Abstract
BUF/Mna rats develop thymomas spontaneously, which histologically mimic human thymomas. Although neoplasms in this rat strain contain a large number of immature lymphocytes, the phenotype has not been sufficiently assessed. We characterized T cell phenotypes in tumors from BUF/Mna rats in the present study. We also analyzed BUF/Mna-Rnu/+ rats, a heterozygous strain with suppressive thymomagenesis, and compared the histology and T cell maturation with those from the BUF/Mna rats. A total of 11 BUF/Mna and 10 BUF/Mna-Rnu/+ rats were used. Three-color flow cytometry was performed with anti-CD3, CD4, and CD8 antibodies to identify infiltrated lymphocytes, while tumor histology was evaluated with hematoxylin-eosin staining. The weight ratios of the entire thymic tissue including thymoma as compared to the BUF/Mna and BUF/Mna-Rnu/+ rat bodies were 0.8±0.8% and 1.2±1.8%, respectively. Histological findings for both rat congenic strains showed abundant lymphocytes surrounding large polygonal neoplastic thymic epithelia, which was compatible with the type B1 classification of the World Health Organization for human thymoma. CD4+CD8+ T cells accounted for 73.7±8.0% of the cells in tumors from BUF/Mna and 67.2±9.4% in those from BUF/Mna-Rnu/+ rats. Further, CD3-CD4-CD8+ T cells, intermediate between CD4-CD8-and CD4+CD8+ cells, accounted for 47.7±17.5% and 38.0±14.0% of the cells in tumors from the BUF/Mna and BUF/Mna-Rnu/+ strains, respectively. Thus, the proportion of developing thymic lymphocytes in and histology of thymomas from BUF/Mna and BUF/Mna-Rnu/+ rats were similar. These results suggest that both BUF/Mna and BUF/ Mna-Rnu/+ strains are suitable animal models for human thymoma to understand the development of immature thymic lymphocytes.
Keywords: Thymus, thymoma, animal model, rat, T cells
Introduction
A human thymoma is a neoplasm of thymic epithelial cells frequently associated with a large number of immature thymic lymphocytes [1]. This type of tumor shows poor nuclear atyp-ism, while malignant progression such as dissemination or metastasis is observed. Since autoimmune diseases including myasthenia gravis occur in some thymoma patients, immu-nological alteration caused by the neoplasm has been speculated [2,3]. It is also reported that immature T cells with the CD3-CD4+CD8- phenotype accumulate in thymomas as compared to the normal thymus [4]. Previously, we identified the contribution of neoplastic thymic epithelial cells to thymic lymphocyte development in vitro [5], however, the biological characteristics of human thymomas remain unclear.
BUF/Mna rats were first reported as an animal model of human thymoma, in 1977 [6]. All of the animals in this strain develop spontaneous thymomas, which consists of neoplastic thymic epithelia with proportions of non-neoplastic lymphocytes. Tsr-1, the genetic region responsible for regulating thymomagenesis, has been identified on chromosome 7 by microsatellite analyses [7]. The major proportion of lymphocytes in BUF/Mna ratthymomas have an immature phe-notype expressing both CD4 and CD8, thus they are called double-positive cells [8]. However, T cell development from CD4-CD8- to CD4+CD8+ via CD3-CD4-CD8+ cells in thymomas is obscure. We assessed this stage of development in BUF/Mna rat thymoma in the present study using flow cytometry. We also analyzed the BUF/Mna-Rnu/+ rat strain, in which thymoma-genesis is moderately suppressed by introduction of a rat nude genetic region, and compared the histology and maturity of lymphocytes from that strain with those from BUF/Mna rat [9].
Materials and Methods
Animal
BUF/Mna and BUF/Mna-Rnu/+ rats were obtained from the animal laboratory of the Department of Surgical Pathology, Fujita Health University Medical School [6-9]. The rats were sacrificed at various ages from 2 to 22 months (average 12 months). Rats younger than 3 months (2 BUF/Mna rats, 1 BUF/Mna-Rnu/+ rat) were used as young controls. Thymomas were extirpated through a median sternotomy with the surrounding thymic tissue. Animal care was performed under supervision of the Animal Research Committee in accordance with the Guidelines for Animal Experiments of our institute.
Histological evaluation
Resected thymomas were fixed in 10% formalin and embedded in paraffin. Histological examinations were performed with hematoxylin-eosin staining by a pathologist.
Antibodies
FITC-conjugated anti-CD3, PE-conjugated anti-CD4, and biotin-conjugated anti-CD8 antibodies were purchased from Serotec (Oxford, UK). FITC-conjugated control mouse IgG and Streptoavidin -PE-Cy5 were from BD Bioscience (Tokyo, Japan).
Preparation of lymphocytes and flow cytometry
Lymphocytes were isolated by mechanically teasing and pressing the thymoma against a stainless steal mesh. The cells were suspended in Hanks balanced salt solution, then separated on a Ficoll-Paque density gradient (GE Healthcare, Uppsala, Sweden) to remove erythrocytes and dead cells. Isolated viable mononuclear cells were suspended and washed once in Hanks balanced salt solution, then counted and applied to flow cytometry as follows. To stain the surface antigens on the lymphocytes, 1 × 106 cells were suspended in 300 μl of PBS and mixed with a combination of 5 μl each of FITC-, PE- and biotin-conjugated monoclonal antibodies (MoAbs). After incubation for 20 minutes at 4°C, the cells were washed twice and suspended in 300 μl of PBS. Streptoavidin-PE-Cy5 was added for three-color flow cytometry. After incubation for 30 minutes at 4°C, the cells were washed twice and subjected to FACS analysis. Data collected from 5 × 104 cells were analyzed using the Cell Quest program.
Statistics
Statistical analysis to compare the average of groups was performed with unpaired t-test using commercially available soft ware Stat View (Abacus Concepts, Inc., Berkeley, CA) and a probability value less than 0.05 was considered significant.
Results
Thymoma weights
The weights of the thymomas with the neighboring thymic tissue varied from 0.29 to 10g in the BUF/Mna (average 3.2±3.5g) and 0.52 to 15. 1g in the BUF/Mna-Rnu/+ (average 4.9±7.3g) rats. The ratios of whole thymic tissue with the thymoma weight to body weight were 0.8±0.8% and 1.2±1.8%, respectively, while those for the young control rats were 0.5% and 0.4%, respectively. We found no significant differences for thymoma weight between the two congenic strains, and no correlation was observed between thymoma weight and rat age.
Pathological findings
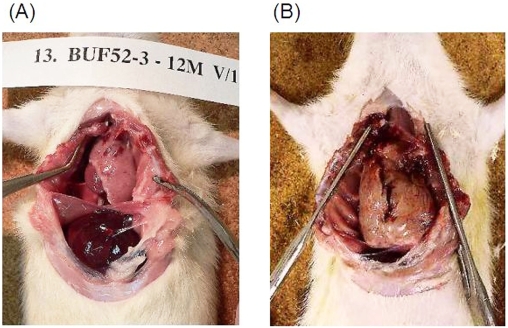
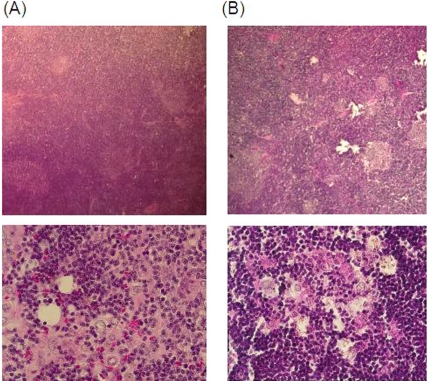
Representative macroscopic findings for BUF/ Mna and BUF/Mna-Rnu/+ rats are shown in Figure 1. Thymomas from both congenic strains were a white-pink color showed a soft elasticity. The border with the residual thymus was generally obscure and no invasion to surrounding organs, such as the lungs, pericardium, and great vessels, was found. No pleural dissemination was detected. Figure 2 shows microscopic findings of thymomas from both strains following hematoxylin-eosin staining, which revealed common features, including enlargement of the cortex area and large-sized rounded neoplastic thymic epithelia associated with abundant lymphocytes. These findings are similar to a type B1 (predominantly cortical type) human thymoma in the World Health Organization Classification [1].
Figure 1.
Characteristic macroscopic finding of spontaneous thymomas from rats. (A) BUF/Mna rat, 12 months old, male, thymoma weight 5.3g. (B) BUF/Mna-Rnu/+ rat, 17 months old, male, thymoma weight 20g. Both thymomas showed a common appearance, including a white-pink color and soft elasticity, without local invasiveness. The difference of these thymomas weight between BUF/Mna-Rnu/+ and BUF/Mna was probably due to the timing of harvest.
Figure 2.
Microscopic findings following hema-toxylin-eosin staining of BUF/Mna (A) and BUF/ Mna-Rnu/+ (B) thymomas. Under low magnification, enlargement of the cortex area with a reduced medulla is shown. Under high magnification, large-sized round neoplastic thymic epithe-lia associated with abundant lymphocytes can be seen.
Flow cytometry of lymphocytes in thymomas
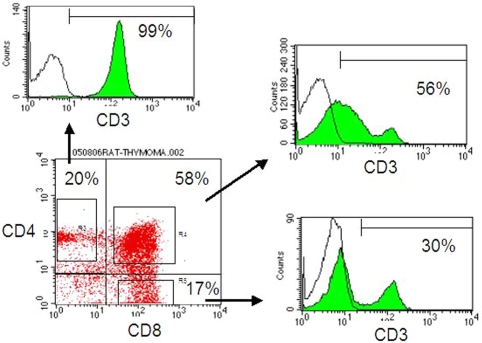
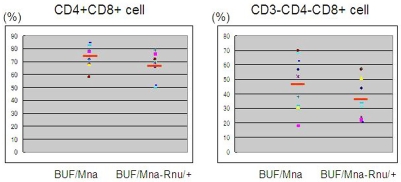
Representative flow cytometry results for lymphocytes in a thymoma from a BUF/Mna rat (12 months) are shown in Figure 3. The major population of thymic lymphocytes had the CD4+CD8+ double phenotype. Although most of the CD4+CD8- T cells were mature T cells expressing CD3, a proportion of CD4-CD8+ cells were immature CD8 single positive cells without CD3 expression, which were intermediate in development from CD4-CD8- to CD4+CD8+ cells. The results of flow cytometry for all rat tumors are summarized in Figure 4. CD4+CD8+ cells accounted for an average 73.7% and 67.2% of total lymphocytes in BUF/Mna and BUF/Mna-Rnu/+ rat thymomas, respectively. The 2 young BUF/Mna control rats had populations comprised of 75% and 69%, respectively, of CD4+CD8+ cells, which was 55% for the young BUF/Mna-Rnu/+ control. In addition, CD3 -CD4-CD8+ cells accounted for 47.7% and 38.0% of the CD4-CD8+ cells in BUF/Mna and BUF/Mna-Rnu/+ thymomas, respectively, while those ratios in the young BUF/Mna control rats were 63% and 48%, and 30% in the young BUF/ Mna-Rnu/+ control. We found no significant difference in the proportion of CD4+CD8+ and CD3-CD4-CD8+ cell subsets between the two congenic strains.
Figure 3.
Representative results of flow cytometry for lymphocytes in thymomas from BUF/Mna rats. The lymphocytes consisted of a major proportion of CD4+CD8+ cells and CD4 or CD8 single positive subsets, while CD4-CD8- cells comprised the minority. All CD4+CD8- cells expressed CD3, while only 30% of the CD4-CD8+ cells expressed CD3. The remaining 70% of CD3-CD4-CD8+ cells were considered to be T cells developing from CD4-CD8- to CD4+CD8+ cells.
Figure 4.
Results of flow cytometric analyses of lymphocytes in thymomas from both strains. CD4+CD8+ cells accounted for 73.7±8.0% and 67.2±9.4% in BUF/Mna and BUF/Mna-Rnu/+ thymomas, respectively. Further, CD3-CD4-CD8+ cells accounted for 47.7±17.5% and 38.0±14.0% of CD4-CD8+ cells in BUF/Mna and BUF/Mna-Rnu/+ thymomas, respectively. Differences between the two strains were not statistically significant (unpaired t-test).
Discussion
Spontaneous thymomas that develop in rats are used as a model of human thymoma, which is a low-grade malignancy arising from thymic epithelium. Microscopic findings and the characteristics of infiltrating lymphocytes in rat thymomas have been reported [8]. In the present study, we analyzed lymphocytes in thymomas from BUF/Mna rats using multi-color flow cy-tometry with CD3, CD4, and CD8 antibodies, and identified active T cell development from CD4-CD8- cells into CD4+CD8+ cells via CD3-CD4-CD8+ cells. This phenomenon was also observed in BUF/Mna-Rnu/+ rat thymomas, in which tumor development is slightly suppressed by introducing of nude genetic region. Further, no significant difference was found in T cell development or maturity between the two con-genic strains. Thus, thymomas from BUF/Mna-Rnu/+ rats had nearly the same immunological features as those from BUF/Mna rats, though the process of thymomagenesis was slightly suppressed [10].
In regards to the pathological features, human thymomas show a varied histology and are currently classified into types A, AB, B1, B2, and B3, according to the World Health Organization Classification [1]. In the present study, thymomas from both BUF/Mna and BUF/Mna-Rnu/+ rats showed a common histological appearance compatible with type B1. In humans, type B1 thymomas are occasionally difficult to distinguish from thymic hyperplasia and recognized to be an intermediate malignancy between type A and type C [11]. However, the presence of neoplastic large round epithelia with a large number of associated lymphocytes, as well as proportional alterations of the cortex and medulla areas, suggested that the thymomas from both strains were neoplastic and not hyperplas-tic. Taguchi and colleague also reported the neoplastic behavior of BUF/Mna rat thymomas using experiments with thymus-implantation under the renal capsule of nude rats [12].
Human thymomas contain abundant CD3-CD4+CD8- T cells that develop into CD4+CD8+ from CD4-CD8- cells, though the proportions vary among patients and are correlated with HLA class II expression levels of neoplastic thymic epithelia [13]. In the present study, the proportion of CD3-CD4-CD8+ cells, which are intermediate between CD4-CD8- and CD4+CD8+ cells, in the rat thymus and thymoma specimens also varied in both strains. According to the intermediate proportion, the developing T cells in BUF/Mna-Rnu/+ rat thymoma were slightly immature as compared to those in BUF/Mna rats, though the difference was not statistically significant. In conclusion, we believe that the both spontaneous thymoma BUF/Mna and BUF/Mna-Rnu/+ rat strains provide suitable animal models for studies of human thymomas, due to similarities in histology and immaturity of infiltrating lymphocytes, while the thymomagenesis is delayed in BUF/Mna-Rnu/+ rat.
References
- 1.Marx A, Ströbel P, Zettl A, Chan JKC, Mueller-Hermelink HK. World Health Organization Classification of Tumors. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology & Genetics Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 152–71. Thymomas. [Google Scholar]
- 2.Buckley C, Douek D, Newsom-Davis J, Vincent A, Willcox N. Mature, long-lived CD4+ and CD8+ T cells are generated by the thymoma in myasthe-nia gravis. Ann Neurol. 2001;50:64–72. doi: 10.1002/ana.1017. [DOI] [PubMed] [Google Scholar]
- 3.Shiono H, Wong YL, Matthews I, Liu JL, Zhang W, Sims G, Meager A, Beeson D, Vincent A, Willcox N. Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmu-nization in the tumor. Int Immunol. 2003;15:903–13. doi: 10.1093/intimm/dxg088. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi Y, Fujii Y, Okumura M, Inada K, Nakahara K, Matsuda H. Accumulation of immature CD3-CD4+CD8- single-positive cell that lack CD69 in epithelial cell tumor of human thymus. Cellular Immunol. 1995;161:181–7. doi: 10.1006/cimm.1995.1025. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Fujii Y, Okumura M, Takeuchi Y, Shiono H, Miyoshi S, Matsuda H, Shirakura R. Neoplastic thymic epithelial cells of human thymoma support T cell development from CD4-CD8- cells to CD4+CD8+ cells in vitro. Clin Exp Immunol. 1998;112:419–26. doi: 10.1046/j.1365-2249.1998.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuyama M, Nagayo T. Development of thymoma and myasthenia in Buffalo/Mna rats. Proc J Cancer Assoc. 1977;36:30. [Google Scholar]
- 7.Oyabu A, Higo K, Ye C, Amo H, Saito M, Yagyu S, Morita H, Maeda K, Serikawa T, Takahashi M, Matsuyama M. Genetic mapping of the thymoma susceptible locus, Tsr-1, in BUF/Mna rats. J Natl Cancer Inst. 1999;91:279–82. doi: 10.1093/jnci/91.3.279. [DOI] [PubMed] [Google Scholar]
- 8.Ezaki T, Kawatsu R, Matsuno K, Kotani M. Characterization of intrathymic and extrathymic T cell development in spontaneous thymoma Buffalo/ Mna rats. Thymus. 1990;16:67–87. [PubMed] [Google Scholar]
- 9.Matsuyama M, Yamada C, Kojima A. Possible single dosage effects of the nude gene: suppression of spontaneous development of thymoma and nephropathy in BUF/Mna-rnu/+ rats. Jpn J Cancer Res. 1987;78:40–4. [PubMed] [Google Scholar]
- 10.Matsuyama M, Kojima A, Katoh H, Kontani K, Kawai M. Establishment of a congenic nude strain of Rats, BUF/Mna-rnu. Immune-deficient Animals in Experimental Medicine. In: Wu B-q, Zheng J., editors. 6th International Workshop on Immune-deficient Animals. Basel: Basel Karger; 1989. pp. 27–31. [Google Scholar]
- 11.Okumura M, Ohta M, Tateyama H, Nakagawa K, Matsumura A, Maeda H, Tada H, Eimoto T, Matsuda H, Masaoka A. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94:624–32. doi: 10.1002/cncr.10226. [DOI] [PubMed] [Google Scholar]
- 12.Taguchi O, Kotani K, Ikeda H, Matsuyama M. An intrinsic thymic epithelial abnormality is responsible for the spontaneous development of predominantly lymphocytic thymomas in BUF/Mna rats. Jpn J Cancer Res. 1992;83:1166–71. doi: 10.1111/j.1349-7006.1992.tb02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue M, Okumura M, Miyoshi S, Shiono H, Fukuhara K, Kadota Y, Shirakura R, Matsuda H. Impaired expression of MHC class II molecules in response to interferon-gamma on human thymoma neoplastic epithelial cells. Clin Exp Immunol. 1999;117:1–7. doi: 10.1046/j.1365-2249.1999.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]