Abstract
Objectives
To describe the prevalence of greater trochanteric pain syndrome (GTPS); to determine whether GTPS is associated with iliotibial band (ITB) tenderness, knee osteoarthritis (OA), body mass index (BMI), or low back pain (LBP); and to assess whether GTPS is associated with reduced hip internal rotation, physical activity, and mobility.
Design
Cross-sectional, population-based study.
Setting
Multicenter observational study.
Participants
Community-dwelling adults (N=3026) ages 50 to 79 years.
Interventions
Not applicable.
Main Outcome Measures
Greater trochanteric tenderness to palpation in subjects with complaints of hip pain and no signs of hip OA or generalized myofascial tenderness.
Results
The prevalence of unilateral and bilateral GTPS was 15.0% and 8.5% in women and 6.6% and 1.9% men. Odds ratio (OR) for women was 3.37 (95% confidence interval [CI], 2.67–4.25), but age and race were not significantly associated with GTPS. In a multivariate model, adjusting for age, sex, ITB tenderness, ipsilateral and contralateral knee OA, BMI, and LBP, ITB tenderness (OR=1.72; 95% CI, 1.34–2.19), knee OA ipsilaterally (OR=3.47; 95% CI, 2.72–4.42) and con-tralaterally (OR=1.74; 95% CI, 1.32–2.28), and LBP (OR=2.79; 95% CI, 2.22–3.50) were positively related to GTPS. In this complete model, BMI was not associated with GTPS (OR=1.10; 95% CI, 0.80–1.52 when comparing ≥ 30 with <25kg/m2). Hip internal rotation range of motion did not differ based on GTPS status. After multivariate adjustment, GTPS did not alter physical activity score, but bilateal GTPS was significantly associated with a higher 20-meter walk time and chair stand time.
Conclusions
The higher prevalence of GTPS in women and in adults with ITB pain or knee OA indicates that altered lower-limb biomechanics may be related to GTPS. Slower functional performance in those with GTPS suggests that the study of targeted rehabilitation may be useful. A longitudinal study will be necessary to identify causal factors and outcomes of interventions.
Keywords: Bursitis, Femur, Rehabilitation
Greater trochanteric pain syndrome (GTPS) is defined as tenderness to palpation over the greater trochanter with the patient in the side-lying position.1–3 In contrast to the term greater trochanteric bursitis, which implies presence of inflammation, referring to this clinical entity as GTPS is preferable for 2 reasons: (1) pain in this region frequently is not associated with signs of inflammation such as warmth, erythema, or swelling, and (2) the etiology is not fully known and may relate to myofascial pain rather than inflammation. Pain generators may be associated with the gluteus maximus, medius, or minimus bursae; muscle attachments; or overlying tissue such as the iliotibial band (ITB).2,4,5
The myofascial attachments and bursae associated with the greater trochanter may be affected by altered lower-limb biomechanics. Physical medicine and rehabilitation and orthopedics textbooks cite that osteoarthritis (OA) of the lumbar spine, hip, or knee; ITB tightness or tendonitis; or strain of the hip external rotators may contribute to trochanteric pain by adding stress to the area.4,6,7 Other experts8 have written that trochanteric bursitis is more prevalent in women, frequently associated with mechanical back strain and obesity and often with reduced hip internal rotation range of motion (ROM). However, others7 suggest that marked loss of hip internal rotation is typically not associated with trochanteric pain. One reason for the differences of opinion regarding these and other factors hypothesized to be associated with GTPS is that none have been analytically assessed.3,9
The prevalence of GTPS in adults with musculoskeletal low back pain (LBP) has been reported to be 20% to 35%.10–12 Studies differ regarding whether GTPS may11,13 or may not14 be more prevalent in women than men. However, numerous sources have recognized that there is a lack of data regarding the prevalence of GTPS.4,9
One study15 suggested that GTPS was the cause of LBP, based on findings that Oswestry Disability Index scores for female patients with LBP improved after greater trochanteric steroid injection. However, considering that the corticosteroid is systemically absorbed, this uncontrolled study may not answer whether there is an association between back pain and GTPS. Although there have been small studies of GTPS in back pain clinics,12 we are unaware of any study examining the prevalence and epidemiology of GTPS in the general population. 9 For providers of musculoskeletal care, it is important to understand factors associated with GTPS to minimize significant decline of physical function and quality of life.
The long delay between presentation and diagnosis of hip external rotator muscle tears, 1 cause of GTPS, was recently found to be caused by underrecognition by physicians.16 Such underrecognition may be because of a lack of education or attention. Frequently, GTPS is associated with pain at night, pain when standing greater than 15 minutes, or radiating pains and paresthesias.11 These complaints may lead to ordering tests such as magnetic resonance imaging or electromyography to assess for radiculopathy instead of conducting a well-informed physical examination.
Understanding whether GTPS is associated with sex, body mass index (BMI), or other lower-limb musculoskeletal diagnoses would enable future study of risk factors for incident and progressive GTPS. Additionally, the characterization of activity level or hip flexibility that could affect pathomechanics at other joints in the kinetic chain, such as the knee or spine, would also guide rehabilitation considerations.
To address these needs, this study assessed the prevalence of GTPS in a community-based population complaining of lower-limb pain and whether GTPS was associated with 4 purported risk factors: (1) ipsilateral ITB pain, (2) knee OA, (3) obesity, and (4) LBP. A secondary aim was to determine whether hip internal rotation ROM or levels of physical activity and physical performance are limited in adults with GTPS.
METHODS
Participants
The Multicenter Osteoarthritis (MOST) Study is a longitudinal study of persons 50 to 79 years old at a high risk of developing symptomatic knee OA or who already had knee OA. Subjects are drawn from the community. Although the purpose of the MOST study is to assess risk factors for incident and progressive knee OA, the availability of standardized data on GTPS diagnosis and associated features in a large community-based sample makes it a useful resource for analysis. The present report uses data from the baseline examination of the MOST study in which subjects underwent a musculoskeletal examination. Institutional review board approval was obtained at each of the investigators’ institutions before initiating recruitment and research protocols.
Subject recruitment was conducted by MOST study personnel who contacted adults aged 50 to 79 years by mass mailings as well as through printed advertisements in the counties surrounding the 2 clinical sites. Potential subjects who indicated interest were then contacted by telephone for screening. Subjects were recruited if they had a history of knee pain, injury, or surgery or were overweight. Exclusion criteria included bilateral knee replacement, cancer, or rheumatologic disease.
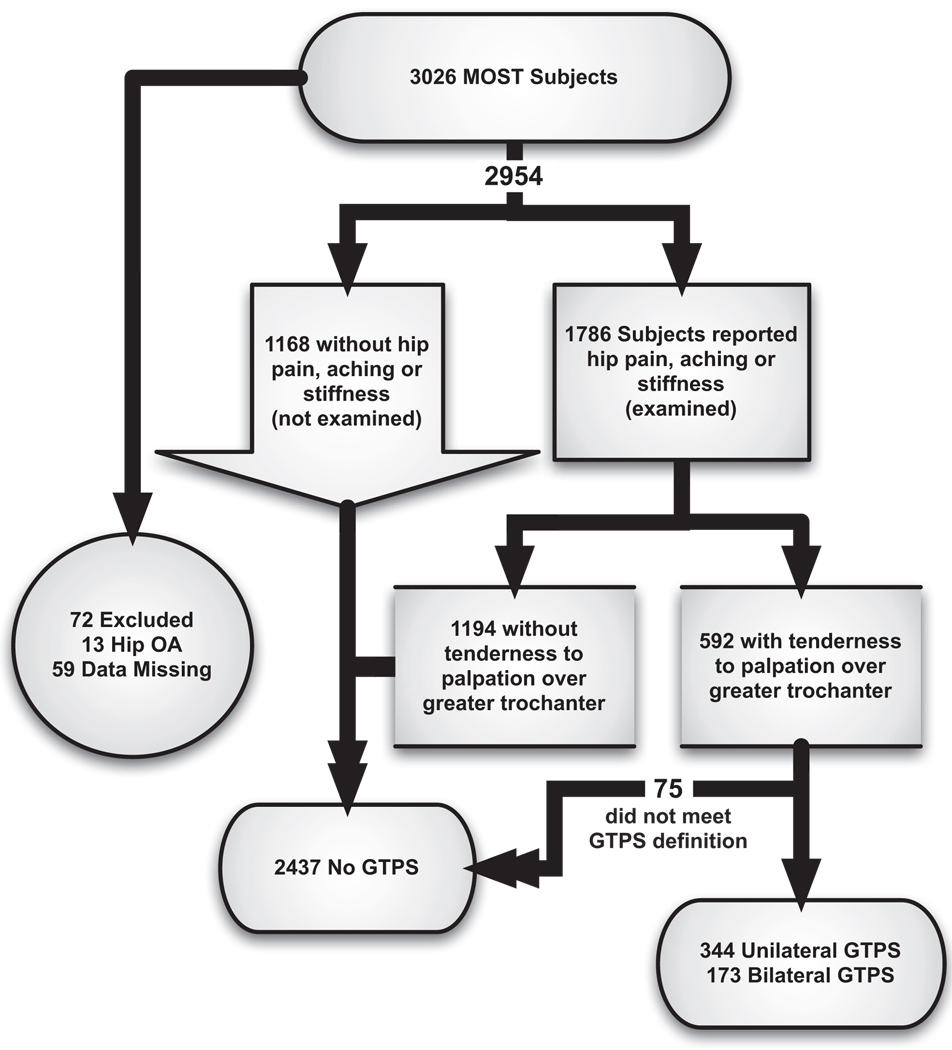
Demographic variables and a report of knee pain, hip pain, and LBP were assessed through questionnaires administered by telephone and in the clinic. At the baseline MOST visit, the subset of 1786 subjects who indicated “pain, aching, or stiffness” located on the lateral aspect of either hip on most days over the last month on a pain diagram underwent a physical examination. Subjects with a prosthetic hip were excluded from the physical examination. We used this physical examination to acquire information on tenderness over the greater trochanter. Subjects who indicated no lateral hip pain were considered not to have GTPS.
Physical Examination
GTPS was defined as tenderness on physical examination in the absence of generalized myofascial tenderness to palpation. Examiners used a Chatillon CMD 10-1 dolorimetera to calibrate finger pressure17,18 to 1.4 and 3.0kg of pressure before palpating subjects’ greater trochanters. Examiners asked subjects “is this tender or painful” while applying 1.4 to 3.0kg of thumb-tip pressure over the lateral and posterior aspects of each greater trochanter with the subject in the lateral decubitus position.2 A positive response to this question was defined as tenderness in the greater trochanter region. Generalized myofascial tenderness was defined by an affirmative response to the same question when 1.4kg of pressure was applied over the soft tissue 2cm proximal to the medial joint line of the knee as well as at 2 or more of the following points: left and right proximal trapezius and left and right extensor mass immediately distal to the lateral epicondyle of an elbow.
ITB tenderness also was assessed and defined as an affirmative response to “is this tender or painful” when applying 1.4kg of pressure with the thumb over each ITB just proximal to the lateral femoral condyle with the subject in the lateral decubitus position. Internal rotation of each hip was measured from neutral, using a 25.4cm (10-in) long-arm goniometer, measuring to the nearest 1°, with subjects seated on the edge of the examination table with hips and knees flexed to 90°. All subjects were examined using a standardized, written protocol, with regular reliability checks performed to ensure consistency between sites and examiners.
LBP was defined as being “bothered by back pain most or all of the time” over the last 30 days. Subjects also were asked “During the past 30 days, have you limited your activities due to back pain?”
Radiograph protocol for the assessment of knee OA in the MOST study has been described previously. Knee OA was defined as at least 1 definite osteophyte visible at standard image size on posteroanterior knee radiographs.19
BMI was calculated as weight (in kilograms) per squared height, and obesity was defined as a BMI of 30kg/m2 or higher. Subjects were encouraged to empty their bladders and bowels, empty their pockets, and remove jewelry before stepping on the scale with paper shorts and a shirt. Weight was measured with a standard medical beam balance with the certified examiner standing behind the subject and following a written protocol. Weight was recorded to the nearest 0.1kg immediately after the measurement. The scale was calibrated monthly with a 50-kg weight for accuracy as well as 5-, 10-, 15-, and 20-kg weights for linearity calibration. Additionally, the scale was calibrated annually by the local Department of Weights and Measures.
Height was measured with a wall-mounted Harpenden stadiometerb and followed a written protocol. Subjects stood without shoes (barefoot or thin stockings) with their heels together and scapulae, buttocks, and both heels touching the wall plate with their head in the Frankfort horizontal plane. A standardized script and positioning protocol were used to measure height on full inspiration to the nearest 1mm. A 0.5-kg soft weight was placed on the headboard to standardize pressure on the head during measurement. The measurement was repeated twice, and, if these differed by more than 3mm, 2 additional measurements were taken. The stadiometer was calibrated daily with a 600-mm rod.
The activity level was measured using the Physical Activity Score for the Elderly (PASE).20 Locomotor function was measured as the time (in seconds) required to walk 20m. The twenty-meter walk test (20MWT) has been shown to be reliable when performed in a standardized fashion in a corridor free of obstructions and distractions.21 Functional mobility was measured as the time (in seconds) required for a person to stand from a seated position in a chair 5 times without using their arms.22
Statistical Methods
SASc was used for the analyses. The diagnosis of GTPS, as defined in the Physical Examination section, was treated as dichotomous for limb-specific analyses (present or absent) and trichotomous (neither, unilateral, or bilateral) for person-specific analyses. In addition to analyzing BMI as a continuous measure, a trichotomous categorical variable for BMI was defined using World Health Organization and National Institutes of Health definitions (<25, ≥25 and <30, ≥30kg/m2).23,24 Categorical variables were summarized using frequencies, proportions, and odds ratios (ORs); 95% confidence intervals (CIs) were calculated (sex, ITB pain, knee OA, knee pain, LBP). The Pearson chi-square test was used to compare proportions. Adjusting for age and sex as covariates and subject as a repeated-factor, limb-specific GTPS status was regressed on (1) ITB pain, (2) ipsilateral knee OA, and (3) contralateral knee OA using logistic regression with generalized estimating equations to adjust for correlation within subject.
Adjusting for age and sex, person-specific GTPS status was regressed on (1) BMI as a continuous variable, (2) BMI category, and (3) LBP using logistic regression with generalized estimating equations to adjust for correlation within subject. Then, we assessed the association between limb-specific GTPS status and each of the possible risk factors while adjusting for each other and age and sex using multiple logistic regression. Continuous variables were summarized with means and standard deviations (SDs) and intergroup comparison of age was evaluated using the Student t test. The effect of person-specific GTPS on (1) physical activity, (2) chair-rise time, and (3) 20MWT time were individually assessed by linear regression of each of these on person-specific GTPS. Each of these models was adjusted for age and sex, and least squares means were estimated for the groups with and without GTPS. The Scheffé procedure was used in a multiple-comparison procedure. These activity and function analyses were repeated, additionally adjusting for LBP, person-specific knee pain, and BMI. For all analyses, significance level was set at .05.
RESULTS
Prevalence of GTPS
A total of 5735 lower limbs from 2954 subjects were eligible for analysis of GTPS (fig 1). The subjects’ mean age ± SD was 62.4±8.1 years, and 60.1% were women. Of these subjects, 517 (17.6%) had GTPS. Specifically, 344 had unilateral and an additional 173 had bilateral GTPS, a prevalence of 11.7% and 5.9%, respectively (table 1). The prevalence of unilateral and bilateral GTPS was 15.0% and 8.5% in women and 6.6% and 1.9% in men. Comparing subjects with and without GTPS, the OR for GTPS in women compared with men was 3.32 (95% CI, 2.63–4.19). Age and race were not found to be significantly associated with GTPS status (see table 1).
Fig 1.
Subject distribution.
Table 1.
Demographic Distribution of GTPS
| Characteristic | GTPS Case Subjects (n=517) |
Control Subjects (n=2437) |
Intergroup Comparisons (P) |
|---|---|---|---|
| Mean age ± SD (y) | 62.8±8.0 | 62.3±8.1 | .198 |
| Sex (% women) | 80.7 | 55.7 | <.001 |
| Race (%) | .372 | ||
| White | 82.6 | 83.4 | |
| African American | 15.3 | 15.3 | |
| All others | 2.1 | 1.3 |
Biomechanic Correlates
ITB tenderness
Logistic regression adjusting for age and sex revealed that ipsilateral ITB tenderness was significantly associated with GTPS, with an OR of 2.54 (95% CI, 2.03–3.17). After multivariate adjustment for all other variables, ITB tenderness continued to be significantly associated with GTPS with an OR of 1.72 (95% CI, 1.34–2.19).
Knee OA
Controlling for age and sex, ipsilateral knee OA was significantly associated with GTPS, with an OR of 4.33 (95% CI, 3.43–5.48). Contralateral knee OA was also significantly associated with GTPS status with an OR of 2.19 (95% CI, 1.66–2.88). After multivariate adjustment for all other variables, ipsilateral (OR=3.47; 95% CI, 2.72–4.42) and contralateral (OR=1.74; 95% CI, 1.32–2.28) knee OA continued to be significantly associated with GTPS.
Body mass index
Logistic regression of GTPS on BMI, adjusted for age and sex, revealed that BMI was significantly associated with GTPS (P<.001). Compared with subjects with a BMI less than 25kg/m2, the OR for GTPS was 1.34 (95% CI, 1.09–1.66) in those with a BMI of 25 or greater and less than 30kg/m2 and 1.54 (95% CI, 1.15–2.07) for those with a BMI of 30kg/m2 or higher. However, after adjustment for ITB tenderness, ipsilateral and contralateral knee OA, LBP, as well as age and sex, BMI no longer was associated with GTPS status with an OR of 1.10 (95% CI, 0.80–1.52) comparing BMI equal to or greater than 30kg/m2 with a BMI less than 25kg/m2.
Low back pain
The presence of LBP most or all of the time was significantly associated with GTPS with an OR of 3.44 (95% CI, 2.76–4.28) after adjustment for age and sex. After adjustment for all other variables in the complete model, the association remained significant with an OR of 2.79 (95% CI, 2.22–3.50). Similarly, functionally significant LBP that limited activities over the past 30 days was associated with GTPS with an OR of 3.15 (95% CI, 2.54–3.92).
Impairments
Mean hip internal rotation ROM did not differ between hips with GTPS (36.7° from neutral; 95% CI, 36.1°–37.3°) and without GTPS (36.8° from neutral; 95% CI, 36.5°–37.1°) in those who underwent physical examination (P=.731). The presence of self-reported knee pain was associated with GTPS status with an age- and sex-adjusted OR of 2.75 (95% CI, 2.22–3.41).
Person-Specific Function
After adjustment for age and sex, GTPS did not significantly alter least mean square estimates of PASE score: no GTPS, 176.4 (95% CI, 173.1–179.6); unilateral GTPS, 169.0 (95% CI, 160.3–177.8); and bilateral GTPS, 171.9 (95% CI, 159.6–184.2). No significant difference was found among PASE in multiple comparisons comparing no GTPS with unilateral (P=.307) and bilateral GTPS (P=.790). Unilateral GTPS also did not alter 20MWT time. However, compared with subjects without GTPS, mean 20MWT time was 1.5±0.3 seconds greater in subjects with bilateral GTPS (P<.001). Similarly, the time to complete 5 sit-to-stand tasks was 0.9±0.2 seconds greater in subjects with unilateral and 1.5±0.3 seconds greater in subjects with bilateral GTPS (P<.001). Additionally, controlling for BMI did not change the overall associations detected.
DISCUSSION
This study was useful in both identifying the prevalence of GTPS in a non-clinic– based population as well as assessing the validity of common teachings regarding GTPS. The GTPS prevalence of 17.6% in this community-based sample of older adults at high risk of knee OA contrasts with the 20% to 35% reported for spine clinic patients presenting with LBP. We found the following were associated with GTPS: female sex, ITB tenderness, knee OA or knee pain, and LBP.
Reasons for the differences in prevalence may relate to the more strict definition of GTPS used in this study or the broader population enrolled in this sample, defined as self-report of outer hip pain in the absence of clinical hip arthritis, who had tenderness to palpation over the greater trochanter and no generalized myofascial tenderness on physical examination. We used this highly specific case definition to maximize the validity of our results. However, this may have been more specific than the clinical impression of GTPS described in prior work.1,3
The increased odds for GTPS in women was consistent with prior reports, but we could not confirm a report associating GTPS with limited hip internal ROM.8 Although the mechanism for increased GTPS in women is unclear,5 this association could relate to anatomy (such as the flared pelvic rim in women altering the pull of the ITB), physiology (hormonal effects on bursal irritation or pain generators), or differences in activity between men and women.
The increased odds for GTPS with obesity and overweight status after adjustment for sex and age appeared to be explained by the combined effect of ITB tenderness, ipsilateral and contralateral knee OA, and LBP. The absence of an association between obesity and GTPS differed from prior reports,8 possibly because of the larger sample size and multivariate adjustment used in our study.
The associations with ITB pain, knee OA, and LBP also were consistent with orthopedic and physical medicine and rehabilitation teachings.6,7 Presumably, a tight ITB could explain tenderness on palpation over the lateral femoral condyle as well as potentially cause irritation over the greater trochanter. Additionally, knee or back pain may relate to GTPS through compensatory movements from 1 musculoskeletal problem causing symptoms at additional locations in the kinetic chain.
In addition to pain, people with GTPS appear to have a slowed gait and ability to rise from a chair. The impact on these functional activities may be significant to patients’ quality of life. However, this study did not detect an impact on participation in activities measured by the PASE instrument. The contrast between differences in function, but not in activity score, may relate to subjects with GTPS limiting their activities or to the reduced activity level in obese subjects.
A recent review of GTPS reiterated prior review articles in stating that “additional research is sorely required to shed light on … this commonly encountered syndrome.”9(p124) This study was useful in investigating the validity of teachings about GTPS that previously had not been analytically assessed or proven.9 The study of GTPS in a community-based, rather than a clinic-based, sample was useful in clarifying the demographics of this condition.
Study Limitations
One limitation of this study was that the population studied was recruited for the MOST study because of characteristics that might predispose them to knee OA including obesity, knee injury, knee surgery, and knee pain. The presence of this recruitment bias may have influenced the prevalence of GTPS in our sample. However, this bias also may have enabled our findings to be more generalizeable to community-dwelling adults who seek medical care. This may be more pertinent to clinical providers who serve patients with lower-body pain complaints.
Another limitation was that this cross-sectional study cannot establish causal relations. To elucidate whether ITB tightness, knee OA, obesity, or LBP lead to or are caused by GTPS, longitudinal studies would be necessary. The initial findings of this study may be useful not only in clinical care but also in generating hypotheses for mechanistic studies or therapeutic trials to benefit patients with GTPS.
CONCLUSIONS
The higher prevalence of GTPS in people who report hip pain in the absence of knee or generalized pain (24% of women, 9% of men) indicates that GTPS is common, and greater clinical awareness may identify patients for primary prevention and therapy. Slower functional performance in those with GTPS suggests that the study of targeted rehabilitation may be useful to minimize the impact of GTPS. A longitudinal study will be necessary to identify causal factors and outcomes of interventions.
Acknowledgments
Supported by the National Institute of Child Health and Human Development, National Institutes of Health (grant no. 5K12HD001097-08) and National Institute on Aging, National Institutes of Health (grant nos. 1 U01 AG18832, 1 U01 AG18820, 1 U01 AG19069, 1 U01 AG18947).
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
Suppliers
Ametek U.S. Gauge Div, Chatillon Brand Products, 8600 Somerset Dr, Largo, FL 33773.
Holtain Ltd, Crosswell, Crymych, Pembrokeshire SA41 3UF, UK.
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513.
References
- 1.Shapira D, Nahir M, Shcharf Y. Trochanteric bursitis: a common clinical problem. Arch Phys Med Rehabil. 1986;67:815–817. [PubMed] [Google Scholar]
- 2.Little H. Trochanteric bursitis: a common cause of pelvic girdle pain. CMAJ. 1979;120:456–458. [PMC free article] [PubMed] [Google Scholar]
- 3.Shbeeb MI, Matteson EL. Trochanteric bursitis (greater trochanter pain syndrome) Mayo Clin Proc. 1996;71:565–569. doi: 10.4065/71.6.565. [DOI] [PubMed] [Google Scholar]
- 4.Lequesne M. From “periarthritis” to hip “rotator cuff” tears. Trochanteric tendinobursitis. Joint Bone Spine. 2006;73:344–348. doi: 10.1016/j.jbspin.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Bird PA, Oakley SP, Shnier R, Kirkham BW. Prospective evaluation of magnetic resonance imaging and physical examination findings in patients with greater trochanteric pain syndrome. Arthritis Rheum. 2001;44:2138–2145. doi: 10.1002/1529-0131(200109)44:9<2138::AID-ART367>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 6.Frontera WR, Silver JK. Essentials of physical medicine and rehabilitation. Philadelphia: Hanley & Belfus; 2002. [Google Scholar]
- 7.Callaghan JJ, Rosenberg AG, Rubash HE. The adult hip. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 8.Klippel JH, Dieppe P. Rheumatology. 2nd ed. St. Louis: Mosby; 1998. [Google Scholar]
- 9.Alvarez-Nemegyei J, Canoso JJ. Evidence-based soft tissue rheumatology: III: Trochanteric bursitis. Clin Rheumatol. 2004;10:123–124. doi: 10.1097/01.rhu.0000129089.57719.16. [DOI] [PubMed] [Google Scholar]
- 10.Swezey RL. Pseudo-radiculopathy in subacute trochanteric bursitis of the subgluteus maximus bursa. Arch Phys Med Rehabil. 1976;57:387–390. [PubMed] [Google Scholar]
- 11.Collee G, Dijkmans BA, Vandenbroucke JP, Rozing PM, Cats A. A clinical epidemiological study in low back pain. Description of two clinical syndromes. Br J Rheumatol. 1990;29:354–357. doi: 10.1093/rheumatology/29.5.354. [DOI] [PubMed] [Google Scholar]
- 12.Tortolani PJ, Carbone JJ, Quartararo LG. Greater trochanteric pain syndrome in patients referred to orthopedic spine specialists. Spine J. 2002;2:251–254. doi: 10.1016/s1529-9430(02)00198-5. [DOI] [PubMed] [Google Scholar]
- 13.Anderson TP. Trochanteric bursitis: diagnostic criteria and clinical significance. Arch Phys Med Rehabil. 1958;39:617–622. [PubMed] [Google Scholar]
- 14.Gordon EJ. Trochanteric bursitis and tendinitis. Clin Orthop Relat Res. 1961;(20):193–202. [PubMed] [Google Scholar]
- 15.Sayegh F, Potoupnis M, Kapetanos G. Greater trochanter bursitis pain syndrome in females with chronic low back pain and sciatica. Acta Orthop Belg. 2004;70:423–428. [PubMed] [Google Scholar]
- 16.Cormier G, Berthelot JM, Maugars Y. SRO (Societe de Rhumatologie de l’Ouest). Gluteus tendon rupture is underrecognized by French orthopedic surgeons: results of a mail survey. Joint Bone Spine. 2006;73:411–413. doi: 10.1016/j.jbspin.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Buskila D, Neumann L, Hershman E, Gedalia A, Press J, Sukenik S. Fibromyalgia syndrome in children—an outcome study. J Rheumatol. 1995;22:525–528. [PubMed] [Google Scholar]
- 18.Smythe HA, Buskila D, Urowitz S, Langevitz P. Control and “fibrositic” tenderness: comparison of two dolorimeters. J Rheumatol. 1992;19:768–771. [PubMed] [Google Scholar]
- 19.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. doi: 10.1016/j.rdc.2004.07.005. vii. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Simonsick EM, Gardner AW, Poehlman ET. Assessment of physical function and exercise tolerance in older adults: reproducibility and comparability of five measures. Aging (Milano) 2000;12:274–280. doi: 10.1007/BF03339847. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan M, Winkleby MA. Identifying patients for weight-loss treatment: an empirical evaluation of the NHLBI obesity education initiative expert panel treatment recommendations. Arch Intern Med. 2000;160:2169–2176. doi: 10.1001/archinte.160.14.2169. [DOI] [PubMed] [Google Scholar]
- 24.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]