Abstract
Basement membranes possess a complex three dimensional topography in the nanoscale and submicron range which have been shown to profoundly modulate a large menu of fundamental cell behaviors. Using the topographic features found in native vascular endothelial basement membranes as a guide, polyurethane substrates were fabricated containing anisotropically ordered ridge and groove structures and isotropically ordered pores from 200 nm to 2000 nm in size. We investigated the impact of biomimetic length-scale topographic cues on orientation/elongation, proliferation and migration on four human vascular endothelial cell-types from large and small diameter vessels. We found that all cell-types exhibited orientation and alignment with the most pronounced response on anisotropically ordered ridges ≥ 800 nm. HUVEC cells were the only cell-type examined to demonstrate a decrease in proliferation in response to the smallest topographic features regardless of surface order. On anisotropically ordered surfaces all cell types migrated preferentially parallel to the long axis of the ridges, with the greatest increase in cell migration being observed on the 1200 nm pitch. In contrast, cells did not exhibit any preference in direction or increase in migration speed on isotropically ordered surfaces. Overall, our data demonstrate that surface topographic features impact vascular endothelial cell behavior and that the impact of features varies with the cell behavior being considered, topographic feature scale, surface order, and the anatomic origin of the cell being investigated.
Keywords: biomimetic material, cell morphology, cell proliferation, ECM, endothelial cell, nanotopography
Introduction
A universal characteristic of epithelial and vascular endothelial cells is that they anchor themselves to their underlying stroma through a unique specialization of the extracellular matrix, the basement membrane [1, 2]. Basement membranes have many features that greatly influence endothelial cell function, including specific proteins and functional groups, a reservoir of growth factors and other trophic agents, and a complex three-dimensional topography. Topographic cues that simulate the feature types and dimensions of native basement membranes have been documented to influence an array of fundamental cell behaviors in a variety of cell types, including endothelial cells [3-10].
We have previously reported quantitative analyses of the topographic features of basement membrane of several different tissues and species and have found that the basement membrane topography is largely conserved across species and tissues [11, 12 ]. Most recently, we have defined the basement membrane topographic features for four separate vessel sites in the Rhesus Macaque [13]. We found significant differences in the dimensions of basement membrane features examined depending on the site of origin and hypothesized that these variations in the biophysical microenvironment may contribute to known endothelial heterogeneity. Several recent studies suggest that alterations in the substratum (synthetic or biologically derived) influence essential endothelial cell behaviors [14-16]. Despite these observations, vascular prosthetics which include the design of engineered vascular tissue and vascular stents have only recently incorporated topography as an essential component of the final engineered product [7, 17, 18].
In order to develop an improved vascular prosthetic it is essential to determine the response of endothelial cells and their interaction with different substrate materials. Much interest in now focused on alterations of the biophysical attributes of cellular scaffolds in order to improve cell behavior and survival and success of implantable devices. Our strategy is to use the biophysical environment of vasculature characterized in previous studies, to guide the fabrication and design of improved biomimetic substrates containing both submicron and nanoscale anisotropic features to investigate the heterogeneous response of different endothelial cell types.
The goal of our study is to investigate the impact of submicron and nanoscale topographic cues on several fundamental endothelial cell behaviors including orientation and alignment to topographic features, proliferation, and migration. Due to the heterogeneity of endothelial cells from different vascular sites, we chose endothelial cells from four different anatomic locations to represent both veins and arteries from large and small vessels. These studies have relevance to our fundamental understanding of vascular endothelial cell matrix interactions in health and disease. Specifically, they will aid in the development of improved plasticware design for in vitro studies utilizing endothelial cells, contribute to the development of novel strategies in tissue engineering and will advance the development of cardiovascular prosthetics.
Materials and Methods
Cell Culture
We selected human endothelial cells that represent both large and small diameter arteries and veins. Specifically, we studied human umbilical vein endothelial cells (HUVEC), human dermal microvascular endothelial cells (HmVEC-d), human aortic endothelial cells (HAEC) (Lonza, Walkersville, MD) and human saphenous vein endothelial cells (HSaVEC-c) (PromoCell, Heidelberg, Germany). HUVEC, HAEC, and HSaVEC cells were cultured in Endothelial Cell Basal medium (EBM) with the EGM-2 bullet kit containing Hydrocortisone, hFGF-B, VEGF, R3-IGF, Ascorbic Acid, heparin, fetal bovine serum (FBS), hEGF, and GA-1000 (Lonza, Walkersville, MD). HmVEC-d cells were cultured in EBM medium with the EGM-2 MV Bulletkit (hEGF, hydrocortisone GA-1000, FBS, VEGF, hFGF-B, R3-IGF1 and Ascorbic acid). All cells were incubated at 37° C and 5% CO2. Endothelial cells for all experiments were utilized between passages 3-8.
Fabrication of Micro-and Nanoscale Surfaces
Patterned silicon surfaces utilized as masters were prepared at the Center for Nanotechnology (University of Wisconsin) as previously described[8, 19]. Each substrate contains an array of six 2×2 mm areas (400 nm, 800 nm, 1200 nm, 1600 nm, 2000 nm and 4000 nm pitch patterned with parallel grooves and ridges (pitch=groove + ridge width) or holes (pitch=lateral center to center distance) separated by flat control surfaces. Scanning electron microscope (SEM) measurements confirmed similar size distributions of the ridge and groove substrates and hole to ridge substrates of 1:1 and a groove depth of 300 nm for each pitch. These silicon masters were used as templates for replication of the patterns in NOA81 polyurethane (Norland Optical Adhesives, Cranbury, NJ) through soft lithography. Polydimethylsiloxane (PDMS) stamps were generated as previously described[9].
NOA81 polyurethane surfaces
A dime-sized amount of NOA81 (Norland Optical, Cranbury, NJ) was added to 35 mm tissue culture plates. Plates were placed in a spin coater for 40 seconds at 4000 rpm. PDMS topographically patterned stamps were placed firmly onto these coated surfaces and cured with 365 nm bulbs for 2 hours in a XL-1500 UV crosslinker. In preparation for tissue culture, all polyurethane surfaces were sterilized by UV light for 15 minutes.
Orientation and Alignment
Endothelial cells were plated at a density of 15,000 cells/cm2 in 35 mm plates containing 6-pack polyurethane substrates having topographic features ranging from 400-4000 nm pitch and intervening control planar regions. After 24 hours, cells were fixed for 20 minutes in 4% paraformaldehyde in 1x phosphate buffered saline (PBS, pH 7.2) followed by staining with TRITC-phalloidin (Sigma, St. Louis, MO) for detailed analysis of orientation and elongation. TRITC-phalloidin binds to actin filaments and provides an outline of the cells for analysis. Using Zeiss KS300 software (Zeiss, Germany), we determined the specific angle of orientation of cells in relationship to the anisotropic surfaces (parallel ridges and grooves). Cells were considered aligned with the ridges and grooves when this angle was between 0 and 10 degrees. Cell elongation is defined as the ratio between the length and breadth of each cell. Cells were considered elongated if this factor was > 1.3.
Proliferation
All endothelial cells were plated at a density of 30-40,000 cells per 35 mm plate containing the patterned polyurethane (NOA 81) 6-pack substrate. Twenty-four hours after plating, cells on each pattern 400-4000 nm pitch and planar control were stained for 30 minutes with a 1 μM concentration of SYTO-11 green-fluorescent nucleic acid stain (Invitrogen, Carlsbad, CA). Cells were imaged and counted at 10x magnification using an inverted Zeiss Axiovert 200M microscope (Carl Zeiss, Germany) equipped with a high resolution digital camera. Endothelial cells were cultured for 5 days at which time the cells were fixed for 20 minutes in 4% paraformaldehyde-PBS. Nuclei were stained with 90 nM concentration of DAPI (Invitrogen, Carlsbad, CA), and imaged using an Axiovert 200 inverted microscope. Cell counts were obtained from images using KS300 image analysis software (Carl Zeiss, Germany). Five substrates were used for each experiment and each experiment was repeated in triplicate.
Migration
Endothelial cells were seeded onto patterned polyurethane surfaces and allowed to adhere in an incubator for 2 hours at 37 degrees and 5% CO2. Plates were transferred to a Zeiss incubated microscope stage to maintain cell culture conditions. Using Axiovision software and an automated stage, areas containing each topographically patterned region (400 nm-4,000nm pitch) and planar control were identified and marked. Sequential images of each marked area were taken every 10 minutes over a 12 hour period at 10x magnification using a Zeiss Axiovert 200 microscope (Carl Zeiss, Germany). The time-lapse imaging allowed for direct observation of individual endothelial cells. For each image, individual cells were identified that remained within the field of view and did not come into contact with another cell or undergo cell division during the course of the experiment. The center of mass was identified and the X and Y coordinates were recorded using a cell tracker module in Axiovision 4.6 (Carl Zeiss, Germany). From the resulting data, cell trajectories, total distance, the rate of migration, and the ratio of distance in of cells in the direction of the topography were calculated. A minimum of 25-50 cells per topographic feature or planar control were analyzed for each endothelial cell type.
Statistics
Data were analyzed using one way analysis of variance (ANOVA). When variability was determined to be significant (p<0.05), the un-paired student’s t-test was utilized to determine significance (p<0.05) between groups. Within the figures significance is denoted by the following *=<0.05, **= p<0.01, *** p<0.001.
Results
We first investigated the orientation and alignment response of several different endothelial cell lines from differing anatomic locations which include a range of vascular sites. Cells were plated onto topographic features ranging from 400 to 4000 nm pitch (pitch=ridge + groove width). Twenty-four hours post-plating cells were fixed and the actin filaments were stained with TRITC-phalloidin which provides an outline of the cell for analysis of orientation and alignment relative to the underlying topography. Representative images of endothelial cells from both vein (HUVEC) and aorta (HAEC) are shown in Figure 1. Both HUVEC and HAEC cells are flat and round on the planar control (A, C). Panels B and D demonstrate the morphological response to the ridges and grooves of the underlying topography. To further investigate whether the orientation response is specific to the surface order of the topographic features, we analyzed the orientation and alignment response of HUVEC and HmVEC-d cells to substrates containing isotropically ordered substrates with holes. Both selected endothelial cell-types exhibited no observable alignment or orientation response to the substrate (data not shown).
Figure 1. Nanoscale topographic cues influence endothelial cell orientation and alignment.

HUVEC and HAEC cells representing both large diameter vein and arterial vessels were plated at a density of 10,000 cells/cm2 in 60 mm plates containing 6-pack polyurethane substrates with planar control surfaces and topography ranging from 400-4000 nm. After 24 hours, cells were fixed and stained with TRITC-phalloidin to label for filamentous actin (red) and DAPI for the nucleus (blue) to observe cell morphology. Representative images were taken with a Zeiss Axiovert 200 on planar (HUVEC panel A, HAEC panel C) and 4000 nm pitch (HUVEC panel B, HAEC panel D) at 40x magnification. Both HUVEC and HAEC cells exhibit a flattened, round morphology on the planar control. In contrast both endothelial cell-types demonstrate orientation with the underlying topography of ridges and grooves.
To quantitate our morphological observations, images from all four endothelial cell lines were analyzed to generate percentage of cells that exhibit both orientation and elongation relative to the ridge and groove topographic patterns. Quantitative results of our analysis of all four vascular endothelial cell-types are shown in figure 2. HUVEC cells (A) exhibit 40% or higher orientation with the greatest alignment response occurring on features between 800 and 1200 nm pitch (p<0.001). In panel B, HmVEC-d cells also show about 40% of the population on each topographic pattern respond and orient with the patterns, with no significant difference observed in alignment of cells on the different feature scales. In contrast, both the HAEC and HSaVEC cells demonstrate a differential response depending on the scale of the topographic features. HAEC cells exhibit a lower percentage of cells aligned on the smallest feature size of 400 nm pitch but still significantly different from the planar control (p<0.05) (Figure 2, C). A statistically significant decreased number of cells are aligned on the 400 nm pitch compared to larger feature sizes. HSaVEC cells also demonstrate decreased alignment on the 400 nm pitch compared to larger scale topographies (D). A statistically significant number of cells were aligned on all topographically patterned feature sizes compared to planar controls.
Figure 2. Endothelial cells demonstrate a heterogeneous orientation and alignment response to nano, submicron and micron scale topographic cues.
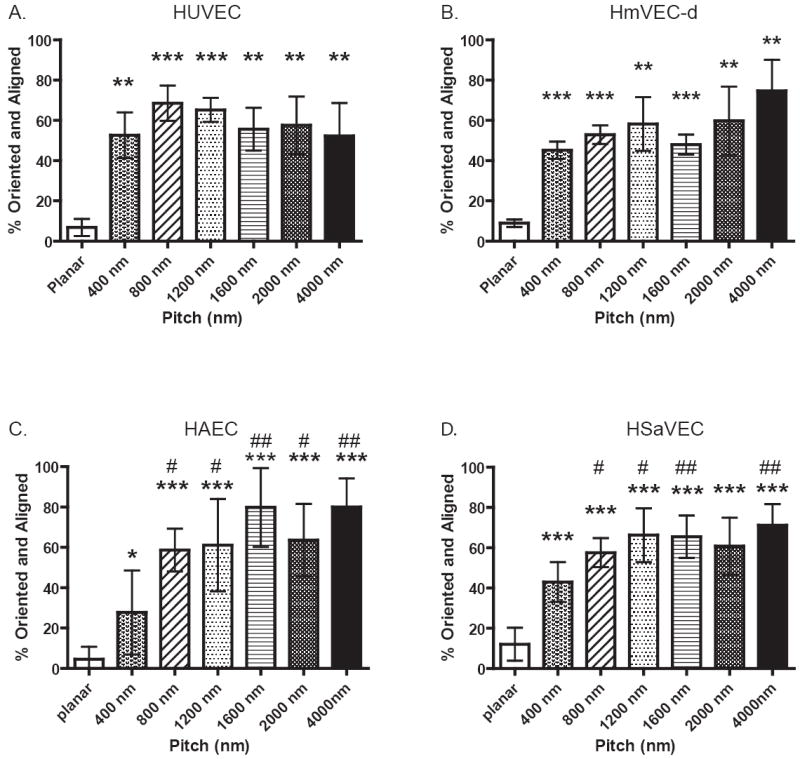
Cells plated on topography for 24 hours were fixed, stained with TRITC-phalloidin, and imaged with a Zeiss Axiovert 200. Images were taken of cells on each topographic surface for detailed analysis and calculation of the percentage of cells oriented (<10 degrees) and elongation (>1.3).). HUVEC (A), HmVEC-d (B), HAEC (C) and HSaVEC (D) all demonstrate significant differences in the response to topographic cues when compared to planar controls. Significant differences between planar controls and topography are marked as follows: * = p<0.05, ** = p<0.01, *** = p<0.001. For the HAEC and HSaVEC cells significant differences were also observed when comparing 400 nm pitch to the 800 nm through 4000 nm pitch and are marked # = p<0.05, ## = p<0.01. (Data shown as Mean ± SEM)
Another behavioral endpoint that was measured on each cell line was the impact of topographic cues on the rate of proliferation. Cells were imaged and counted after 24 hours and 5 day time points to calculate a percent increase in cell number. A statistically significant (p< 0.05)two-fold decrease in proliferation for HUVEC cells was found on the smallest ridge and groove topographies (400 nm and 800 nm pitch) compared to planar controls after 5 days in culture (Figure 3 A). No significance was observed between planar control and topographies above 1200 nm. Significance was also observed between 400 and 800 nm when compared to the 4000 nm pitch. Of all the endothelial cell-types investigated, only the HUVEC cells demonstrated a measurable statistically significant decrease in proliferation on nanoscale topographic patterns at the day 5 time point. Longer time points of up to 14 days with the HmVEC-d, HAEC, and HSaVEC cells also demonstrated no significant changes in proliferation between planar control and the topographic cues (data not shown).
Figure 3. HUVEC’s demonstrate decreased proliferation on nanoscale topography.
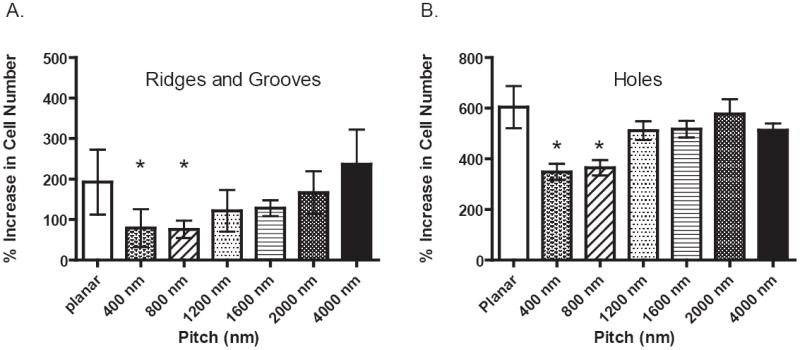
Cells on nanoscale features (400 nm to 4000 nm pitch) including anisotropic ridges and grooves, holes and control planar region were counted after 1 and 5 days in culture. Significant decreases in proliferation were only observed in HUVEC cells (A) on the 400 nm and 800 nm pitch (p<0.05) with a 2 fold reduction in cell number when compared to both the 4000 nm pitch and planar control on the ridge and groove topographic features. HUVEC cells also demonstrate a significant decrease (p<0.05) in proliferation on a different geometry (holes) but within the same nanoscale range. The scale of topography (400-4000 nm) did not impact proliferation of HmVEC-d, HAEC or HSaVEC cells. These data indicate a heterogeneous response of endothelial cells to topographic cues. (Data shown as Mean ± SEM)
To confirm that the scale of the features and not the geometric shape or surface order is responsible for the significant decrease in HUVEC proliferation, experiments were also conducted with the substrates containing anisotropically ordered holes. A significant two-fold decrease in proliferation on both the 400 nm and 800 nm pitch for HUVEC cells (p< 0.05) was confirmed (Figure 3 B). Our proliferation data demonstrates that feature size-scale is a key biophysical cue in the regulation of HUVEC proliferation regardless of surface order.
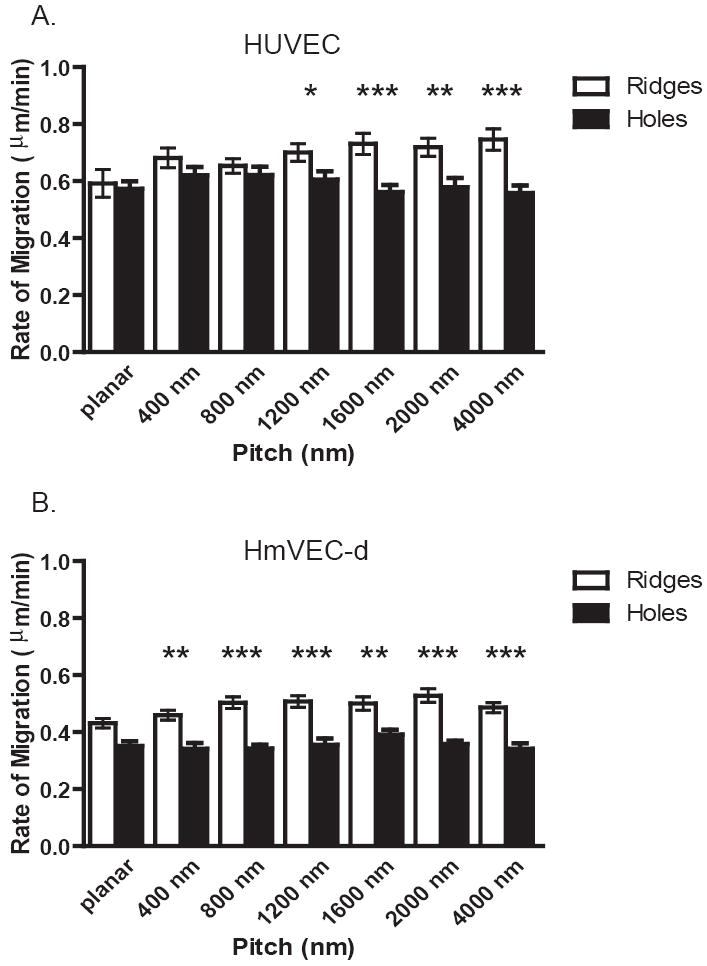
We also initiated experiments to characterize the effects of nano and micron scale topography on migration of vascular endothelial cells. Images of cells were taken every 10 minutes over a 12 hour period. Total distance traveled was determined to calculate the rate of migration on planar control and on topographically patterned surfaces. Figure 4, illustrates the rate of migration determined for each endothelial cell type and the influence of topographic cues of differing size scales. HUVEC cells (panel A) demonstrate no significant difference in rates between planar, 400 nm and 800 nm pitch (0.6 μm/min). Differences are observed on the larger pitch sizes starting at 1200 nm (p<0.05) up through 4000 nm (p<0.01). HmVEC-d cells (panel B) exhibit a similar response, however, significant differences in rate of migration include 800 nm pitch (p<0.01) through 4000 nm pitch (p<0.01) surfaces. The HAEC cells (panel C) demonstrate no discernable difference in migration rate between planar control and any of the topographically patterned surfaces. HSaVEC cells (panel D) show significant response for the 800 nm (p<0.01) through 4000 nm pitch (p<0.05) when compared to planar controls.
Figure 4. Endothelial rate of migration is differentially influenced by nano to micron scale topographic cuing.

Cumulative distance of at least 50 cells over a 12 hour period was determined using particle tracking within Zeiss Axiovision software for each cell line including HUVEC (A), HmVEC-d (B), HAEC (C) and HSaVEC (D). HAEC cells were the only cell line analyzed that did not demonstrate significant differences between planar control and topographically patterned substrates. Significant differences are marked as follows: * =<0.05, ** = p<0.01, *** = p<0.001. Note that no differences were seen on 400 nm pitch surfaces for all cell-types examined (Data shown as Mean ± SEM).
To further investigate the impact of feature size on migration, HUVEC migration rates were analyzed on surfaces with isotropically ordered holes. Both HUVEC and HmVEC-d cells demonstrated no significant difference in the rate of migration regardless of size scale compared to the planar control. However, the rate of HUVEC and HmVEC-d migration on these isotropically patterned surfaces was found to be significantly reduced relative to migration on the anisotropically patterned surfaces of parallel ridges and grooves (Figure 5). HUVEC migration exhibited a significant decrease on 1200 nm (p<0.05) through 4000 nm (p<0.001) pitch of up to twenty-five percent (panel A). HmVEC-d migration rate was found to be significantly reduced by up to thirty percent throughout each size scale analyzed from 400nm (p<0.01) to 4000 nm (p<0.001) pitch (panel B).
Figure 5. HUVEC and HmVEC-d rate of migration is influenced by the geometry of nanoscale topographic cues.
Cumulative distance on isotropic holes from 25-50 cells over a 12 hour period was determined using particle tracking within Zeiss Axiovision software for the HUVEC and HmVEC-d cell lines. For HUVEC cells (A) Significantly decreased migration rates were observed on 1200 nm through the 4000 nm pitch holes when compared to HUVEC’s on ridge and groove topography. HmVEC-d cells (B) had significant reduction in migration rates on holes compared to ridges and grooves on 400 nm through 4000 nm pitch. Significant differences are marked as follows * p=<0.05, **p=0.01, ***p=<0.001 (Data shown as Mean ± SEM).
The ability of each of the topographic cues to guide the direction of migration was also analyzed. Representative trajectories of HUVEC cells on planar control (A), 400 nm (B), 1200 nm (C) and 4000 nm (D) ridge and groove substrates are shown in Figure 6. Cells on the planar substrate show random directional migration. HUVEC cells on the 400 nm pitch exhibit some contact guided migration with the ridges and grooves. Cells on the 1200 nm and 4000 nm pitch have trajectories almost exclusively in the direction of the topographic cues. For quantitative analysis, cell trajectories were re-analyzed and the total direction in Y (parallel to the topographic cues) vs. the direction in X (perpendicular to the ridges and grooves) was calculated (Figure 7). HUVEC cells demonstrated the highest level of contact guided migration on each topography with a 2.5-3 fold increase in the ratio above 1200 nm (p<0.001) (Figure 7 A). HmVEC-d cells followed the same trend with the highest level of contact guidance with 2.5 fold increase at the largest pitch sizes of 2000 nm (p<0.001) and 4000 nm (p<0.01) (Figure 7 B). However, no significant difference was observed on the 400 nm pitch when compared to planar control. HAEC cells also showed a small level of contact guided migration for each topography analyzed when compared to planar control surfaces (Figure 7 C). HSaVEC cells also demonstrate an increase in contact guided migration as pitch size increased up to a 3-fold difference (Figure 7 D). All four cell-types examined exhibited contact guidance of the cells trajectory during 12 hours of migration. Upon examination of endothelial cells on our isotropically ordered porous topographic features, a directional vector in terms of migration was not identified (data not shown). In summary, despite some heterogeneity between endothelial cell lines, all cells exhibited a contact guidance response to anisotropically ordered topographic cues.
Figure 6. Trajectories of HUVEC cells on topographic cues suggest contact guided migration.
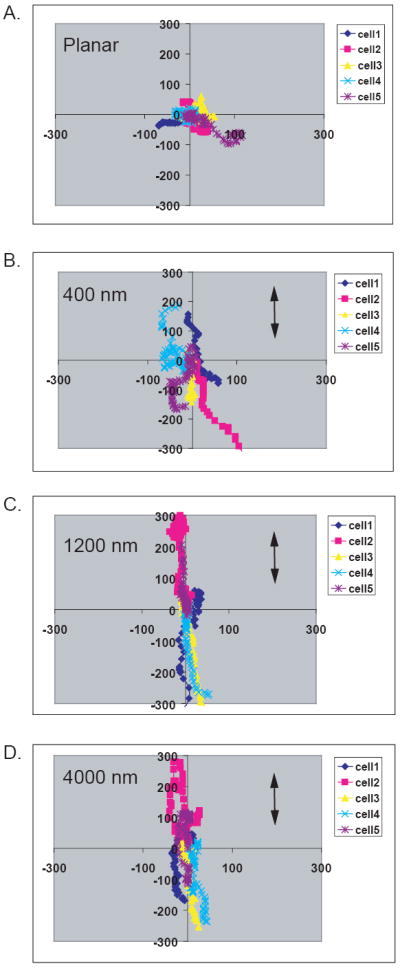
Representative diagrams of five individual cell trajectories of HUVEC cells on planar control (A), 400 nm (B), 1200 nm (C) and 4000 nm (D) pitch. For each plot, 5 individual cells are represented by a different color and the trajectory of the center of mass of each cell is graphed over time. As the feature sizes increased, cell movement was almost exclusively along the y axis (parallel to the topography), with limited movement along the X axis (perpendicular to topography). In contrast, cell movement on flat surfaces was random with respect to the axes.
Figure 7. Ratio of contact guided migration differs between endothelial cell-types.

Cumulative distance in both the X (perpendicular to topography) and Y axis (parallel to topography) was calculated using particle tracking Zeiss Axiovision software. From the data, a ratio of contact guided migration was calculated for each cell line including HUVEC (A), HmVEC-d (B), HAEC (C), and HSaVEC (D). Significant differences are marked as follows: * =<0.05, ** = p<0.01, *** = p<0.001. (Data shown as Mean ± SEM)
Discussion
In aggregate, the data clearly demonstrate the differential response of vascular endothelial cells to topographic cues having biologically relevant size scales. It has long been reported that endothelial heterogeneity including structure and function exists between tissues, within an organ, and within the same vasculature [20-23]. Differential changes in cell shape, regulation of fluid flow [24-26], response to chemical cues and activation of intracellular signaling pathways have previously been reported as having an impact in the endothelial differences observed. We propose that the biophysical environment consisting of topographic cues may also contribute to endothelial heterogeneity. Our findings support these earlier studies documenting heterogeneity in attributes of vascular endothelial cells obtained from differing anatomic sites.
Endothelial behaviors including orientation and alignment response, proliferation and migration are all important for the remodeling of matrix, formation of new vessels during both development and wound healing as well as for the interaction of native endothelial cells with implanted prosthetic scaffolds. These essential processes may be initiated and driven by changes in endothelial cell shape [27, 28]. For example, during capillary morphogenesis endothelial precursor cells exhibit a spindle shaped morphology and orient themselves into cord-like structures in preparation of the formation of capillaries. Basement membrane is formed separating the lumen from the rest of the matrix [29-31]. These changes are believed to be integrin mediated RhoGTPase, Cdc42 and Rac 1 activation through collagen and other ECM molecules such as fibronectin at the surface of the endothelial cell, which promotes a signaling cascade of events resulting in changes in cytoskeletal rearrangement, migration and proliferation of cells which leads to the sprouting and assembly of new vessels [13, 32-34].
The specific biophysical cues by which changes in cell shape contribute to downstream molecular signaling events are not understood. It is well known that fluid shear forces influence endothelial cell shape and alignment in vitro and in vivo. Specifically, cells are oriented and elongated in the direction of fluid flow [35]. In our studies, we investigated a single biophysical cue from the topographic features of the underlying substrate to investigate the impact on cell shape. We have found during the course of our studies with vascular endothelial cells, that cells from each anatomic site have a unique orientation and alignment response to the ridge and groove topographic cues. This may help explain some of the reported disagreement in the literature as to the optimal size features for cell alignment. There is no one size fits all approach and each cell-type will have a unique response to topographic cues. For example, previous reports from our laboratory have demonstrated the differential response of cells from the same tissue including corneal epithelial cells and stromal fibroblasts to both feature size and depth [8, 36]. Recent reports from Biela et. al. also demonstrate the heterogeneity of vascular endothelial cells, smooth muscle cells and fibroblasts to topographic cues [6]. Feature size-scale, shape, depth, surface order as well as other biophysical and chemical attributes of a selected substrate material will all influence how the cell will respond and should be considered important elements to incorporate into the future design of vascular prosthetics.
Changes in cell shape from orientation and alignment of cells have been implicated in alterations in cell cycle which would directly influence the proliferative state of the cell. Micropatterning of adhesive islands has previously been shown to impact cell shape and influence the endothelial cell cycle [37]. Results from Roca-Cusachs et al. demonstrated that cell spreading and the resulting change in nuclear shape enhanced proliferation of endothelial cells. Enhanced proliferation has been suggested as a method for increasing efficacy of vascular stents or other implantable devices. The faster the implanted material is covered with an endothelial cell layer, the less likely issues with restenosis and thrombosis will occur [18]. Current methodologies have focused on prevention through coating of the implant with biologically active molecules such as peptides, antibodies and growth factors. Our studies suggest that incorporation of topographic features also needs to be considered in the design of improved prosthetics.
In our studies only HUVEC cells demonstrated a change in proliferation on the smallest size features, 400 nm and 800 nm respectively. Unlike studies that report increased cell proliferation on nanometer scale topography [9, 38, 39], we did not observe significant increases in cell proliferation compared to planar control. However, this observation is similar to what we have reported for primary corneal epithelial cells and fibroblasts. Corneal epithelial cells and fibroblasts demonstrate the same decrease in proliferation on the smallest features; noting that the suppression in fibroblast proliferation was not observed until 14 days of culture [9]. Suppression in proliferative response of HUVEC cells was also observed on the smallest features using isotropically ordered substrates suggesting that this effect is independent of surface order.
The migration of endothelial cells during development or wound repair requires both initial adhesion and subsequent release from the cell substrate [40, 41]. Regulation of this complex biological process is believed to be influenced by interactions between specific cell surface receptors and extracellular matrix molecules. In large vessels, endothelial cell migration is necessary for vascular repair to prevent thrombosis and in small vessels to reduce ischemia [42]. Previous studies have suggested that the biophysical properties of the substrate including topography [6] and compliance have an impact on cell migration.
We found that all of the endothelial cell-types had preferential migration along the ridges and grooves with the most significant response at the higher topographic features examined. We also demonstrate differences in rates of migration between the various topographies, with the exception of HAEC cells. Topographic cues from the extracellular matrix likely participate in modulating neovascularization and wound repair processes. HAEC cells would not be expected to be involved in sprouting and repair as often as endothelial cells from microvasculature which may explain our observations. Our studies have focused on the impact of topographic cues in isolation whereas cells migrating in vivo must simultaneously integrate other biophysical and chemical cues that would influence rate and direction including haptotatic migration and shear stress from blood flow [42, 43].
In summary, vascular implants must be engineered to improve initial adhesion, orientation and alignment, migration of cells, and proliferation [44]. It has been demonstrated that coverage of vascular implants with endothelial cells prior to implantation increases the graft patency and ultimate success of the implant [7, 45]. However, several issues are commonly encountered with synthetic polymer and metal substrates whereby the cells cannot completely populate the substrate causing an immunologic response or are sheared off during normal blood flow. Past solutions have included surface modifications of the substrate with ECM coating or plasma treatment with oxygen or argon [7]. Although each approach has advantages, we believe that it is essential to integrate topographic features to achieve optimal performance.
Conclusions
Overall, our data document a heterogeneous response of endothelial cells to topography suggesting that cells from large and small vessels respond differentially to topographic cues. We investigated several essential endothelial cell behaviors including orientation and alignment to the underlying substrate, proliferation and migration. The greatest response in orientation and alignment in all cell-types was observed on topographic ridges greater than 800 nm. Interestingly, only HUVEC cells demonstrate a decrease in proliferation in response to the smallest topographic features regardless of surface order. On anisotropically ordered surfaces all cell-types migrated preferentially parallel to the long axis of the ridges, with the greatest increase in cell migration observed on the 1200 nm pitch. In addition, we demonstrate that surface order has a similar effect on some cell behaviors (inhibition of proliferation) but can elicit a differential response on others (cell migration). These studies have relevance to our fundamental understanding of vascular endothelial cell matrix interactions in health and disease and indicate the importance of incorporating topographic cues into substrates used for in vitro studies and in the development of improved cardiovascular prosthetics.
Acknowledgments
This project was supported by the National Institute of Health through grants from the National Heart Lung and Blood Institute (1RO1HL079012-01A) and the National Eye Institute (1RO1EY016134-01A2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood) 2007;232:1121–9. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 2.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 3.Barbucci R, Lamponi S, Magnani A, Pasqui D. Micropatterned surfaces for the control of endothelial cell behaviour. Biomol Eng. 2002;19:161–70. doi: 10.1016/s1389-0344(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 4.Chung TW, Liu DZ, Wang SY, Wang SS. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials. 2003;24:4655–61. doi: 10.1016/s0142-9612(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 5.Uttayarat P, Chen M, Li M, Allen FD, Composto RJ, Lelkes PI. Microtopography and flow modulate the direction of endothelial cell migration. Am J Physiol Heart Circ Physiol. 2008;294:H1027–35. doi: 10.1152/ajpheart.00816.2007. [DOI] [PubMed] [Google Scholar]
- 6.Biela SA, Su Y, Spatz JP, Kemkemer R. Different sensitivity of human endothelial cells, smooth muscle cells and fibroblasts to topography in the nano-micro range. Acta Biomater. 2009 doi: 10.1016/j.actbio.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Tajima S, Chu JS, Li S, Komvopoulos K. Differential regulation of endothelial cell adhesion, spreading, and cytoskeleton on low-density polyethylene by nanotopography and surface chemistry modification induced by argon plasma treatment. J Biomed Mater Res A. 2008;84:828–36. doi: 10.1002/jbm.a.31539. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro-and nanostructured substrates. J Cell Sci. 2003;116:1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J Biomed Mater Res A. 2006;79:185–92. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl KA, Foley JD, Nealey PF, Murphy CJ. Nanoscale topography modulates corneal epithelial cell migration. J Biomed Mater Res A. 2005;75:603–11. doi: 10.1002/jbm.a.30467. [DOI] [PubMed] [Google Scholar]
- 11.Abrams G, Teixeria A, Nealey P, Murphy C. The effects of Substratum Topography on Cell Behavior. In: Dillow A, Lowman A, editors. Biomimetic Materials and Design: Interactive Biointerfacial Strategies, Tissue Engineering, and Drug Delivery. New York: Marcel Dekker; 2002. pp. 91–136. [Google Scholar]
- 12.Brody S, Anilkumar T, Liliensiek S, Last JA, Murphy CJ, Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12:413–21. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liliensiek SJ, Nealey P, Murphy CJ. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A. 2009;15:2643–51. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol. 2005;4:9. doi: 10.1186/1475-2840-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, et al. Biomechanical properties of native basement membranes. Febs J. 2007;274:2897–908. doi: 10.1111/j.1742-4658.2007.05823.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsai SH, Liu YW, Tang WC, Zhou ZW, Hwang CY, Hwang GY, et al. Characterization of porcine arterial endothelial cells cultured on amniotic membrane, a potential matrix for vascular tissue engineering. Biochem Biophys Res Commun. 2007;357:984–90. doi: 10.1016/j.bbrc.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008;4:192–201. doi: 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–64. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–43. [PubMed] [Google Scholar]
- 21.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 22.Auerbach R. Vascular endothelial cell differentiation: organ-specificity and selective affinities as the basis for developing anti-cancer strategies. Int J Radiat Biol. 1991;60:1–10. doi: 10.1080/09553009114551401. [DOI] [PubMed] [Google Scholar]
- 23.Gumkowski F, Kaminska G, Kaminski M, Morrissey LW, Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24:11–23. [PubMed] [Google Scholar]
- 24.Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci U S A. 1999;96:13203–7. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grisham JW, Nopanitaya W, Compagno J, Nagel AE. Scanning electron microscopy of normal rat liver: the surface structure of its cells and tissue components. Am J Anat. 1975;144:295–321. doi: 10.1002/aja.1001440304. [DOI] [PubMed] [Google Scholar]
- 26.Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 2001;36:258–64. doi: 10.1016/s0165-0173(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 27.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DIC, Whitesides GM, et al. Engineering cell shape and function. Science. 1994;264:696–98. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 28.Ingber DE. Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res. 2002;91:877–87. doi: 10.1161/01.res.0000039537.73816.e5. [DOI] [PubMed] [Google Scholar]
- 29.Drake CJ, Little CD. VEGF and vascular fusion: implications for normal and pathological vessels. J Histochem Cytochem. 1999;47:1351–6. doi: 10.1177/002215549904701101. [DOI] [PubMed] [Google Scholar]
- 30.Vernon RB, Sage EH. Between molecules and morphology. Extracellular matrix and creation of vascular form Am J Pathol. 1995;147:873–83. [PMC free article] [PubMed] [Google Scholar]
- 31.Aloisi M, Schiaffino S. Growth of elementary blood vessels in diffusion chambers. II. Electron microscopy of capillary morphogenesis. Virchows Arch B Cell Pathol. 1971;8:328–41. doi: 10.1007/BF02893542. [DOI] [PubMed] [Google Scholar]
- 32.Whelan MC, Senger DR. Collagen I initiates endothelial cell morphogenesis by inducing actin polymerization through suppression of cyclic AMP and protein kinase A. J Biol Chem. 2003;278:327–34. doi: 10.1074/jbc.M207554200. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Senger DR. Matrix-specific activation of Src and Rho initiates capillary morphogenesis of endothelial cells. FASEB J. 2004;18:457–68. doi: 10.1096/fj.03-0948com. [DOI] [PubMed] [Google Scholar]
- 34.Davis GE, Bayless KJ, Mavila A. Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anat Rec. 2002;268:252–75. doi: 10.1002/ar.10159. [DOI] [PubMed] [Google Scholar]
- 35.Nerem RM. Shear force and its effect on cell structure and function. ASGSB Bull. 1991;4:87–94. [PubMed] [Google Scholar]
- 36.Fraser SA, Ting YH, Mallon KS, Wendt AE, Murphy CJ, Nealey PF. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum-rich and serum-free media. J Biomed Mater Res. 2008;86:725–35. doi: 10.1002/jbm.a.31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farre R, Navajas D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys J. 2008;94:4984–95. doi: 10.1529/biophysj.107.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Bauer S, Schlegel KA, Neukam FW, von der Mark K, Schmuki P. TiO2 nanotube surfaces: 15 nm--an optimal length scale of surface topography for cell adhesion and differentiation. Small. 2009;5:666–71. doi: 10.1002/smll.200801476. [DOI] [PubMed] [Google Scholar]
- 39.Rebollar E, Frischauf I, Olbrich M, Peterbauer T, Hering S, Preiner J, et al. Proliferation of aligned mammalian cells on laser-nanostructured polystyrene. Biomaterials. 2008;29:1796–806. doi: 10.1016/j.biomaterials.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 40.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 41.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–57. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 42.Hsu S, Thakar R, Liepmann D, Li S. Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces. Biochem Biophys Res Commun. 2005;337:401–9. doi: 10.1016/j.bbrc.2005.08.272. [DOI] [PubMed] [Google Scholar]
- 43.Davis GE, Senger DR. Extracellular matrix mediates a molecular balance between vascular morphogenesis and regression. Curr Opin Hematol. 2008;15:197–203. doi: 10.1097/MOH.0b013e3282fcc321. [DOI] [PubMed] [Google Scholar]
- 44.Vartanian KB, Kirkpatrick SJ, Hanson SR, Hinds MT. Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun. 2008;371:787–92. doi: 10.1016/j.bbrc.2008.04.167. [DOI] [PubMed] [Google Scholar]
- 45.Meinhart J, Deutsch M, Zilla P. Eight years of clinical endothelial cell transplantation. Closing the gap between prosthetic grafts and vein grafts ASAIO J. 1997;45:M515–M21. [PubMed] [Google Scholar]


