Abstract
Stretch reflexes have been considered one of the simplest circuits in the human nervous system. Yet, their role is controversial given that they assist or resist an imposed perturbation depending on the task instruction. Evidence shows that a loud acoustic stimulus applied prior to an impending movement elicits a movement-direction dependent muscle activity. In our study, we found that a perturbation can also trigger this early onset of movement, if applied during movement preparation. These responses were also perturbation direction dependent. This suggests an interaction of between the limb-stabilizing stretch reflexes and the voluntary activity.
I. INTRODUCTION
Many theories of motor control assume that stretch sensitive reflexes serve to stabilize the posture of a limb [1]. While this is generally true for short latency reflexes, a number of groups have demonstrated that longer latency stretch reflexes, occurring 50–100 ms after perturbation onset in upper limb muscles, can serve to assist an imposed limb displacement, arguing against the stabilizing properties of this response [2, 3] and leading to confusion regarding the functional role of long latency stretch reflexes.
An important distinction of the studies demonstrating an assistive stretch reflex is that their protocols required subjects to have a specific motor goal, such as moving to a previously specified target, prior to perturbation onset. Hence, it is possible that the long-latency reflexes observed in these studies represented an early release of a pre-planned motor program rather than a specific response to the perturbation. It previously has been demonstrated that startling acoustic stimuli can trigger the early release of a planned motor response [4]. These startle-induced reactions, often termed StartReact responses, are characterized by the early release of motor activity in all muscles involved in the pre-planned motor response. In the upper limb, the earliest activation in these muscles occurs at a latency of approximately 70 ms, which is within the range of the long-latency stretch reflex and before the onset of typically recorded voluntary reaction times. Responses to startling acoustic stimuli are thought to be mediated through subcortical pathways [5] and can reliably be identified by activation of the sternoclaidomastoid (SCM) muscle in addition to the muscles involved in the pre-planned motor behavior [6].
The purpose of this study was to determine if postural perturbations can elicit StartReact responses similar to those elicited by startling acoustic stimuli. Such a finding would be consistent with the proposition that the assistive “stretch reflexes” reported in previous studies represent the early release of pre-planned motor actions rather than specific responses to the imposed postural disturbance. It also would clarify the role of the stretch reflex responses typically observed in the absence of a prepared motor program and provide insight to how this stretch reflex interacts with prepared motor actions to guide transitions from postural control to movement control.
II. METHODS
A. Subjects
Experiments were performed on 17 able-bodied subjects (8 female, 9 male; aged: 24–34) with no known neurological disorders. All protocols were approved by the Northwestern University Institutional Review Board and required informed consent.
B. Equipment
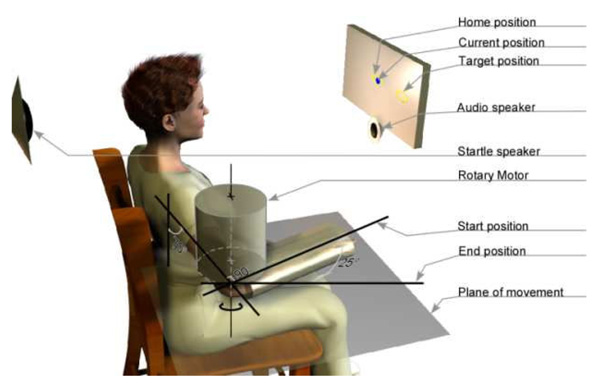
Subjects were seated comfortably with the trunk secured to an adjustable chair (Biodex, NY) using padded straps. The right forearm of each subject was positioned in the horizontal plane at a nominal posture of 70° shoulder abduction, 0° shoulder flexion and 90° elbow flexion with the forearm fully pronated, as shown in Figure 1. The wrist joint was immobilized in neutral position using a rigid custom-made plastic cast. The cast was attached to a rotary motor [BSM90N-3150, Baldor Electric Company, WV], aligned such that the motor axis was in line with the elbow flexion/extension axis. The rotary motor was attached to a 10:1 gear head AD140-010-PO/BaldorBSM90N3 (Apex Dynamics, Taiwan ROC). This allowed the encoder to record position with a resolution of 3.6 × 10−3 °. The rotary motor was used to apply position perturbations, apply background torque bias, and simulate compliant environments using custom-written software on MATLAB/XPC (Mathworks, MA). Physical stops limited the actuator to 20° of flexion and 45° of extension from the nominal position. Software limits were implemented to prevent motion 10° before these limits.
Figure 1.
Setup used for the experiment. The shoulder straps and the lap belt used for restraining the subject are not shown in the figure.
Surface electromyographic (EMG) activity was recorded from biceps (BIC), triceps lateral head (TRI), and the left sternoclaidomastoid (SCM) using bipolar Ag/AgCl electrodes (Noraxon Dual Electrodes, #272, Noraxon USA Inc., AZ). EMGs were amplified and conditioned using a Bortec AMT-8 (Bortec Biomedical Ltd., Canada), with a band-pass filter of 10–1,000 Hz. The resulting signals were anti-alias filtered using 5th order Bessel filters with a 500 Hz cut-off frequency and sampled at 2500 Hz using a PCI-DAS1602/16 (Measurement Computing, MA).
Visual feedback of the current elbow angle, the starting position (90° elbow flexion), and the target position (65° elbow flexion) were provided on a computer monitor placed 25 cm in front of the subject.
Auditory pulses were presented as cue and imperative signals to prepare to move and to make the movement, respectively. Both cue and imperative signals were presented via Sonalert SC628ND speakers (Mallory Sonalert Products Inc., IN) placed 25 cm in front of the subject. The startling acoustic signal was presented via a piezo-dynamic siren (M85PDS; MG Electronics, NY) placed 20 cm directly behind the head of the subject. The peak intensities of cue, imperative and startling acoustic signals near the subject’s ears were approximately 80dB, 80dB and 118dB, respectively. The intensities were tested using a digital sound lever meter, (Model 407730 Extech Instruments Corp, MA). The duration of these auditory signals was set to 40ms.
C. Protocols
Maximum voluntary contractions (MVC) of the biceps and triceps muscles were recorded while the subject was attached to the manipulandum, prior to the experiment.
The experiment consisted of a training phase and a testing phase. In both phases the rotary motor was set to simulate a compliant environment (stiffness = 0). Six of the subjects were instructed to exert an extension bias (2 Nm) against the motor throughout the experiment; the rest had no bias.
In the training phase, subjects made repeated fast ballistic motions to the 25° extension target. The cue was provided only when the subjects had held the nominal position ± 1° for 0.5–1 s. This hold time was randomized between trials. After a randomized time interval of 2.5–3.5 s following the cue, an imperative signal was provided. Subjects were instructed to reach for the target, “as soon as, and as fast as” they could. Subjects were also told that the reactions times were more crucial than the accuracy of the reach. Training was concluded when the subject’s reaction time was consistently within 2 standard deviations from the mean reaction time for 10 consecutive trials. A maximum of 40 training reaches were necessary to reach this condition across all subjects.
During the testing phase, the cue and imperative signals were provided in the same manner as in the training phase. Subjects exerting no bias performed a total of 300 reaching trials, split into 5 blocks of 60 trials each. To avoid fatigue, subjects exerting a bias were limited to a total of 180 reaching trials, split into 5 blocks of 36 trials each. A minimum of a 1-minute break was enforced between blocks; the subjects had the option of resting between trials as well. In random, non-consecutive catch trials, the imperative signal was presented concomitantly with one of the following stimuli:
SAS – a startling acoustic signal,
EXT – an elbow extension perturbation
FLEX – an elbow flexion perturbation
Ramp-and-hold perturbations were used for flexion and extension. These had a displacement of 6°, a velocity of 60°/s, and a hold time of 250 ms after the end of the ramp. A total of 20 trials were recorded for each of the 3 catch trials conditions for subjects exerting no voluntary bias. In the case of subjects exerting a voluntary bias, 10 trials were recorded per catch trial condition. In all non-catch trials (labeled REACH), subjects completed a voluntary reach, as in the training phase.
In the subjects exerting a voluntary bias force, we obtained the responses to perturbations during posture maintenance as well. The subjects were told, “Do not intervene” (DNI). Twenty perturbations per direction were presented in a randomized order.
III. DATA ANALYSIS
All data analyses, except the statistics, were done using MATLAB (Mathworks, MA). The EMG data were rectified before further analysis. For BIC and TRI EMG, the background (BGD, averaged over 500ms before the onset of the cue signal) was removed.
For each trial, the EMG activity in each muscle was quantified by calculating the onset and the amplitude of the EMG activity. The onset was calculated as the first point two standard deviations above the background, at least 50 ms after the imperative signal. The 50 ms threshold eliminated short-latency reflex components in the EMG signal. This automated process produced an 8±7 ms delay compared to detecting onsets by visual inspection. The amplitude was quantified by the average amplitude above or below background over two separate windows. The short latency response (SLR) amplitude was computed over a 20 – 50 ms window and the long latency response (LLR) amplitude was computed over a 50 – 100 ms window after the imperative signal onset. All amplitudes were normalized to BGD. Outliers in background, amplitude and onset measures were excluded (10% or less).
We classified trials as startle-evoking (+) if the onset of activity in the SCM was earlier than 150 ms; otherwise they were labeled non-startle evoking (−). For the REACH trials, we did not include the trials with activity in the sternoclaidomastoid muscle.
All statistical analyses were performed using R project[7]. We performed a repeated measures analysis of variance with subject as a random factor, and the trial type (REACH, SAS±, EXT±, FLEX±) as the fixed, interacting factors. TukeyHSD post-hoc analysis was conducted to obtain the contrasts. Statistical significance was tested against a p-value of 0.05 or a confidence interval of 95%.
IV. RESULTS
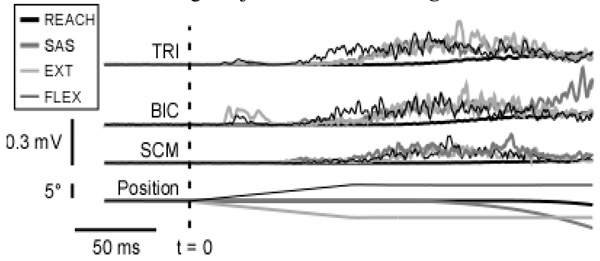
All but 2 subjects showed responses in the sternoclaidomastoid muscles to SAS trials. These 2 subjects were not included in any further analysis. Sample data from one of the remaining subjects is shown in Figure 2.
Figure 2.
Sample data from a representative subject shows the EMG activities in the triceps, biceps and the left sternoclaidomastoid muscles for REACH, SAS+, EXT+ and FLEX+ trials.
All subjects showed a tri-phasic pattern of activity in the TRI and BIC muscles for the REACH task. As expected, this pattern was maintained in the SAS trials. In the FLEX and EXT trials, we still saw a tri-phasic pattern of activity. However, since these two types of trials involved ramp-and-hold perturbations, the tri-phasic pattern could not be tested for consistency. As the perturbations had a ramp of 100 ms duration, we considered only the first 100 ms of activity to be free of all confounding factors. Since the onsets of activity in the BIC (antagonist) were closer to 100 ms, the rest of the results are focused mainly only on the agonist.
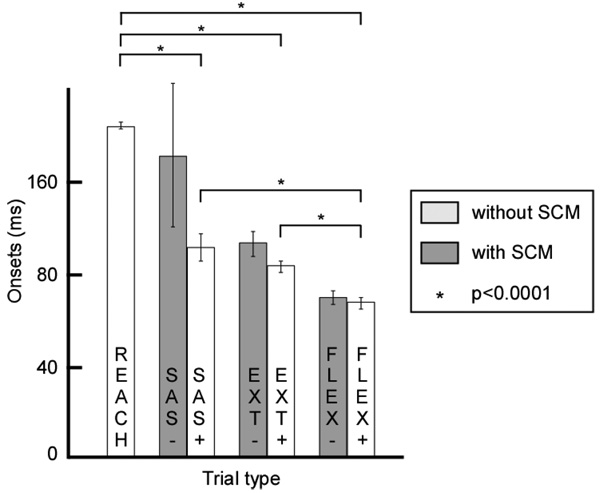
A. Onset of Agonist (TRI) activity
Across all subjects, the onset of TRI activity in each of the three catch trial types with SCM activity were earlier than in REACH trials, as shown in Figure 3. The onset of the agonist activity in SAS+ trials (81±3.5 ms) occurred earlier than in REACH (140±2ms). These latencies are consistent with those of the StartReact responses [4].
Figure 3.
The onsets of activity in the triceps show differences between the different trial types. The onsets modulate with the direction of perturbation but not with the presence or absence of sternoclaidomastoid activity. Trial types with significant differences are noted with asterisks.
The agonist onsets in EXT+ (78±2.5 ms) were not statistically different from SAS+ trials. However, the onsets in FLEX+ trials (65±2 ms) were significantly earlier than the other catch type trials (p<0.0001, TukeyHSD post-hoc analysis). This is consistent with the behavior of the long latency stretch reflex to postural perturbations. This suggests that perturbations applied prior to movement still evoke a limb stabilizing reflex response.
The onset latencies are not significantly different between perturbation trials with and without an SCM response. In conjunction with latency differences between FLEX± and EXT± trials, this suggests that the initial response to perturbation is a postural response. The later part of the response (from 81 ms; observed in SAS+ trials), however, includes an early release of the voluntary movement.
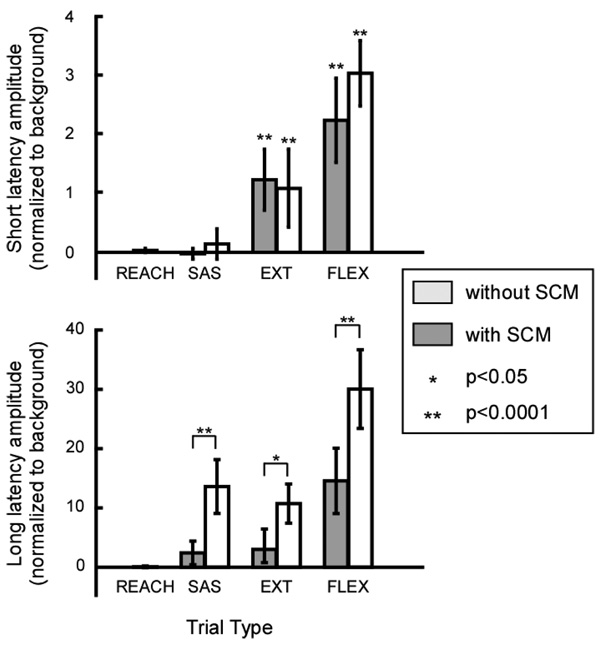
B. Short latency response (SLR) of the agonist
The amplitude of the triceps SLR (Figure 4) was significantly different from zero only for the FLEX or EXT trials, as expected (p<0.0001, t-test). There was no significant difference between trials with and without SCM response (p>0.05, TukeyHSD post-hoc test). Since the short latency reflex is spinal, this result leads us to believe that the spinal reflexes are not involved in the startle pathways.
Figure 4.
The short and long latency amplitudes (normalized to the background) show significant differences between trial types. The short latency response is significant only when a stretch perturbation is applied. The long latency response shows interactions between perturbations and early release of planned movement.
C. Long latency response (LLR) of the agonist
The amplitude of the triceps LLR (Figure 4) was significantly different between the catch trials evoking an SCM response and the REACH trials (p<0.0001, TukeyHSD post-hoc test). This result was expected, since much of the LLR comes from the early onset of voluntary activity.
We also observed that the LLRs in FLEX trials had significantly greater amplitudes than those in EXT or SAS trials (p<0.0001, TukeyHSD post-hoc test). This suggests a perturbation-dependent modulation of the response and is consistent with the responses to postural perturbations.
LLR amplitudes were greater in trials with an SCM response than in trials without. This result was significant (p<0.03 for EXT trials and p<0.0001 for FLEX and SAS trials, TukeyHSD post-hoc tests), suggesting that the perturbations and acoustic startles may elicit responses through similar neural pathways.
D. Probability of Startle
The probability of eliciting a startle, indicated by a response in the sternoclaidomastoid within 150 ms of the onset of the imperative signal, was significantly greater than zero for all three catch trials, across all subjects. This response has been used as an indicator of startle. SAS (0.74±0.15) trials had a greater probability of evoking a startle than EXT (0.64±0.2) or FLEX (0.67±0.2), although this effect was not statistically significant (p>0.1).
V. DISCUSSION
This study investigated the response elicited by an unexpected mechanical perturbation applied prior to execution of a ballistic movement. Previous observations of such responses have shown early onsets of activity in the agonist muscles. This has been variously interpreted as a long-latency reflex modulation to the impending direction of motion [2, 8], or as triggered reactions [1, 9]. These studies have hitherto not examined possible contributions from startle pathways.
Our data show that a joint perturbation evoked muscular responses with characteristics similar to those evoked by acoustic startles. In addition, some of the elements of the response (short and long latency responses in the agonist) were dependent on the direction of the perturbation. This leads us to believe that the response to stretch perturbations evoke a startle-like response that interacts with the stretch reflex response within the long latency window. Further analysis over smaller windows of time would help clarify the time window of this interaction.
Though not shown here, the latency of onsets in the sternoclaidomastoid were 20 ms earlier in the acoustic startle trials than in the perturbation trials. This difference was not statistically significant. These differences might be in part because the perturbations characteristics are not matched to those of the acoustic startle signal. Studies to look at the effects of stimulus intensities (perturbation velocity, for instance) would help clarify this difference.
One could argue that the activity observed in the sternoclaidomastoid is a postural response to perturbation. This is unlikely considering the absence of this activity during voluntary reaches and its presence during both acoustic startles and perturbations.
In conclusion, evidence presented in this paper leads us to believe that mechanical perturbations evoke the startle circuits that release a preplanned movement that interacts with a long-latency reflex component.
Acknowledgments
This work was supported in part by the NIH K25-D044720.
Contributor Information
Vengateswaran J. Ravichandran, Biomedical Engineering Department, Northwestern University, Chicago, IL 60611 USA, (phone: 312-238-1268; fax: 312-238-2208; vennjr@u.northwestern.edu)
Jonathan B. Shemmell, Sensory Motor Performance Program, The Rehabilitation Institute of Chicago, IL 60611 USA, (jshemmell@northwestern.edu)
Eric J. Perreault, Biomedical Engineering, and the Physical Medicine and Rehabilitation Departments, Northwestern University, Chicago, IL 60611 USA, and with the Sensory Motor Performance Program, The Rehabilitation Institute of Chicago, Chicago, IL 60611, (e-perreault@northwestern.edu)
REFERENCES
- 1.Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976 Sep;vol. 39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- 2.Koshland GF, Hasan Z. Electromyographic responses to a mechanical perturbation applied during impending arm movements in different directions: one-joint and two-joint conditions. Exp Brain Res. 2000 Jun;vol. 132:485–499. doi: 10.1007/s002210000356. [DOI] [PubMed] [Google Scholar]
- 3.Pruszynski JA, Kurtzer I, Scott SH. Rapid motor responses are appropriately tuned to the metrics of a visuospatial task. J Neurophysiol. 2008 Jul;vol. 100:224–238. doi: 10.1152/jn.90262.2008. [DOI] [PubMed] [Google Scholar]
- 4.Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol. 1999 May 1;vol. 516(Pt 3):931–938. doi: 10.1111/j.1469-7793.1999.0931u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothwell JC, MacKinnon CD, Valls-Sole J. Role of brainstem-spinal projections in voluntary movement. Mov Disord. 2002;vol. 17 Suppl 2:S27–S29. doi: 10.1002/mds.10054. [DOI] [PubMed] [Google Scholar]
- 6.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp Brain Res. 2003 Oct;vol. 152:510–518. doi: 10.1007/s00221-003-1575-5. [DOI] [PubMed] [Google Scholar]
- 7.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 8.Rothwell JC, Traub MM, Marsden CD. Influence of voluntary intent on the human long-latency stretch reflex. Nature. 1980 Jul 31;vol. 286:496–498. doi: 10.1038/286496a0. [DOI] [PubMed] [Google Scholar]
- 9.Newell KM, Houk JC. Speed and accuracy of compensatory responses to limb disturbances. J Exp Psychol Hum Percept Perform. 1983 Feb;vol. 9:58–74. doi: 10.1037//0096-1523.9.1.58. [DOI] [PubMed] [Google Scholar]