Abstract
Background
Chlamydia trachomatis (Ct) and Chlamydophila (Chlamydia) pneumoniae (Cpn) are known triggers of reactive arthritis (ReA). These chlamydial species exist in a persistent metabolically active infection state in the synovium suggesting that persistent chlamydiae may be susceptible to antimicrobial agents. The goal of this study was to investigate whether a six-month course of combination antibiotics is an effective therapy for patients with chronic Chlamydia-induced ReA.
Methods
This study was a 9-month, double-blind, triple-dummy prospective trial assessing a 6-month course of combination antibiotics as a treatment for Chlamydia-induced ReA. Eligible patients were age 18 to 70 years, fulfilled the European Spondyloarthropathy Study Group (ESSG) Criteria, and had disease duration equal to or longer than 6 months. Subjects had to be polymerase chain reaction (PCR)-positive for Ct or Cpn in order to be randomized to therapy; randomization was performed in a 1:1:1 fashion. Treatment was for 6 months; the 3 groups included doxycycline 100mg twice daily and rifampin 300mg daily, azithromycin 500mg daily × 5 days then twice weekly and rifampin 300mg daily, or matching placebos. The primary efficacy endpoint was to assess the number of responders in the combination antibiotic group vs. placebo at month 6 compared to baseline. Responders were defined as those subjects who improved 20% or more in at least 4 of 6 variables without worsening in any one variable.
Results
80 subjects were screened and 42 were randomized to treatment (27 to combination antibiotics and 15 to placebo). Subjects in each group had similar demographics and baseline characteristics. At month 6, 17/27 subjects (63%) randomized to combination antibiotics were responders compared to 3/15 (20%) on placebo (P-value = 0.01). Secondary efficacy endpoints showed similar results with significant improvement in the modified swollen joint count, tender joint count, physician global assessment (P-values 0.0007, 0.002, and 0.0009, respectively), and a trend with the erythrocyte sedimentation rate (P-value = 0.07) in those patients on combination antibiotics compared to placebo. 6/27 (22%) subjects on combination antibiotics experienced complete resolution of their symptoms whereas 0/15 subjects on placebo achieved this endpoint. There were significantly more subjects who became PCR negative at month 6 in the active therapy group than in the placebo group (P-Value = 0.03). Adverse events (AE's) were mild; there were no significant differences between the groups.
Conclusion
These data suggest that a 6-month course of combination antibiotics is an effective therapy for chronic Chlamydia-induced ReA.
Keywords: reactive arthritis, combination antibiotic treatment, Chlamydia trachomatis, Chlamydia pneumoniae
Chlamydia trachomatis (Ct), and various species in the Genera Salmonella, Shigella, Campylobacter, and Yersinia, all are known triggers of reactive arthritis (ReA).1 A number of studies also indicate that Chlamydophila (Chlamydia) pneumonaie (Cpn) is another, albeit less frequent, causative agent in ReA.2,3 C. trachomatis and C. pneumoniae are both commonly acquired asymptomatically4,5 making the causative trigger less clinically apparent in many cases.6 Polymerase chain reaction (PCR) technology occasionally has demonstrated the presence of chromosomal DNA from the known triggering organisms in the synovial tissue of patients with the post-dysentery form of ReA.7-10 This same technology has demonstrated the routine presence of both C. trachomatis and C. pneumoniae DNA in synovial tissue from patients with the post-chlamydial arthritis11-13. One important difference is that these chlamydial species exist in a persistent metabolically active infection state, whereas the post-enteric organisms do not (with the possible exception of Yersinia14), suggesting that persistent chlamydiae may be uniquely susceptible to antimicrobial agents.
Persistent chlamydial organisms exist in a morphologically aberrant but metabolically active state in synovial tissue.12,15 The pattern of gene expression is significantly different in the persistent infection state compared to that characteristic of normal active infection. During persistence, expression of the major outer membrane protein gene (omp1) and several genes required for the cell division process are severely down-regulated. This is coupled with differential up-regulation of the three paralog genes specifying the C. trachomatis heat shock proteins (HSP)-60 (Ct110, Ct604, and Ct755)16.
The use of long-term antibiotic treatment for patients with ReA is controversial. Several published studies have indicated that prolonged antimicrobial monotherapy is not efficacious17-20. Indeed, in vitro data confirm these findings21-24. Yet other studies suggest there might be a benefit, specifically with Chlamydia-induced ReA25,26 and that this treatment approach might also work better in early disease.27 Conversely, a recent study suggested no benefit with a 4 month course of doxycycline in Chlamydia-induced ReA.28 Rifampin has excellent tissue penetration, which is mandatory when treating obligate intracellular pathogens such as chlamydiae. Rifampin also has been shown to attenuate all chlamydial gene transcription, including the HSP's.29 The latter may prime the infected cell for eradication,30,31 allow for proper apoptosis,32,33 and/or eliminate the immunogenic source.34 Combining this effect with antibiotics that block chlamydial protein synthesis (e.g., doxycycline or azithromycin) may allow for successful eradication of the cell harboring persistently infecting intracellular organisms. Interestingly, the same in vitro data cited above suggest synergistic eradication of the persistent chlamydial infection with a combination of azithromycin and rifampin.22
A recent pilot study from our group suggested that prolonged treatment with a combination of doxycycline and rifampin significantly improves symptoms of chronic undifferentiated spondyloarthropathy (with a special focus on Chlamydia) compared to doxycycline alone.35 The goal of the present study was to further investigate whether a six-month course of combination antibiotics, one of which is rifampin, is an effective therapy for the treatment of patients with chronic Chlamydia-induced ReA.
Patients and Methods
Patients
Eligible patients were 18 to 70 years of age, fulfilled a modified European Spondyloarthropathy Study Group (ESSG) Criteria36 with a modification described below, and had disease duration equal to or longer than 6 months. Patients were excluded if they had current psoriasis, a history of ankylosing spondylitis or inflammatory bowel disease (IBD), previous exposure to antibiotics (> 2 weeks) as a potential treatment for their ReA, or a history of sensitivity or allergic reaction to rifampin, doxycycline, or azithromycin. Other standard exclusions applied. Concurrent treatment with disease-modifying antirheumatic drugs (DMARDs) and biologic agents were not exclusions.
For this study, the ESSG criteria were modified as inclusion criteria to increase the likelihood of specifically recruiting post-chlamydial ReA patients. The ESSG criteria include a past or present diagnosis of IBD and a present diagnosis of psoriasis. We used these as exclusion criteria since we did not want to enroll patients with IBD-related spondyloarthritis or patients with psoriatic arthritis. “An episode of acute diarrhea occurring within one month before arthritis” is another ESSG criterion. This, too, was used an exclusion in order to eliminate patients with post-dysentery ReA. The ESSG inclusion criteria were otherwise the same.
Study Design
This study was a 9-month, double-blind, triple-dummy prospective trial conducted at 4 centers in the US and Canada (ClinicalTrials.gov Identifier: NCT00351273). All participating sites received approval from their governing institutional review board (or equivalent); all patients provided informed written consent. This study was supported by a grant from the National Institutes of Health (AR-053646).
Individuals were recruited and screened from April 2006 through December 2007. At screening, their medical history was recorded and the modified ESSG criteria were carefully reviewed. Other information recorded included a complete physical examination, swollen and tender joint counts (SJC; TJC), a questionnaire regarding their duration and severity of low back and peripheral joint pain and stiffness, a Health Assessment Questionnaire (HAQ), HLA-B27 status, and a history of any known chlamydial exposures with detailed timing of that event (to both C. trachomatis and C. pneumonaie). Because of the study population, a previously established 76 SJC and 78 TJC scoring system (to include the distal interphalangeal and carpometacarpal joints) was utilized.37 A second modified swollen joint count was also recorded at all visits; this modified 76 SJC included dactylitis. Any subject with dactylitis had that added to the SJC; one swollen digit was counted equal to one swollen joint (this digit, however, was not counted twice). Dactylitis was not quantified; it was recorded in a qualitative manner only. A review of any recent pelvis or dedicated sacroiliac films by the PI was performed to assess for radiographic sacroiliitis.
At the screening visit all subjects had a blood sample taken and shipped from the clinical sites to APH's laboratory at ambient temperature via overnight courier. In those subjects with active synovitis and who gave consent, synovial tissue was obtained at the same time by blind synovial biopsy using a Parker-Pearson needle.38 The site biopsied was the knee in all subjects, with the exception of one sample obtained from the wrist by open surgical procedure. These samples were immediately snap-frozen in liquid nitrogen and stored at -80°C until shipping to APH's laboratory on dry ice, again using overnight courier.
In APH's laboratory, PCR was employed to assess chlamydial DNA in peripheral blood mononuclear cells (PBMCs) or synovial tissue, and these assays targeting C. trachomatis and C. pneumoniae have been described extensively.12,13,15,39,40 Screening PCR assays were performed in duplicate by each of two individuals, and standard analyses included assays targeting at least two different DNA sequences from each organism. The C trachomatis-directed assays targeted omp1 and the 16S rRNA genes. Assays to assess the presence of C pneumoniae DNA targeted the homologous genes in that organism; C trachomatis-directed primer systems do not amplify sequences in C pneumoniae and vice-versa. Samples were considered positive if both duplicates in all assays agreed. In the case of discrepancies, a third set of assays targeting several other genes was used for resolution. Extreme care was taken to avoid contamination of PCR-related materials.
Treatment and Testing
Those subjects who were PCR-positive for either chlamydial species in PBMCs and/or synovial tissue were randomly assigned (1:1:1) to 1 of 3 treatment groups; randomization was stratified by age (less than or greater than 40 years) and disease duration (0.5 to 2 years or more than 2 years) in order to achieve a more homogeneous patient population in each of the 3 groups. Group 1 received doxycycline 100mg by mouth twice daily and rifampin 300mg by mouth daily plus azithromycin placebo; Group 2 received azithromycin 500mg by mouth daily for 5 days and then 500mg by mouth twice weekly and rifampin 300mg by mouth daily plus doxycycline placebo; Group 3 received azithromycin, doxycycline, and rifampin placebos. All three groups were treated for a total of 6 months and followed for 3 months after completion of the study drugs (total of 9 months). The purpose of the month 9 visit was to assess if the patient's condition had worsened after discontinuing their study medications.
In addition to the study drugs, subjects were allowed to take oral corticosteroids (less than/equal to 10mg/day prednisone or equivalent) and/or nonsteroidal anti-inflammatory drugs if they were on stable doses for more than 4 weeks prior to randomization; DMARDs and biologic agents were allowed if on stable doses more than 12 weeks prior to randomization. The doses of these medications could not be increased; however, if the subject experienced clinical improvement during the study, downward adjustment of these background medications was allowed.
Study subjects were evaluated at screening, baseline, and then at months 1, 3, 6, and 9. C. trachomatis and C. pneumonaie serum titers were checked at baseline, but were not rechecked at any point during the study. Urogenital chlamydial testing was not performed. Disease activity assessments were performed at each study visit. Study subjects were queried at each visit as to whether they felt their disease was in remission. Disease remission was a strict, but subjective, determination by the study subject requiring that the patient felts their disease-related symptoms were “100% resolved”. At each visit, subjects had an ESR; a hsCRP was performed at baseline and months 1, 3, and 6. Safety assessments were monitored routinely, including completion of an adverse event form, at each visit. Adverse events were predefined as any new medical diagnosis or condition; serious adverse events were predefined as those that included hospitalization, intravenous antibiotics, or death.
PCR results were followed in a blinded fashion throughout the study. All randomized subjects had a PBMC PCR for each chlamydial species repeated at months 1, 6, and 9 in addition to initial screening. If they consented, each subject who had a positive synovial tissue PCR at screening had a repeat synovial biopsy of the same joint at month 6.
Statistical Analysis
We estimated a total sample size of 42 subjects, calculated with an 80% power to detect a 73% improvement in the group receiving combination antibiotics and a 20% improvement in the placebo group with a significance level of 0.05. 73% of the patients who received combination antibiotics in our pilot trial were considered responders; only 13% in the monotherapy group responded.34 Since the therapy in the pilot trial was not blinded to the patient, we increased the expected placebo response rate in this study. Patients were randomized in a 2:1 fashion (combination antibiotics vs. placebo). The study was not powered to detect an efficacy difference between the two active combination antibiotic groups.
The primary endpoint in this study was to assess the number of responders in the combination antibiotic group vs. placebo at month 6 compared to baseline. Responders were defined as those subjects who improved 20% or more in at least 4 of 6 variables without worsening in any one variable. These 6 variables included a modified 76 SJC (as described above), 78 TJC, and the 4 component questionnaire (average duration of morning stiffness in their low back per day over the past week, current low back pain visual analog scale (VAS), current peripheral joint pain VAS, and global VAS). This questionnaire was utilized in previous ReA studies.35,41
Efficacy and safety analyses were performed on an intention-to-treat (ITT) basis. Subjects who prematurely withdrew or who were lost to follow-up for any reason were included in the ITT population and were considered non-responders. For the primary efficacy analysis, the Fisher's Exact Test 2×2 contingency table was used to compare the difference in responders in those on combination antibiotics vs. those on placebo at month 6.
Baseline study subject demographics were analyzed using the Fisher's Exact Test 2×2 contingency table or unpaired t-tests depending on whether the data were categorical or quantifiable. The secondary efficacy analyses included each component of the responder determination in an independent fashion; other secondary efficacy analyses included the Physician global VAS, ESR, hsCRP, and the HAQ. These were analyzed using unpaired t-tests. Remission rates and PCR results were analyzed in a similar fashion to the primary endpoint using the Fisher's Exact Test.
Results
Study Subject Characteristics
Study data were collected between April 2006 and October 2008. 80 subjects total were screened, with 42 enrolled and randomized to treatment. 12 subjects were randomized to receive doxycycline and rifampin, 15 subjects were randomized to azithromycin and rifampin, and 15 subjects were randomized to matching oral placebos. Table 1 summarizes the demographics and baseline characteristics of those subjects on combination antibiotics vs. those on placebo. More males than females were enrolled, and the mean age ranged from 44.2 years to 49 years. These subjects had chronic disease with mean disease duration of greater than 10 years. The only baseline subject characteristic with a statistical difference between study groups was that subjects randomized to combination antibiotics were statistically more likely to have a history of iritis. The majority of randomized subjects were had positive chlamydial serologies at baseline; specifically 35/42, 9/42, 2/42, and 1/42 subjects were positive for Cpn IgG, Cpn IgA, Ct IgG, and Ct IgA, respectively.
Table 1. Baseline Characteristics and Demographics.
| Characteristic | Combination Antibiotics (n = 27) | Placebo (n = 15) | P-value |
|---|---|---|---|
| Age, years | 44.2 +/- 12.3 | 49.0 +/- 16.4 | 0.28 |
| Male, no. (%) | 15 (56%) | 9 (60%) | 1.0 |
| Race | 18 Caucasian; 9 African American |
9 Caucasian; 5 African American; 1 other |
0.73 |
| Disease Duration, years | 10.4 +/- 12.1 | 14.2 +/- 14.2 | 0.37 |
| SJC (of 66 joints) | 3.4 +/- 2.4 | 3.8 +/- 2.7 | 0.67 |
| TJC (of 68 joints) | 5.0 +/- 4.3 | 7.9 +/- 7.4 | 0.12 |
| Duration of Morning Low Back Stiffness, hours | 1.7 +/- 1.4 | 1.0 +/- 0.9 | 0.12 |
| Patient Global VAS (0: worst; 100: best) | 44.9 +/- 23.2 | 40.3 +/- 22.9 | 0.54 |
| Physician Global VAS (0: best; 100: worst) | 59.9 +/- 12.8 | 61.6 + 15.8 | 0.70 |
| HAQ DI Score | 0.77 +/- 0.46 | 0.93 +/- 0.61 | 0.33 |
| Axial Arthritis, no. (%) | 20 (74%) | 12 (80%) | 1.0 |
| Peripheral Arthritis, no. (%) | 26 (96%) | 13 (87%) | 0.29 |
| Active Enthesitis, no. (%) | 17 (63%) | 9 (60%) | 1.0 |
| Active Dactylitis, no. (%) | 7 (26%) | 6 (40%) | 0.49 |
| Active Iritis, no. (%) | 3 (11%) | 0 (0%) | 0.54 |
| History of Iritis, no. (%) | 7 (26%)* | 0 (0%)* | 0.04 |
| Active KB, no. (%) | 3 (11%) | 0 (0%) | 0.54 |
| History of KB, no. (%) | 6 (22%) | 3 (20%) | 1.0 |
| Active CB, no. (%) | 0/15 (0%) | 1/9 (11%) | 0.38 |
| History of CB, no. (%) | 2/15 (13%) | 2/9 (22%) | 0.61 |
| Active Urethritis, no. (%) | 2 (7%) | 1 (7%) | 1.0 |
| History of Chlamydia trachomatis, no. (%) [at any point] | 14 (52%) | 5 (33%) | 0.34 |
| History of Chlamydia trachomatis, no. (%) [within 1 month of arthritis] | 3 (11%) | 1 (7%) | 1.0 |
| History of known Chlamydia pneumoniae infection, no. (%) | 0 (0%) | 0 (0%) | 1.0 |
| Use of NSAIDs, no. (%) | 20 (74%) | 11 (73%) | 1.0 |
| Use of corticosteroids, no. (%) | 4 (15%) | 0 (0%) | 0.28 |
| Use of DMARDs, no. (%) | 7 (26%)# | 3 (20%)## | 1.0 |
| Radiographic Sacroiliitis**, no. (%) | 19/20 (95%) | 8/10 (80%) | 0.25 |
| HLA-B27 Positive, no. (%) | 11/24 (46%) | 3/13 (23%) | 0.29 |
Statistically significant
DMARDs included: sulfasalazine (n=6); methotrexate (n=1)
DMARDs included: sulfasalazine (n=1); methotrexate (n=1); hydroxychloroquine (n=1)
Definition of sacroliliitis: asymmetric appearance with at least grade II unilateral changes. Routine radiographs of the sacroiliac joints were not part of the study protocol. These data were only collected if the study subjects had a previous radiograph performed as part of their routine clinical care. 30/42 (71%) of the study subjects had previous radiographs of the sacroiliac joints available for review.
The demographics and baseline characteristics of all 80 subjects screened were similar to the 42 subjects enrolled and randomized. Specifically, the mean age was 45.5 years (range 21-70 years) with mean disease duration of 9.9 years (range 0.5-40 years). Comparing baseline demographics and characteristics of the two combination antibiotic groups also yielded similar results. No statistically significant differences existed between the two active treatment groups (data not shown).
Treatment
All randomized study subjects received at least one dose of the study drugs and were included in the ITT and safety analysis. 34/42 subjects (81%) completed the 6 months of treatment; 3/27 subjects (11%) on the combination antibiotics and 5/15 (33%) in the placebo arm discontinued the study medications prematurely. Of the 3 subjects on combination antibiotics discontinuing early, 2 were on doxycycline and rifampin (reasons for discontinuation were peripheral edema and lost to follow-up) and 1 was on azithromycin and rifampin (reason for discontinuation was lost to follow-up). Regarding the 5 subjects on placebo, the reasons for discontinuation were lack of efficacy (1), nausea and diarrhea (1), seizures (1), withdrawal of consent (1), and lost to follow-up (1).
Efficacy
The primary endpoint was achieved in 17/27 (63%) of the subjects on combination antibiotics vs. 3/15 (20%) on placebo (P-value = 0.01). Regarding the six individual components that determined responder status, 5/6 improved significantly at 6 months compared to baseline in the combination antibiotic group (P-values <0.0001, <0.0001, 0.002, 0.01, 0.008 for the modified SJC, TJC, back pain VAS, peripheral joint pain VAS, and patient global VAS, respectively). The sixth component (AM low back stiffness) revealed a trend toward improvement at 6 months compared to baseline in those subjects on combination antibiotics (P-value = 0.068). In the placebo group, 0/6 components improved significantly at month 6 compared to baseline. Responders did not differ from non-responders regarding their baseline characteristics including age, sex, disease duration, axial and/or peripheral joint involvement, history of known chlamydial infections, or HLA-B27 status (data not shown).
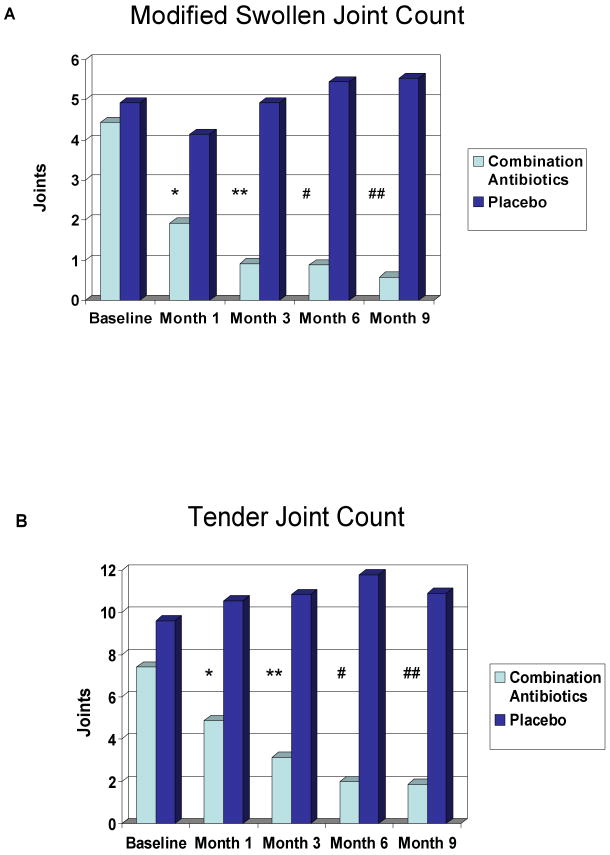
Regarding a head to head comparison of the modified SJC and TJC in those subjects on combination antibiotics vs. placebo, statistically significant differences were present in the modified SJC and TJC at month 6 (Figure 1). The modified SJC and the TJC were statistically significantly different at month 1 and at all subsequent time points in those subjects taking combination antibiotics vs. placebos (Figure 1).
Figure 1.
A. Modified Swollen Joint Count Legend:
P-values: *=0.0001; **<0.0001; #=0.0007; ##=0.0005
Fig 1A: Modified swollen joint counts in subjects on combination antibiotics versus placebo. There is a statistically significant difference at months 1, 3, 6, & 9.
B. Tender Joint Count Legend:
P-Values: *=0.0009; **<0.0001; #=0.002; ##=0.0004
Fig 1B: Tender joint counts in subjects on combination antibiotics versus placebo. There is a statistically significant difference at months 1, 3, 6, & 9.
All other secondary endpoints demonstrated either statistically significant improvement in the combination antibiotic treatment subjects vs. those on placebo or a trend towards improvement (Table 2). We found no significant worsening in any of the endpoints from months 6 to 9. In terms of dactylitis, 7/27 (26%) subjects randomized to combination antibiotics and 4/15 (27%) placebo subjects had this finding at baseline; all 7 subjects on combination antibiotics had resolution of their dactylitis at month 6, yet only 2/4 placebo subjects experienced resolution. Regarding background DMARD usage, downward adjustments were allowed if the subject felt better. Of the 7 subjects on DMARD's at baseline in the combination antibiotic group, 4 discontinued their DMARD during the trial (all 4 on sulfasalazine; 2 discontinued at month 1, 1 at month 3, and 1 at month 6); 0/3 placebo subjects discontinued their background DMARD because of clinical improvement. Four subjects randomized to active therapy were on chronic corticosteroids at baseline; one discontinued use, one decreased the daily usage, and the other two remained on a stable dose throughout the trial. A final secondary endpoint followed was remission. 6/27 subjects (22%) randomized to combination antibiotics felt that their disease went into complete remission during the trial, whereas no subject in the placebo arm achieved remission (P-value = 0.07). All subjects who achieved remission reached this mark by month 3 or month 6, and all 6 remained in remission at month 9. The average disease duration of the 6 subjects who achieved remission was 8.5 years. 5/6 of the subjects who achieved remission were randomized to the azithromycin and rifampin group.
Table 2. Secondary Endpoints.
| Combination Antibiotics (n=27) | Placebo (n=15) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 1 | Month 3 | Month 6 | Month 9 | Baseline | Month 1 | Month 3 | Month 6 | Month 9 | P-Value* | |
| Physican Global VAS | 63.9 | 35.2 | 23.2 | 16.2 | 17.7 | 60.2 | 52.9 | 49.4 | 45.7 | 43.8 | 0.0009; 0.0003 |
| HAQ-DI | 0.84 | 0.79 | 0.68 | 0.71 | 0.57 | 1.1 | 0.92 | 0.87 | 0.99 | 0.92 | 0.19; 0.13 |
| ESR | 25.1 | 17.8 | 17.7 | 12.7 | 14.0 | 18.9 | 25.2 | 19.8 | 17 | 18.4 | 0.07; 0.02 |
| hsCRP | 1.07 | 0.56 | 0.63 | 0.41 | TNP | 0.42 | 0.27 | 0.55 | 0.34 | TNP | 0.11 |
P-values at month 6 and month 9 (combination antibiotics vs. placebo).
TNP = test not performed
A post-hoc analysis was performed to determine the number of subjects who improved 50% and 70% or more in at least 4 of the 6 same variables that were utilized to determine responders. Of the 27 subjects randomized to combination antibiotics, 11/27 (41%) were 50% responders and 7/27 (26%) were 70% responders. 5/15 subjects randomized to azithromycin and rifampin and 2/12 randomized to doxycycline and rifampin were 70% responders. 5/6 of the subjects who felt their disease went into remission were 70% responders. Regarding the 3 placebo responders, 1/15 (7%) met the 70% response criteria.
PCR Analyses
All subjects had to be PCR-positive for either C. trachomatis or C. pneumoniae on PBMC or synovial tissue in order to be randomized to treatment. Of the 80 subjects screened, 45 met these criteria. Three PCR-positive subjects withdrew consent prior to randomization, leaving 42 PCR-positive subjects for randomization. 26/80 (33%) subjects screened had a synovial biopsy; of these 16/26 (62%) were PCR-positive for chlamydiae, and 15/16 PCR positive subjects were randomized to treatment. Of the 15 subjects randomized based on positive synovial tissue analysis, 5 were also PCR positive on PBMC. 27/42 subjects (64%) were randomized based on a PCR-positive PBMC analysis for chlamydiae (no synovial tissue was obtained in these 27 subjects).
Of the 27 subjects randomized based on the PBMC PCR-positivity for chlamydiae, 17 were on active therapy and 10 on placebo (Table 3). 12/17 subjects on active therapy became PCR-negative at month 6, and 3/10 on placebo cleared at month 6 (P-value = 0.057) [subjects without a blood sample at month 6 were considered PCR failures]. 7/15 subjects randomized based on a positive PCR synovial tissue analysis had a synovial biopsy repeated in the same joint after 6 months of treatment; 6 of these were randomized to combination antibiotics and one to placebo. 4/6 subjects on active therapy were PCR-negative in synovial tissue at month 6; the subject on placebo with synovial tissue available at month 6 remained PCR-positive. Combining the PBMC and available synovial tissue PCR data, 16/23 (70%) subjects in the combination antibiotic and 3/11 (27%) on placebo cleared at month 6 (P-value = 0.03).
Table 3. PCR Results.
| Combination Antibiotics (n=27) | Placebo (n=15) | ||||
|---|---|---|---|---|---|
| [17 PBMC +; 10 Synovial Tissue +] | [10 PBMC +; 5 Synovial Tissue +] | ||||
| Screening PCR | Month 6 PCR | Screening PCR | Month 6 PCR | ||
| PBMC + for Ct, no. | 12 | 3 | 7 | 5 | |
| PBMC + for Cpn, no. | 3 | 2 | 2 | 1 | |
| PBMC + for Ct & Cpn, no. | 2 | 0 | 1 | 1 | |
| PBMC Clearance at Month 6, no. (%) | n/a | 12/17 (71%) | n/a | 3/10 (30%) | |
| Synovial Tissue + for Ct, no. | 6 | 2 of 4 | 3 | 0 of 1 | |
| Synovial Tissue + for Cpn, no. | 3 | 0 of 2 | 1 | TNP | |
| Synovial Tissue + for Ct & Cpn, no. | 1 | TNP | 1 | TNP | |
| Synovial tissue Clearance at Month 6, no. (%) | n/a | 4/6 (66%) | n/a | 0/1 (0%) | |
TNP = test not performed
n/a = not applicable
As stated, there were a total of 17 responders who received combination antibiotics and 3 responders in the placebo group. 2/17 responders on active therapy who were randomized based on a PCR-positive synovial tissue analysis did not have a repeat synovial biopsy at month 6, leaving 15 subjects with PCR data at baseline and month 6. 13/15 (87%) of these responders became PCR-negative for chlamydiae at month 6. Of the 3 placebo responders, 1 became PCR-negative at month 6.
PBMC PCR analyses were performed again at month 9 (3 months after completion of the study drugs). Regarding the 12/17 subjects whose PBMC PCR became negative at month 6, all but two remained negative at month 9.
Safety
Table 4 lists the serious adverse events (SAE's) and any adverse events that occurred more than once in either the combination antibiotic or placebo group. Although both SAE's occurred in the placebo group, neither was felt to be related to the study drugs (seizures [1], stabbing [1]). None of the adverse events were statistically more likely to occur in those subjects on combination antibiotics compared to those on placebo. The most common adverse events in those subjects taking combination antibiotics were gastrointestinal in nature (nausea, diarrhea, abdominal pain, GERD). Only one study subject had to discontinue their study medications due to adverse gastrointestinal events; this subject was on placebo.
Table 4. Adverse Events.
| Adverse Event | Combination Antibiotics (n=27) | Placebo (n=15) | P-Value |
|---|---|---|---|
| Serious Adverse Events (SAE's), no. (%) | 0 (0%) | 2 (13%) | 0.12 |
| Subjects with Any Adverse Event (AE), no. (%) | 22 (81%) | 10 (66%) | 0.45 |
| Nausea | 6 (22%) | 1 (7%) | 0.39 |
| Abdominal Pain | 3 (11%) | 1 (7%) | 1.0 |
| Diarrhea | 5 (19%) | 1 (7%) | 0.40 |
| GERD | 2 (7%) | 0 (0%) | 0.53 |
| Arthralgia | 2(7%) | 1 (7%) | 1.0 |
| Rash | 2 (7%) | 0 (%) | 0.53 |
| Viral/Upper Respiratory Infection | 3 (11%) | 1 (7%) | 1.0 |
| Vaginal Candidiasis, no./females (%) | 2/12 (17%) | 1/6 (17%) | 1.0 |
Discussion
This randomized 9-month prospective, blinded study demonstrated that a 6 month course of combination antibiotics resulted in a significantly higher response rate in subjects with chronic Chlamydia-induced ReA compared to placebo. Although cases of acute ReA often remit spontaneously, the fact that all of the subjects in this study had disease duration of at least 6 months (with mean >10 years) makes it extremely unlikely that their ReA would improve or resolve spontaneously. Many of the individual clinical response measurements (e.g., modified SJC, TJC, Physician Global VAS, ESR) also significantly improved in the subjects who received active therapy compared to placebo. Twenty-two percent of the subjects on combination antibiotics felt that their ReA symptoms completely resolved. Finally, only those subjects who were PCR positive for chlamydiae were randomized to therapy, and there were significantly more subjects who became PCR negative at month 6 in the active therapy group than in the placebo group.
Importantly, the results presented here constitute the first blinded study to indicate a benefit of prolonged combination antimicrobial therapy in patients with chronic Chlamydia-induced ReA. The results of previous trials assessing antimicrobial therapy in the setting of ReA have been equivocal. One previous trial which suggested that antimicrobials are efficacious in the setting of ReA analyzed this treatment approach in acute ReA only and not in chronic patients.25 Notably, all previous trials evaluated antimicrobial monotherapy (rather than combination therapy) and only one28 attempted to restrict enrollment to patients with the post-chlamydial form of ReA. It seems likely to us that these two important changes in the study protocol resulted in improved efficacy. Our data suggest that it is advantageous to attempt attenuation of chlamydial production of HSP's with rifampin, which binds to the beta-subunit of prokaryotic RNA polymerase and thereby prevents initiation of transcription of HSP's29 in combination with antibiotics that block protein synthesis to ensure eradication of the persistent form of this organism. This is best accomplished when treated with a prolonged combination of antibiotics, as is the case with other persistent intracellular organisms (i.e., Mycobacterium tuberculosis and Helicobacter pylori).
Probably the most significant aspect of the present trial is that it suggests an avenue of effective therapy in a patient cohort with chronic arthritis not responsive to NSAIDs or DMARDs. Persistently infecting chlamydiae are recognized to be the driving force underlying Chlamydia-induced ReA, and organisms in that infectious state have been demonstrated to be refractory to antibiotic treatment.21 The data presented here indicate that while these viable, persistently infectious organisms respond poorly to single antibiotic treatment, they do appear to be susceptible to combination antimicrobial therapy. Thus, a cure theoretically exists. Our study subjects had mean disease duration of more than 10 years; more than 20% of patients on active therapy felt that their disease process did completely resolve and 26% of these same subjects met a 70% or greater response criteria by post-hoc analysis. This observation is strengthened by the fact that significantly more subjects on active therapy than those on placebo became PCR-negative at month 6. Further, there was no indication of clinical worsening from months 6 to 9, demonstrating that the response was sustained after cessation of antibiotic therapy.
The most common adverse events in subjects on active therapy were gastrointestinal in nature. This is in keeping with the known side effects of the medications studied. Doxycycline is known to cause GERD and esophagitis42 and azithromycin can cause nausea, abdominal pain, and diarrhea; this has been demonstrated in trials assessing the long-term administration of azithromycin in other disease states.43 These adverse events were mild in nature and no subject on active therapy had to discontinue their study drugs because of gastrointestinal complaints.
Some questions remain to be addressed. This study did not determine which combination of antibiotics is the most effective, and it was not powered to compare the two different antibiotic regimens. In spite of this limitation, 5/6 subjects who felt their disease went into remission were on the combination of azithromycin and rifampin and 33% of the subjects randomized to this treatment strategy achieved remission; these data suggest that this combination might be superior to the other tested. As stated, in vitro data has shown that this same combination of antibiotics is capable of eradicating chlamydiae in a cell culture model 8 days after infection.22 The most appropriate dosing for long-term administration remains unknown, particularly with azithromycin, and the proper duration of therapy can still be questioned. Of course, alternative antimicrobial combinations may well exist that could be more efficacious. The administration of long-term antibiotics also poses the risk of bacterial resistance. This could be true for the target organism itself or normal flora. The month 6 PCR data are reassuring that this is not the case for the former, but we have no long-term data in these patients regarding the latter. 12/17 subjects randomized based on a positive PBMC PCR cleared their PBMC PCR at month 6; 2 of these 12 again had a positive PBMC PCR at month 9 (both for C trachomatis). Although the numbers are small, this suggests possible reinfection from a sexual partner who is an asymptomatic carrier for that organism. More data are needed in this regard. Also, the vast majority of patients with ReA have peripheral arthritis and many have axial involvement. This was true of the patients in our trial, but 3 (7%) subjects had no peripheral arthritis (only axial arthritis) at screening. The relapsing course of the arthritis in some patients with chronic ReA is a possible explanation, but an alternate diagnosis, such as ankylosing spondylitis, cannot be excluded in these 3 subjects. Finally, the utility of diagnostic testing with PCR in similar patients in a clinical setting is still unproven. However, recent data suggest that synovial sample PCR analysis is the method of choice to establish the diagnosis of Chlamydia-induced arthritis in patients with ReA and undifferentiated oligoarthritis.44 This same study demonstrated that there is no correlation with chlamydial specific serological response. This was also true in our study. Other data agree that chlamydial serological testing is subjective45; it has also been demonstrated that chlamydial serologies have poor reproducibility46, may not be chlamydial species specific47, and perhaps not even chlamydial specific48. Because of the trial design, we have no data to address whether combination antibiotic treatment would be effective in undifferentiated SpA patients who are negative for chlamydiae on PCR testing. Potential for false positive PCR tests exists, particularly in those patients who are PCR-positive on PBMC for C pneumoniae. In this trial, 7 subjects were randomized based on a positive PBMC PCR for this respiratory pathogen (5 to active therapy, 2 to placebo). Of the 5 subjects on active therapy, only 1 was a responder. Perhaps the results could be improved upon by restricting treatment to those subjects who are PCR positive for C trachomatis.
The results of this study are encouraging for the management of chronic post-Chlamydia ReA. These data suggest that the potential for eradication of this persistent infection exist, and improvement in the clinical sequelae that are the result of these infections can be achieved in a substantial number of patients. Clearly, more studies are needed. A diagnostic test that is specific for Chlamydia-induced ReA would be important in designing such studies. The most efficacious combination of antimicrobial therapy, including dosing and duration, as a potential cure for Chlamydia-induced ReA warrants further study.
Acknowledgments
Supported by grant AR-053646 from the US National Institutes of Health.
References
- 1.Carter JD, Hudson AP. Reactive Arthritis: Clinical Aspects and Medical Management. Rheum Dis Clin North Am. 2009;35(1):21–44. doi: 10.1016/j.rdc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Laitko S, Treharne J, Eggens U, Wu P, Distler A, Sieper J. Chlamydia pneumoniae – a new causitive agent of reactive arthritis and undifferentiated oligoarthritis. Ann Rheum Dis. 1994;53(2):100–5. doi: 10.1136/ard.53.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hannu T, Puolakkainen M, Leirisalo-Repo M. Rheumatology (Oxford) 5. Vol. 38. 1999. Chlamydia pneumoniae as a triggering infection in reactive arthritis; pp. 411–4. [DOI] [PubMed] [Google Scholar]
- 4.Manavi K. A review on infection with Chlamydia trachomatis. Best Pract Res Clin Obstet Gynaecol. 2006;20(6):941–51. doi: 10.1016/j.bpobgyn.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Miyashita N, Niki Y, Nakajima M, Fukano H, Matsushima T. Prevalence of asymptomatic infection with Chlamydia pneumoniae in subjectively healthy adults. Chest. 2001;119(5):1416–9. doi: 10.1378/chest.119.5.1416. [DOI] [PubMed] [Google Scholar]
- 6.Carter JD, Gérard HC, Espinoza LR, Ricca LR, Valeriano-Marcet J, Snelgrove J, Oszust C, Vasey FB, Hudson AP. Chlamydiae as Etiologic Agents for Chronic Undifferentiated Spondyloarthropathy. Arthritis Rheum. 2009;60(5):1311–1316. doi: 10.1002/art.24431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun J, Tuszewski M, Eggens U, Mertz A, Schauer-Petrowskaja C, Döring E, Laitko S, Distler A, Sieper J, Ehlers S. Nested polymerase chain reaction strategy simultaneously targeting DNA sequences of multiple bacterial species in inflammatory joint diseases. I. Screening of synovial fluid samples of patients with spondyloarthropathies and other arthritides. J Rheumatol. 1997;24(6):1092–100. [PubMed] [Google Scholar]
- 8.Granfors K, Jalkanen S, Toivanen P, Koski J, Lindberg AA. Bacterial lipopolysaccharide in synovial fluid cells in Shigella triggered reactive arthritis. 1992;19(3):500. [PubMed] [Google Scholar]
- 9.Nikkari S, Merilahti-Palo R, Saario R, Soderstom KO, Granfors K, Skurnik M, Toivanen P. Yersinia-triggered reactive arthritis. Use of polymerase chain reaction and immunocytochemical in the detection of bacterial components from synovial specimens. Arthritis Rheum. 1992;35(6):682–7. doi: 10.1002/art.1780350613. [DOI] [PubMed] [Google Scholar]
- 10.Nikkari S, Rantakokko K, Ekman P, et al. Salmonella-triggered reactive arthritis: use of polymerase chain reaction, immunocytochemical staining, and gas-chromatography-mass spectrometry in the detection of bacterial components from synovial fluid. Arthritis Rheum. 1999;42(1):84–9. doi: 10.1002/1529-0131(199901)42:1<84::AID-ANR11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 11.Taylor-Robinson D, Gilroy CB, Thomas BJ, Keat AC. Detection of chlamydia trachomatis DNA in joints of reactive arthritis patients by polymerase chain reaction. Lancet. 1992;340(8811):81–2. doi: 10.1016/0140-6736(92)90399-n. [DOI] [PubMed] [Google Scholar]
- 12.Gerard HC, Branigan PJ, Schumacher HR, Jr, Hudson AP. Synovial chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25(4):734–42. [PubMed] [Google Scholar]
- 13.Gerard HC, Schumacher HR, El-Gabalawy H, Goldbach-Mansky R, Hudson AP. Chlamydia pneumoniae present in the human synovium are viable and metabolically active. Microb Pathog. 2000;29(1):17–24. doi: 10.1006/mpat.2000.0360. [DOI] [PubMed] [Google Scholar]
- 14.Gaston JS, Cox C, Granfors K. Clinical and experimental evidence for persistent Yersinia infection in reactive arthritis. Arthritis Rheum. 1999;42(10):2239–42. doi: 10.1002/1529-0131(199910)42:10<2239::AID-ANR29>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Gerard HC, Wang Z, Whittum-Hudson JA, et al. Cytokine and chemokine mRNA produced in synovial tissue chronically infected with Chlamydia trachomatis and C. pneumoniae. J Rheumatol. 2002;29(9):1827–35. [PubMed] [Google Scholar]
- 16.Gerard HC, Whittum-Hudson JA, Schumacher HR, Hudson AP. Differential expression of three Chlamydia trachomatis hsp60-encoding genes in active vs. persistent infections. Microb Pathog. 2004;36(1):35–9. doi: 10.1016/j.micpath.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, Mottonen T, Hakola M, Korpela M, Sanila M, Parviainen J, Uksila J, Vainionpaa R, Toivanen A. Effect of a three month course of ciprofloxacin on the outcome of reactive arthritis. Ann Rheum Dis. 2000;59(7):565–70. doi: 10.1136/ard.59.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kvien TK, Gaston JS, Bardin T, Butrimiene I, Dijkmans BA, Leirisalo-Repo M, Solakov P, Altwegg M, Mowinckel P, Plan PA, Vischer T. Three month treatment of reactive arthritis with azithromycin: a EULAR double blind, placebo controlled study. Ann Rheum Dis. 2004;63(9):1113–9. doi: 10.1136/ard.2003.010710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieper J, Fendler C, Laitko S, Sorensen H, Gripenberg-Lerche C, Hiepe F, Allen R, Keitel W, Groh A, Uksila J, Eggens U, Granfors K, Braun J. No benefit of long-term ciprofloxacin in patients with reactive arthritis and undifferentiated oligoarthritis: a three-month, multicenter, double-blind, randomized, placebo-controlled study. Arthritis Rheum. 1999;42(7):1386–96. doi: 10.1002/1529-0131(199907)42:7<1386::AID-ANR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Wakefield D, McCluskey P, Verma M, Aziz K, Gatus B, Carr G. Ciprofloxacin treatment does not influence course or relapse rate of reactive arthritis and anterior uveitis. Arthritis Rheum. 1999;42(9):1894–7. doi: 10.1002/1529-0131(199909)42:9<1894::AID-ANR14>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Dreses-Werringloer U, Padubrin I, Jurgens-Saathoff B, Hudson AP, Zeidler H, Kohler L. Persistence of Chlamydia trachomatis is induced by ciprofloxacin and ofloxacin in vitro. Antimicrob Agents Chemother. 2000;44(12):3288–97. doi: 10.1128/aac.44.12.3288-3297.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dreses-Werringloer U, Padubrin I, Zeidler H, Kohler L. Effects of azithromycin and rifampin on Chlamydia trachomatis infection in vitro. Antimicrob Agents Chemother. 2001;45(11):3001–8. doi: 10.1128/AAC.45.11.3001-3008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother. 2003;47(2):636–42. doi: 10.1128/AAC.47.2.636-642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrissey I, Salman H, Bakker S, Farrell D, Bebear CM, Ridgway G. Serial passage of Chlamydia spp. In sub-inhibitory fluoroquinolone concentrations. J Antimicrob Chemother. 2002;49(5):757–61. doi: 10.1093/jac/dkf031. [DOI] [PubMed] [Google Scholar]
- 25.Lauhio A, Leirisalo-Repo M, Lahdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis, with special reference to Chlamydia arthritis. Arthritis Rheum. 1991;34(1):6–14. doi: 10.1002/art.1780340103. [DOI] [PubMed] [Google Scholar]
- 26.Yli-Kerttula T, Luukkainen R, Yli-Kerttula U, Mottonen T, Hakola M, Korpela M, Sanila M, Uksila J, Toivanen A. Effect of three month course of ciprofloxacin on the late prognosis of reactive arthritis. Ann Rheum Dis. 2003;62(9):880–4. doi: 10.1136/ard.62.9.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz J, Mosquera JA, Romero S. Long-term evaluation (7 years) of antimicrobial therapy in early reactive arthritis. Arthritis Rheum. 1999;42(9) S334 abstract. [Google Scholar]
- 28.Putschky N, Pott HG, Kuipers JG, Zeidler H, Hammer M, Wollenhaupt J. Comparing 10-day and 4-month doxycycline courses for treatment of Chlamydia trachomatis-reactive arthritis: a prospective, double-blind trial. Ann Rheum Dis. 2006;65(11):1521–4. doi: 10.1136/ard.2005.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engel JN, Pollack J, Perara E, Ganem D. Heat shock response of murine Chlamydia trachomatis. J Bacteriol. 1990;172(12):6959–72. doi: 10.1128/jb.172.12.6959-6972.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zugel U, Kaufmann SH. Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev. 1999;12(1):19–39. doi: 10.1128/cmr.12.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qoronfleh MW, Gustafson JE, Wilkinson BJ. Conditions that induce Staphylococcus heat shock proteins also inhibit autolysis. FEMS Microbiol Lett. 1998;166(1):103–7. doi: 10.1111/j.1574-6968.1998.tb13189.x. [DOI] [PubMed] [Google Scholar]
- 32.Dean D, Powers VC. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect Immun. 2001;69(4):2442–7. doi: 10.1128/IAI.69.4.2442-2447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Airenne S, Surcel HM, Tuukkanen J, Leinonen M, Saikku P. Chlamydia pneumoniae inhibits apoptosis in human epithelial and monocyte cell lines. Scand J Immunol. 2002;55(4):390–8. doi: 10.1046/j.1365-3083.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 34.Curry AJ, Portig I, Goodall JC, Kirkpatrick PJ, Gaston JS. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol. 2000;121(2):261–9. doi: 10.1046/j.1365-2249.2000.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter JD, Valeriano J, Vasey FB. A Prospective, Randomized 9-Month Comparison of Doxycycline vs. Doxycycline and Rifampin in Undifferentiated Spondyloarthropathy – with Special Reference to Chlamydia-Induced Arthritis. J Rheum. 2004;31(10):1973–80. [PubMed] [Google Scholar]
- 36.Dougados M, van der Linden S, Juhlin R, Huitfeldt B, Amor B, Calin A, Cats A, Dijkmans B, Olivieri I, Pasero G, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218–27. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- 37.Dougados M, vam der Linden S, Leirisalo-Repo M, Huitfeldt B, Juhlin R, Veys E, Zeidler H, et al. Sulfasalazine in the treatment of spondylarthropathy. A randomized, multicenter, double-blind, placebo-controlled study. Arthritis Rheum. 1995;38(5):618–27. doi: 10.1002/art.1780380507. [DOI] [PubMed] [Google Scholar]
- 38.Schumacher HR, Jr, Kulka JP. Needle biopsy of the synovial membrane – experience with the Parker-Pearson technique. N Engl J Med. 1972;286(8):416–9. doi: 10.1056/NEJM197202242860807. [DOI] [PubMed] [Google Scholar]
- 39.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282(5389):754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 40.Kalman S, Mitchell W, Marathe R, Lammel C, Fan J, Hyman RW, Olinger L, Grimwood J, Davis RW, Stephens RS. Comparative genomes of Chlamydia pneumoniae and C trachomatis. Nat Genet. 1999;21(4):385–389. doi: 10.1038/7716. [DOI] [PubMed] [Google Scholar]
- 41.Clegg DO, Reda DJ, Weisman MH, Cush JJ, Vasey FB, Schumacher HR, Jr, et al. Comparison of sulfasalazine and placebo in the treatment of reactive arthritis (Reiter's Syndrome). A Department of Veterans Affairs Cooperative Study. Arthritis Rheum. 1996;39(12):2021–7. doi: 10.1002/art.1780391211. [DOI] [PubMed] [Google Scholar]
- 42.Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management. Drug Saf. 2000;22(3):237–49. doi: 10.2165/00002018-200022030-00007. [DOI] [PubMed] [Google Scholar]
- 43.Saiman L. The use of macrolide antibiotics in patients with cystic fibrosis. Curr Opin Pulm Med. 2004;10(6):515–23. doi: 10.1097/01.mcp.0000142101.53084.f0. [DOI] [PubMed] [Google Scholar]
- 44.Siala M, Gdoura R, Younes M, Fourati H, Cheour I, Meddeb N, Bargaoui N, Baklouti S, Sellami S, Rihl M, Hammami A. Detection and frequency of Chlamydia trachomatis DNA in synovial samples from Tunisian patients with reactive arthritis and undifferentiated oligoarthritis. FEMS Immunol Med Microbiol. 2009;55(2):178–86. doi: 10.1111/j.1574-695X.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- 45.Dowell SF, Peeling RW, Boman J, Carlone GM, Fields BS, Guarner J, Hammerschlag MR, Jackson LA, Kuo CC, Maass M, Messmer TO, Talkington DF, Tondella ML, Zaki SR. C pneumoniae Workshop Participants. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada) Clin Infect Dis. 2001;33(4):492–503. doi: 10.1086/322632. [DOI] [PubMed] [Google Scholar]
- 46.Peeling RW, Wang SP, Grayston JT, Blasi F, Boman J, Clad A, Freidank H, Gaydos CA, Gnarpe J, Hagiwara T, Jones RB, Orfila J, Persson K, Puolakkainen M, Saikku P, Schachter J. Chlamydia pneumoniae serology: interlaboratory variation in microimmunofluorescence assay results. J Infect Dis. 2000;181(Suppl 3):S426–9. doi: 10.1086/315603. [DOI] [PubMed] [Google Scholar]
- 47.Ozanne G, Lefebvre J. Specificity of the microimmunofluorescence assay for the serodiagnosis of Chlamydia pneumoniae infections. Can J Microbiol. 1992;38(11):1185–9. doi: 10.1139/m92-194. [DOI] [PubMed] [Google Scholar]
- 48.Haralambieva I, Iankov I, Petrov D, Ivanova R, Kamarinchev B, Mitov I. Crossreaction between the genus-specific lipopolysaccharide antigen of Chlamydia spp. and the lipopolysaccharides of Porphyromonas gingivalis, Escherichia coli O119 and Salmonella newington: implications for diagnosis. Diagn Microbiol Infect Dis. 2001;41(3):99–106. doi: 10.1016/s0732-8893(01)00299-1. [DOI] [PubMed] [Google Scholar]