 10.1113/jphysiol.2008.161943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kremer B, Clark CM, Almqvist EW, Raymond LA, Graf P, Jacova C, Mezei M, Hardy MA, Snow B, Martin W, Hayden MR. Influence of lamotrigine on progression of early Huntington disease: a randomized clinical trial. Neurology. 1999;53:1000–1011. doi: 10.1212/wnl.53.5.1000. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain: part III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MC, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing the D1 dopamine receptor gene. Proc Natl Acad Sci U S A. 1991;88:4205–4209. doi: 10.1073/pnas.88.10.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucetti C, Gambaccini G, Bernardini S, Dell’Agnello G, Petrozzi L, Rossi G, Bonuccelli U. Amantadine in Huntington’s disease: open-label video-blinded study. Neurol Sci. 2002;23 (Suppl 2):S83–S84. doi: 10.1007/s100720200081. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Duplication of biochemical changes of Huntington’s chorea by intrastriatal injections of glutamic and kainic acids. Nature. 1976;263:517–519. doi: 10.1038/263517a0. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Monette R, Small DL, Mealing G, Morley P. A fluorescence confocal assay to assess neuronal viability in brain slices. Brain Res Brain Res Protoc. 1998;2:99–108. doi: 10.1016/s1385-299x(97)00020-2. [DOI] [PubMed] [Google Scholar]
- Murman DL, Giordani B, Mellow AM, Johanns JR, Little RJ, Hariharan M, Foster NL. Cognitive, behavioral, and motor effects of the NMDA antagonist ketamine in Huntington’s disease. Neurology. 1997;49:153–161. doi: 10.1212/wnl.49.1.153. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247 (Suppl 5):V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Ratzliff AH, Howard AL, Santhakumar V, Osapay I, Soltesz I. Rapid deletion of mossy cells does not result in a hyperexcitable dentate gyrus: implications for epileptogenesis. J Neurosci. 2004;24:2259–2269. doi: 10.1523/JNEUROSCI.5191-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington disease. Proc Natl Acad Sci U S A. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Mody I. Developmental regulation and neuroprotective effects of striatal tonic inhibition. 2008 Neuroscience Meeting Planner, Society for Neuroscience; Washington DC, USA. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Coyle JT. Neurochemical sequelae of kainate injections in corpus striatum and substantia nigra of the rat. Life Sci. 1977;20:431–436. doi: 10.1016/0024-3205(77)90384-8. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Kitai ST. Dopaminergic regulation of striatal efferent pathways. Curr Opin Neurobiol. 1994;4:915–919. doi: 10.1016/0959-4388(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tanaka Y, Furuta T, Yanagawa Y, Kaneko T. The effects of cutting solutions on the viability of GABAergic interneurons in cerebral cortical slices of adult mice. J Neurosci Methods. 2008;171:118–125. doi: 10.1016/j.jneumeth.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Taverna S, Canciani B, Pennartz CM. Membrane properties and synaptic connectivity of fast-spiking interneurons in rat ventral striatum. Brain Res. 2007;1152:49–56. doi: 10.1016/j.brainres.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci. 2008;28:5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. GABAergic inhibition in the neostriatum. Prog Brain Res. 2007;160:91–110. doi: 10.1016/S0079-6123(06)60006-X. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]