Abstract
Primary small cell carcinoma (SmCC) of the breast is very rare and may be difficult to distinguish from me-tastatic small cell carcinoma. Confident histopathological diagnosis of a primary breast SmCC requires the demonstration of an in situ component. We report a case of primary small cell carcinoma of the breast with coexisting carcinoma in situ in which the invasive carcinoma and in situ component both expressed neuroendocrine markers and Thyroid transcription factor-1 (TTF-1) by immunohistochemistry. Expression of neuroendocrine markers and TTF-1 in the in situ component allowed a highly confident diagnosis of primary small cell carcinoma of the breast. To our knowledge there is only one previous report of TTF-1 expressing in situ carcinoma associated with a primary SmCC of the breast.
Keywords: Small cell carcinoma, Breast, Thyroid transcription factor-1, Neuroendocrine, Carcinoma in situ
Introduction
SmCC most commonly arises in the lung but may arise at other anatomical sites including the breast. Diagnosing a breast tumour as a primary SmCC may be difficult as the H&E appearance and immunohistochemical staining profile, including immunoreactivity for TTF-1, are nearly identical to that of SmCC arising at other sites [1, 2]. Confident diagnosis of primary SmCC of the breast requires the exclusion of non-mammary primary sites by clinical examination and imaging studies, and/or histopathological demonstration of an in situ component [2]. The immunohistochemical features of in situ carcinoma co-existing with SmCC of the breast are not well described. Ersahin etal [3] reported a case of SmCC of the breast with admixed in situ carcinoma in which the invasive carcinoma expressed TTF-1 and neuroendocrine markers, but the in situ component did not express TTF-1 or neuroendocrine markers. There is one previously reported case of TTF-1 expression by in situ carcinoma associated with a primary SmCC of the breast [4].
Case presentation
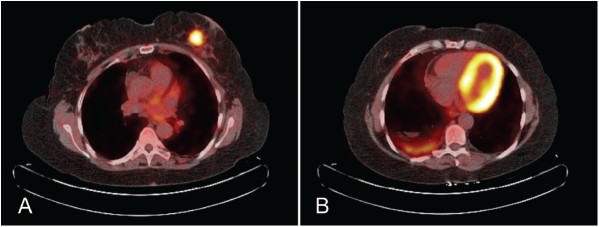
A 61-year-old woman presented with a newly diagnosed 3cm left breast mass and bulky left axillary lymphadenopathy. A non-smoker, her previous history included longstanding erythema nodosum and a right-sided pleurodesis twelve months previously for idiopathic recurrent pleural effusions. Her family history included a paternal aunt diagnosed with breast cancer in her 80s. Mammography and ultrasound demonstrated a 3cm spiculated lesion in the left breast and axillary lymphadenopathy. A needle core biopsy of the breast mass was diagnosed as small cell carcinoma. Pre-operative staging was performed; bone scintigraphy was negative for metastatic disease, and a computed tomography (CT) scan of the chest and upper abdomen showed cystic changes with fluid levels in the right lower lobe lung region, in addition to the breast tumour and lymphadenopathy. A PET/CT scan revealed a markedly fluoro-deoxy-glucose (FDG) avid 3cm left breast mass (Figure 1A), at least four enlarged FDG avid left axillary lymph nodes, and a FDG avid left internal mammary lymph node. There was also moderately intense irregular FDG uptake in the basal right lower lobe lung region consistent with an ongoing inflammatory process (Figure 1B) and similar in appearance to a PET/CT scan performed 12 months previously following the pleurodesis. The PET/CT findings were interpreted as those of a primary breast cancer and the patient underwent a left breast wide local excision, level II axillary clearance and excision of the PET-positive left internal mammary lymph node. During the course of investigations the breast tumour was noted to have increased rapidly in size. Four weeks after surgery the development of right upper quadrant pain prompted a liver ultrasound, which revealed multiple liver metastases. Serum CEA was 2625.1 ug/L (ref. <5.0 ug/L). She commenced chemotherapy one month post-operatively, with Carboplatin and Etoposide from days 1-3 on a three weekly schedule, and a plan for 4-6 cycles. The first cycle was well tolerated apart from ongoing hepatic pain. However, after two weeks she rapidly deteriorated and died, less than three months after the initial presentation.
Figure 1.
PET/CT demonstrating (A) the markedly FDG avid 3cm lesion in the left breast and (B) moderate irregular FDG avidity in the basal right lower lobe lung region consistent with inflammatory changes.
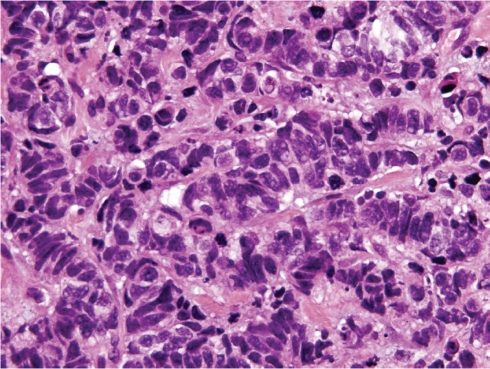
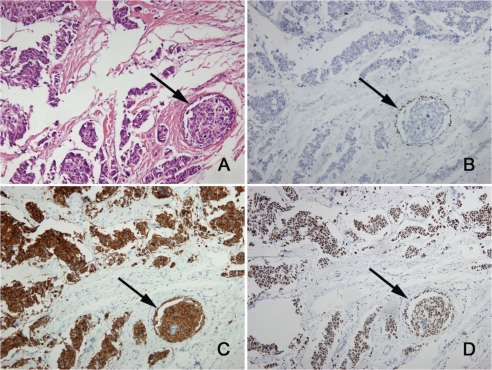
The excised breast tumour measured 45mm, was clear of the surgical margins, and was firm, irregularly shaped, and pink to pale fawn in colour. On H&E sections the tumour had the typical appearance of a SmCC with sheets, irregular nests and trabeculae of small to medium sized cells with minimal cytoplasm, hyperchromatic angulated nuclei, even chromatin and inconspicuous nucleoli (Figure 2). Mitoses were numerous and there was extensive lymphatic invasion. There was a minor in situ component (<5% of the tumour) within and around the SmCC (Figure 3), confirmed by immunohistochemistry for myoepithelial cell markers p63 (Figure 3B) and myosin heavy chain, and there was focal Paget's disease of the nipple. The in situ carcinoma cells were morphologically similar to the invasive carcinoma. Nine axillary lymph nodes examined were all involved by metastatic tumour. By immunohistochemistry the invasive carcinoma cells were positive for neuroendocrine markers chromogranin, synaptophysin (Figure 3C) and CD56, and were positive for TTF -1 (Figure 3D), E-cadherin, CAM5.2, CK7 and CK20. The invasive carcinoma cells were negative for oestrogen receptor, progesterone receptor, HER2, and GCDFP-15. The in situ carcinoma showed the same staining profile as the invasive carcinoma, including expression of neuroendocrine markers and TTF-1 (Figures 3C &3D).
Figure 2.
The tumour composed of irregular nests and trabeculae of small to medium sized cells with minimal cytoplasm, hyperchromatic angulated nuclei, even chromatin and inconspicuous nucleoli (H&E, ×600).
Figure 3.
In situ carcinoma (arrows) surrounded by small cell carcinoma (×200). (A) H&E. Immunohistochemistry for (B) myoepithelial marker p63 confirms the presence of in situ carcinoma, (C) neuroendocrine marker synaptophysin shows positive staining of the in situ carcinoma and the small cell carcinoma, and (D) TTF-1 shows positive staining of the in situ carcinoma and the small cell carcinoma.
Discussion
The clinical presentation and imaging features of primary SmCC of the breast are generally similar to the more common types of invasive carcinoma of the breast although in some cases the breast lump is described as rapidly growing [5]. Stage at presentation is on average higher than for usual breast carcinomas with more than 50% of patients having lymph node metas-tases [6].
The main differential diagnosis of primary SmCC of the breast is metastatic SmCC from other sites. In the presented case, pleural inflammation led to abnormal findings in the right lower lobe lung region on CT, initially raising the possibility of a lung primary. PET/CT is an accepted method of evaluating patients with malignancy of unknown primary, and is superior to conventional imaging alone [7]. PET/CT demonstrated much greater avidity in the breast lesion and its regional lymph nodes compared to the lung (Figure 1), and the breast lesion was therefore considered to be the primary tumour, leading to initial surgical management.
The H&E and immunohistochemistry features of primary SmCC of the breast are nearly identical to SmCC at other sites [1, 2]. In some cases there is a dimorphic appearance with a mixture of SmCC and more common histopathological types of breast carcinoma [5]. By immunohistochemistry cytokeratin CAM5.2 is usually positive [5]. Neuroendocrine markers including chromo-granin, synaptophysin and CD56 are variably positive and are not required to make the diagnosis [5]. TTF-1 immunoreactivity may occur in primary SmCC of the breast [8]. TTF-1 was previously thought to be a specific marker for primary lung carcinomas, but subsequent reports revealed 11-80% of extrapulmonary SmCCs to be TTF-1 positive [9, 10]. Primary SmCC of the breast is usually CK7 positive and CK20 negative, differing from SmCC of the lung which is usually CK7 negative and CK20 negative [5]. In the presented case the tumour was CK7 positive and CK20 positive, a profile not previously reported. Immunoreactivity for oestrogen receptor and progesterone receptor are variable in primary SmCC of the breast, with more than 50% of cases positive for one or both markers [6]. Oestrogen receptor immunoreactivity is supportive evidence that a SmCC is a breast primary, however progesterone receptor immunoreactivity has been reported in non-mammary SmCC and should be interpreted with caution [5]. HER2 immunoreactivity has not been reported in primary SmCC of the breast.
The presence of in situ carcinoma strongly supports the diagnosis of a primary breast carcinoma. However, in situ carcinoma has only been recorded as present in 60% of primary SmCCs of the breast [6]. Of particular note in the reported case, the in situ carcinoma was focally immunoreactive for neuroendocrine markers and TTF-1. This strongly suggests that the in situ carcinoma was a direct precursor of the SmCC and further supports the diagnosis of a primary SmCC of the breast.
Although there are no established protocols for the treatment of primary SmCC of the breast, more recent cases have been managed with breast conservation surgery, and chemotherapy regimes normally used for the treatment of SmCC of the lung [6]. The prognosis of primary SmCC of the breast may be worse than more common types of breast carcinoma [1].
In conclusion, it is important to distinguish primary SmCC of the breast from metastatic SmCC because the management and prognosis may differ. In this setting, the finding of an in situ component is strong evidence for a breast primary and we believe this evidence is further strengthened by expression of TTF-1 and/or neuroendocrine markers by the in situ component.
Acknowledgments
We thank Associate Professor L. Irving, Dr R.H. De Boer, Mr R. Millar, Associate Professor M. Gonzales, and the Alfred Hospital Department of Nuclear Medicine.
References
- 1.Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: a report of three cases and review of the literature. J Clin Pathol. 2005;58:775–778. doi: 10.1136/jcp.2004.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen PP. Philadelphia: Lippincott Williams & Wilkins; 2001. Rosen's Breast Pathology. [Google Scholar]
- 3.Ersahin C, Bandyopadhyay S, Bhargava R. Thyroid transcription factor-1 and “basal marker"–expressing small cell carcinoma of the breast. IntJ Surg Pathol. 2009;17:368–372. doi: 10.1177/1066896909340275. [DOI] [PubMed] [Google Scholar]
- 4.Salman WD, Harrison JA, Howat AJ. Small-cell neuroendocrine carcinoma of the breast. J Clin Pathol. 2006;59:888. doi: 10.1136/jcp.2005.032607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: a clinicopa-thologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000;24:1231–1238. doi: 10.1097/00000478-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita S, Hirano A, Komine K, Kobayashi S, Kyoda S, Takeyama H, Uchida K, Morikawa T, Nagase J, Sakamoto G. Primary small-cell neuroendocrine carcinoma of the breast: Report of a case. SurgToday. 2008;38:734–738. doi: 10.1007/s00595-007-3716-0. [DOI] [PubMed] [Google Scholar]
- 7.Dong MJ, Zhao K, Lin XT, Zhao J, Ruan LX, Liu ZF. Role of fluorodeoxyglucose-PET versus fluorodeoxyglucose-PET/computed tomography in detection of unknown primary tumor: a meta-analysis of the literature. Nucl Med Commun. 2008;29:791–802. doi: 10.1097/MNM.0b013e328302cd26. [DOI] [PubMed] [Google Scholar]
- 8.Shin SJ, DeLellis RA, Rosen PP. Small cell carcinoma of the breast–additional immunohistochemical studies. Am J Surg Pathol. 2001;25:831–832. doi: 10.1097/00000478-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann O, Dietel M. Expression of thyroid transcription factor-1 in pulmonary and extrapulmonary small cell carcinomas and other neuroendocrine carcinomas of various primary sites. Histopathology. 2000;36:415–420. doi: 10.1046/j.1365-2559.2000.00890.x. [DOI] [PubMed] [Google Scholar]
- 10.Ordonez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24:1217–1223. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]