Abstract
Accessory pathway (AP) ablation is one of the most satisfying invasive electrophysiology procedures associated with high success rates and relatively few complications. Nevertheless, when APs are found on the cardiac septum, ablative procedures become complex, and unique pitfalls need to be avoided.
These difficulties with septal ablation are magnified in the pediatric population. The relatively small heart, rapid nodal conduction, and proximity of the arterial system specifically complicate septal ablation in children. The electrophysiologist must use every tool in his or her armamentarium, including exact delineation of pathway location, identification of pathway potentials, detection of the presence of pathway slant, etc. In addition, an exact knowledge of the complex anatomy of the cardiac septum, including the posteroseptal space, the aortic cusp region, and the proximity of the AV conduction system and coronary vessels, becomes mandatory.
In this review, we describe the developmental anatomy and regional anatomy of septal accessory pathways. We then discuss approaches to map specific to pathways in particularly problematic regions at or near the septum, including venous and aortic cusp related accessory pathways.
Keywords: Septal, Accessory Pathway, Children, Complications, Electrophysiology
Introduction
Accessory atrioventricular pathway ablation has become a common occurrence in the field of electrophysiology. Septal accessory pathways (APs) are, however, known to be difficult to ablate as compared to those in other locations [1]. Because of the difficulty, it becomes clinically relevant for the practicing electrophysiologist to understand the anatomy, development, and practical methods for performing successful ablations, especially in the posteroseptal region. The posteroseptal region of the heart is probably the most complex area among those that harbor AV accessory fibers. There is no sharp true demarcation between this region and its surrounding regions including the midseptal, left posterior paraseptal, and right posterior paraseptal (Figure 1) [2]. Furthermore, a higher recurrence rate and potential for AV block with ablation have been reported in patients with septal APs [1]. Therefore, it is imperative to understand the anatomy and development of this area in order to successfully and safely ablate pathways in this location.
Figure 1.
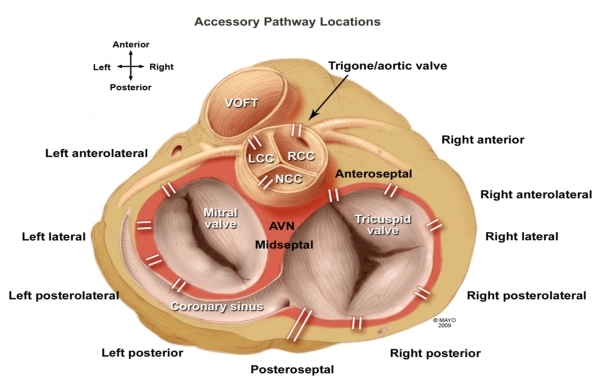
Atrial ventricular accessory pathways may occur anywhere along the atrial ventricular annulus. Note the posteroseptal region is not a part of the true septum. Note also the position of the aortic annulus interspersed between the right and left anteroseptal regions. Midseptal pathways are among the most difficult to ablate because of the proximity of the AV node. VOFT = ventricular outflow tract; LCC = left coronary cusp; RCC = right coronary cusp; NCC = noncoronary cusp; AVN = AV node
Anatomy
Previous surgical experience has divided the septum into anterior and posterior divisions. The point of division is made up of the annuli of the two AV valves, the aortic annulus, the membranous ventricular septum, and atrial septum, i.e. the right fibrous trigone [1]. The posterior AV septum is bound by the posteromedial aspects of the left and right atrium, the superior process of the posterior confluences of the left and right ventricular walls, and the epicardium over the crux of the heart [1]. The right atrial boundary also contains the orifice of the coronary sinus (CS) [1]. The smaller anterior portion of the AV septum is bounded posteriorly by the right fibrous trigone; laterally by the epicardial side of the right atrium and the annulus fibrosus; medially and anteriorly by the epicardium that is reflected from the aortic root and by the infundibulum to the right atrium; and inferiorly by the muscular ventricular septum and the right ventricular infundibulum.
As further research into the septal regions has advanced, previous clinical designations of the septal regions are not regarded as entirely accurate. Dean et al have reported that the mid septum is the only true muscular septal area between the offset attachments of the mitral and tricuspid valves, while the previously named anterior and posterior septal areas are those regions that are anterior and posterior to the true septum [1]. This true septum corresponds roughly to the location of the triangle of Koch. The triangle is of great value to the electrophysiologist because it marks the approximate location of the AV node and bundle of His. Bounded by the tendon of Todaro (linear tissue that runs from the central fibrous body to the Eustachian ridge), the tricuspid annulus, and the orifice of the coronary sinus, the AV node is situated at the apex of this triangle. The APs that occur within the septum typically occur circumferentially along the annuli of the mitral and tricuspid valves (Figure 2) [1].
Figure 2.
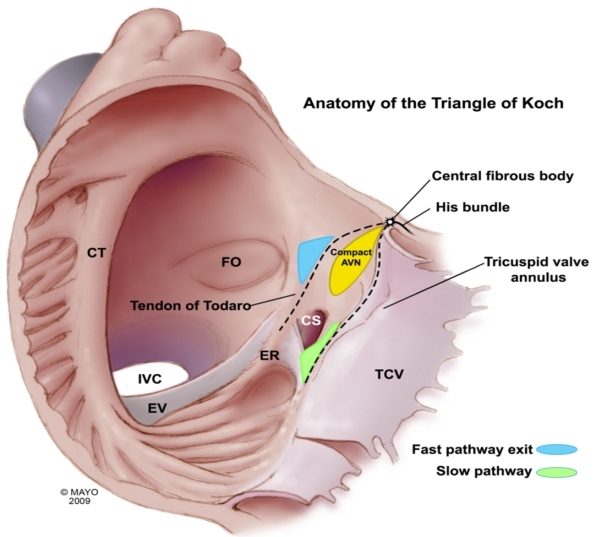
The true interatrial septum near the tricuspid annulus consists of the anterior and midseptal regions. Midseptal pathways are inserted into the triangle of Koch region, close to the compact AV node. Accurate assessment of pathway slant and ablation preferably of the ventricular insertion site along with the use of cryoenergy may all be required to minimize the risk of AV block when ablating pathways in this region. CT = cristoterminalis ; FO = Forman ovale; EV = Eustachian valve; IVC = inferior vena cava; TCV = tricuspid valve; CS = coronary sinus; ER = Eustachian Ridge; AVN = AV node
Development and Embryology
Atrial septation during cardiac development is an extremely complex process. The concept of converting a single myocardial tube into four cardiac chambers involves multiple mechanisms, including looping, the formation of extra cardiac mesenchyme, the segmental transformation of endocardial endothelium into valvuloseptal (cushion) mesenchyme, and finally the remodeling of the myocardial epithelium into muscular partitions [3]. It is important to note that the septation process is reflected in the position of the primitive conduction system. At the level of the atrium, the sinus venosus and sinoatrial transition to form the sinoatrial node [4]. During this period of development, multiple accessory pathways run through the areas that are to become the right and left cardinal veins, pulmonary veins, coronary sinus, and right atrioventricular ring [4]. However, in the mature adult heart, this primitive conduction tissue is not recognizable anymore, and usually undergoes electrical insulation via formation of the annulus fibrosus. When insulation does not occur appropriately, the result is the persistence of APs [5,6]. Most APs are found within the parietal atrioventricular junctional areas, including the paraseptal region [5]. These APs consist mostly of myocardium and are rarely specialized cells. Since they are derived from the same precursor, many of their characteristics are similar to normal cardiac myocytes [5]. However, refractory periods during cardiac repolarization differ in length and provide the ability for faster conduction or faster reexcitation. AP origin on the atrial aspect is thick while insertion on the ventricular side is through thin branches that are electrically connected on both sides by gap junctions [5]. Accessory atrioventricular pathways are thought to develop secondary to disruption of the electrical insulation between the atria and the ventricles. The cushion tissue forms the internal scaffolding which will become the valve leaflets and contribute to the formation of the annulus fibrosis for electrical insulation [5,6]. Recent studies in this area from Gittenberger-de Groot et al have shown the importance of the epicardially-derived cells (EDCs) in the formation of this fibrous tissue. By inhibiting migration of EDCs in quail embryos, Gittenberger-de Groot et al observed the persistence of broad bundles of accessory atrioventricular canal myocardial connections that resulted in ventricular pre-excitation [7].
Posteroseptal Pathways
Anatomy and Electrocardiography
In many studies, ablation of the posteroseptal region has been identified as being more difficult than for pathways located in other areas [8,9]. Of note, ablation in this area has been associated with longer procedure times, longer radiation exposure, and the need for more radiofrequency (RF) pulses to accomplish successful results [9]. Additionally, ablation of these APs has been known to require RF energy within the CS, including the middle cardiac veins and diverticulum; therefore, it is a special importance to understand the complexity of this region.
The posteroseptal region is specifically located between the posterosuperior process of the left ventricle and inferior wall of the right atrium [2], i.e. the crux. The crux anatomically corresponds to the area where the four cardiac chambers reach their maximum proximity posteriorly. The posterior septum is bounded by three distinct areas: 1) midseptum (anterior and superior borders); 2) right posterior paraseptal (right lateral border); and 3) left posterior paraseptal (left lateral border) [2].
The posteroseptal region can be anatomically divided into right and left regions that correspond to the annuli of the tricuspid and mitral valves, respectively. A posteroseptal AP is defined as one located posterior or apical to the orifice of the CS. Surface EKGs typically show the following preexcitation features: 1) R/S ratio > 1 in V2; 2) negative delta wave in III; and 3) R/S ratio < 1 in III [10,11]. Furthermore, nearly all posteroseptal pathways have negative P waves in the three inferior leads and positive P waves in lead I. Studies have shown difficulty in discriminating left- from right-sided posteroseptal pathways [12]; however, it has been suggested that a positive delta wave in V1 can further differentiate a left-sided from a right-sided posteroseptal pathway [2,12]. Additionally, the frontal axis of the delta wave in a right-sided pathway was -30 to 50, and -60 or greater in left-sided pathways [2].
Mapping and Ablation
The comments presented below may be applied for mapping and ablation anywhere on the cardiac septum but are perhaps best exemplified when dealing with the posteroseptal space. Specific differences in other septal locations are presented in the appropriate sections that follow.
Exact Mapping
Although precise mapping is desirable for pathway ablation anywhere in the heart, it is essential when targeting APs on the septum. In general, electrophysiologists use various criteria for targeting pathway locations for energy delivery [13,14]. However, some of these approaches are best avoided when ablating on the septum:
Shortest AV interval. Caution should be used when solely targeting the shortest AV (atrial pacing) or V-A interval (ventricular pacing). Depending on the pacing site, the V-A interval may be 0 ms at sites far away from the actual AP. In those instances, the short interval is simply a reflection of simultaneous activation of the atrium and ventricle at that time (pseudo interval) picked up by the electrode on the annulus. The situation can be clarified by pacing close to the suspected AP site, but this is not straightforward to do with septal pathways [13,15,16].
Earliest ventricular activation during atrial pacing or earliest atrial activation during ventricular pacing/orthodromic reciprocating tachycardia. Although this technique can be applied here again on the septum, it is not ideal. For example, the earliest atrial activation for a downward slanted posteroseptal pathway may be very close to the compact AV node, and safe ablation could have been done more inferiorly on the annulus itself [17-19].
The pathway potential. In general, discerning a pathway potential and targeting this signal for ablation is a superior method, but this is most acutely valid with septal pathways. Pacing maneuvers, so as to separate the candidate signal (possible pathway potential) from the atrial electrogram, ventricular electrogram, and in some instances His bundle electrogram needs to be done to validate the signal. Once certain of the nature of the signal and after establishing adequate contact, the pathway potential can be targeted for energy delivery (Figure 3, Figure 4) [20-24].
Figure 3.
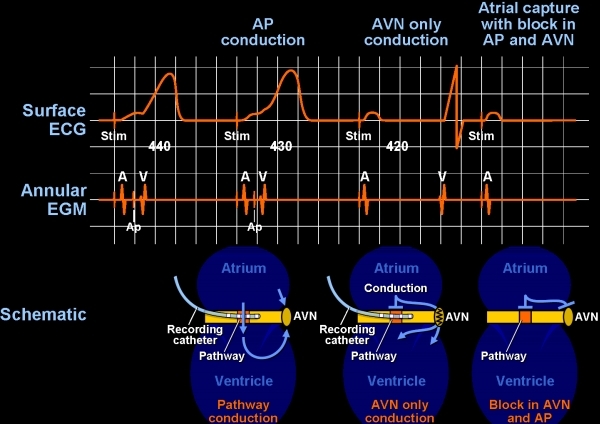
Accurate diagnosis of a candidate potential as an accessory pathway potential is important for any accessory pathway ablation, but it is critical for safe ablation of septal pathways. The suspicious potential in this patient with an antegrade conducting pathway is noted between the atrial (A) and ventricular (B) electrograms. The operator must distinguish the AP potential from a fragmented portion of the atrium or ventricular electrograms. The most important, however, is to make sure this is not part of the atrial electrogram, since targeting such an electrogram will be meaningless. Pacing at a more rapid rate causes block in the accessory pathway and subsequently block in the AV node, as well. The atrial electrogram is seen to be easily dissociated from the accessory pathway potential (Ap) thus ensuring that it was not a fragmented portion of the V. AP = accessory pathway; EGM = electrogram; ECG = electrocardiogram;
Figure 4.
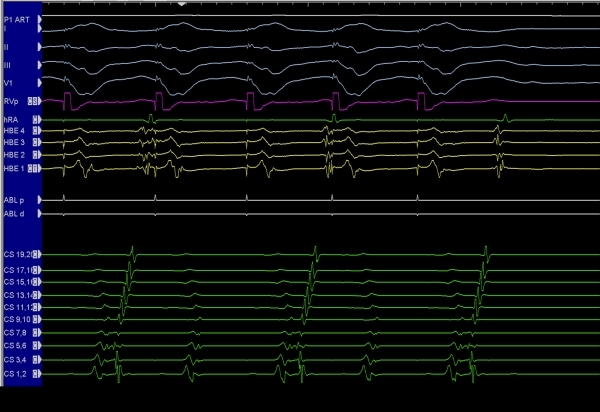
Ventricular pacing in a patient with a left-sided accessory pathway, and having failed multiple prior ablation attempts. Fragmented electrograms are seen on the coronary sinus electrode (CS5, 6) etc. Ventricular pacing results in V-A block. The first two components of the complex signal cannot be dissociated from the ventricular electrogram, and thus likely represent complex ventricular signals. Atrial extrastimuli can be given during ventricular pacing to further distinguish the last two components of the complex electrograms as to whether they represent fragmented atrial electrograms or pathway potential and an atrial electrogram.
Another concept that should be thoroughly understood when attempting ablation on the septum is that of pathway slant. This basic concept has been well-illustrated by Gonzales et al [25,26]. Briefly, pacing on either side of the AP results in (for example) a retrograde conducting left-sided AP. Pacing on either side of the ventricular insertion will result in variation in the local V-A interval measured at an electrode on the annulus. When pacing along the slant (closer to the ventricular insertion), the V-A interval is shorter and vice versa. This technique, however, needs to be modified when assessing supero-inferior slant in the septum with pacing sites chosen superior to and inferior to the insertion site of the pathway. The value of knowing this technique well is that electrophysiologists may then chose to ablate along the slant in such a way as to avoid potential collateral damage. For instance, if a posteroseptal pathway is slanted so that the atrial insertion is more superior and the ventricular insertion is more inferior, ablation can be targeted at the ventricular insertion site (more posteriorly) to minimize the risk of AV nodal damage [14,27,28].
While the principles described above (along with the accompanying figures and explanations) elucidate the salient concepts needed for careful ablation of any septal pathway, there are certain peculiar issues that arise with posteroseptal pathway ablation, especially for epicardial pathways involving the coronary sinus myocardium (Figure 5, Figure 6). The most important of these specific issues is detailed below where we discuss the potential complication of coronary artery injury followed by a brief description of techniques to avoid this complication.
Figure 5.
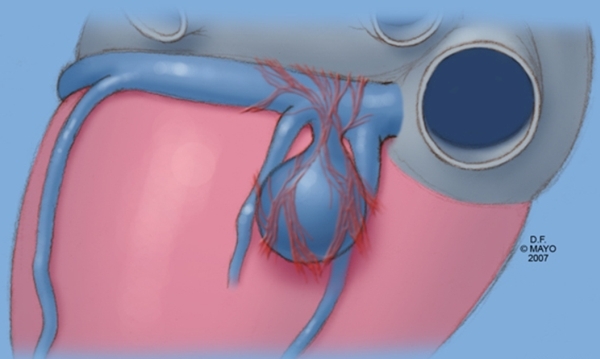
Illustration of myocardial fibers on a coronary sinus diverticulum. In this instance, the ostium of the diverticulum is located between the middle cardiac vein and a posterior ventricular vein. Note that the myocardial fibers essentially are a continuation of the usual fibers found in the proximal coronary sinus. When these fibers connect to both the atrial myocardium and ventricular myocardium, a complex epicardial accessory pathway is created. One approach to ablation of these types of pathways involves circumferential ablation at the mouth of the diverticulum.
Figure 6.
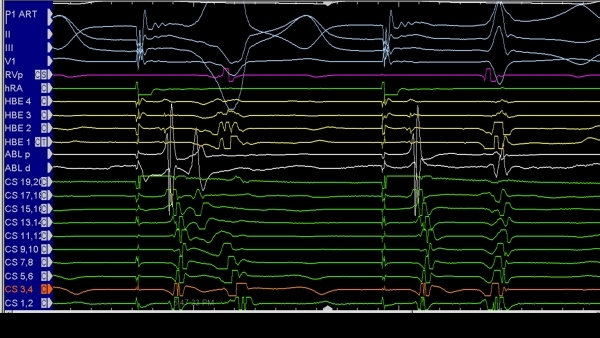
Intracardiac electrocardiograms during encircling type ablation done at the ostium of a coronary sinus diverticulum. The last portion of the circumference is being ablated (ABLT and ABLD ablation catheters proximal and distal electrodes) with block in accessory pathway conduction resulting. Tachycardia was no longer inducible in this patient. CS = coronary sinus; HBE = His bundle electrogram; RV = right ventricle, HRA = High right atrial; ECG leads I, II, III, and V1
Complications of Ablation - Coronary Artery Injury
RF catheter ablation can lead to rare but serious consequences. Coronary artery injury after ablation may present acutely or several weeks after an ablation procedure. Stenosis of the coronary arteries immediately and during long-term follow-up after ablation has been described in animal models [29-31]. However, the overall incidence of coronary injuries during ablation procedures is extremely low [32]. In one large retrospective study, clinically evident coronary injury incidence is estimated to be 0.06% - 0.1% in adults [33,34]. This low risk is surprising given the close proximity of the coronary arteries to common sites of ablation and may be due to under-recognition and underreporting [32].
Patients typically present with chest discomfort during and after ablation, likely secondary to pericardial irritation. Several authors have attributed this presentation to coronary vasospasm because of reversible ST segment elevation but with normal angiography [32,33,35,36]. The mechanism for this is unclear; however, spasm is thought to occur from thermal energy transmitted during ablation [37]. Conversely, in children coronary angiograms taken after ablation have shown stenosis of coronary arteries occurring in as little as 30 minutes from the conclusion of the procedure [36]. In animal models, the application of RF energy directly on a coronary artery causes acute edema with wall thickening and luminal narrowing [209]. In a matter of days, medial necrosis with loss of intimal and elastic tissue with the subsequent development of severe intimal hyperplasia develops [29,38]. In the setting of delayed coronary injury, the presumption is that in addition to intimal hyperplasia, ablation damages the arterial wall and endothelium, which provides a nidus for progressive thrombus formation weeks later [32].
Multiple case reports have documented that ablation of a posteroseptal pathway has led to injury of the coronary arteries [20,39,42]. Again, the complex anatomy of this region and the pathways that traverse it may provide the insight to these observations. In children, a study of postmortem pediatric cardiac histology has demonstrated that the distance between the endocardium at the AV groove and the coronary arteries shown to be shortest within the CS and in the posteroseptal region, generally less than 4 cm [43]. In adults, a significant coronary artery is found within 2 mm of the best ablation site in the CS or its branches in nearly two-thirds of patients, and ablation at this site causes coronary stenosis in two-thirds of patients [29]. Furthermore, Hasdemir et al have examined the anatomic relationship of the coronary arteries to the tricuspid and mitral annulus and finds that the right coronary artery (RCA) is < 5 mm from the cavotricuspid isthmus in 8% of patients, and the left circumflex artery is ≤ 2 mm from the lateral mitral annulus in 24 % of patients [44]. The CS and its branches are also in close proximity to the distal circumflex and the posterolateral branches of the RCA, as well [31]. These anatomic relationships explain why the usual ablation of posteroseptal pathways, specifically right-sided, usually results in damage to the distal RCA, as previously reported in adults.
Avoiding Collateral Damage
Right coronary artery occlusion
As mentioned before, ablation attempts into the middle cardiac vein and/or the coronary sinus can result in damage to a branch of the RCA (Figure 7). There are some reports describing coronary occlusion following RF deliveries in that region, prompting urgent angioplasty [39-42]. The acute injury can be secondary to spasm, edema, thrombosis with complete obstruction, coronary embolization, or direct intimal trauma. Chronic occlusion can also occur and is secondary to progressive scarring.
Figure 7.
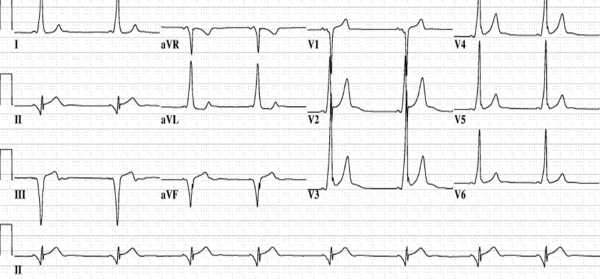
Classic electrocardiographic pattern in a patient with a posteroseptal accessory pathway. The delta wave transition occurs between V1 and lead II.22 The delta wave is negative in II, III, and AVF, placing the pathway in the posteroseptal region. However, the pathway can be further localized to the middle cardiac vein or posterior vein, since the delta wave is immediately negative in lead II. (See text for details)
With the intention of preventing this serious complication, anticipated angiography should be done. In cases that the artery is more than 5 mm away from the ablation site, energy can be safely delivered [45]. If the artery is closer, an intravascular ultrasound catheter should be placed to monitor for damage. When it is extremely close, cryoenergy can be used instead of RF. Cryoablation is safer and yields good acute success rates for AP ablation [46-48], although it is also associated with higher recurrences (Figure 8).
Figure 8.
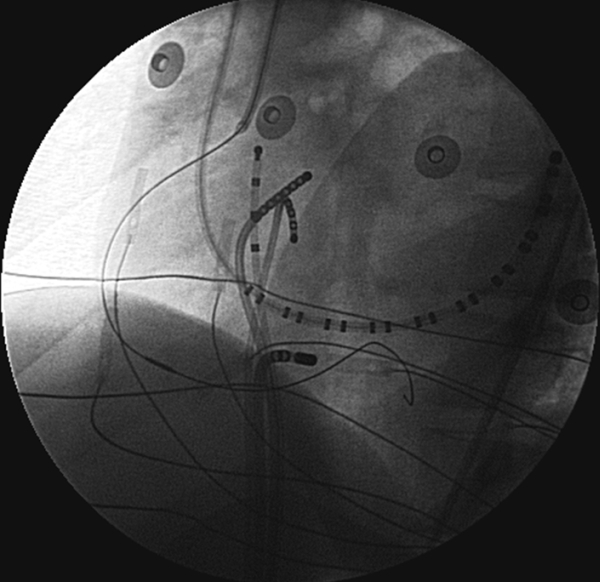
Left anterior oblique fluoroscopic image showing an ablation catheter placed near the ostium of the middle cardiac vein. A guiding sheath has engaged the right coronary artery and an intravascular ultrasound probe placed into the arterial system. The probe can be moved so as to image the relevant heart rate when applying radiofrequency energy within a proximate vein.
PJRT - Permanent Form of Junctional Reciprocating Tachycardia
These accessory pathways are often found near or on the septum [49] and can be difficult to target for ablation. The characteristic of these APs is decremental conduction with long conduction times. As a result, distinguishing this arrhythmia (PJRT) from AV node reentry or atrial tachycardia may not be straightforward. Typical techniques, including parahisian pacing, PVCs to preexcited tachycardia, etc., may not be revealing because of the decremental conduction these pathways show. However, the electrophysiologist can use the decremental nature of these pathways as an advantage when trying to elucidate the tachycardia mechanism. For instance, PVCs may routinely post-excite the tachycardia or terminate the tachycardia, despite being delivered at a time of His bundle refractoriness. Moreover, because of the long ventriculoatrial conduction time, AP signals can in general be easier to see and establish in PJRT compared to regular AP-related tachycardias [50-52].
Midseptal Pathways
Ablation at the midseptal region septum is particularly risky because of the intricate conduction system. Inadvertent second- and third-degree AV block has been reported, especially in children, because of the relative proximity of the AV node to the posteroseptal region [34,45,53]. In the Pediatric Radiofrequency Ablation Registry reported in 1996, ablation of midseptal APs was more frequently associated with accidental AV block (10.4 %) in comparison to other septal locations (anteroseptal 2.7 % and posteroseptal 1 %) [54]. One out of the three described deaths was secondary to complete AV block and subsequent cardiogenic shock while attempting ablation of a mid-septal AP [53]. More recently, also in the pediatric group, it was demonstrated that high-grade AV block occurred in 10% of mid-septal AP ablations and in 5% of anteroseptal AP ablations [55]. Although in most of those cases the high probability of block was anticipated, the ablation was necessary due to refractoriness to medication and highly symptomatic arrhythmia. Thus, although with anteroseptal pathways we may see a His bundle electrogram on the catheter, the greatest care must be used when ablating on the mid septum because of the anatomic location of the compact AV node in this region. RF energy should never be applied as first line therapy on the midseptal region, and attempts with cryoablation first is generally prudent. Further, because the AV node is an atrial structure, ablation energy delivery should be exactly at the annulus or at ventricular locations when performed near the midseptal region, and it is critical to define accurately the location of the pathway prior to delivering either kind of energy.
Anteroseptal Pathways
An anteroseptal pathway is a less common cause of AV re-entrant tachycardia and accounts for 9% of all encountered APs [54,55]. The penetrating bundle of His is a well-insulated structure since it carries a sleeve of fibrous annular tissue for variable distances into the ventricle, losing this insulation as the right bundle exits to the myocardium. As a result, ablation is generally better tolerated on the His bundle itself compared to the fragile and easily ablated compact AV node. Anatomically, since the His bundle is a ventricular and anterior structure, whereas the compact AV node is a midseptal and atrial structure, more caution is needed whenever ablating lower than and more atrially than the anteroseptal region. As with midseptal pathways, it is generally preferable to attempt ablation with cryoenergy after the pathway has been mapped in this region (Figure 9) [46,55].
Figure 9.
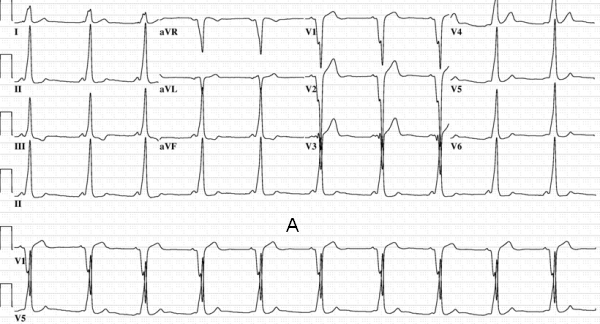
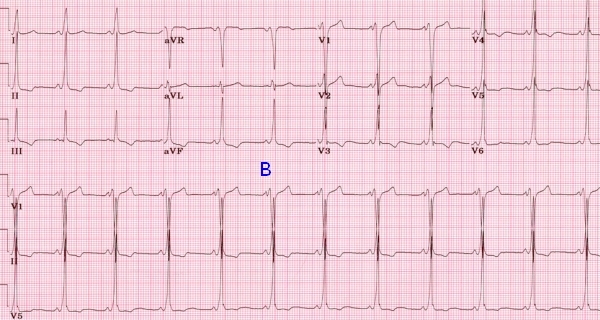
Panel A: Characteristic electrocardiogram in a patient with an anteroseptal pathway. Strongly positive delta waves in leads II, III, and AVF are noted. The delta wave is isoelectric/negative in lead V1. Successful ablation was performed at a location on the anteroseptal annulus where a His bundle electrogram was being recorded. Ablation was very anterior, and thus not in the vicinity of the compact AV node. Further, the ablation was performed targeting the ventricular insertion, as well. Panel B: In contrast, this preexcited electrocardiogram was obtained in a patient with successful ablation on the supravalvar aortic cuspal region. Although the delta waves are positive in leads II, III, and AVF, note the slightly positive delta wave in lead V1 suggesting that the pathway is not on the true septum (see text for details).
One variant of anteroseptal pathways are pathways that involve aortic cuspal ablations.
Aortic Cuspal Ablation
There are rare situations in which an anteroseptal AP could be ablated from the noncoronary aortic cusp [56,-58]. The substrate for ablation is still unclear, but myocardium potentials have been encountered in the supravalvar area [59]. Therefore, bridges across the aortic valve connecting the atrial and ventricular myocardium could constitute the AP. Another possibility is that they transverse the fibrous trigone in this region of the heart, but the aortic cusps would still be the better approach for ablation [59]. In this context, it is very important to remember mapping the aortic valve region whenever attempting ablation of an anteroseptal pathway. Correct identification of the pathway potential would allow successful ablation of the substrate while avoiding collateral AV conduction damage (Figure 10) [58,60].
Figure 10.
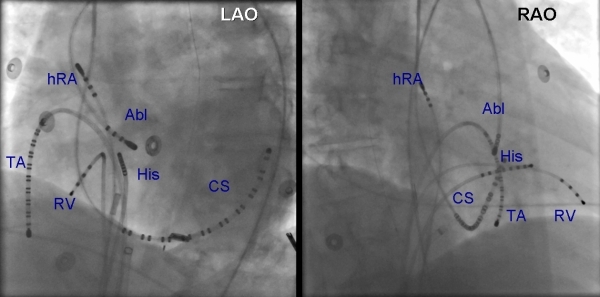
Left anterior oblique (LAO) and right anterior oblique (RAO) projection of a patient with successful ablation of an accessory pathway in the supravalvar aortic cusp region. ABL ablation catheter in the left coronary cusp/noncoronary cusp junction. HRA = high right atrium; TA = tricuspid annulus catheter; CS = coronary sinus catheter; RV = right ventricular catheter.
Uncommon Septal Accesory Pathways
In addition to true atrial ventricular accessory pathways that happen to be located on the septum or the aortic valve region described above, certain pathway variants may present as potential septal accessory pathways:
Fasciculoventricular tracts [61] are not true atrial ventricular bypass tracts. Rather, they represent early excitation of the ventricle as a result of the absence of the usual fibrous insulating coat of the His bundle and right bundle branch. This results in a short H-V interval and the presence of preexcitation on the electrocardiogram. However, since conduction is still through the AV node, the H-V interval will not shorten nor become negative with decremental atrial pacing, and the extent of preexcitation does not change with either the atrial pacing site or with concurrent use of adenosine or with decremental pacing. Fasciculoventricular tracts are benign and not associated with any reentrant arrhythmia and should not be targeted for ablation.
Another rare variant involves the so-called nodal fascicular or nodal ventricular tracts (Figure 11). These tracts may be associated with tachycardia, and earliest atrial activation is found in the atria exactly at the site of early atrial activation with retrograde nodal conduction [62,63]. Ablation can be successfully performed without AV nodal damage targeting the nodal fascicular or nodal ventricular fiber or in some instances in the region of the CS ostium [64,65]. True atrial ventricular tracts with decremental conduction (Mahaim) fibers are generally thought not to occur on the septum; however, some nodal fascicular tracts, especially when ablated in the posteroseptal region, may have represented true AV bypass tracts involving a small portion of the atrial myocardium ventricular to the tendon of Todaro. Although very rare, these variants should be kept in mind when approaching patients with septal accessory bypass tracts.
Figure 11.
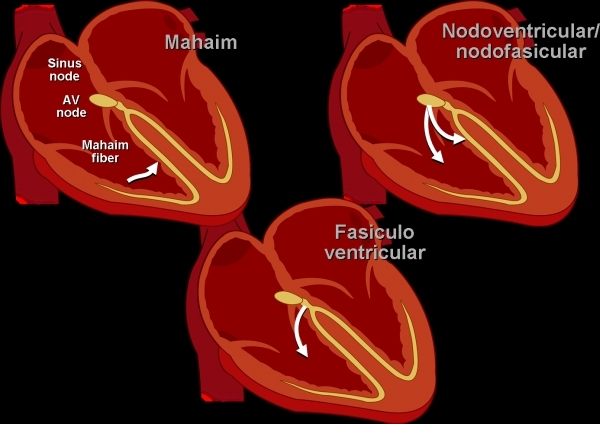
Figurative illustration of unusual pathways. Mahaim fibers are true atrial ventricular connection, however, the connection occurs through an AV node and Hispurkenje-like structure (Mahaim fiber). Fasciculoventricular tracts are not true accessory pathways, but rather “breaches in the insulation” of the proximal His bundle and right bundle region such that early activation of the basal portion of the right ventricle occurs with a short H-V interval and a suggestion of preexcitation. Nodal ventricular and nodal fascicular fibers are rare accessory pathways that may participate in tachycardia (see text for details).
Summary
Although mapping and ablation of accessory bypass tracts in patients with symptomatic tachycardias is a highly rewarding, successful, and safe procedure, septal pathways remain challenging. Posterior septal pathways, especially when epicardial and involving the coronary sinus myocardium or middle cardiac venous myocardium can be very difficult to ablate because of the proximity of the arterial system. Accurate knowledge of the anatomy involved, use of cryoenergy, adjunctive coronary angiography and intravenous ultrasound, and careful mapping within the veins or venous diverticula may lead to success.
Midseptal pathways are particularly likely to be complicated with damage to the compact AV node. In patients with anteroseptal pathways, careful delineation of the pathway potential with energy delivery sometimes involving the aortic cusps should be considered.
References
- Tai CT, et al. Characteristics and radiofrequency catheter ablation of septal accessory atrioventricular pathways. Pacing Clin Electrophysiol. 1999;22:500. doi: 10.1111/j.1540-8159.1999.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Jazayeri MR, et al. Posteroseptal accessory pathways: an overview of anatomical characteristics, electrocardiographic patterns, electrophysiological features, and ablative therapy. J Interv Cardiol. 1995;8:89. doi: 10.1111/j.1540-8183.1995.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Markwald R. Overview: Formation and Early Morphogenesis of the Primary Heart Tube. In: Clark EB, et al., editors. Developmental Mechanisms of Heart Disease. Armonk, NY: Futura Publishing Company, Inc; 1995. p. 149. [Google Scholar]
- Groot AG-d, et al. Overview: Cardiac Morphogenesis: Outflow Tract, Aortic Arch, and Conduction System. In: Clark EB, et al., editors. Etiology and Morphogenesis of Congenital Heart Disease: Twenty Years of Progree in Genetics and Developmental Biology. Armonk, NY: Futura Publishing Company, Inc; 2000. p. 191. [Google Scholar]
- Ho SY. Accessory atrioventricular pathways: getting to the origins. Circulation. 2008;117:1502. doi: 10.1161/CIRCULATIONAHA.107.764035. [DOI] [PubMed] [Google Scholar]
- Kolditz DP. Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways. Circulation. 2008;117:1508. doi: 10.1161/CIRCULATIONAHA.107.726315. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, et al. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- Kuck K, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways. Lancet. 1991:1557. doi: 10.1016/0140-6736(91)93258-b. [DOI] [PubMed] [Google Scholar]
- Schluter M, et al. Catheter ablation using radiofrequency current to cure symptomatic patients with tachyarrhythmias related to an accessory atrioventricular pathway. Circulation. 1991;84:1644. doi: 10.1161/01.cir.84.4.1644. [DOI] [PubMed] [Google Scholar]
- Lorga Filho A, et al. Electrocardiographic identification of mid-septal accessory pathways in close proximity to the atrioventricular conduction system. Pacing Clin Electrophysiol. 1996;19:1984. doi: 10.1111/j.1540-8159.1996.tb03266.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez LM, et al. The 12-lead electrocardiogram in midseptal, anteroseptal, posteroseptal and right free wall accessory pathways. Am J Cardiol. 1993;72:1274. doi: 10.1016/0002-9149(93)90296-o. [DOI] [PubMed] [Google Scholar]
- Tai CT, et al. Electrocardiographic and electrophysiologic characteristics of anteroseptal, midseptal, and para-Hisian accessory pathways. Implication for radiofrequency catheter ablation. Chest. 1996;109:730. doi: 10.1378/chest.109.3.730. [DOI] [PubMed] [Google Scholar]
- Dhala AA, et al. Transcatheter ablation of posteroseptal accessory pathways using a venous approach and radiofrequency energy. Circulation. 1994;90:1799. doi: 10.1161/01.cir.90.4.1799. [DOI] [PubMed] [Google Scholar]
- Takenaka S, et al. Characteristics and radiofrequency ablation in posteroseptal and left free-wall subepicardial accessory pathways. J Electrocardiol. 2005;32:69. doi: 10.1016/j.jelectrocard.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Soejima Y, et al. Radiofrequency catheter ablation of posteroseptal atrioventricular accessory pathways-location-specific electrographic characteristics of successful ablation sites. Jpn Circ J. 1997;61:46. doi: 10.1253/jcj.61.46. [DOI] [PubMed] [Google Scholar]
- Guiraudon GM. Surgical ablation of posterior septal accessory pathways in the Wolff-Parkinson-White syndrome by a closed heart technique. J Thorac Cardiovasc Surg. 1986;92:406. [PubMed] [Google Scholar]
- Tebbenjohanns J, et al. Direct angiography of the coronary sinus: impact on left posteroseptal accessory pathway ablation. Pacing Clin Electrophysiol. 1996;19:1075. doi: 10.1111/j.1540-8159.1996.tb03416.x. [DOI] [PubMed] [Google Scholar]
- Weiss C, et al. Prospective evaluation of the coronary sinus anatomy in patients undergoing electrophysiologic study. Clin Cardiol. 1999;22:537. doi: 10.1002/clc.4960220810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatzoulis KA, et al. Radiofrequency catheter ablation of posteroseptal accessory pathways--results of a step-by-step ablation approach. J Interv Card Electrophysiol. 2001;5:193. doi: 10.1023/a:1011489710747. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, et al. Catheter ablation of paroxysmal supraventricular tachycardia. Circulation. 2007;116:2465. doi: 10.1161/CIRCULATIONAHA.106.655746. [DOI] [PubMed] [Google Scholar]
- Jackman WM, et al. Localization of left free-wall and posteroseptal accessory atrioventricular pathways by direct recording of accessory pathway activation. Pacing Clin Electrophysiol. 1989;12:204. doi: 10.1111/j.1540-8159.1989.tb02648.x. [DOI] [PubMed] [Google Scholar]
- Arruda MS, et al. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol. 1998;9:2. doi: 10.1111/j.1540-8167.1998.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Wen MS, et al. Radiofrequency ablation therapy of the posteroseptal accessory pathway. Am Heart J. 1996;132:612. doi: 10.1016/s0002-8703(96)90246-x. [DOI] [PubMed] [Google Scholar]
- Kusano KF, et al. Catheter ablation of an epicardial accessory pathway via the middle cardiac vein guided by monophasic action potential recordings. Europace. 2001;3:164. doi: 10.1053/eupc.2001.0161. [DOI] [PubMed] [Google Scholar]
- Otomo K, et al. Reversing the direction of paced ventricular and atrial wavefronts reveals an oblique course in accessory AV pathways and improves localization for catheter ablation. Circulation. 2001;104:550. doi: 10.1161/hc3001.093499. [DOI] [PubMed] [Google Scholar]
- Gonzalez MD, et al. Transseptal left heart catheterization for cardiac ablation procedures. J Interv Card Electrophysiol. 2001;5:89. doi: 10.1023/a:1009818210563. [DOI] [PubMed] [Google Scholar]
- Bashir Y, et al. Radiofrequency current delivery by way of a bipolar tricuspid annulus-mitral annulus electrode configuration for ablation of posteroseptal accessory pathways. J Am Coll Cardiol. 1993;22:550. doi: 10.1016/0735-1097(93)90063-7. [DOI] [PubMed] [Google Scholar]
- Moulton K. Ablation of posteroseptal accessory atrioventricular pathways. In: Huang Y, editor. Radiofrequency Catheter Ablation of Cardiac Arrhythmias. New York: Futura Publishing Company, Inc; 1995. p. 289. [Google Scholar]
- Aoyama H, et al. Comparison of cryothermia and radiofrequency current in safety and efficacy of catheter ablation within the canine coronary sinus close to the left circumflex coronary artery. J Cardiovasc Electrophysiol. 2005;16:1218. doi: 10.1111/j.1540-8167.2005.50126.x. [DOI] [PubMed] [Google Scholar]
- Bokenkamp R, et al. Effects of intracardiac radiofrequency current application on coronary artery vessels in young pigs. J Cardiovasc Electrophysiol. 2000;11:565. doi: 10.1111/j.1540-8167.2000.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Paul T, et al. Coronary artery involvement early and late after radiofrequency current application in young pigs. Am Heart J. 1997;133:436. doi: 10.1016/s0002-8703(97)70185-6. [DOI] [PubMed] [Google Scholar]
- Roberts-Thomson KC. Coronary artery injury due to catheter ablation in adults: presentations and outcomes. Circulation. 2009;120:1465. doi: 10.1161/CIRCULATIONAHA.109.870790. [DOI] [PubMed] [Google Scholar]
- Calkins H, et al. Radiofrequency catheter ablation of accessory atrioventricular connections in 250 patients. Abbreviated therapeutic approach to Wolff-Parkinson-White syndrome. Circulation. 1992;85:1337. doi: 10.1161/01.cir.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Scheinman MM, et al. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol. 2000;23:1020. doi: 10.1111/j.1540-8159.2000.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Dinckal H, et al. Left anterior descending coronary artery occlusion after left lateral free wall accessory pathway ablation: what is the possible mechanism? Europace. 2003;5:263. doi: 10.1016/s1099-5129(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Schneider HE. Incidence of coronary artery injury immediately after catheter ablation for supraventricular tachycardias in infants and children. Heart Rhythm. 2009;6:461. doi: 10.1016/j.hrthm.2009.01.029. [DOI] [PubMed] [Google Scholar]
- Spar DS, et al. Coronary Artery Spasm During Radiofrequency Ablation of a Left Lateral Accessory Pathway. Pediatr Cardiol. 2010;31:724. doi: 10.1007/s00246-010-9670-4. [DOI] [PubMed] [Google Scholar]
- D'Avila A, et al. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implications for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing Clin Electrophysiol. 2002;25:1488. doi: 10.1046/j.1460-9592.2002.01488.x. [DOI] [PubMed] [Google Scholar]
- de Paola AA, et al. Mechanical reperfusion of acute right coronary artery occlusion after radiofrequency catheter ablation and long-term follow-up angiography. J Invasive Cardiol. 2003;15:173. [PubMed] [Google Scholar]
- Duong T, et al. Acute right coronary artery occlusion in an adult patient after radiofrequency catheter ablation of a posteroseptal accessory pathway. J Invasive Cardiol. 2004;16:657. [PubMed] [Google Scholar]
- Khanal S, et al. Right coronary artery occlusion as a complication of accessory pathway ablation in a 12-year-old treated with stenting. Catheter Cardiovasc Interv. 1999;46:59. doi: 10.1002/(SICI)1522-726X(199901)46:1<59::AID-CCD16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Strobel GG, et al. Successful pediatric stenting of a nonthrombotic coronary occlusion as a complication of radiofrequency catheter ablation. Pacing Clin Electrophysiol. 2001;24:1026. doi: 10.1046/j.1460-9592.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- Al-Ammouri I, et al. Proximity of coronary arteries to the atrioventricular valve annulus in young patients and implications for ablation procedures. Am J Cardiol. 2006;97:1752. doi: 10.1016/j.amjcard.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Hasdemir C, et al. Angiographic analysis of the anatomic relation of coronary arteries to mitral and tricuspid annulus and implications for radiofrequency ablation. Am J Cardiol. 2007;100:666. doi: 10.1016/j.amjcard.2007.03.082. [DOI] [PubMed] [Google Scholar]
- Asirvatham SJ. Difficulties with ablation for arrhythmias in children. Indian Pacing Electrophysiol J. 2008;8:S55. [PMC free article] [PubMed] [Google Scholar]
- Collins KK, et al. Cryoablation of accessory pathways in the coronary sinus in young patients: a multicenter study from the Pediatric and Congenital Electrophysiology Society's Working Group on Cryoablation. J Cardiovasc Electrophysiol. 2007;18:592. doi: 10.1111/j.1540-8167.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- Gist KM, et al. Acute success of cryoablation of left-sided accessory pathways: a single institution study. J Cardiovasc Electrophysiol. 2009;20:637. doi: 10.1111/j.1540-8167.2008.01375.x. [DOI] [PubMed] [Google Scholar]
- Tuzcu V. Cryoablation of accessory pathways in children. Pacing Clin Electrophysiol. 2007;30:1129. doi: 10.1111/j.1540-8159.2007.00824.x. [DOI] [PubMed] [Google Scholar]
- Gaita F, et al. Catheter ablation of permanent junctional reciprocating tachycardia with radiofrequency current. J Am Coll Cardiol. 1995;25:648. doi: 10.1016/0735-1097(94)00455-Y. [DOI] [PubMed] [Google Scholar]
- Manita M, et al. Electrophysiological characteristics and radiofrequency ablation of accessory pathways with slow conductive properties. Circ J. 2004;68:1152. doi: 10.1253/circj.68.1152. [DOI] [PubMed] [Google Scholar]
- Gaita F, et al. Cryoenergy catheter ablation: a new technique for treatment of permanent junctional reciprocating tachycardia in children. J Cardiovasc Electrophysiol. 2004;15:263. doi: 10.1046/j.1540-8167.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- Meiltz A, et al. Permanent form of junctional reciprocating tachycardia in adults: peculiar features and results of radiofrequency catheter ablation. Europace. 2006;8:21. doi: 10.1093/europace/euj007. [DOI] [PubMed] [Google Scholar]
- Kugler JD, et al. Pediatric radiofrequency catheter ablation registry success, fluoroscopy time, and complication rate for supraventricular tachycardia: comparison of early and recent eras. J Cardiovasc Electrophysiol. 2002;13:336. doi: 10.1046/j.1540-8167.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- Schaffer MS, et al. Inadvertent atrioventricular block during radiofrequency catheter ablation. Results of the Pediatric Radiofrequency Ablation Registry. Pediatric Electrophysiology Society. Circulation. 1996;94:3214. doi: 10.1161/01.cir.94.12.3214. [DOI] [PubMed] [Google Scholar]
- Mandapati R, et al. Radiofrequency catheter ablation of septal accessory pathways in the pediatric age group. Am J Cardiol. 2003;92:947. doi: 10.1016/s0002-9149(03)00975-5. [DOI] [PubMed] [Google Scholar]
- Balasundaram R, et al. Successful targeted ablation of the pathway potential in the noncoronary cusp of the aortic valve in an infant with incessant orthodromic atrioventricular reentrant tachycardia. J Cardiovasc Electrophysiol. 2009;20:216. doi: 10.1111/j.1540-8167.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- Huang H, et al. Catheter ablation of anteroseptal accessory pathway in the non-coronary aortic sinus. Europace. 2006;8:1041. doi: 10.1093/europace/eul122. [DOI] [PubMed] [Google Scholar]
- Suleiman M, et al. Successful cryoablation in the noncoronary aortic cusp for a left anteroseptal accessory pathway. J Interv Card Electrophysiol. 2008;23:205. doi: 10.1007/s10840-008-9294-2. [DOI] [PubMed] [Google Scholar]
- Tabatabaei N, et al. Supravalvular arrhythmia: identifying and ablating the substrate. Circ Arrhythm Electrophysiol. 2009;2:316. doi: 10.1161/CIRCEP.108.847962. [DOI] [PubMed] [Google Scholar]
- Suleiman M. The Non-Coronary Cusp as a Site for Successful Ablation of Accessory Pathways - Electrogram Characteristics in Three Cases. J Interv Card Electrophysiol. 2010 doi: 10.1111/j.1540-8167.2010.01811.x. Accepted, publication pending. [DOI] [PubMed] [Google Scholar]
- Sternick EB, et al. Fasciculoventricular pathways: clinical and electrophysiologic characteristics of a variant of preexcitation. J Cardiovasc Electrophysiol. 2003;14:1057. doi: 10.1046/j.1540-8167.2003.03206.x. [DOI] [PubMed] [Google Scholar]
- Mantovan R, et al. Orthodromic tachycardia with atrioventricular dissociation: evidence for a nodoventricular (Mahaim) fiber. Pacing Clin Electrophysiol. 2000;23:276. doi: 10.1111/j.1540-8159.2000.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Hluchy J, et al. Electrophysiologic characteristics and radiofrequency ablation of concealed nodofascicular and left anterograde atriofascicular pathways. J Cardiovasc Electrophysiol. 2000;11:211. doi: 10.1111/j.1540-8167.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- Abbott JA, et al. Coexistent Mahaim and Kent accessory connections: diagnostic and therapeutic implications. J Am Coll Cardiol. 1987;10:364. doi: 10.1016/s0735-1097(87)80020-7. [DOI] [PubMed] [Google Scholar]
- Kottkamp H, et al. Variants of preexcitation--specialized atriofascicular pathways, nodofascicular pathways, and fasciculoventricular pathways: electrophysiologic findings and target sites for radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 1996;7:916. doi: 10.1111/j.1540-8167.1996.tb00466.x. [DOI] [PubMed] [Google Scholar]


