Summary
Natural killer (NK) cells are lymphocytes with the capacity to produce cytokines and kill target cells upon activation. NK cells have long been categorized as members of the innate immune system and as such have been thought to follow the ‘rules’ of innate immunity, including the principle that they have no immunologic memory, a property thought to be strictly limited to adaptive immunity. However, recent studies have suggested that NK cells have the capacity to alter their behavior based on prior activation. This property is analogous to adaptive immune memory; however, some NK cell memory-like functions are not strictly antigen-dependent and can be demonstrated following cytokine stimulation. Here we discuss the recent evidence that NK cells can exhibit properties of immunologic memory, focusing on the ability of cytokines to non-specifically induce memory-like NK cells with enhanced responses to restimulation.
Keywords: natural killer cell, innate immunity, memory, cytokines, interferon γ
ntroduction
An efficient host immune response encompasses both fast acting innate immunity as well as slower, but more specific, adaptive immunity. The innate immune system is diverse and comprises a variety of cells including natural killer (NK) cells, neutrophils, macrophages, dendritic cells (DCs), as well as soluble factors such as complement (1, 2). The importance of innate effectors is perhaps best evidenced by patients with defects in innate immunity, who suffer from uncontrolled and often fatal infections as well as other chronic diseases (3–5). The adaptive immune response is typified by antigen-specific T and B lymphocytes that provide long-lasting protection against a wide variety of pathogens and is the basis for the protective effect of immunizations (2, 6). While these two systems are often discussed separately, neither arm of the immune system works in isolation. For example, adaptive immune T-cell responses depend upon recognition of non-self molecules by innate antigen-presenting cells (APCs). Not only do APCs prime T-cell responses, but they also help the adaptive immune system to distinguish foreign antigens from host antigens (7). Thus, innate and adaptive immunity are not always easily separable as distinct components of immune responses.
Likewise, our concepts of the roles and functions of innate and adaptive immunity are constantly evolving. For instance, the discovery of germline encoded pattern recognition receptors on innate immune cells, which when triggered can orchestrate discrete immune responses, indicates that innate immunity is not as non-specific as previously thought (8). Similarly, memory CD8+ T cells have been found to efficiently produce cytokines during the ‘innate’ response, thereby aiding the host in the early defense against pathogens (9). Thus, some features of innate and adaptive immune cells are more similar than previously recognized.
Over the years, one classical distinction between these two arms of the immune response has remained, which is the restriction of immunologic memory to adaptive immune antigen-specific T and B lymphocytes. By virtue of their capacity to somatically rearrange antigen-specific receptors, T and B cells express a near limitless number of receptors and can recognize a wide array of pathogen-derived antigens. Memory provides both passive immunity in the form of circulating antibodies as well as long-lasting active immunity with expansion and activation of specific memory lymphocytes upon secondary antigen exposure (2, 6). The functional outcome of this memory is that the host is armed with a pool of lymphocytes and soluble mediators that provide enhanced protection against subsequent infection. By contrast, innate immune effectors lack the capacity to somatically rearrange receptors and do not have the same diversity of antigen-specific receptors as adaptive lymphocytes. Moreover, innate immune cells are not thought to ‘adapt’ or change the way in which they respond to subsequent immunologic challenges, i.e. they respond similarly to successive challenges.
Recent studies of innate immune NK cells, however, have challenged the notion that the functional outcome of immunologic memory is limited to adaptive immunity (10–12). NK cells are lymphocytes of the innate immune system that protect the host against a wide variety of infections, including viruses, bacteria, and parasites, and are also thought to participate in tumor immunosurveillance (13–15). They mediate their effects via two broad effector mechanisms: production of immunoregulatory cytokines and killing target cells such as infected or tumor cells (16, 17). NK cell-derived cytokines, such as IFN-γ, not only activate innate immune responses and promote pathogen clearance but also influence the subsequent development of adaptive immune Th1 responses (18, 19). While NK cells lack the capacity to somatically rearrange antigen-specific receptors, they express germline-encoded activating and inhibitory NK cell receptors (NKRs), which recognize major histocompatibility complex (MHC) class I and class I-like molecules (20–22). In addition to regulating NK cell activation, these receptors are critical for the maintence of NK cell tolerance to self (23–28). This process of NK cell tolerance, termed licensing, requires engagement of inhibitory NKRs with self-MHC class I for acquisition of functional competence by NK cells. Licensing ensures that NK cells lacking appropriate inhibitory receptors to self are not fully functional and thereby detrimental to the host.
While NK cell responses to activation have been extensively studied, whether or not NK cells can ‘learn’ from these prior experiences and exhibit memory-like responses has only recently been addressed. There is now convincing evidence that indeed NK cells can change their behavior based on prior activation and that this is seen in response to both specific and non-specific activation (10–12).
Mechanisms of NK cell activation
When questioning whether or not NK cells can exhibit enhanced responses to later activation, a key component of immunologic memory, it is important to consider how NK cells are activated during an immune response. Unlike adaptive lymphocytes, in most cases a single NK cell is capable of responding to a wide variety of stimuli, and NK cells have limited capacity for recognition of pathogen-encoded ligands (akin to foreign antigen recognition by T cells). NK cells can be triggered via two primary mechanisms: cytokine stimulation and engagement of activating NKRs. Together or in isolation, both of these signaling mechanisms can result in NK cell effector functions including cytokine production and induction of cytotoxicity.
A wide variety of cytokines, especially IL-12, IL-15, and IL-18, have been shown in numerous studies to activate NK cells in vitro and in vivo. NK cell responses to cytokines appear to differ depending on the stimulation. For example, costimulation of NK cells with IL-12 and IL-18 efficiently induces production of IFN-γ, but not IL-10, which is produced following stimulation with IL-15 or IL-2 plus IL-12 (29, 30). Whereas activation of NK cells with IL-15 alone poorly induces IFN-γ, this cytokine induces production of granzyme B and perforin, key components of the cytotoxic machinery of NK cells (31). In addition, while bulk populations of NK cells respond to multiple cytokines, it is also possible that there might be specialized subsets of NK cells responsive to only certain cytokines as evidenced by the recent finding of IL-22-producing NK (NK-22) cells (32–36). NK-22 cells are found in both mouse and humans in mucosa-associated lymphoid tissue and specifically respond to IL-23 (32). In humans, another NK cell subset enriched in lymph nodes, CD56bright NK cells, constitutively express the high-affinity heterotrimeric IL-2Rαβ γ receptor, allowing them to respond to small amounts of IL-2 produced by activated T cells (37, 38). CD56bright NK cells also express chemokine receptors distinct from the CD56dim NK cell subset, which is more abundant in the periphery (39, 40). Thus, in vivo, NK cell responses to cytokines likely depend upon many factors including the local cytokine milieu as well as the type of NK cells present in a given microenvironment.
Cytokine activation of NK cells in vivo appears to be largely dependent on cross-talk with APCs, especially DCs (41–44). In addition to cytokines, NK and DC cross-talk also depends on cell-cell contact, in part due to trans-presentation of IL-15 via the IL-15Rα chain by DCs, but also related to synapse formation for efficient cytokine delivery (44–48). A recent study by Beuneu et al. (49) used two-photon imaging to evaluate in situ interaction between NK cells and DCs in response to a Toll-like receptor 3 (TLR3) agonist and compared this to T-cell interactions with antigen-pulsed DCs. While T cells formed stable, prolonged interactions with antigen-pulsed DCs, NK cells were highly motile and formed multiple brief communications with DCs in vivo (49). The authors suggest that these short-lived contacts allow NK cells to sample and integrate multiple signals, including cytokines, from the immunologic microenvironment.
Many early NK cell responses to infection appear to be mediated by cytokines and NK-APC cross-talk. For example, Salmonella-infected macrophages activate NK cells in vitro via production of IL-2, IL-12, IL-15, and IL-18 and adhesion-molecule mediated cell-contact (50). Blockade of IL-12 during infections as diverse as murine cytomegalovirus (MCMV) and Toxoplasma gondii inhibits NK cell IFN-γ production in vivo (51, 52). Thus, cytokine activation of NK cells, particularly in the context of NK-APC cross-talk, represents a common mechanism of non-specific NK cell activation.
NK cell receptors are also important for NK cell activation through integration of signals from inhibitory and activating NKRs (22, 53). Inhibitory receptors bind to self-MHC class I and class-I like molecules and signal through immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Activating NK receptors are structurally related to inhibitory receptors but lack ITIMs and associate with signaling molecules such as DAP12, CD3ζ, or FcRγ, which signal through immunoreceptor tyrosine-based activating motifs (ITAMs) or the YINM-bearing DAP10 (53). Activation of NK cells via NKRs results in cytotoxic activity and cytokine production.
While many ligands for activating NKRs remain unknown, examples of both pathogen and host-encoded ligands have been discovered. In the mouse, Ly49H is an activating receptor that specifically recognizes the MCMV-encoded cell-surface ligand m157 (54–58), and Ly49H is responsible for genetic NK-mediated resistance to MCMV infection in certain mouse strains (59, 60). Another activating murine NKR, Ly49P, has also been proposed to bind to the MCMV-derived antigen M04 in the context of specific MHC class I molecules (61). In humans, there is evidence that certain activating natural cytotoxicity receptors (NCRs) can bind to conserved viral hemagglutinins; however, this interaction is not specific, since NCRs also recognize unidentified ligands upregulated by multiple tumors (62, 63). In addition, human NK cells expressing the activating CD94/NKG2C receptor are expanded during CMV infection, although it is unclear if there is specific recognition of a viral ligand by this receptor (64, 65). By contrast, the activating NKG2D receptor expressed by most human and mouse NK cells is unique in its ability to recognize ‘altered or induced self’, including multiple host-encoded ligands induced by genotoxic stress and tumor cells (66, 67). Thus, NK cell receptors represent an important mechanism for stimulation of specific (in the case of Ly49H) as well as relatively non-specific (such as NKG2D) NK cell activation.
Memory-like functions of NK cells
While the concept of immunologic memory has been well described in T and B adaptive immune lymphocytes, several recent studies suggest that NK cells can also exhibit properties of memory, both specific and non-specific (10–12). O’Leary et al. (10) demonstrated that in the absence of T and B cells, NK cells can mediate a hapten-specific contact hypersensitivity (CHS) response, a classic example of adaptive immune-mediated delayed type hypersensitivity reaction. A CHS response was present in recombination-activating gene 2 (Rag2)-deficient and sever combined immunodeficient (SCID) mice lacking T and B cells but possessing NK cells, whereas the response was absent in Rag2-deficient, γc-deficient mice which lack T, B, and NK cells. Moreover, adoptive transfer of NK cells from hapten-sensitized mice into naive mice resulted in a CHS reaction when recipients were challenged with the original hapten (10). This observation is consistent with a memory-like response mediated by NK cells and suggests that NK cells are sufficient for CHS. It is unclear whether there was direct recognition of haptenated protein by NK cells; however, the response was specifically seen after the transfer of liver NK cells expressing the Ly49C/I+ receptor (10). This receptor specificity suggests two possibilities. The first is that NK cell licensing via inhibitory receptors for self-MHC (Ly49C recognition of H2Kb in this case) is a pre-requisite for development of NK cells with memory-like functions. A second possibility is that there are alterations in NK cell recognition of haptenated cells specific to Ly49C-positive NK cells. While the exact mechanism remains unclear, these studies suggested that NK cells are capable of mediating a specific recall response that was traditionally a hallmark property of adaptive immunity.
In our own studies of NK cell ‘memory’, we explored the possibility that previously stimulated NK cells would display a component of classical immunological memory, i.e. an enhanced response upon secondary challenge. Moreover, of the two primary and complementary mechanisms to activate NK cells, cytokines and NKRs, we chose to investigate the functional consequences of cytokine stimulation of NK cells. We utilized an in vivo adoptive transfer system to address the question of whether stimulation of NK cells via cytokines alone renders a memory-like effect (11). In this system, freshly isolated splenic NK cells were activated with IL-12 and IL-18 and cultured overnight in the presence of a low-dose of IL-15 as a survival factor. NK cells were then labeled with carboxyfluorescein diacetate succinamidyl ester (CFSE) and adoptively transferred into naive hosts in parallel with control-treated NK cells, which received only IL-15. The combination of IL-12 and IL-18 induced >90% of cells to produce IFN-γ, ensuring that nearly all transferred cells were secreting IFN-γ at the time of transfer. However, by one week after transfer, previously activated NK cells returned to a resting state and did not constitutively produce IFN-γ (11). At that timepoint, preactivated NK cells did not exhibit a distinct phenotype and expressed similar levels of multiple activation and cytokine receptors as control-transferred cells. One notable difference between preactivated and control cells is that cells stimulated with cytokines went on to divide in vivo within 72 h of transfer (11, authors’ unpublished observations). Transferred NK cells were seen in multiple organs, and similar proliferation was observed in the spleen, liver, and lymph nodes. These data suggest that following a response to local inflammation, NK cells may have the capacity to traffic to distant sites where they can then proliferate and reside.
While preactivated NK cells did not continuously produce IFN-γ at one week following adoptive transfer, they demonstrated a significantly more robust response to re-activation in vitro than control-transferred or endogenous host NK cells (11). This was observed when cells were re-stimulated ex vivo with cytokines (such as IL-12 + IL-15), or via engagement of the activating NKRs NK1.1 and Ly49H, activation signals to which they had never been previously exposed. This effect was NK-intrinsic and not related to alteration of the host environment, since co-transfer of preactivated and control-treated congenic NK cells into the same host resulted in similar findings (authors’ unpublished observations) (Fig. 1). Enhanced response to NK cell re-stimulation by preactivated cells persisted greater than four weeks (authors’ unpublished observations) (Fig. 1), a relatively long time for NK cells considering that their half-life had been estimated to be approximately one to two weeks (68, 69). These findings suggest that based on a prior experience, NK cells fundamentally change the way that they respond to later activation, a central property of immunologic memory. This property is best described as ‘memory-like’, since it represents a non-specific activation of a long-term memory type response.
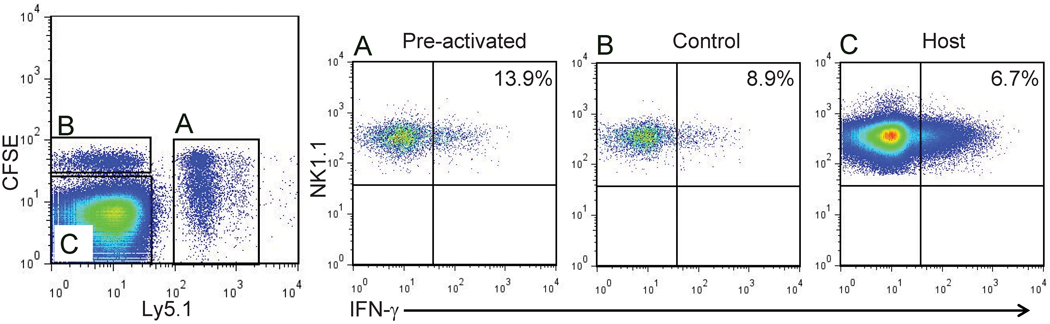
Fig. 1. Enhanced IFN-γ production by memory-like NK cells is maintained for at least 4 weeks.
Cytokine-activated (A) Ly5.1+ CFSE-labeled NK cells and control-treated (B) Ly5.2+ CFSE-labeled NK cells were adoptively co-transferred into a naive Ly5.2 host. Four weeks after adoptive transfer, NK cell were re-stimulated in vitro for four hours with IL-12 and IL-15. Three populations of NK cells were identified based on CFSE and Ly5.1 expression (left panel, gated on NK cells): (A) preactivated NK cells were significantly more likely to produce IFN-γ when compared to (B) control-transferred or (C) host NK cells (representative flow plots shown).
One potential explanation for gain of memory-like responses by NK cells in this system is that proliferation in vivo might allow for changes in daughter NK cells enabling them to produce IFN-γ. However, this was not the case, and identification of the original parental NK cells and daughter generations by CFSE dilution clearly showed that all preactivated NK cell generations had a similar memory-like phenotype (11). These data suggest that NK memory-like responses are heritable and passed on to daughter cells that were never previously activated.
NK cell memory-like responses were also recently evaluated in the context of receptor activation (12). While the overall NK cell receptor repertoire is limited, some NKRs have been found to specifically bind pathogen-encoded ligands, akin to foreign antigens as discussed. The most well-described of these interactions is the activating Ly49H receptor, which is expressed on approximately 50% of NK cells from certain mouse strains, including C57BL/6 mice, and is specific for the MCMV-encoded ligand m157 (54–58, 70). We previously demonstrated that Ly49H+ NK cells specifically undergo a rapid proliferative phase with significant expansion followed by a contraction phase two weeks after infection (71). Expansion of Ly49H+ NK cells is driven by signals delivered directly through Ly49H and observed even in the absence of cytokines such as type I interferons (72). Using a model where splenic NK cells were adoptively transferred into DAP12-deficient mice, Sun et al. (12) recently found that following MCMV infection, previously activated Ly49H+ NK cells persist greater than two months. Interestingly, these NK cells were more responsive to activation in vitro 70 days after initial MCMV infection and expressed a more ‘mature’ phenotype with low levels of CD27 and higher levels of Ly6C, KLRG1, and CD43 (12). In addition, adoptive transfer of Ly49H+ NK cells that were previously activated during MCMV infection provided superior protection compared to naïve NK cells against MCMV challenge of DAP12−/− newborn mice which, for the most part, lack functional Ly49H (12, 73, 74). These authors suggest that Ly49H NK cells differentiate into antigen-specific ‘memory’ NK cells with characteristics more typical of memory T cells than cytokine-induced memory-like NK cells (12, 73). It will be interesting to learn whether the protective effect of Ly49H+ MCMV-induced memory NK cells extends to other infections or is specific to m157-expressing MCMV, as would be predicted based on adaptive immune models of memory. Moreover, it will be of interest to determine if memory Ly49H+ NK cells control reactivation of MCMV, which undergoes latency. However, MCMV can mutate such that m157-deficient viral clones can emerge as early as 3 weeks after infection (75), suggesting that MCMV could escape from antigen-specific memory NK cells.
These studies point toward a previously unrecognized attribute of NK cells and innate immunity, the capacity to exhibit memory-like immune responses. It appears that memory-like NK cells can be generated via activation with cytokines or through engagement of activating receptors. The relationship between these two mechanisms of activation and relevance in vivo remains to be seen, although it is clear that NK cells acquire the ability to mediate enhanced responses based on prior activation.
Cytokine-induced NK cell memory-like responses versus priming or arming
While NK cells have the capacity to quickly produce cytokines and are able to kill target cells within hours, it would obviously be detrimental to the host if they did so at all times. Instead, NK cells require a signal to boost their immunoregulatory functions, i.e. positive signals via NKRs or cytokine receptors are required to optimally trigger NK cells by other stimuli, a concept termed ‘priming or arming’. The Diefenbach laboratory (44) recently demonstrated that interactions with DCs and trans-presentation of IL-15 to NK cells following in vivo activation with TLR ligands and certain infections primes ex vivo NK cell cytokine production and killing. Work by Fehniger et al. (31) provided a potential mechanism for IL-15-induced changes by showing that activation of NK cells releases a post-transcriptional block in granzyme B and perforin production, ‘arming’ NK cells with these proteins and thus enhancing NK cell cytotoxicity In addition to IL-15, in vivo IL-18 was also recently shown to be required for optimal NK cell activation ex vivo (76). Thus, it is important to address whether cytokine-induced memory-like NK responses actually represent prolonged priming/arming.
While both memory-like responses and priming/arming can be induced by cytokine signaling, there are several key differences. First, cytokine-induced memory-like responses are seen weeks after the initial activation event [11) (Fig. 1). Priming and arming have been studied in the context of immediate activation of NK cells and conceptually designate an event that takes place just prior to NK cell effector functions, i.e. cytokine production or cytotoxicity. Second, cytokine-induced memory-like responses are heritable. Priming and arming are dependent on direct signaling and would only be expected to affect those NK cells directly activated by cytokines and not their progeny. Finally, memory-like NK cells do not constitutively express high levels of activation receptors or granzyme B, as is seen with priming and arming respectively (11, 31, 44). Therefore, cytokine-induced memory-like responses can be distinguished from priming and arming based on the length of response, heritability, and activation state of NK cells. That memory-like cells do not constitutively produce cytokines or granzyme B protein suggest that short-term priming/arming is complementary to long-term memory-like responses and may represent the initial component of the memory-like effect.
Specificity of NK cell memory-like responses
NK cell memory-like responses have been described as specific for a particular activation signal, as in the case of NK-mediated hapten-induced CHS (10), specific to an NK cell receptor, as seen with m157-induced MCMV responses (12), and also non-specific in response to cytokines (11). This brings to question the requirement for specificity with regards to immunologic memory.
By their nature, T and B lymphocytes are antigen-specific and thus current concepts of immunologic memory built around these adaptive immune cells require specificity. However, it can be argued that the requirement for antigen-specificity by adaptive immune cells does not preclude the possibility that other routes are possible to achieve a biologic effect of immunologic memory, i.e. a robust response to later activation. In fact, non-specific memory responses by T cells were recently proposed by Noble, who suggested the possibility of CD8+ T-cell antigen-nonspecific ‘memory of danger’ as another form of T-cell memory, allowing the host to exhibit enhanced CD8+ memory T-cell responses to general inflammation rather than specific antigen challenges (77, 78). While the concept of non-specific T-cell memory may be controversial, it is well-established that memory CD8+ T cells are activated not just by antigen but also by cytokine stimulation, suggesting that it is beneficial even for the adaptive immune system to acquire the capacity to respond non-specifically during the immune response (79).
How would non-specific NK cell memory-like responses benefit the host? As discussed, many early NK cell responses to infection are dependent on cytokines and interactions with APCs. Even in the context of MCMV, which encodes a ligand (m157) specific for an NK cell activating receptor (Ly49H), early NK cell proliferation and IFN-γ production are independent of Ly49H stimulation (80). Thus, there are many situations during which the host may benefit from an experienced pool of NK cells ready to robustly respond to an immunologic challenge (Fig. 2).
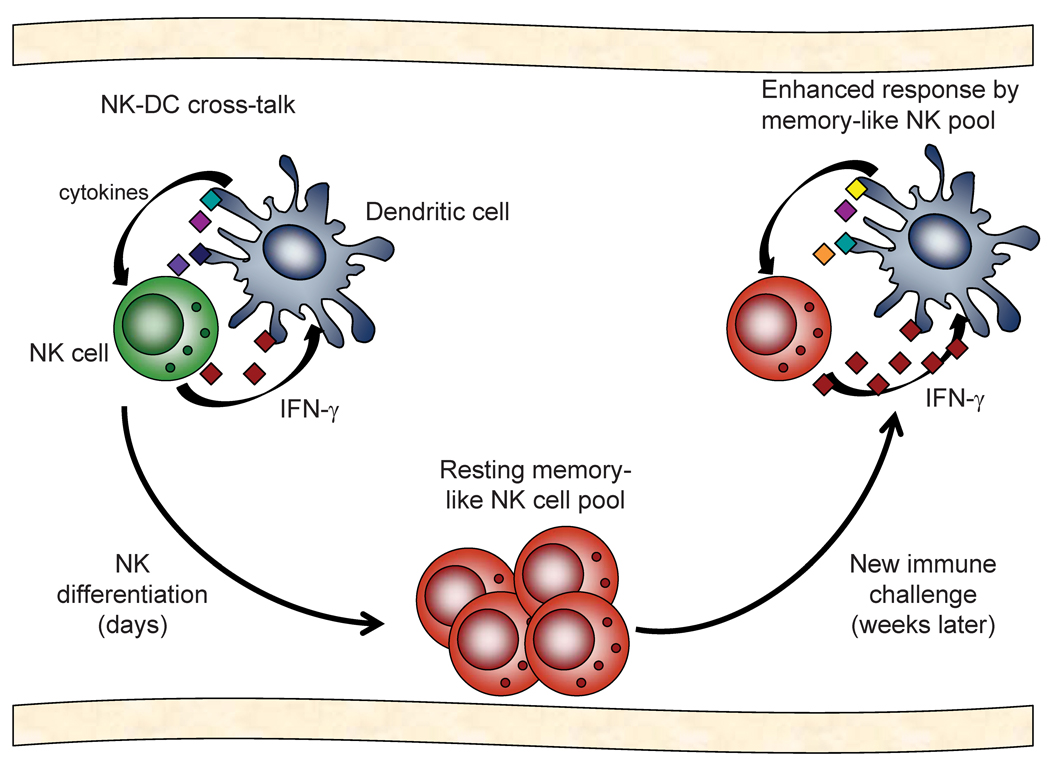
Fig. 2. Model of cytokine-induced NK cell memory-like responses.
NK and dendritic cell (DC) cross-talk involves cytokine stimulation as well as cell-contact signals. Following activation and production of IFN-γ, some NK cells might differentiate into memory-like cells, a process that takes less than one week. These memory-like NK cells do not constitutively produce IFN-γ and are maintained in a resting state. However, weeks later, when these NK cells are activated again via NK-DC cross-talk, this pool of memory-like NK cells could provide enhanced IFN-γ and therefore potentially improved protection against pathogens.
The finding that previously cytokine-activated NK cells were more responsive to re-stimulation, via both non-specific cytokine and specific activating NKRs including Ly49H (11), suggests that non-specific NK activation could later influence more specific NK cell responses. In this regard, it is again important to note that MCMV clones with defective m157 expression can escape NK cell control during the course of a single infection (75). Thus, ultimately, both antigen-specific and nonspecific routes to differentiating a long-lasting pool of NK cells able to respond more robustly than naive NK cells could function in complementary roles in vivo to benefit the host.
Potential mechanisms of cytokine-induced memory-like responses
Cytokine-induced memory-like responses result in enhanced IFN-γ production by NK cells that were previously activated. Regulation of IFN-γ by NK cells is complex and multiple post-transcriptional regulatory mechanisms play a role in the production of this protein (81). Indeed, results from an IFN-γ reporter mouse in which yellow fluorescent protein is expressed by cells transcribing IFN-γ suggest that Ifng transcription is turned on very early during NK cell development, even at the stage of NK1.1− NK cell bone marrow progenitors (82). These NK cells and NK precursors do not, however, constitutively produce IFN-γ protein, indicating translational control. Such post-transcriptional control of NK cell effector molecules is not limited to just IFN-γ, and NK cell perforin and granzyme B have also been shown to be controlled at this level (31), suggesting that translational regulation may be a common mechanism to direct NK cell function. Consistent with translational control in NK cells, basal IFN-γ transcript levels were the same in memory-like and control NK cells, though it is possible that only a subset of previously activated NK cells represent the memory-like pool, diluting any possible differences between naïve and memory-like NK cells (11).
The finding that cytokine-induced memory-like responses are NK-intrinsic and heritable suggest a mechanism whereby NK cell responsiveness is permanently altered, such as an epigenetic change. In T cells, the ability to efficiently produce IFN-γ is thought to be related to epigenetic alterations in and around the Ifng locus (83, 84). The Ifng gene has also been interrogated in bulk NK cells, and in contrast to naive T cells, NK cells appear to have an ‘open’ locus more similar to Th1 polarized T cells than naive T cells (83, 85). This information, combined with the knowledge that basal IFN-γ transcription is the same, argues against epigenetic changes at the Ifng locus. This does not preclude the possibility of heritable epigenetic changes in other genes that regulate IFN-γ transcription or translation, of currently unknown modifications to the Ifng gene that might allow for enhanced transcription upon activation, or that the subset of activated NK cells representing the memory-like pool has epigenetic alterations in the Ifng as compared to naive NK cells.
Future directions
Many questions regarding the routes of NK cell activation that result in memory-like effects remain. Given the data that Ly49H activation induces long-lasting NK cell responses, it is logical to hypothesize that other activating NK cell receptors might induce similar functions. For example, the activating receptor NKG2D recognizes multiple host-encoded stress-induced ligands and memory-like responses to activation through NKG2D may represent a common mechanism enabling NK cells to better recognize transformed and stressed cells.
Cytokine-induced memory-like NK cells exhibit enhanced IFN-γ responses. However, are NK cells activated to produce cytokines other than IFN-γ capable of memory-like responses? For example, NK-22 cells produce IL-22 in response to IL-23 stimulation (32–36). Are these NK cells capable of retaining a similar memory of the capacity to produce IL-22? Similarly, one could hypothesize that the functional outcome of memory-like responses depends upon the prior response mediated by an NK cell. Might initial activation via NK cell cytotoxic pathways be capable of generating memory-like cells with the capacity for enhanced killing?
Further investigation of the functional properties and discovery of characteristics distinguishing cytokine-induced memory-like NK cells will be important to studying potential mechanisms of enhanced NK cell function. For example, knowledge of other cytokines more efficiently produced by these cells or distinguishing phenotypic markers might spur investigations of common pathways regulating these properties.
These are all important questions as we begin to evaluate the consequences of NK cells that are better prepared to respond to immunologic challenges. Furthermore, the discovery of memory-like properties by NK cells suggests it is also worth considering whether other innate immune cells might have the capacity to ‘remember’. Finally, evaluation of the mechanisms of NK cell memory-like responses may have the potential to provide insights into the workings of not only innate immunity but also adaptive immune memory.
Conclusions
The most important function of NK cells is to provide host defense against infections and transformed cells. The findings of NK cell memory-like functions, both specific and non-specific, will hopefully add to our understanding of NK cell defenses and lead to strategies that will enable us to manipulate NK cell and innate immune responses. In addition to further dissection of murine NK cell memory-like responses, evaluation of the relevance (and existence!) of such responses in humans will also be important to understanding how this discovery might aid in human disease.
Acknowledgments
Work in the Yokoyama lab is supported by the Howard Hughes Medical Institute and grants AI34385, AI33903, AI51345, AI57160, and AR48335 from the National Institutes of Health. M.A.C. is supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 HD043010 from the NICHD. We thank J. Elliott and H. Jonsson for critical review of the manuscript.
References
- 1.Medzhitov R, Janeway C., Jr Innate immunityN Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KTP, Walport M. Janeway's Immunobiology. 7th edn. New York: Taylor & Francis, Inc.; 2007. [Google Scholar]
- 3.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 4.Bustamante J, et al. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory states the examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- 6.Crotty S, Ahmed R. Immunological memory in humans. Semin Immunol. 2004;16:197–203. doi: 10.1016/j.smim.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- 9.Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18:338–343. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 11.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 14.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9:568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- 18.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304–316. doi: 10.1038/nri1055. [DOI] [PubMed] [Google Scholar]
- 21.Stewart CA, Vivier E, Colonna M. Strategies of natural killer cell recognition and signaling. Curr Top Microbiol Immunol. 2006;298:1–21. doi: 10.1007/3-540-27743-9_1. [DOI] [PubMed] [Google Scholar]
- 22.Bryceson YT, Long EO. Line of attack: NK cell specificity and integration of signals. Curr Opin Immunol. 2008;20:344–352. doi: 10.1016/j.coi.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 25.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalifour A, et al. A Role for cis Interaction between the Inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, et al. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc Natl Acad Sci USA. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anfossi N, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Fehniger TA, et al. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 30.Grant LR, et al. Stat4-dependent, T-bet-independent regulation of IL-10 in NK cells. Genes Immun. 2008;9:316–327. doi: 10.1038/gene.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 35.Sanos SL, et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 37.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 38.Fehniger TA, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 39.Campbell JJ, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 40.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ. Evidence for NK cell subsets based on chemokine receptor expression. J Immunol. 2006;177:7833–7840. doi: 10.4049/jimmunol.177.11.7833. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez NC, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 42.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- 43.Moretta L, et al. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–228. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 44.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 48.Brilot F, Strowig T, Roberts SM, Arrey F, Munz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Rα. J Clin Invest. 2007;117:3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114:3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 50.Lapaque N, Walzer T, Meresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. J Immunol. 2009;182:4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 51.Orange JS, Biron CA. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 52.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown MG, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 55.Daniels KA, Devora G, Lai WC, O'Donnell CL, Bennett M, Welsh RM. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, et al. Susceptibility to mouse cytomegalovirus is associated with deletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nat Genet. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- 57.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 59.Cheng TP, et al. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics. 2008;60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fodil-Cornu N, et al. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kielczewska A, et al. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J Exp Med. 2009;206:515–523. doi: 10.1084/jem.20080954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 63.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Guma M, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2006;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 65.Guma M, Angulo A, Lopez-Botet M. NK cell receptors involved in the response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 2006;298:207–223. doi: 10.1007/3-540-27743-9_11. [DOI] [PubMed] [Google Scholar]
- 66.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koka R, et al. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 70.Scalzo AA, Yokoyama WM. Cmv1 and natural killer cell responses to murine cytomegalovirus infection. Curr Top Microbiol Immunol. 2008;321:101–122. doi: 10.1007/978-3-540-75203-5_5. [DOI] [PubMed] [Google Scholar]
- 71.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 72.Geurs TL, Zhao YM, Hill EB, French AR. Ly49H engagement compensates for the absence of type I interferon signaling in stimulating NK cell proliferation during murine cytomegalovirus infection. J Immunol. 2009;183:5830–5836. doi: 10.4049/jimmunol.0901520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun JC, Lanier LL. Natural killer cells remember: an evolutionary bridge between innate and adaptive immunity? Eur J Immunol. 2009;39:2059–2064. doi: 10.1002/eji.200939435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Orr MT, et al. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.French AR, et al. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 2004;20:747–756. doi: 10.1016/j.immuni.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 76.Chaix J, et al. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noble A. Do we have memory of danger as well as antigen? Trends Immunol. 2009;30:150–156. doi: 10.1016/j.it.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Leggat JA, et al. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J Allergy Clin Immunol. 2008;122:1014–1021. doi: 10.1016/j.jaci.2008.08.011. e1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sprent J, Zhang X, Sun S, Tough D. T-cell turnover in vivo and the role of cytokines. Immunol Lett. 1999;65:21–25. doi: 10.1016/s0165-2478(98)00119-9. [DOI] [PubMed] [Google Scholar]
- 80.Dokun AO, Chu DT, Yang L, Bendelac AS, Yokoyama WM. Analysis of in situ NK cell responses during viral infection. J Immunol. 2001;167:5286–5293. doi: 10.4049/jimmunol.167.9.5286. [DOI] [PubMed] [Google Scholar]
- 81.Young HA, Bream JH. IFN-gamma: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- 82.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang S, Aune TM. Histone hyperacetylated domains across the Ifng gene region in natural killer cells and T cells. Proc Natl Acad Sci USA. 2005;102:17095–17100. doi: 10.1073/pnas.0502129102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hatton RD, et al. A distal conserved sequence element controls Ifng gene expression by T cells and NK cells. Immunity. 2006;25:717–729. doi: 10.1016/j.immuni.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Tato CM, Martins GA, High FA, DiCioccio CB, Reiner SL, Hunter CA. Cutting Edge: Innate production of IFN-γ by NK cells is independent of epigenetic modification of the IFN-γ promoter. J Immunol. 2004;173:1514–1517. doi: 10.4049/jimmunol.173.3.1514. [DOI] [PubMed] [Google Scholar]