Abstract
The diagnosis of endometrial hyperplasia and endometrial type adenocarcinoma arising within the uterine cavity has long been rested on morphologic criteria. Although distinction between normal endometrial epithelium from adenocarcinoma is usually straightforward, the separation between normal and hyperplastic endometrium, particularly those cases without atypia, can be a diagnostic challenge. The same is true in separation of hyperplastic endometrium with atypia from endometrial-type endometrial adenocarcinoma. Type 2 3α-/type 5 17β-hydroxysteroid dehydrogenase (HSD) (AKR1C3) is a multifunctional enzyme involved in androgen, estrogen, progesterone, and pros-taglandin metabolism. Its expression has been shown in the epithelium of the renal tubules, urothelial epithelium, and endothelial cells in normal tissues as well as in prostatic adenocarcinoma. The proliferation and maintenance of endometrial epithelium is dependent on both estrogen and progesterone; and AKR1C3-mediated steroid metabolism may play a critical role in the maintenance of viable normal and abnormal endometrial epithelium. We studied the expression of AKR1C3 in 33 endometrial biopsy specimens including 13 cases of normal proliferative endometrium, 8 cases of hyperplastic endometrium with and without atypia, and 12 cases of primary endometrial adenocarcinoma of endometrial type. We demonstrated a uniform, diffuse, and strong expression of AKR1C3 in normal endometrial epithelium but not in endometrial stromal cells. In contrast, the expression of AKR1C3 is reduced in both hyperplastic and carcinomatous endometrial epithelium. These findings suggest that AKR1C3 may play important roles in the physiology of endometrial cells and that suppressed AKR1C3 expression may represent a feature that allows differentiation of hyperplastic and neoplastic endometrial epithelium from normal endometrial epithelium. However, reduced AKR1C3 expression cannot distinguish hyperplastic endometrium from endometrial adenocarcinoma of endometrial type. The biologic and pathological roles of AKR1C3 in endometrial epithelium require further investigation.
Keywords: Aldo-keto reductase, endomtrial cancer, estrogen, progesterone, prostaglandin
Introduction
Normal endometrial function requires an orchestrated interplay between different steroid hormones, including estrogen and progesterone [1]. Based on biochemical and clinical studies, the concentration of 17β-estradiol in endometrial carcinoma tissue is significantly higher than its concentration in normal endometrium [2] and excess or prolonged estrogen exposure unopposed by progesterone increases the risk of endometrial carcinoms [3, 4]. On the other hand, progesterone is absolutely necessary for maintaining the decidual phenotype before menstruation and during pregnancy through supporting endometrial cell survival [5]. There is increasing evidence that progestagen supplementation can antagonize estrogen-activated cell proliferation and protect against the development of endometrial cancer [4]. Enzymes that are responsible for intratumoral steroid hormone biosynthesis and metabolism have been suggested to play cardinal roles in steroid-dependent epithelial neoplasm such as breast cancer [6]. However, the roles of steroid hormone metabolizing enzymes in endometrial carcinoma remain unclear.
The aldo-keto reductases (AKRs) comprise a functionally diverse 15 gene families [7]. Members of the AKRsuperfamily are generally mono-meric (37 kD), cytosolic, and NAD(P)(H)-dependent oxidoreductases that share a common (α/β)8-barrel structural motif. This family of enzymes convert carbonyl groups to primary or secondary alcohols (http://www.med.upenn.eud/akr) [8]. Natural substrates for these enzymes include steroids, prostaglandins (PGs), and lipid aldehydes [9]. In humans, at least four AKR1C isoforms exist; they are known as AKR1C1 [20α (3α)-hydroxysteroid dehydrogenase (HSD)] [10], AKR1C2 (type 3 3α-HSD) [11, 12], AKR1C3 (type 2 3α/type 5 17β-HSD) [13, 14], and AKR1C4 (type 1 3α-HSD) [12].
AKR1C3 was originally cloned from human prostate [14] and placental cDNA libraries [15]. AKR1C3 has 3α-HSD, 17β -HSD, and 11-ketoprostaglandin reductase activities [16] which catalyze androgen and PG metabolism [11, 14, 16]. AKR1C3 also converts estrone (weak estrogen) to 17β-estrodiol (potent estrogen) and progesterone to 20α-hydroxyl-progesterone through reductive activity, and the reverse reactions through its oxidative activity [17]. As a result, AKR1C3 is capable of indirectly governing ligand access to various nuclear receptors, including androgen receptor (AR), estrogen receptor (ER), progesterone receptor (PR), and peroxisome proliferator-activated receptor (PPAR) [18], and regulating trans-activation activities of these nuclear receptors.
The presence of AKR1C3 has been demonstrated in both steroid-dependent and non-steroid-dependent cells including the Leydig cells [19], urothelial epithelium, epithelium of the renal tubules [20], and endometrial cells [21]. Deregulated expression of AKR1C3 has been demonstrated in multiple types of cancers, including breast cancer [22], lung cancer [23], prostate cancer [24-27], and Wilms’ tumor [28]. In contrast to earlier reports suggesting that AKR1C3 expression is significantly elevated in endometrial hyperplasia and endometrial adenocarcinoma of endometrial type [21, 29], we demonstrated immunohistochemical evidence of reduced expressions of AKR1C3 in hyperplas-tic and carcinomatous endometrial epithelium as compared to proliferative phase endometrial epithelium.
Materials and Methods
Materials
Mouse anti-AKR1C3 monoclonal antibody was produced in our laboratory [30]. Biotinylated goat-anti mouse IgG antibody and horseradish peroxidase (HRP)-conjugated streptavidin were obtained from Vector Laboratories (Burlingame, CA). Stable diaminobenzidine tetrahydrochlo-ride (DAB) and goat serum were purchased from Invitrogen (Carlsbad, CA). Hematoxylin and per-mount mounting media were obtained from Sigma-Aldrich (St. Louis, MO).
Human Tissues
Archival, formalin-fixed, paraffin-embedded non-hyperplastic/non-neoplastic, hyperplastic, and malignant human endometrium specimens were procured in the Departments of Pathology and Obstetrics and Gynecology at the University of Oklahoma Health Sciences Center. Human tissue specimens were obtained and processed with Institutional Review Board (IRB) approval. A total of 33 endometrial biopsy specimens, all from different patients, were obtained for this study. This consortium included 13 cases of control proliferative endometrium which is defined as proliferative endometrium without hyperplasia, neoplasia, or atrophy, 8 cases of hyperplastic endometrium with and without atypia, and 12 cases of primary endometrial adenocar-cinoma of endometrial type. Out of the 8 cases of hyperplastic endometrium, atypia is present in 6 of the 8 cases. In the cases with adenocar-cinoma, the cases ranged from International Federation of Gynecology and Obstetrics (FIGO) grade 2 to 3 and nuclear grade 2 to 3. The age of these patients ranged from 17 to 94 years old and all of the endometrial samples without evidence of hyperplasia, neoplasia, or atrophy (control endometrium) were obtained from women between 17 and 51 year of ages.
Immunohistochemistry of Tissue Sections
Immunohistochemistry of human tissue sections was performed as per our previously reported procedures [25] in duplicates. Briefly, tissue sections cut about 4-6 μm were mounted and baked at 60 °C for 1 hr. Sections were de-paraffinized with xylene and re-hydrated in graded ethanol followed by rinses with 0.1 M Tris-HCl (pH 7.6). Endogenous peroxidase activity was blocked by incubating the tissue sections with 1.6% H2O2 in methanol for 30 min. Antigen retrieval was performed with 0.01 M sodium citric acid buffer (pH 6.0) at 95 °C for 1 hr. Non-specific binding was blocked by incubating the tissue sections with 0.1 M Tris-HCl containing 10% goat serum for 2 hr. AKR1C3 was then detected by incubating the sections with mouse anti-AKR1C3 monoclonal antibody (clone NP6G6.A6) at a 1:200 dilution in the above blocking solution in a moist chamber at 4 °C overnight. Negative controls were performed in parallel in the absence of the primary antibody. After washes with 0.1 M Tris-HCl, the sections were treated with 1:400 dilution of biotinylated horse anti-mouse secondary antibody and incubated at room temperature for 2 hr. Following another rinses with 0.1 M Tris-HCl, antibody binding was detected by incubating the tissue sections with HRP-conjugated strepta-vidin at room temperature for 30 min. DAB-H2O2 substrate was then added to the slides and incubated at room temperature for an additional 4 min. Tissue sections were counter stained lightly with hematoxylin, dehydrated in graded alcohol, cleared in xylene, and mounted with Permount Mounting Media for visualization by light-microscopy.
Histological and Pathological Evaluation of Endometrium Specimens
The diagnoses were confirmed and the stained sections were evaluated independently by two pathologists (KMF and VZ) using a conventional light microscope. The percentage of positive cells within the entire population of epithelial cells were evaluated and assigned to one of the following categories: negative to positivity < 5%, positivity >5% but < 25%, positivity >25% but < 75%, and 100% positivity. The intensity of im-munoreactivity was also evaluated for being weak, moderate, and strong for every case.
Results
Endometrium without Evidence of Hyperplasia, Neoplasia, or Atrophy (control endometrium)
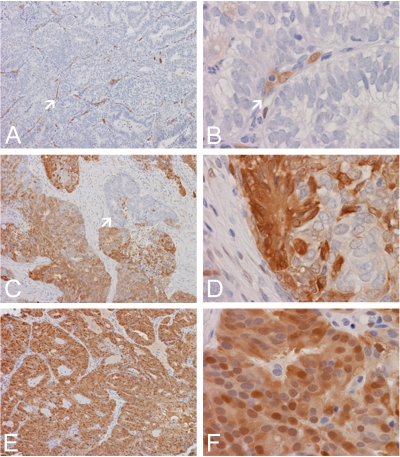
A total of 13 biopsy specimens were studied. Of these specimens, 6 of them contained only unremarkable endometrium and findings in the remaining specimens include stromal breakdown (Table 1). Immunoreactivity was evaluated and demonstrated 100% immunoreactivity (Table 1 and Table 2) in all of the epithelial cells (Figure 1). Stromal cells were consistently negative. Endothelial cells, as we have reported before [20, 25], were also strongly positive with both nuclear and cytoplasmic immunoreactivity. Strong immunoreactivity in the epithelium was noted in all of these specimens.
Table 1.
Percentage of positivity in proliferative, hypeplastic, and neoplastic endometrial tissue
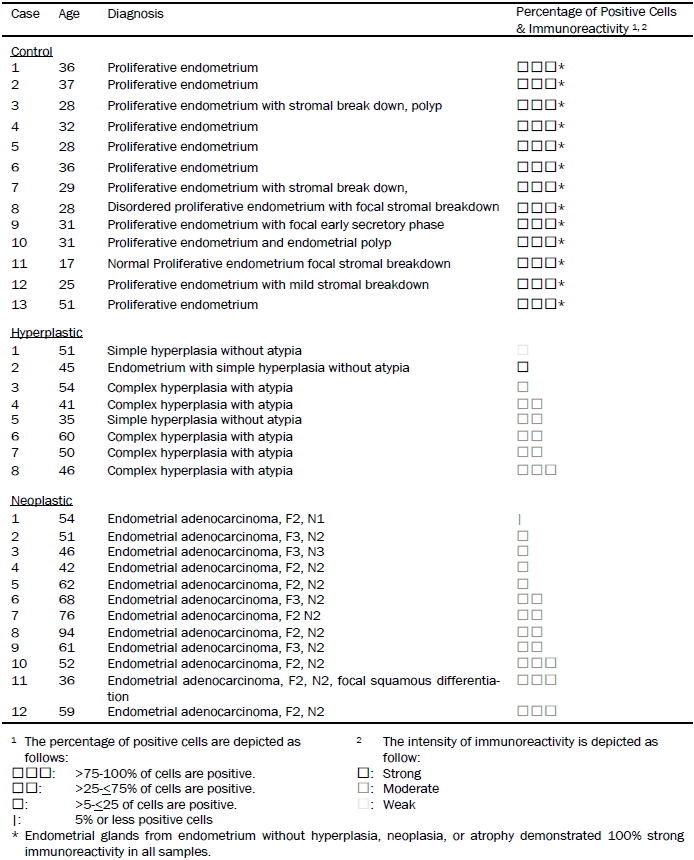 |
Table 2.
Summary of immunoreactivities
| Diagnosis | Percentage of positive cells | No. of cases |
|---|---|---|
| Proliferative endometrium without evidence of hyperplasia or neoplasia (13 cases) | >75% to 100%* | 13 (100%) |
| ≤75%, >25% | 0 (0%) | |
| ≤25%, >5% | 0 (0%) | |
| ≤5% | 0 (0%) | |
| Endometrial hyperplasia with or without atypia (8 cases) | >75% to 100% | 1 (12.5%) |
| -≤75%, >25% | 4 (50%) | |
| ≤25%, >5 | 3 (37.5%) | |
| ≤5% | 0 (0%) | |
| Endometrial adenocarcinoma, endometrial type (12 cases) | >75% to 100% | 3 (25%) |
| ≤75%, >25% | 4 (33.3%) | |
| ≤25%, >5 | 4 (33.3%) | |
| ≤5% | 1 (8.3%) |
Endometrial glands from endometrium without hyperplasia, neoplasia, or atrophy demonstrated 100% strong immunoreactivity in all samples.
Figure 1.
Expression of AKR1C3 in normal and hyperplastic endometrium. (A) Normal proliferative endometrial glands and blood vessels (arrow) are uniformly immunoreactive for AKR1C3 but endometrial stromal cells are negative. (B) Both nuclear and cytoplasmic immunoreactivity are noted in both endometrial glands and endothelial cells of blood vessels (arrow). (C) In this case of complex hyperplasia with atypia, only about 30% of the hyperplastic glands are immunoreactive for AKR1C3. Both positive (arrow) and negative areas are included here. (D) In some areas of this case, weak immunoreactivity limited to scant cells is demonstrated (arrow). (E) In this case of complex hyperplasia with atypia, there is immunoreactivity in practically in all of the hyperplastic cells. Note that a strip of residual normal endometrium is present (arrow) and shows stronger immunoreactivity than the hyperplastic cells. (F) Both cytoplasmic and nuclear immunoreactivity are noted in the hyperplastical glands. Stromal cells are largely negative. (Original magnification for panel A, C, and E is 10×, for panel B, D, and F is 60×).
Figure 2.
Expression of AKR1C3 in endometrial adenocarcinoma of endometrial type. (A) In this case of adenocarcinoma, there is no immumoreactiv-ity for AKR1C3 in the carcinomatous cells but enodothelial cells of blood vessels are immunoreactive (arrow). (B) Both nuclear and cytoplasmic immunoreactivity are demonostrated in blood vessels (arrow). (C) In this case of adenocarcinoma, there is widespread positive immunoreactivity with small clusters of non-immunoreactive area (arrow). (D) Small clusters of non-immunoreactive cells are present among positive cells. (E) In this case of adenocarcinoma, practically all carcinomatous cells are immunoreactive. (F) Both nuclear and cytoplasmic immunoreactivity are demonstrated. (Original magnification for panel A, C, and E is 10×, for panel B, D, and F is 60×).
Hyperplastic Endometrium with and without Atypia
A total of 8 biopsy specimens of hyperplastic endometrium were studied including 3 specimens without atypia and 5 specimens with atypia (Table 1). When immunoreactivity was positive, it was present only in epithelial cells and endothelial cells. Stromal cells were consistently negative. Out of these specimens, only 1 specimen contained 100% immunoreactivity that was diagnosed as complex hyperplasia with atypia. A range of immunoreactivity was demonstrated in the remaining specimens (Table 1) from 10% to 75%; and an intensity of immunoreactivity ranged from weak to moderated (Figure 1). The immunoreactivity in all but one was weaker than the control endometrium. There was 1 specimen (12.5%) with 75% to 100% positive immunoreactivity, 4 specimens (50%) with 25% to 75% positive immunoreactivity, 3 specimens (37.5%) with 5% to 25% positive immunoreactivity, and no specimen under 5% immunoreactivity (Table 2). The percentage of positive cells did not correlate with whether atypia was present.
Endometrial Adenocarcinoma
A total of 12 biopsy specimens of primary endometrial adenocarcinoma arising from the endometrium were studied; and these specimens classified as FIGO grades 2 and 3, and nuclear grades 2 and 3 (Table 1). When immunoreactivity was positive, they were present only in epithelial cells and endothelial cells. Stromal cells were consistently negative. In 3 of these specimens (25%), 100% positive immunoreactivity was present in all tumor cells. In 4 of these specimens (33.3%), there was immunoreactivity in 25% to 75% of the tumor cells. In another 4 of these specimens (33.3%), there was immunoreactivity in 5% to 25% of the tumor cells. In one of the cases (8.3%), there was less than 5% positive immunoreactivty in tumor cells. (Table 2)
Discussion
The expression patterns of AKR1C3 were studied in 13 specimens of endometrium without evidence of hyperplasia, neoplasia or atrophy, 8 specimens of hyperplasia with and without atypia, and 12 cases of primary endometrial adenocarcinoma arising from the endometrium. All of the specimens were obtained from biop sies. A strong, 100% immunoreactivity was demonstrated in the endometrial epithelium without hyperplastic or neoplastic changes. Variable degree of attenuated immuoreactivities was demonstrated in endometrial epithelium with hyperplasia and neoplasia (adenocarcinoma). Consistently, the intensities of immunoreactivity in hyperplastic and neoplastic endometrial epithelium are lower than that of endometrial epithelium without evidence of hyperplastic or neoplastic changes. The endometrial stromal cells are uniformly negative; and there is no difference in immunoreactivities in the stromal cells among the three types of specimens. These results suggest that the levels of AKR1C3 expression are reduced in hyperplastic and neoplastic epithelium.
Our data are different from previous reports [29, 31]. Ito et al. showed that AKR1C3 immunoreactivity is detected in 50% and 69% of endometrial hyperplasia and endometrial carcinoma, respectively, as compared to 19% and 25% immunoreactivity in proliferative and secretory phases of endometrium, respectively [29]. In his paper, the authors did not describe the number regarding to the normal endometrium nor did the authors provide any photomicrographs of negative examples. In another study, Šmuc at al. demonstrated up-regulated expression of AKR1C3 in endometrial adenocarcinoma [29, 31]. The authors claimed that up regulation of AKR1C3 in 8 out of 16 samples of adenocarcinoma based on levels of mRNA. However, the ratio of epithelial cells to stromal cells is greatly increased in adenocarcinoma and many of these tumors do not contain significant amount of stromal cells as compared to normal endometrium. As a result, there wound be more mRNA in carcinoma tissue per unit weight even though the level of total RNA per cell remains the same as normal endometrial epithelium. In their study of 16 specimens, there were 6 cases with significant increase, 4 with significantly decreased expression, and 6 with minimal increase or decrease in expression if the cut off point is set at RNA ratio for tumor and normal at 5 (for increased expression) and 0.5 (for decreased expression) respectively. Stastistically, this is not an evidence of increased expression. [31]
Intratumoral steroid hormone metabolism and biosynthesis is important in the etiology and progression of endometrial adenocarcinoma. In situ estrogen metabolism, including synthesis and catabolism, has recently been thought to play important roles in the development and progression of various human estrogen-dependent neoplasms including endometrial cancer. These reports have shown that local estrogen biosynthesis can be regulated by aro-matase and types 1 and 2 17β-HSD in endometrial carcinoma. Intratumoral production of estrogen occurs as a result of the aromatization of androgens such as testosterone into estrogens; and this reaction is catalyzed by the cyto-chrome P450 aromatase enzyme [32] in the stromal cells or fibroblasts of endometrial carcinoma [33]. The reversible conversions of 17β-estrodiol and estrone can be catalyzed by types 1 and 2 17β-HSD. The 17β-reduction of biologically less active estrone is catalyzed to 17β-estrodiol by type 1 17β-HSD [34], and the oxidation of 17β-estrodiol to estrone is catalyzed by type 2 17β-HSD [35]. It was reported that type 1 17β-HSD immunoreactivity and mRNA were absent in normal and hyperplastic endometrium and in endometrial carcinoma [36, 37], and type 2 17β-HSD expression was detected in normal endometrium (secretory phase) but was decreased in hyperplastic endometrium and endometrial carcinoma [37].
AKR1C3 has also been shown to metabolize progesterone. Based on radiochemical assays, AKR1C3 interconverts progesterone and 20α-hydroxyprogesterone [17]. Protective roles of progesterone in anti-endometrial cancer remain undefined. It has been suggested that progesterone exerts a potent anti-estrogenic effect in epithelial cells of the endometrium by inducing type 2 17β-HSD expression [38]. In contrast, although a strong positive correlation between type 2 17β-HSD and PR expression in the cytoplasm and the nuclei of endometrial carcinoma cells [37], the same report also showed a suppressed expression of type 2 17β-HSD in carcinoma. Since AKR1C3 may regulate the ligand availability for the PR, AKR1C3-mediated progesterone metabolism may affect estrogen action, but require further validation.
Androgens (i.e. androstenedione and 5α-dihydrotesterone), androgen metabolizing enzymes (i.e. 5α-reductases) and the AR have been identified in the endometrium [39]. AKR1C3 can convert androstenedione to testosterone [15]; and this pathway has been suggested for estrogen accumulation in the diseased endometrium [40]. However, with lower AKR1C3 expression in endometrial cancer, this pathway may be impaired in endometrial adenocarcinoma. The role of AKR1C3-mediated androgen conversion in regulating other steroid hormones metabolism in endometrial adenocar-noma requires further study.
The orphan nuclear receptor PPARγ plays important roles in the regulation of lipid homeostasis, adipogenesis, insulin resistance, and the development of various organs. AKR1C3 has 11-ketoprostaglandin reductase activity and forms 9α, 11β-PGF2α, and the reaction depletes PGD2 substrate available for converting to 15-deoxy-Δ12,14-PGJ2, the naturally occurring PPARγ ligand [41]. Ito el al. reported that that PPARγ immunoreactivity is significantly lower in endometrial adenocarcinoma as compared to secretory phase endometrium and endometrial hyperplasia [1]. Although PGJ2 inhibits cell proliferation in endometrial carcinoma cell lines [42] and PGF2α stimulates endometrial cancer cell line growth and aggressiveness through elevated expression of VEGF [43], roles of altered AKR1C3 expression in regulating PG metabolites in endometrial adenocarcinoma need to be further evaluated.
Based on enzyme kinetics, AKR1C3 catalyzes conversions between estrone and 17β-estrodiol, progesterone and 20α-hydroxyprogesterone, androstenedione and testosterone [17]. In addition, AKR1C3 can reduce 5α-dihydrotestosterone (5α-DHT) through its 3α-HSD activity [14] and accumulate 9α 11β-PGF2α through its 11-ketoprostaglandin reductase activities [16]. Based on these observations, AKR1C3 is capable of metabolizing multiple steroid hormones (estrogen, progesterone, androgen, and PG); and reduction or accumulation of these steroid metabolites may have significant impacts on intratumoral hormone balance with altered expression of AKR1C3 in en-dometrialadenocarcinoma. AKR1C3-mediated steroid hormone metabolisms and their consequences of pathological development remain undefined, and need to be further studied in the development of endometrial cancer.
Acknowledgments
This work was supported in part by and internal grant awarded to Vladislav Zakharov from the Department of Pathology of the University of Oklahoma Health Sciences Center, and grants 1R01-CA90744 and P30-ES013508 awarded to TMP and Department of Veterans Affairs Merit Review Award to HKL.
Glossary
Abbreviations
- AKR
aldo-keto reductase
- AR
androgen receptor
- ER
estrogen receptor
- HSD
hydroxysteroid dehydrogenase
- PG
prostaglandin
- PR
progesterone receptor
- PPAR
peroxisome proliferator activating receptor
References
- 1.Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma–new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54:667–679. doi: 10.1507/endocrj.kr-114. [DOI] [PubMed] [Google Scholar]
- 2.Berstein LM, Tchernobrovkina AE, Gamajunova VB, Kovalevskij AJ, Vasilyev DA, Chepik OF, Turkevitch EA, Tsyrlina EV, Maximov SJ, Ashrafian LA, Thijssen JH. Tumor estrogen content and clinico-morphological and endocrine features of endometrial cancer. J Cancer Res Clin Oncol. 2003;129:245–249. doi: 10.1007/s00432-003-0427-9. [DOI] [PubMed] [Google Scholar]
- 3.Legro RS, Kunselman AR, Miller SA, Satyaswaroop PG. Role of androgens in the growth of endometrial carcinoma: an in vivo animal model. Am J Obstet Gynecol. 2001;184:303–308. doi: 10.1067/mob.2001.109734. [DOI] [PubMed] [Google Scholar]
- 4.Voigt LF, Weiss NS, Chu J, Daling JR, McKnight B, van Belle G. Progestagen supplementation of exogenous oestrogens and risk of endometrial cancer. Lancet. 1991;338:274–277. doi: 10.1016/0140-6736(91)90417-n. [DOI] [PubMed] [Google Scholar]
- 5.Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T, Miki Y, Nakamura Y, Moriya T, Ito K, Ohuchi N, Sasano H. Sex steroid-producing enzymes in human breast cancer. Endocr Relat Cancer. 2005;12:701–720. doi: 10.1677/erc.1.00834. [DOI] [PubMed] [Google Scholar]
- 7.Jez JM, Flynn TG, Penning TM. A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol. 1997;54:639–647. doi: 10.1016/s0006-2952(97)84253-0. [DOI] [PubMed] [Google Scholar]
- 8.Jez JM, Bennett MJ, Schlegel BP, Lewis M, Penning TM. Comparative anatomy of the aldo-keto reductase superfamily. Biochem J. 1997;326:625–636. doi: 10.1042/bj3260625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyndman D, Bauman DR, Heredia VV, Penning TM. The aldo-keto reductase superfamily homepage. Chem Biol Interact. 2003;143-144:621–631. doi: 10.1016/s0009-2797(02)00193-x. [DOI] [PubMed] [Google Scholar]
- 10.Hara A, Matsuura K, Tamada Y, Sato K, Miyabe Y, Deyashiki Y, Ishida N. Relationship of human liver dihydrodiol dehydrogenases to hepatic bile-acid-binding protein and an oxidoreductase of human colon cells. Biochem J. 1996;313:373–376. doi: 10.1042/bj3130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dufort I, Soucy P, Labrie F, Luu-The V. Molecular cloning of human type 3 3a-hydroxysteroid dehydrogenase that differs from 20a-hydroxysteroid dehydrogenase by seven amino acids. Biochem Biophy Res Communication. 1996;228:474–479. doi: 10.1006/bbrc.1996.1684. [DOI] [PubMed] [Google Scholar]
- 12.Deyashiki Y, Ogasawara A, Nakayama T, Nakanishi M, Miyabe Y, Sato K, Hara A. Molecular cloning of two human liver 3a-hydroxysteroid/ dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. Biochem J. 1994;299:545–552. doi: 10.1042/bj2990545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna M, Qin KN, Wang RW, Cheng KC. Substrate specificity, gene structure, and tissue-specific distribution of multiple human 3a-hydroxysteroid dehydrogenases. J Biol Chem. 1995;270:20162–20168. doi: 10.1074/jbc.270.34.20162. [DOI] [PubMed] [Google Scholar]
- 14.Lin HK, Jez JM, Schlegel BP, Peehl DM, Pachter JA, Penning TM. Expression and characterization of recombinant type 2 3a-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3a/17b-HSD activity and cellular distribution. Mol Endocrinol. 1997;11:1971–1984. doi: 10.1210/mend.11.13.0026. [DOI] [PubMed] [Google Scholar]
- 15.Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17b-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:568–574. doi: 10.1210/endo.140.2.6531. [DOI] [PubMed] [Google Scholar]
- 16.Matsuura K, Shiraishi H, Hara A, Sato K, Deyashiki Y, Ninomiya M, Sakai S. Identification of a principal mRNA species for human 3a-hydroxysteroid dehydrogenase isoform (AKR1C3) that exhibits high prostaglandin D2 11-ketoreductase activity. J Biochem (Tokyo) 1998;124:940–946. doi: 10.1093/oxfordjournals.jbchem.a022211. [DOI] [PubMed] [Google Scholar]
- 17.Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K. Human 3a-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000;351:67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penning TM, Steckelbroeck S, Bauman DR, Miller MW, Jin Y, Peehl DM, Fung KM, Lin HK. Aldo-keto reductase (AKR) 1C3: Role in prostate disease and the development of specific inhibitors. Mol Cell Endocrinol. 2006;248:182–191. doi: 10.1016/j.mce.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Ashley RA, Yu Z, Fung KM, Frimberger D, Kropp BP, Penning TM, Lin HK. Developmental Evaluation of Aldo-keto Reductase 1C3 Expression in the Cryptorchid Testis. Urology. 2009 doi: 10.1016/j.urology.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 20.Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in adult genitourinary system. J Histochem Cytochem. 2008;56:853–861. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol. 2006;248:126–135. doi: 10.1016/j.mce.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Lewis MJ, Wiebe JP, Heathcote JG. Expression of progesterone metabolizing enzyme genes (AKR1C1, AKR1C2, AKR1C3, SRD5A1, SRD5A2) is altered in human breast carcinoma. BMC Cancer. 2004;4:27. doi: 10.1186/1471-2407-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan Q, Mumford JL, Shen M, Demarini DM, Bonner MR, He X, Yeager M, Welch R, Chanock S, Tian L, Chapman RS, Zheng T, Keohavong P, Caporaso N, Rothman N. Oxidative damage-related genes AKR1C3 and OGG1 modulate risks for lung cancer due to exposure to PAH-rich coal combustion emissions. Carcinogenesis. 2004;25:2177–2181. doi: 10.1093/carcin/bgh240. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Y, Suzuki T, Nakabayashi M, Endoh M, Sakamoto K, Mikami Y, Moriya T, Ito A, Takahashi S, Yamada S, Arai Y, Sasano H. In situ androgen producing enzymes in human prostate cancer. Endocr Relat Cancer. 2005;12:101–107. doi: 10.1677/erc.1.00914. [DOI] [PubMed] [Google Scholar]
- 25.Fung KM, Samara ENS, Wong C, Metwalli A, Krlin R, Bane B, Liu CZ, Yang JT, Pitha JT, Culkin DJ, Kropp BP, Penning TM, Lin HK. Increased expression of type 2 3a-hydroxysteroid dehydro-genase/type 5 17b-hydroxysteroid dehydrogenase (AKR1C3) and its relationship with androgen receptor in prostate carcinoma. Endocr Relat Cancer. 2006;13:169–180. doi: 10.1677/erc.1.01048. [DOI] [PubMed] [Google Scholar]
- 26.Stanbrough M, Bubley G, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 27.Wako K, Kawasaki T, Yamana K, Suzuki K, Jiang S, Umezu H, Nishiyama T, Takahashi K, Hamakubo T, Kodama T, Naito M. Expression of androgen receptor through androgen-converting enzymes is associated with biological aggressiveness in prostate cancer. J Clin Pathol. 2008;61:448–454. doi: 10.1136/jcp.2007.050906. [DOI] [PubMed] [Google Scholar]
- 28.Azzarello JT, Lin H-K, Gherezghiher A, Zakharov V, Yu Z, Kropp BP, Culkin DJ, Penning TM, Fung K-M. Expression of AKR1C3 in renal cell carcinoma, papillary urothelial carcinoma, and Wilms’ tumor. Int J Clin Expt Pathol. 2010 in press. [PMC free article] [PubMed] [Google Scholar]
- 29.Ito K, Utsunomiya H, Suzuki T, Saitou S, Akahira J, Okamura K, Yaegashi N, Sasano H. 17b-hydroxysteroid dehydrogenases in human endo-metrium and its disorders. Mol Cell Endocrinol. 2006;248:136–140. doi: 10.1016/j.mce.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 30.Lin HK, Steckelbroeck S, Fung KM, Jones AN, Penning TM. Characterization of a monoclonal antibody for human aldo-keto reductase AKR1C3 (type 2 3a-hydroxysteroid dehydro-genase/type 5 17b-hydroxysteroid dehydrogenase); immunohistochemical detection in breast and prostate. Steroids. 2004;69:795–801. doi: 10.1016/j.steroids.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Smuc T, Rizner TL. Aberrant prereceptor regulation of estrogen and progesterone action in endometrial cancer. Mol Cell Endocrinol. 2009;301:74–82. doi: 10.1016/j.mce.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Nimrod A, Ryan KJ. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab. 1975;40:367–372. doi: 10.1210/jcem-40-3-367. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K, Sasano H, Harada N, Ozaki M, Niikura H, Sato S, Yajima A. Aromatase in human endometrial carcinoma and hyperplasia. Immunohistochemical, in situ hybridization, and biochemical studies. Am J Pathol. 1995;146:491–500. [PMC free article] [PubMed] [Google Scholar]
- 34.Puranen T, Poutanen M, Ghosh D, Vihko P, Vihko R. Characterization of structural and functional properties of human 17 b-hydroxysteroid dehydrogenase type 1 using recombinant enzymes and site-directed mutagenesis. Mol Endocrinol. 1997;11:77–86. doi: 10.1210/mend.11.1.9872. [DOI] [PubMed] [Google Scholar]
- 35.Wu L, Einstein M, Geissler WM, Chan HK, Elliston KO, Andersson S. Expression cloning and characterization of human 17b-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20a-hydroxysteroid dehydrogenase activity. J Biol Chem. 1993;268:12964–12969. [PubMed] [Google Scholar]
- 36.Husen B, Psonka N, Jacob-Meisel M, Keil C, Rune GM. Differential expression of 17b-hydroxysteroid dehydrogenases types 2 and 4 in human endometrial epithelial cell lines. J Mol Endocrinol. 2000;24:135–144. doi: 10.1677/jme.0.0240135. [DOI] [PubMed] [Google Scholar]
- 37.Utsunomiya H, Suzuki T, Kaneko C, Takeyama J, Nakamura J, Kimura K, Yoshihama M, Harada N, Ito K, Konno R, Sato S, Okamura K, Sasano H. The analyses of 17b-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J Clin Endocrinol Metab. 2001;86:3436–3443. doi: 10.1210/jcem.86.7.7661. [DOI] [PubMed] [Google Scholar]
- 38.Yang S, Fang Z, Gurates B, Tamura M, Miller J, Ferrer K, Bulun SE. Stromal PRs mediate induction of 17b-hydroxysteroid dehydrogenase type 2 expression in human endometrial epithelium: a paracrine mechanism for inactivation of E2. Mol Endocrinol. 2001;15:2093–2105. doi: 10.1210/mend.15.12.0742. [DOI] [PubMed] [Google Scholar]
- 39.Carneiro MM, Morsch DM, Camargos AF, Reis FM, Spritzer PM. Androgen receptor and 5a-reductase are expressed in pelvic endometriosis. Bjog. 2008;115:113–117. doi: 10.1111/j.1471-0528.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 40.Bukulmez O, Hardy DB, Carr BR, Auchus RJ, Toloubeydokhti T, Word RA, Mendelson CR. Androstenedione up-regulation of endometrial aromatase expression via local conversion to estrogen: potential relevance to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2008;93:3471–3477. doi: 10.1210/jc.2008-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-D12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARg. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 42.Ota K, Ito K, Suzuki T, Saito S, Tamura M, Hayashi S, Okamura K, Sasano H, Yaegashi N. Peroxisome proliferator-activated receptor g and growth inhibition by its ligands in uterine endometrial carcinoma. Clin Cancer Res. 2006;12:4200–4208. doi: 10.1158/1078-0432.CCR-05-1833. [DOI] [PubMed] [Google Scholar]
- 43.Sales KJ, List T, Boddy SC, Williams AR, Anderson RA, Naor Z, Jabbour HN. A novel angiogenic role for prostaglandin F2a-FP receptor interaction in human endometrial adenocarcinomas. Cancer Res. 2005;65:7707–7716. doi: 10.1158/0008-5472.CAN-05-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]