Abstract
Oligodendrocyte progenitor cells first proliferate to generate sufficient cell numbers and then differentiate into myelin-producing oligodendrocytes. The signal transduction mediators that underlie these events, however, remain poorly understood. The tyrosine phosphatase Shp1 has been linked to oligodendrocyte differentiation as Shp1-deficient mice show hypomyelination. The Shp1 homologue, Shp2, has recently been shown to regulate astrogliogenesis but its role in oligodendrocyte development remains unknown. Here we report that Shp2 protein levels were developmentally regulated in oligodendrocytes, with Shp2 phosphorylation being promoted by oligodendroglial mitogens but suppressed by laminin, an extracellular matrix protein that promotes oligodendroglial differentiation. In contrast, oligodendrocyte progenitors were found to be unresponsive to mitogens following Shp2, but not Shp1, depletion. In agreement with previous studies, Shp1 depletion led to decreased levels of myelin basic protein in differentiating oligodendrocytes, as well as reduced outgrowth of myelin membrane sheets. Shp2 depletion in contrast did not prevent oligodendrocyte differentiation but promoted expanded myelin membrane outgrowth. Taken together these data suggest that Shp1 and Shp2 have distinct functions in oligodendrocyte development: Shp2 regulates oligodendrocyte progenitor proliferation and Shp1 regulates oligodendrocyte differentiation. Adhesion to laminin may additionally provide extrinsic regulation of Shp2 activity and thus promote the transition from progenitor to differentiating oligodendrocyte.
Keywords: Shp1, Shp2, oligodendrocyte, myelin, tyrosine phosphatase
Introduction
Appropriate myelination is critical for central nervous system (CNS) function and homeostasis. Specialized glial cells termed oligodendrocytes carry out CNS myelin ensheathment, a complex process orchestrated by a series of glial intrinsic and extrinsic mechanisms (Colognato & ffrench-Constant 2004). For instance, oligodendrocytes arise from oligodendrocyte progenitor cells (OPCs) that are characterized in part by their ability to proliferate and thus generate appropriate numbers of oligodendrocytes; this process depends on receptor tyrosine kinase activation by extrinsic growth factors. To differentiate, OPCs undergo a series of gross phenotypic changes that include withdrawal from the cell cycle, increased gene expression to produce myelin components, and a large expansion in cell size with concomitant membrane synthesis, all regulated by a variety of cell surface receptors. While many individual factors have been elucidated that control the steps of OPC development, the downstream signal transduction pathways that coordinate these events remain poorly understood.
The non-receptor protein tyrosine phosphatase Shp1 is one such downstream signaling mediator that has been implicated in oligodendrocyte function, as mice that have a complete loss-of-function mutation in the gene that encodes Shp1 (called motheaten) show developmental hypomyelination and decreased oligodendrocyte differentiation (Massa et al. 2000, Wishcamper et al. 2001, Massa et al. 2004). It remains unknown, however, what role Shp1 plays in OPC function. Shp1 consists of two SH2 domains that impart its ability to dock various signaling effectors, and a C-terminal phosphatase domain that provides its enzymatic function (Poole & Jones 2005). Intriguingly, Shp2, a tyrosine phosphatase that has high sequence and domain homology to Shp1, has recently been implicated as a key regulatory protein for both CNS neurogenesis and gliogenesis (Gauthier et al. 2007, Ke et al. 2007). In particular, mice engineered to lack Shp2 in developing embryonic brains were found to have fewer OPCs (Ke et al. 2007). Shp2 has furthermore been implicated in human brain development, as approximately 50% of cases of Noonan's Syndrome, a collection of congenital abnormalities that include growth defects, developmental delays, mild mental retardation, and cognitive deficits, are the result of activating mutations in the gene that encodes Shp2 (Neel et al. 2003). Recent work has demonstrated that one such Shp2 mutation, when expressed in CNS neural stem cells, can lead to inappropriate neurogenesis at the expense of astrogliogenesis (Gauthier et al. 2007). Shp2 has also been found to be essential for neural stem cell proliferation, likely due to a Shp2 requirement in order to transmit growth factor signaling downstream of receptor tyrosine kinases (Ke et al. 2007).
In the current study, we investigated whether Shp2 and/or Shp1 possessed a regulatory role in OPC development, in particular focusing on whether these phosphatases altered the ability of OPCs to respond to extrinsic developmental cues such as growth factors and extracellular matrix (ECM) proteins. We report that Shp1 and Shp2 have distinct roles in OPC development such that Shp1 is required for normal OPC differentiation but Shp2 is required for normal OPC proliferation. We furthermore found that a variety of OPC mitogens triggered Shp2 phosphorylation while the pro-differentiation ECM protein laminin suppressed Shp2 phosphorylation. Together, these findings confirm and expand roles for Shp1 in the oligodendrocyte lineage and identify Shp2 as a novel signaling effector in OPC development, while further implicating dysregulated gliogenesis as a potential contributor to cognitive impairments observed in Noonan's syndrome.
Materials and Methods
Cell culture
Disassociated rat neonatal cortices were cultured (37°C, 7.5% CO2) in high glucose DMEM with 10% fetal calf serum (FCS) on PDL-coated flasks. Medium was changed every 3–4 days. By day 10–14, mixed glial cultures consisting of oligodendrocyte precursor cells and microglia on an astrocyte monolayer were obtained. Purified oligodendrocyte precursor cells (OPCs) were isolated from mixed glial cultures using a modification of the mechanical dissociation and differential adhesion method described by McCarthy and de Vellis (McCarthy & de Vellis 1980, Colognato et al. 2004). For immunocytochemistry, purified OPCs were added to PDL or laminin-coated 8-well chamber slides in Sato's medium with 0.5% FCS and allowed to differentiate for indicated time periods. To evaluate protein levels or protein phosphorylation, cells were grown on Nunclon tissue culture dishes coated with PDL or laminin. Human placental laminin (a mixture of laminins enriched for laminin-211, Chemicon) was used to coat surfaces of slides and dishes at 10 mg/ml in PBS for 4 hours at 37°C. Surface coating with PDL (Sigma) was performed similarly but instead diluted in dH20 to obtain 10 mg/ml. Following coating, surfaces were blocked with 10μg/ml heat-inactivated BSA (endotoxin-free, Sigma) for 30 minutes at 37°C and washed with PBS.
Protein analysis
Cells were lysed on ice in 1% Trition-X-100, 0.1% SDS, 10 mM Tris pH 7.4, 5 mM EDTA, and 150mM NaCl that contained a cocktail of protease and phosphatase inhibitors (Calbiochem). Cell lysates were scraped and transferred to microfuge tubes and incubated on ice for 15 minutes, then centrifuged at 13,200 rpm to remove insoluble material. For experiments examining phosphorylation events, cells were lysed for 10 minutes at 95°C in pre-heated buffer of 1% SDS, 20 mM Tris pH 7.4 with protease and phosphatase inhibitors. Protein concentration was determined (detergent compatible protein assay, BioRad) and lysates were boiled for 5 minutes in LSB, 3% βME. Proteins were separated by SDS-PAGE using 10% or 12% acrylamide minigels (0.75 mm thickness) and blotted onto 0.45 μm nitrocellulose. Membranes were blocked in Tris buffered saline with 0.1 % Tween20 (TBS-T) that contained 4% BSA (blocking buffer) for 1 h, followed by primary antibodies in blocking buffer overnight at 4°C. Membranes were washed in TBS-T, incubated for 1 h in HRP-conjugated secondary antibodies (Amersham) diluted 1:3000 in blocking buffer, washed in TBS-T, and developed using enhanced chemiluminescence (Amersham). Experiments were performed a minimum of 3 times. Relative protein levels were normalized to actin as a loading control. Relative levels of phospho-proteins were normalized to the corresponding protein.
BrdU incorporation
Indirect immunocytochemistry was used to visualize bromo-D-uridine (BrdU) incorporation in cycling OPCs undergoing S-phase. At 24 hours post-transfection OPCs were incubated for 8 hours with Sato's medium containing 10μM BrdU and one of the following OPC mitogens: 10 ng/ml FGF, 10 ng/ml PDGF, or 100 ng/ml neuregulin-1. Cells were washed to remove unincorporated BrdU, then fixed with ethanol:acetic acid (95:5) for 30 min at −20°C; BrdU incorporation was subsequently detected using manufacturer's instructions (Roche). Each condition was performed in duplicate and cells were scored as either BrdU positive or negative from a minimum of 4 different fields in each well. A minimum of 500 cells were evaluated within each well. Results were expressed as percent BrdU-positive cells and normalized to control siRNA-transfected cells without added mitogen.
Microscopy and image acquisition
Slides were visualized using either a Zeiss Axioplan upright fluorescence microscope fitted with 10 times eyepiece magnification using 20 times (0.5 N.A.) and 40 times (0.75 N.A.) objectives, or, using a Zeiss Axioplan inverted fluorescence microscope fitted with 10 times eyepiece magnification using 10 times (0.3 N.A.), 20 times (0.5 N.A.), and 40 times (0.75 N.A.) objectives. Images were acquired using a Zeiss Axiocam MRM digital camera and Zeiss Axiovision imaging software (inverted microscope).
Immunocytochemistry
Cells were fixed either in 4% PFA (15 minutes) or 100% MeOH (5 minutes at 20°C). Block and primary antibody incubations were in PBS, 10% donkey serum (with 0.05% Triton in block following PFA fixations). Nuclei were stained with DAPI and cells were mounted in SlowFade (Molecular Probes).
Morphometric analysis
Differentiated oligodendrocytes were evaluated for MBP immunoreactivity and scored as MBP(+) or MBP(−) according to pixel intensity. To ensure consistent analyses across different experiments, MBP(+) cells and MBP(−) cells were determined using intensity thresh-holding relative to background intensity (Axiovision, Zeiss). Once MBP(+) cells had been chosen by intensity threshholding, morphometric analysis to determine the area coverage of MBP(+), sheet-bearing cells was performed on the subset of MBP(+) cells that exhibited myelin membrane sheet formation. Thus, cells that were MBP(+) by intensity threshold, but showed immunoreactivity solely in the cell body or in processes, were excluded from the morphometric analysis as non-sheet-bearing cells. Cells that showed any degree of MBP-immunoreactive sheets were considered as sheet-positive; these cells were analyzed by tracing the outer perimeter to determine total area cell coverage (Axiovision Interactive Measurement Module, Zeiss). A minimum of 4 fields per condition were evaluated by morphometric analysis. Statistics were performed using 2-tailed Student's t-tests; significance was set at p<0.05 (*, significant), p<0.01 (**, very significant) and p<0.001 (***, highly significant).
Reagents
Antibodies
The following antibodies were used for immunocytochemistry: rat anti-MBP (Serotec), mouse anti-CNP (Sigma), rabbit anti-NG2 (Chemicon), rabbit anti-Shp1 (Santa Cruz), rabbit anti-Shp2 (Santa Cruz). Secondary antibodies: FITC-, Texas Red-, or CY3-conjugated donkey antibodies against rabbit, mouse, and rat IgG (Jackson Immunoresearch). The following antibodies were used for Western blotting: mouse anti- β-actin (Sigma); rabbit anti-Shp1 (Santa Cruz); rabbit anti-Shp2 (Santa Cruz); rabbit anti-phosphorylated Y542 Shp2 (Cell Signaling); mouse anti-phosphorylated ERK1/2 (Sigma, clone MAPKYT); rabbit phosphorylated anti-ERK1/2 (R&D Biosystems); rabbit anti-ERK1/2 (Cell Signaling); rabbit anti-phosphorylated Histone H3 ((Upstate); mouse anti-CNP (Sigma, clone 11-5B); rat anti-MBP aa 82–87 (AbD Serotec, clone 12).
Proteins
Human recombinant PDGF-A and FGF2 were used at 10 ng/ml (Peprotech). Recombinant protein comprising the EGF-like domain of neuregulin-1 was used at 100 ng/ml (Peprotech).
siRNA Transfection
Pools of 4 siRNA duplexes designed to target rat Shp1 or Shp2 mRNA were used; siRNA non-targeting pool for rat (siCONTROL) was used as a negative control condition (Dharmacon). Prior to transfection, OPCs were removed from mixed glial cultures at approximately 10–14 days in vitro. Cells were cultured overnight on uncoated Petri dishes in SATO with 20 ng/ml PDGF and 20 ng/ml FGF2. siRNAs were transfected into OPCs using the Nucleofector electroporation system with the rat oligodendrocyte transfection reagent as per manufacturer's instructions (Amaxa). Cells were seeded directly onto dishes or chamber slides and changed to differentiation medium (SATO + 0.5% FCS) or proliferation conditions (see BrdU incorporation assay) approximately16 hours later.
Results
Shp1/2 expression and activity in developing oligodendrocytes
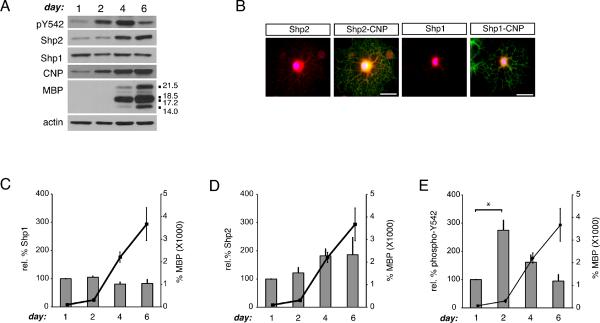
While the non-receptor protein phosphatase Shp1 has been linked to oligodendrocyte development (Massa et al. 2000), Shp2 expression and phosphorylation has not been characterized previously in oligodendroglial cells. To compare protein levels of Shp1 and Shp2 during oligodendrogenesis, rat oligodendrocyte progenitor cells were allowed to differentiate for 1, 2, 4, or 6 days. Cell lysates were obtained at each time point and Western blot analysis using Shp1- and Shp2-specific antibodies was performed (Fig. 1). Shp1, as reported previously (Massa et al. 2000, Massa et al. 2004), was observed in oligodendrocyte lysates and remained at constant levels during development (Fig. 1A, C). Shp2 protein was found in oligodendrocytes at all stages, and its levels were found to increase approximately 2-fold during the time course (Fig. 1A, D). To monitor Shp2 phosphorylation we used an antibody specific for the phosphorylated Y542 of Shp2, a residue that is phosphorylated in response to, among other things, receptor tyrosine kinase activity (Araki et al. 2003). Phosphorylation at Shp2 Y542 leads to loss of basal Shp2 inhibition and is thus correlated with increased tyrosine phosphatase activity. Shp2 Y542 phosphorylation changed during oligodendrocyte development such that the maximal level of phosphorylated Shp2 (relative to total Shp2 protein) was observed at day 2 of differentiation (Fig. 1A, E; p=0.0142, n=3). 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNP) and myelin basic protein (MBP) protein levels were also monitored as stage-specific markers for oligodendrocyte development. Relative levels of phosphorylated Shp2 peaked just prior to MBP expression onset and then dropped to coincide with enhanced MBP production (Fig. 1A, E). In addition, immunocytochemistry was used to visualize Shp2 and Shp1 in conjunction with oligodendrocyte lineage marker, CNP (Fig. 1B) and MBP (Fig. S1D). Shp2 immunoreactivity was observed in oligodendrocyte cell bodies as well as processes whereas Shp1 immunoreactivity was found primarily in the cell body. Proteins that are insoluble in cold Triton-X-100 are typically associated either with specialized membrane domains termed lipid rafts or with cytoskeletal-associated protein complexes. To test whether Shp2 might be regulated in terms of its spatial associations, we examined Triton-X-100 soluble and insoluble protein fractions obtained from oligodendrocytes differentiated for 1 or 4 days (Supplementary Figure S1). Shp2 protein was found to be primarily Triton-X-100 soluble at day 1 (Fig. S1; 86.0±5.2%, n=3, p=0.0069), but by day 4 a significant fraction was found to be Triton-X-100 insoluble (Fig. S1; 34.4±2.3%, n=3, p=0.0071). In addition, the majority of phosphorylated Shp2 (at Y542, relative to total Shp2) was observed in the Triton-X-100 insoluble pool at both day 1 (Fig. S1; 89.5±5.7%, n=3, p=0.0068) and day 4 (Fig. S1; 85.1±2.9%, n=3, p=0.0022). Together, these observations suggest that Shp2 spatial associations may be regulated such that active Shp2 is found primarily in Triton-X-100 insoluble protein fractions, and relative proportions of Shp2 found in this pool change during oligodendrocyte differentiation.
Figure 1. Shp2 phosphorylation is temporally regulated during oligodendrogenesis.
(A) OPCs were differentiated for 1, 2, 4, or 6 days post-withdrawal of mitogens to determine protein levels of Shp1, Shp2, and phosphorylated Shp2 (pY542) in comparison to oligodendrocyte stage-specific myelin proteins (CNP and MBP). A representative set of Western Blots is shown. Actin blots are provided as a control for equal protein loading. (B) Representative images depicting Shp1 and Shp2 immunoreactivity (red) in oligodendrocytes colabeled with CNP immunoreactivity (green) to indicate oligodendrocyte lineage specificity and DAPI (blue) to indicate nuclei (scale bars = 50 microns). (C) Densitometry to determine levels of Shp1 protein during oligodendrocyte development, relative to actin. Bars represent the mean (± sem) relative change in Shp1 protein from 3 independent experiments. Black line graph (right axis) represents the mean (± sem) change in MBP protein from 3 independent experiments. (D) Densitometry to determine levels of Shp2 protein during oligodendrocyte development, relative to actin. Bars represent the mean (± sem) relative change in Shp2 protein from 3 independent experiments. Black line graph (right axis) represents the mean (± sem) change in MBP protein from 3 independent experiments. (E) Densitometry to determine levels of Shp2 phosphorylation (pY542) during oligodendrocyte development, relative to total Shp2 protein. Bars represent the mean (± sem) relative change in Shp2 phosphorylation from 3 independent experiments (*p<0.05). Black line graph (right axis) represents the mean (± sem) change in MBP protein from 3 independent experiments.
Oligodendroglial mitogens promote Shp2 phosphorylation
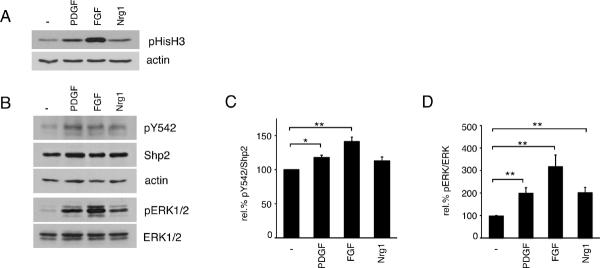
In order to determine if Shp2 phosphorylation was modulated by activation of oligodendrocyte receptor tyrosine kinases, we applied exogenous stimulation to oligodendrocyte progenitor cells using three known mitogens: platelet derived growth factor (PDGF), fibroblast growth factor 2 (FGF2), or neuregulin-1 (Nrg1) (Baron et al. 2005). Oligodendrocyte progenitor cells were serum-starved overnight (~20 hours) and then treated with mitogen for 30 minutes after which cell lysates were obtained and evaluated using Western Blot analysis. First, phosphorylated Histone H3 (pHisH3) immunoreactivity was used to verify mitogenic response to each growth factor (Fig. 2A). As expected, each mitogen caused an increase in the level of phosphorylated HistoneH3, with FGF mediating the largest response followed by PDGF, then Nrg1. The same lysates were evaluated to determine the degree of Shp2 Y542 phosphorylation (Fig. 2B). Levels of phosphorylated Shp2 Y542 (relative to total Shp2 protein) increased in response to each mitogen relative to control cells (117.9±3.3% with PDGF, p=0.0297; 141.3±6.5% with FGF, p=0.0017; 112.8±5.8% with Nrg1, p=0.0691); the mean relative responses to five independent experiments is shown in Fig. 2C. In agreement with previous studies (Stariha & Kim 2001), levels of phosphorylated ERK1/2 (relative to total ERK1/2 protein) increased in response to each mitogen (Fig. 2D); these increases occurred in concert with Shp2 phosphorylation but did not mimic it precisely (Fig. 2B–D). Relative levels of ERK1/2 phosphorylation increased by 201.2±22.2% in response to PDGF (p=0.0030), 318.8±50.3% in response to FGF (p=0.0020), and 203.4±21.8% in response to Nrg1 (p=0.0017) (Fig. 2D, n=6).
Figure 2. Oligodendroglial mitogens stimulate Shp2 phosphorylation.
(A) Oligodendrocyte progenitor cells were treated with growth factor for 30 minutes to induce proliferation-associated signaling mechanisms. Western blots of cell lysates were performed to monitor phosphorylated Histone H3 (pHisH3) as a readout for cells in M-phase. Actin blots were performed to control for equal protein loading. Growth factor treatments: PDGFA (PDGF), FGF2 (FGF), or neuregulin-1 (Nrg1). (B) Lysates of oligodendrocyte progenitor cells, treated with growth factors as above, were analyzed by Western blot to determine relative protein phosphorylation of Shp2 and ERK1/2. Growth factor stimulation led to increased phosphorylation of Shp2 and ERK1/2. A representative set of blots from 5 independent experiments is shown. (C) Densitometry to determine levels of Shp2 phosphorylation (pY542) induced by growth factor treatment, relative to total Shp2 protein. Bars represent the mean (± sem) relative change in Shp2 phosphorylation from 5 independent experiments (*p<0.05; **p<0.01). (D) Densitometry to determine levels of ERK1/2 phosphorylation induced by growth factor treatment, relative to total ERK1/2 protein. Bars represent the mean relative (± sem) change in ERK1/2 phosphorylation from 6 independent experiments (**p<0.01).
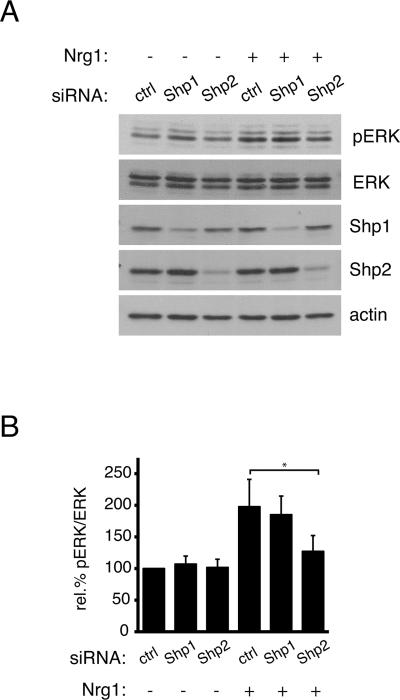
ERK1/2 activation is required for normal proliferation of oligodendrocyte progenitor cells (Frederick et al. 2007), and is predicted to be activated downstream of phosphorylated Shp2, as pY542 has been proposed to provide a docking site for Grb2 (Araki et al. 2003, Frederick et al. 2007). To determine whether loss of Shp2 protein affected ERK1/2 phosphorylation in oligodendrocyte progenitor cells, small interfering RNAs (siRNAs) were used to first deplete cells of Shp1 or Shp2, followed by mitogen treatment as above. Using this approach, we observed substantial loss of Shp1 and Shp2 proteins by 36 hours post-transfection (Fig. 3A; 79.7±16.1% loss of Shp1 and 88.2±1.0% loss of Shp2, n=4). Loss of Shp2, but not Shp1, decreased the ability of cells to activate ERK1/2 phosphorylation in response to a 30 minute treatment with neuregulin-1. Control siRNA treated cells and Shp1 siRNA treated cells showed 197.8±43.1% and 185.2±29.3 increases in relative ERK1/2 phosphorylation, respectively, which were statistically indistinguishable (Fig. 3B; n=4, p=0.6536). Shp2 siRNA treated cells, on the other hand, showed only a 127.2±24.9% increase, which was significantly different from the response of control cells (Fig. 3B; n=4, p=0.0318). Thus, Shp2 was required for normal ERK1/2 activity in response to receptor tyrosine kinase stimulation in oligodendrocyte progenitor cells. These findings furthermore suggested that Shp2 was likely to play a role in oligodendrocyte progenitor proliferation.
Figure 3. Shp2, but not Shp1, is required for MAPK signaling in oligodendrocyte progenitor cells.
(A) Oligodendrocyte progenitors, transfected with Shp1 or Shp2 specific siRNA, were treated with neuregulin1 (Nrg1) for 30 minutes and lysed to evaluate relative ERK1/2 phosphorylation by Western blot. Shp1 and Shp2 knockdown was confirmed using Shp1 and Shp2 immunoreactivity. ERK1/2 phosphorylation, relative to total ERK1/2, was decreased following Shp2, but not Shp1, depletion. A representative set of blots from 4 independent experiments is shown. (B) Densitometry to determine levels of ERK1/2 phosphorylation induced by neuregulin1 treatment, relative to total ERK1/2 protein. Bars represent the mean relative (± sem) change in ERK1/2 phosphorylation from 4 independent experiments (*p<0.05).
Shp2 promotes oligodendrocyte progenitor proliferation
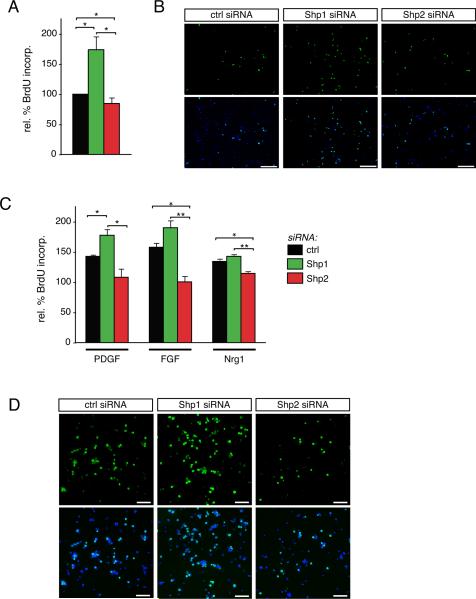
To asses the potential role of Shp1 and/or Shp2 in oligodendrocyte progenitor proliferation we first transfected cells with control, Shp1, or Shp2 siRNA and then maintained them for 24 hours in progenitor maintenance medium to prevent differentiation while allowing for sufficient time for the siRNAs to take effect. We first assessed BrdU incorporation following mitogen withdrawal by switching the cells into DMEM with no added serum or growth factors for 8 hours (Fig. 4A). Under conditions of mitogen withdrawal, oligodendrocyte progenitors in which Shp1 was depleted showed significantly more BrdU incorporation compared to control cells (173.8±21.7% of control cells, n=3, p=0.0422). Conversely, oligodendrocyte progenitors in which Shp2 was depleted showed a small but significant reduction in BrdU incorporation relative to that in control cells (84.7±9.5%, n=3, p=0.0312). Representative images of BrdU immunocytochemistry under these conditions are shown in Fig. 4B. These results suggested that under conditions that typically trigger cell cycle exit i.e. mitogen withdrawal, proliferation was inappropriately elevated in Shp1-deficient cells, but lowered in Shp2-deficient cells.
Figure 4. Shp2 is required for oligodendrocyte progenitor cell proliferation.
(A) Oligodendrocyte progenitors, transfected with Shp1, Shp2, or control (ctrl) siRNA, were grown under serum-free medium for 8 hours to evaluate BrdU incorporation under non-mitogenic conditions. Percent BrdU incorporation relative to cells transfected with control siRNA was calculated. Bars represent the mean (± sem) relative change in BrdU incorporation from 3 independent experiments (*p<0.05). (B) Representative micrographs of BrdU immunocytochemistry (green) and DAPI nuclear stain (blue) following control (ctrl), Shp1, or Shp2 siRNA treatments are shown. Scale bar equals 200 microns. (C) Oligodendrocyte progenitors, transfected with Shp1, Shp2, or control (ctrl) siRNA, were grown in serum-free medium containing PDGF, FGF, or neuregulin-1 for 8 hours to evaluate BrdU incorporation under mitogenic conditions. Percent BrdU incorporation relative to cells transfected with control siRNA was calculated. Bars represent the mean (± sem) relative change in BrdU incorporation from 3 independent experiments (*p<0.05; **p<0.01). (D) Representative micrographs of BrdU immunocytochemistry (green) and DAPI nuclear stain (blue) following FGF-treatment of control (ctrl), Shp1, or Shp2 siRNA transfected cells are shown. Scale bar equals 100 microns.
Next, we assessed the role of Shp1 and Shp2 in oligodendrocyte progenitors that were stimulated for 8 hours with PDGF, FGF2, or neuregulin-1 (Fig. 4C). In control siRNA transfected cells, increased BrdU incorporation was observed following PDGF treatment relative to DMEM alone (142.7±2.7, n=3, p=0.0002). In Shp1-depleted cells, the PDGF-triggered increase in BrdU incorporation was increased significantly compared to control siRNA cells (177.7±9.9% in Shp1 deficient versus 142.7±2.7 in control, n=3, p=0.0415). Shp2 deficient cells, on the other hand, showed substantially less BrdU incorporation relative to control or Shp1-deficient cells, and no significant increase in BrdU incorporation was seen in Shp2 deficient cells above cells in DMEM alone (108.1±14.0% of untreated control cells, n=3, p=0.2956). In the presence of FGF treatment a similar trend was observed: control cells showed increased BrdU incorporation in response to FGF treatment (157.8±7.1%, n=3, p=0.0022), Shp1 deficient cells treated with FGF showed similar BrdU incorporation compared to untreated Shp1-deficient cells (190.0±11.9% in FGF-treated cells versus 173.8±21.7 in untreated cells, n=3, p=0.6020), and Shp1-deficient cells showed a trend towards increased BrdU incorporation relative to control siRNA cells treated with FGF (157.8±7.1% in control FGF-treated cells versus 190.0±11.9% in Shp1-deficient FGF-treated cells, n=3, p=0.1828). Shp2-deficient cells did not show increased BrdU incorporation relative to untreated control cells (Fig. 4C; 100.6±9.4%, n=3, p=0.3610). In the presence of neuregulin-1, however, Shp1 deficient cells (142.9±3.1%, n=3, p=0.2418) did not show increased BrdU incorporation above and beyond that observed in control siRNA cells (134.4±4.4%, n=3). Again, while BrdU incorporation showed a significant increase during neuregulin-1 treatment of control siRNA cells (relative to untreated control cells, n=3, p=0.0024), Shp1 deficient cells were not significantly different than untreated Shp1 deficient cells (n=3, p=0.0626). Finally, Shp2 deficient cells again showed significantly less BrdU incorporation (114.6±3.5% of untreated control cells) than control (n=3, p=0.0372) or Shp1 deficient cells (n=3, p=0.0062). Taken together these data suggest that both Shp1 and Shp2 are involved in the signaling pathways that dictate the choice between cell division and cell differentiation in oligodendrocyte progenitor cells, however, these roles are likely to act in opposition. Loss of Shp1, therefore, leads to enhanced proliferation, even in the absence of added mitogens, suggesting that Shp1 normally acts as a brake on cell division and would be predicted to promote differentiation. Loss of Shp2, on the other hand, leads to loss of proliferation in the presence of all 3 tested mitogens, suggesting that Shp2 is a critical mediator of OPC receptor tyrosine kinase signaling.
The pro-differentiation extracellular matrix molecule, laminin, suppresses Shp2 phosphorylation
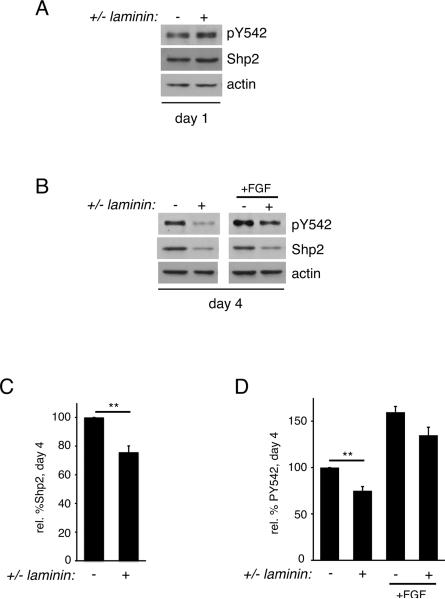
Having identified a role for Shp2 in mediating extrinsic signals that drive oligodendrocyte progenitor cell division, we next addressed whether Shp2 was involved in signaling via laminin, an extrinsic signal that promotes oligodendrocyte progenitor differentiation. Oligodendrocyte progenitor cells were plated on laminin versus control substrate, PDL, for 1 day and cell lysates were assessed for Shp2 phosphorylation (Fig. 5). In contrast to mitogen treatment (Fig. 2B), no significant change in Shp2 phosphorylation at Y542 (or Shp2 protein) was observed following exposure to laminin (Fig. 5A). Next we evaluated long-term exposure to laminin by allowing oligodendrocytes to differentiate for 4 days on laminin or PDL (Fig. 5B–D). After 4 days on laminin, oligodendrocytes showed significantly less Shp2 protein (normalized to actin loading controls, Fig. 5C, 75.8±4.0% that on PDL, n=5, p=0.0037) as well as significantly less phosphorylation at Shp2 Y542 (normalized to Shp2 loading controls, Fig. 5D, 75.0±4.5% that on PDL, n=4, p=0.0067), indicating that laminin led to the suppression of Shp2 function. Treatment of day 4 oligodendrocytes with FGF2 for 30 minutes, however, was able to increase Shp2 Y542 phosphorylation to a similar degree both in oligodendrocytes grown on control or on laminin substrate (Fig. 5D; 159.7±6.2% on control (PDL) versus 134.8±8.6% on laminin, n=3, p=0.1004). These data suggested that the pro-differentiation protein laminin was able to suppress Shp2 phosphorylation during the oligodendrocyte differentiation process. And, together with the relative decrease in Shp2 Y542 phosphorylation that correlated with the appearance of MBP-producing oligodendrocytes (Fig. 1), these findings furthermore suggested that Shp2 may be involved in the regulation of differentiation timing, but was unlikely to be needed for differentiation per se.
Figure 5. The pro-differentiation protein laminin leads to suppression of Shp2 phosphorylation.
(A) Oligodendrocytes differentiated for 1 day on laminin or PDL substrates were evaluated by Western blot to determine Shp2 phosphorylation (pY542) relative to total Shp2 protein. Actin blots were performed to control for equal protein loading. A representative set of blots from 3 independent experiments is shown. (B) Oligodendrocytes differentiated for 4 days on laminin or PDL substrates, followed by 30 minutes of FGF stimulation, were evaluated by Western blot to determine Shp2 phosphorylation (pY542) relative to total Shp2 protein. Actin blots were performed to control for equal protein loading. A representative set of blots from 4 independent experiments is shown. (C) Densitometry to determine levels of Shp2 protein relative to actin. Bars represent the mean (±sem) relative change in Shp2 protein levels from 5 independent experiments (**p<0.01). (D) Densitometry to determine levels of Shp2 phosphorylation (pY542) induced by laminin or FGF conditions, relative to total Shp2 protein. Bars represent the mean (± sem) relative change in Shp2 phosphorylation from 4 independent experiments (**p<0.01).
Shp1, but not Shp2, is required for oligodendrocyte differentiation
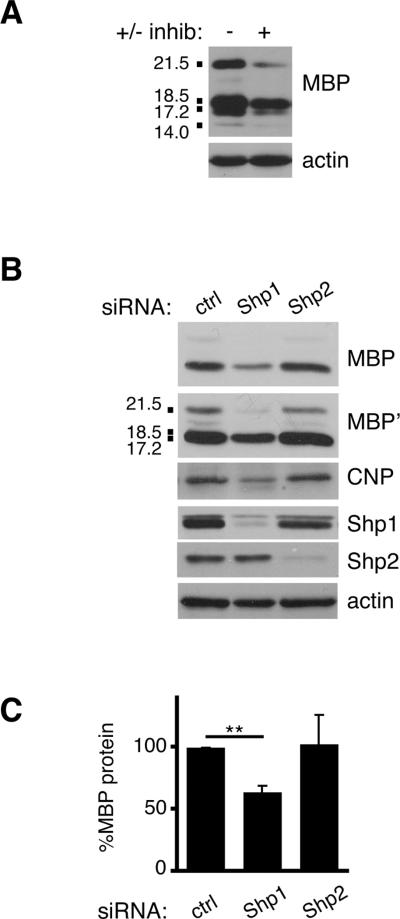
To assess potential requirements for Shp1 and/or Shp2 during oligodendrocyte differentiation, we tested a pharmacological inhibitor of both phosphatases, NSC-87877. NSC-87877 has previously been shown to specifically block both Shp2 and Shp1 phosphatase activity and downstream ERK1/2 phosphorylation and in response to receptor tyrosine kinase stimulation in a range of 10–50 μM (Chen et al. 2006). We tested the effectiveness of NSC-87877 on oligodendrocyte progenitor cells by pre-incubating cells with 5, 10, 20, or 50 μM inhibitor for 30 minutes followed by FGF-2 stimulation (Supplementary Fig. S2). We determined that 20μM inhibitor was effective at blocking Shp2 phosphorylation at Y542 and therefore utilized this concentration for a differentiation experiment (Fig. 6A). Oligodendrocyte progenitor cells were allowed to differentiate for 4 days; at day 1, either NSC-87877 or vehicle control (DMSO) was added and media was changed each day thereafter. Lysates obtained from cells differentiated for 4 days revealed substantially less MBP protein in cells treated with Shp1/2 inhibitor (Fig. 6A; 58.7±24.5%, n=3, p=0.1903), however these changes were highly variable and not statistically significant. Because the inhibitor affects both Shp1 and Shp2 activity we next determined whether more selective removal of either Shp1 or Shp2 would help elucidate a potential role for each phosphatase. We transfected control, Shp1, or Shp2 siRNA into oligodendrocyte progenitor cells, followed by differentiation for 4 days, and evaluated cell lysates by Western Blot analysis to determine (1) siRNA effectiveness at day 4 and (2) levels of proteins normally found in mature oligodendrocytes in each condition (Fig. 6B). First, we observed that both Shp1 and Shp2 siRNA remained effective at day 4 differentiation (Fig. 6B; 84.0±8.9% reduction of Shp1 and 76.8±10.1% reduction of Shp2; n=3). And, both CNP and MBP protein levels were selectively reduced in Shp1, but not Shp2 deficient or control, oligodendrocytes (Fig. 6B,C). Shp1 deficient cells had CNP levels that were 64.9±6.0% that of control cells (n=3, p=0.0043) whereas Shp2 deficient cells had similar CNP levels to control cells (106.5±22.7%, n=3, p=0.7877). Likewise, Shp1 deficient cells had MBP levels 64.1±5.8% of control cells (n=3, p=0.0035) and Shp2 deficient cells had MBP levels indistinguishable from control cells (103.2±23.7%, n=3, p=0.8970). Together these data indicated that Shp1 is required for normal oligodendrocyte differentiation, as shown previously using cells from Shp1-deficient Motheaten mice (Massa et al. 2004, Wishcamper et al. 2001, Massa et al. 2000), yet Shp2 is not required for normal oligodendrocyte lineage progression.
Figure 6. Shp1, but not Shp2, is required for oligodendrocyte expression of myelin proteins.
(A) Oligodendrocytes differentiated for 4 days in the presence of Shp1/2 inhibitor or vehicle control were evaluated by Western blot to determine MBP protein relative to actin loading control. A representative set of blots from 3 independent experiments is shown. MBP isoforms (21.5, 18.5, 17.2, and 14.0 kDa) are indicated. (B) Following transfection with Shp1, Shp2, or control (ctrl) siRNA, oligodendrocytes were differentiated for 4 days and evaluated by Western blot to determine MBP and CNP protein relative to actin loading control. MBP' represents a longer exposure time for the MBP blot shown. Shp1 and Shp2 immunoblots were also performed to assess protein knockdown at day 4. MBP isoforms (21.5, 18.5, 17.2, and 14.0 kDa) are indicated. (C) Densitometry to determine levels of MBP protein in Shp1, Shp2, or control (ctrl) siRNA transfected oligodendrocytes. Bars represent the mean (± sem) change in MBP protein relative to actin from 3 independent experiments (**p<0.01).
Shp1, but not Shp2, is required for the pro-differentiation effect of laminin
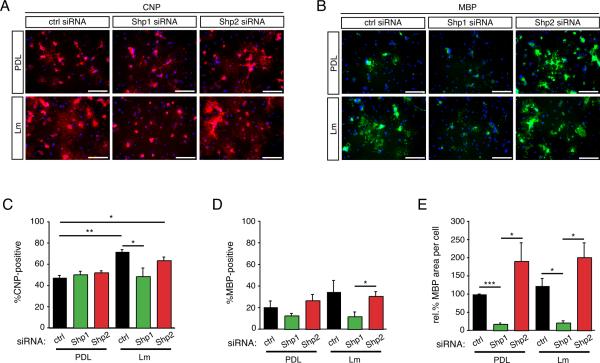
Given that the pro-differentiation factor laminin was able to suppress Shp2 phosphorylation in differentiating oligodendrocytes (Fig. 5B,C), we next sought to determine whether loss of Shp1 or Shp2 modulated oligodendrocyte differentiation in the presence or absence of laminin. To more precisely characterize differentiation we determined both the number of CNP(+) and MBP(+) cells in each condition as well as the degree to which mature oligodendrocytes showed myelin membrane cell expansion, a hallmark of mature oligodendrogenesis (Fig. 7). Interestingly, while overall CNP protein levels were decreased in Shp1 deficient oligodendrocyte after 4 days of differentiation (Fig. 6A) the overall percentage of CNP(+) oligodendrocytes in Shp1 deficient cells was not significantly different (Fig. 7A&C; 50.3±3.0% in Shp1 siRNA versus 47.5±1.9% in control siRNA cells, n=3, p=0.6130). Control and Shp2 deficient cells that were differentiated on laminin substrates, however, showed significantly more CNP(+) oligodendrocytes compared to control cells differentiated on PDL (Fig. 7A&C; 71.9±1.7% in control siRNA cells, n=3, p=0.0042; 63.6±3.1% in Shp2 siRNA, n=3, p=0.0114). Shp1 deficient cells grown on laminin, in contrast, did not show increased numbers of CNP(+) cells (48.6±7.8%, n=3, p=0.9208). These data suggested that Shp1, but not Shp2, is necessary for laminin-induced increases in oligodendrocyte differentiation. Representative images depicting CNP(+) oligodendrocytes grown on PDL and laminin are shown in Fig. 7A.
Figure 7. Shp1, but not Shp2, is required for oligodendrocyte differentiation.
Following transfection with Shp1, Shp2, or control (ctrl) siRNA, oligodendrocytes were differentiated for 4 days on either PDL or laminin (Lm) and evaluated by immunocytochemistry to determine the percentage of CNP or MBP positive cells. (A) Representative micrographs of CNP immunocytochemistry (red) and DAPI nuclear stain (blue). Scale bar equals 100 microns. (B) Representative micrographs of MBP immunocytochemistry (red) and DAPI nuclear stain (blue). Scale bar equals 100 microns. (C) Bars represent the mean percentage (± sem) of CNP(+) cells after differentiation on PDL or laminin (Lm). (D) Bars represent the mean percentage (± sem) of MBP(+) cells after differentiation on PDL or laminin (Lm). (E) Bars represent the mean (± sem) relative change in area per MBP(+) myelin membrane sheet after differentiation on PDL or laminin (Lm). Mean areas are normalized to mean area of control cells on PDL (*p<0.05, **p<0.01, ***p<0.001).
Next, we assessed the percentage of cells in each condition that became MBP(+) after 4 days of differentiation following Shp1 or Shp2 depletion (Fig. 7B & 7D). Overall, fewer cells were MBP(+) at day 4 than were CNP(+), and two trends emerged: Shp1 deficient cells showed fewer MBP(+) cells than did control or Shp2 deficient cells, and these changes in Shp1 deficient cells occurred both on PDL and on laminin. These trends were not as pronounced as those for changes in CNP(+) cells and thus were not statistically significant except in the case of cells grown on laminin in which Shp1 deficient cells (11.8±4.0%) showed significantly less MBP(+) cells compared to Shp2 deficient cells (30.6±4.0%, n=3, p=0.0258).
Loss of Shp1, but not Shp2, leads to decreased myelin membrane cell area
The growth of myelin membrane sheets by mature MBP(+) oligodendrocytes in culture is thought to involve many of the phenotypic changes that occur in vivo during myelin wrapping, such as extensive process growth and plasma membrane expansion. Here we tested whether Shp1 or Shp2 were involved in the myelin membrane expansion of MBP(+) oligodendrocytes, either on PDL or on laminin (Fig. 7B,E). To minimize variability between different experiments, cell area data were compiled such that cell areas were normalized to the mean area of control cells grown on PDL being defined as 100%. A striking decrease in mean MBP(+) myelin sheet area was observed in Shp1 deficient cells relative to control cells, both on PDL (17.6±2.6%, n=3, p=0.0010) and on laminin (21.2±5.0%, n=3, p=0.0218). Interestingly, Shp2-deficient cells showed, on average, larger MBP(+) myelin sheets on both PDL (190.7±50.1, n=3, p=0.2118) and on laminin (201.1±39.5%, n=3, p=0.1796). While not statistically significant, the trend towards increased myelin membrane area in Shp2 deficient cells suggested that Shp2 might normally act as a break for signaling pathways that mediate myelin membrane expansion and/or process extension. Alternatively it may be that in the absence of Shp2, the pro-differentiation Shp1 becomes dominant and leads to enhanced differentiation above that seen in control cells. It should be noted that in the presence of a pharmacological inhibitor that blocks both Shp1 and Shp2, MBP(+) myelin membrane sheet area was decreased, albeit not significantly so, and this decrease was only apparent on laminin substrates (Supplementary Figure S3). These findings indicate that the combined effect of blocking both Shp1 and Shp2 may counteract the more dramatic decrease in cell area observed in Shp1-deficient cells; an alternative explanation, however, could be that pharmacological treatment, while effective in short-term signaling assays (Supplementary Figure S2), is only partially effective at inhibiting Shp1 function in long term differentiation assays.
Discussion
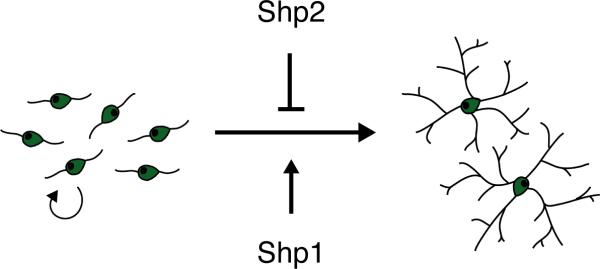
In the current study we investigated potential roles for Shp1 and Shp2 protein tyrosine phosphatases in oligodendrocyte lineage progression. Two major findings emerged: (1) loss of Shp1 interfered with the ability of OPCs to differentiate into oligodendrocytes and (2) loss of Shp2 interfered with the ability of OPCs to proliferate in response to mitogenic growth factors. Thus Shp1 normally would act to promote oligodendrocyte differentiation whereas Shp2, by promoting OPC proliferation, would therefore suppress, or delay, oligodendrocyte differentiation (see Model, Fig. 8). We also identified Shp2 as being negatively regulated by laminin, an extracellular matrix protein that promotes OPC differentiation (Colognato et al. 2007; 2009). Together these findings suggest that the ability to generate appropriate numbers of oligodendrocytes in the developing brain may require a sequential effort from both Shp2 and Shp1 phosphatases. In addition, these data suggest that the timing of Shp1 and Shp2 activity may be critical such that premature suppression or activation of Shp1 and Shp2 may dysregulate oligodendrogenesis. It will therefore be important to identify the exogenous signals that regulate Shp1 and/or Shp2 during brain development.
Figure 8. Model of Shp1 and Shp2 action in oligodendrocyte development.
The Shp1 and Shp2 tyrosine phosphatases may have opposing roles during oligodendrocyte development: Shp2 promotes maintenance of the proliferative OPC phenotype, while Shp1 promotes the differentiation of OPCs into oligodendrocytes. In addition, Shp2 may also act as a negative regulator of oligodendrocyte cell expansion.
Previous studies have found that in the absence of Shp1 gene expression (mice termed motheaten), a variety of developmental abnormalities occur that include impaired CNS myelination (Massa et al. 2000, Wishcamper et al. 2001, Massa et al. 2004). Shp1 also has an important regulatory role in hematopoietic cell function; Shp1-deficient mice have a profound dysregulation of immune cell function of which one consequence is widespread inflammation due to inappropriate cytokine signaling (Zhao et al. 2006, Massa et al. 2000). In oligodendrocytes, the signaling role of Shp1 is less well understood but it has been shown to suppress the activation of the JAK/STAT pathway in response to IL-6 (Massa et al. 2000). And, mice lacking Shp1 expression show a substantial decrease in oligodendrocyte numbers along with hypomyelination (Massa et al. 2000, Wishcamper et al. 2001, Massa et al. 2004, Rossi et al. 1985, Shultz et al. 1983). However, it remains unknown whether the decrease in myelination reflects delayed versus impaired oligodendrogenesis, as these mice die prematurely due to hemorrhage (Rossi et al. 1985, Shultz et al. 1983). Here we report that following Shp1 depletion using siRNA, OPCs do not show normal differentiation as measured by two distinct criteria: decreased expression of mature oligodendrocyte stage-specific proteins (CNP and MBP) and decreased myelin membrane area. In a seeming contradiction, however, the percentage of cells that achieve CNP-positive status is not changed following Shp1 depletion (Fig. 7C), yet CNP protein levels are reduced in total cell lysates from Shp1 depleted cells (Fig. 6B). These findings can be reconciled by the observation that the sizes of mature oligodendrocytes are much smaller in Shp1 deficient cells (Fig. 7E). Oligodendrocyte cell shape typically changes dramatically during differentiation such that cell processes become longer and highly branched; these changes are thought to enable these cells to contact multiple axons in their immediate environment as well as successfully wrap axons during myelination. Thus hypomyelination observed in Shp1-deficient motheaten mice may not simply be due to defective oligodendrogenesis but may also reflect an inability of Shp1-deficient oligodendrocytes to undergo normal morphological changes need to contact and ensheath axons.
Like Shp1, Shp2 has also been implicated as an important regulator of hematopoiesis yet evidence suggests that its role is in opposition to that of Shp1 (Poole & Jones 2005). In the current study we also found that Shp1 and Shp2 often showed opposing roles in oligodendrocyte development. For instance, the loss of Shp1 caused a reduction in the size of mature oligodendrocytes whereas the loss of Shp2 resulted in an increase in oligodendrocyte size (Fig. 6C). And, Shp1-deficient OPCs showed enhanced BrdU incorporation, at least in response to PDGF, while Shp2-deficient OPCs showed decreased BrdU incorporation in response to this and other mitogens (Fig. 4). Given that Shp1 and Shp2 are highly homologous, their small differences in protein binding motifs, particularly in the C-terminal region, may account for these different properties. In addition, Shp1, but not Shp2, has been shown to contain a nuclear localization signal that may contribute to its distinct role (Poole & Jones 2005). It will be interesting to learn whether in oligodendrocytes, Shp1 is able to shuttle to the nucleus and whether disruption of this trafficking could underlie changes in myelin gene expression seen in Shp1-deficient cells.
Loss of function studies in mice have led to the conclusion that Shp1 has important roles in maintaining normal physiology. Shp2, however, is more ubiquitously expressed and a complete loss of Shp2 causes early embryonic lethality (Neel et al. 2003). Thus Cre/Lox gene inactivation strategies have been necessary to elucidate the function of Shp2 in particular tissues or cells. Using a conditional ablation of Shp2 in the developing neuroepithelia Ke and colleagues recently demonstrated that loss of Shp2 during brain development has substantial consequences for neural cell fate decisions such that neurogenesis was impaired and astrogliogenesis was enhanced (Ke et al. 2007). In addition, the group reported a decrease in the ability of cultured Shp2 −/− neural stem cells to generate oligodendrocytes. In the current study we found that depletion of Shp2 in committed oligodendrocyte progenitors, or OPCs, had no effect on the ability of these cells to differentiate into oligodendrocytes, suggesting that the failure in oligodendrocyte generation in Shp2-null neural stem cells was likely due to an earlier failure of neural stem/progenitor cells to generate OPCs. Indeed the authors indicate that Shp2-deficient brains have fewer OPCs as reflected by a large decrease in NG2-positive cells in the developing postnatal cerebral cortex (Ke et al. 2007). A complementary work by Gauthier and colleagues came to similar conclusions: that Shp2 is essential for normal brain development and has a critical role in mediating the switch between neurogenesis, that occurs earlier in development, and gliogenesis, that occurs later in development (Gauthier et al. 2007). Our preliminary studies further indicate that loss of Shp2 function in oligodendroglia may lead to increased cell death in this population, as Shp2-deficient oligodendrocytes show enhanced levels of cleaved caspase 3 (D.P. and H.C., unpublished observations); these data suggest that an altered ability to survive is a potential contributor to the reduced oligodendroglial numbers seen in Shp2-null brains.
In addition to loss-of-function studies, several groups have addressed the role of Shp2 in development through the use of a transgenic mouse model of Noonan's syndrome (Araki et al. 2004). Noonan's syndrome is a genetic abnormality arising, approximately 50% of the time, from mutations in the gene that encodes Shp2; these mutations result in a more active form of the phosphatase. Children with Noonan's syndrome have widespread growth defects and dysplasias, including a higher propensity to develop leukemia. Cognitive impairments are frequently associated with the disease and range from learning disabilities to mental retardation. Gauthier and colleagues used a mouse with a genetic knock-in of a Noonan's syndrome activating mutation to elegantly demonstrate a crucial role for appropriate Shp2 activity during brain development; these mice showed enhanced neurogenesis and the expense of astrogliogenesis (Gauthier et al. 2007). It will be interesting to determine whether these mice have increased numbers of oligodendrocytes, and if so, whether disturbed oligodendrogenesis could contribute to the cognitive impairments observed in children with Noonan's syndrome.
While both studies point to a role for Shp2 in modulating cell fate choice during brain development, the studies differed as to a putative role for Shp2 in mediating the proliferation of neural cell types. Disruption of Shp2 at the gene level led to decreased neural progenitor proliferation in the study by Ke and colleagues yet disruption of Shp2 via RNA interference did not alter neural progenitor proliferation in the study by Gauthier and colleagues (Gauthier et al. 2007, Ke et al. 2007). In the current study we found that depletion of Shp2 using RNA interference caused a significant decrease in the ability of OPCs to respond to oligodendroglial mitogens (Fig. 4). However, not all mitogenic responses of OPCs were similarly disrupted, with the largest change relative to control cells occurring in response to FGF stimulation. Since of the three mitogens used, FGF also showed the most pronounced MAPK signaling response, it may be that FGFR signaling in OPCs is particularly dependent on Shp2's ability to enhance MAPK signaling. Thus it may be that proliferation does not require Shp2 per se but proliferation in response to particular mitogens, or under particular cellular conditions, does require Shp2. Indeed, a study examining fibroblast proliferation found that Shp2 was involved in proliferation in response to FGF and PDGF but not EGF or IGF (Araki et al. 2003).
In the current study we have identified PDGF, FGF, and neuregulin-1 as exogenous growth factors that lead to enhanced Shp2 phosphorylation at Y542. Shp2 phosphorylation at this site leads to enhanced Shp2 phosphatase activity, although the precise mechanism through which this occurs remains controversial. There is universal agreement however regarding the importance of Shp2 for normal Ras/Erk MAPK signaling (Dance et al. 2008). For example, a key substrate for Shp2 is the docking protein Gab1, which when dephosphorylated by Shp2 leads to less recruitment of RasGAP and thus enhancement of Ras-mediated MAPK signaling (Montagner et al. 2005). Our study is the first to link Shp2 to MAPK signaling in oligodendrocyte progenitors and in turn to oligodendrocyte progenitor (OPC) proliferation. Future studies will be needed, however, in order to uncover the downstream targets of Shp2 in OPCs that contribute to Shp2's ability to promote proliferation.
While we have shown that oligodendroglial mitogens depend on Shp2 to signal, we have also identified laminin as mediating the suppression of Shp2 phosphorylation. This is despite the fact that laminin has previously been shown to promote enhanced ERK1/2 phosphorylation in response to soluble neuregulin-1 (Colognato et al. 2002). It may be that laminin's ability to promote ERK1/2 phosphorylation in differentiated oligodendrocytes is Shp2-independent. However, at earlier stages laminin can suppress OPC proliferation (unpublished observations) and has been found to promote the transition from OPC to newly-formed oligodendrocyte (Colognato et al. 2009). Since Shp2 is essential for normal mitogen-driven OPC proliferation, laminin may be able to promote OPC differentiation at least in part by modifying the activation state of Shp2 and altering the ability of OPC mitogens to signal. It will be interesting to learn whether laminins in the neurogenic niche of the subventricular zone (SVZ) (Lathia et al. 2007) also influence Shp2 phosphorylation states, and, possibly Shp2-dependent signaling in neural stem cells and their immediate progeny.
While many extrinsic cues have been identified that can influence the transition between OPC and oligodendrocyte, little is known regarding the integration of multiple signaling inputs and how these translate into cell decisions. Thus the identification of Shp2 as a downstream of multiple signaling inputs, both mitogenic and pro-differentiation, provides new insight into how oligodendrogenesis occurs. We suggest that Shp2 is essential for normal OPC proliferation yet can be suppressed in the presence of pro-differentiation signals. To better understand how Shp2 operates in the context of oligodendroglial development it will be important to address the following questions: What are the binding partners and signaling targets of oligodendrocyte Shp2? Do Noonan's syndrome activating Shp2 mutations lead to oligodendroglial dysfunction? Do Shp1 and Shp2 have overlapping functions and/or targets, and if not, what distinguishes each? A better understanding of Shp2 function may also prove useful in demyelinating conditions such as Multiple Sclerosis (MS). In MS, OPCs have been shown to accumulate, failing to complete the differentiation program and myelinate in chronically-demyelinated lesions (Franklin & Ffrench-Constant 2008). Therefore, identification of signaling effectors such as Shp2 that influence the ability of OPCs to proceed with oligodendrogenesis may prove valuable in understanding OPC dysfunction during Multiple Sclerosis and other demyelinating diseases.
Supplementary Material
Acknowledgements
This study was supported by a National Multiple Sclerosis Society Career Transition Fellowship (H.C.), the National Institute of Neurological Disorders and Stroke (5R01NS054042), and the NY State Department of Health (CART C020929). The authors wish to thank the members of the Colognato lab for helpful advice and discussion.
References
- Araki T, Mohi MG, Ismat FA, et al. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem. 2003;278:41677–41684. doi: 10.1074/jbc.M306461200. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49:467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Chen L, Sung SS, Yip ML, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Colognato H, ffrench-Constant C. Mechanisms of glial development. Curr Opin Neurobiol. 2004;14:37–44. doi: 10.1016/j.conb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Colognato H, Galvin J, Wang Z, Relucio J, Nguyen T, Harrison D, Yurchenco PD, Ffrench-Constant C. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134:1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167:365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008;20:453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Frederick TJ, Min J, Altieri SC, Mitchell NE, Wood TL. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia. 2007;55:1011–1022. doi: 10.1002/glia.20520. [DOI] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Zhang EE, Hagihara K, Wu D, Pang Y, Klein R, Curran T, Ranscht B, Feng GS. Deletion of Shp2 in the brain leads to defective proliferation and differentiation in neural stem cells and early postnatal lethality. Mol Cell Biol. 2007;27:6706–6717. doi: 10.1128/MCB.01225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Rao MS, Mattson MP, Ffrench-Constant C. The microenvironment of the embryonic neural stem cell: lessons from adult niches? Dev Dyn. 2007;236:3267–3282. doi: 10.1002/dvdy.21319. [DOI] [PubMed] [Google Scholar]
- Massa PT, Saha S, Wu C, Jarosinski KW. Expression and function of the protein tyrosine phosphatase SHP-1 in oligodendrocytes. Glia. 2000;29:376–385. [PubMed] [Google Scholar]
- Massa PT, Wu C, Fecenko-Tacka K. Dysmyelination and reduced myelin basic protein gene expression by oligodendrocytes of SHP-1-deficient mice. J Neurosci Res. 2004;77:15–25. doi: 10.1002/jnr.20155. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagner A, Yart A, Dance M, Perret B, Salles JP, Raynal P. A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation. J Biol Chem. 2005;280:5350–5360. doi: 10.1074/jbc.M410012200. [DOI] [PubMed] [Google Scholar]
- Neel BG, Gu H, Pao L. The `Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Rossi GA, Hunninghake GW, Kawanami O, Ferrans VJ, Hansen CT, Crystal RG. Motheaten mice--an animal model with an inherited form of interstitial lung disease. Am Rev Respir Dis. 1985;131:150–158. doi: 10.1164/arrd.1985.131.1.150. [DOI] [PubMed] [Google Scholar]
- Shultz LD, Bailey CL, Coman DR. Hematopoietic stem cell function in motheaten mice. Exp Hematol. 1983;11:667–680. [PubMed] [Google Scholar]
- Stariha RL, Kim SU. Protein kinase C and mitogen-activated protein kinase signalling in oligodendrocytes. Microsc Res Tech. 2001;52:680–688. doi: 10.1002/jemt.1052. [DOI] [PubMed] [Google Scholar]
- Wishcamper CA, Coffin JD, Lurie DI. Lack of the protein tyrosine phosphatase SHP-1 results in decreased numbers of glia within the motheaten (me/me) mouse brain. J Comp Neurol. 2001;441:118–133. [PubMed] [Google Scholar]
- Zhao J, Brooks DM, Lurie DI. Lipopolysaccharide-activated SHP-1-deficient motheaten microglia release increased nitric oxide, TNF-alpha, and IL-1beta. Glia. 2006;53:304–312. doi: 10.1002/glia.20283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.