Abstract
Angiosarcoma involving the serous membrane may mimic mesothelioma; therefore, the term “pseudomesotheliomatous angiosarcoma” has been suggested for this entity. However, the pathogenesis of pseudomesotheliomatous angiosarcoma remains unclear. Here, we report an autopsy case of splenic angiosarcoma, which systemically metastasized to the serous membrane of both the peritoneum and pleura, closely resembling a mesothelioma. The spindle-shaped tumor cells exhibited marked invasion of the lymphatic vessels and invaded the serous membrane causing thickening of the fibrous tissues like desmoplastic mesothelioma. In the present case, immunohistochemical staining showed that the tumor expressed not only the endothelial cell markers, such as CD31, vascular endothelial growth factor receptor 3, and podoplanin (D2-40), but also matrix metalloproteinase-1 (also known as collagenase-1), which is known to increase the invasiveness of mesothelioma cells. MMP-1 expression was not observed in the other cases of angiosarcoma, examined. This tumor might systemically metastasize to the serous membrane via the lymphatic route and might generate the fibrous stroma aided by the matrix metalloproteinase-1.
Keywords: Angiosarcoma, pseudomesothelioma, MMP-1, collagenase-1
Introduction
Angiosarcoma is characterized by aggressive biological behavior and high metastatic rate [1]. Splenic angiosarcoma is highly metastatic, often metastasizing to the liver, lung, and bones through hematogenous dissemination and rarely to the regional lymph nodes [2]. However, the metastasis of splenic angiosarcoma to the serous membrane is quite uncommon.
Angiosarcoma involving the serous membrane could mimic mesothelioma; thus, the term “pseudomesotheliomatous angiosarcoma” has been suggested for this entity [3-5]. Pseudomesotheliomatous angiosarcoma is rare; therefore, its pathogenesis remains largely unclear. In addition, the location of the primary lesion of pseudomesotheliomatous or serosal angiosarcoma is sometimes unclear because multiple tumor sites, i.e., liver and/or lung, have been found to be involved at the time of diagnosis [4].
Some investigators have speculated that serosal angiosarcoma could represent a peculiar malignant mesothelioma differentiating along an abnormal angioblastic pathway [6]. However, others think that most pseudomesotheliomatous angiosarcomas originate near a serosal surface and rapidly spread over the serous membrane, thereby masking their origin [7].
Here, we report a case of splenic angiosarcoma, which exhibited an unusual metastasis to the peritoneum and pleura, closely resembling a mesothelioma. This tumor exhibited marked invasion of the lymphatic vessels in the peritoneum and pleura. Notably, the present angiosarcoma cells expressed matrix metalloproteinase-1 (MMP-1; also known as collagenase-1) that is also expressed by many mesothelioma cells. However, we could not detect MMP-1 expression in the other examined angiosarcoma cells, which did not show metastasis to the serous membrane. Recent studies have highlighted that MMP-1 may play a critical role in the development of mesothelioma [8-10]. We think that both invasion to the lymphatic vessels and MMP-1 expression might be related to the peculiar metastatic pattern of the studied angiosarcoma cells.
Case history
The patient was a 37-year-old, previously healthy male, who presented with low back pain and abdominal bloating. He had no history occupational exposure to asbestos. On the basis of the clinical examination, including computerized tomography (CT), the patient was suspected to have splenomegaly and underwent splenectomy. Splenectomy specimen was 18×16×13 cm in size and weighted 1800g and was diagnosed with primary splenic angiosarcoma after the final pathological examination. At the time of splenectomy, any unusual serous change was observed by CT or even by direct observation of the peritoneum.
A year after splenectomy, the patient had massive ascites and pleural effusion with peritoneal and pleural wall thickening (Figure 1). The patient underwent radiotherapy (45 Gy and 56 Gy to the abdomen and chest, respectively, for a year), but his condition deteriorated, and he died 2 years after splenectomy. After obtaining the consent of the patient's family, an autopsy was performed 1hr after death.
Figure 1.
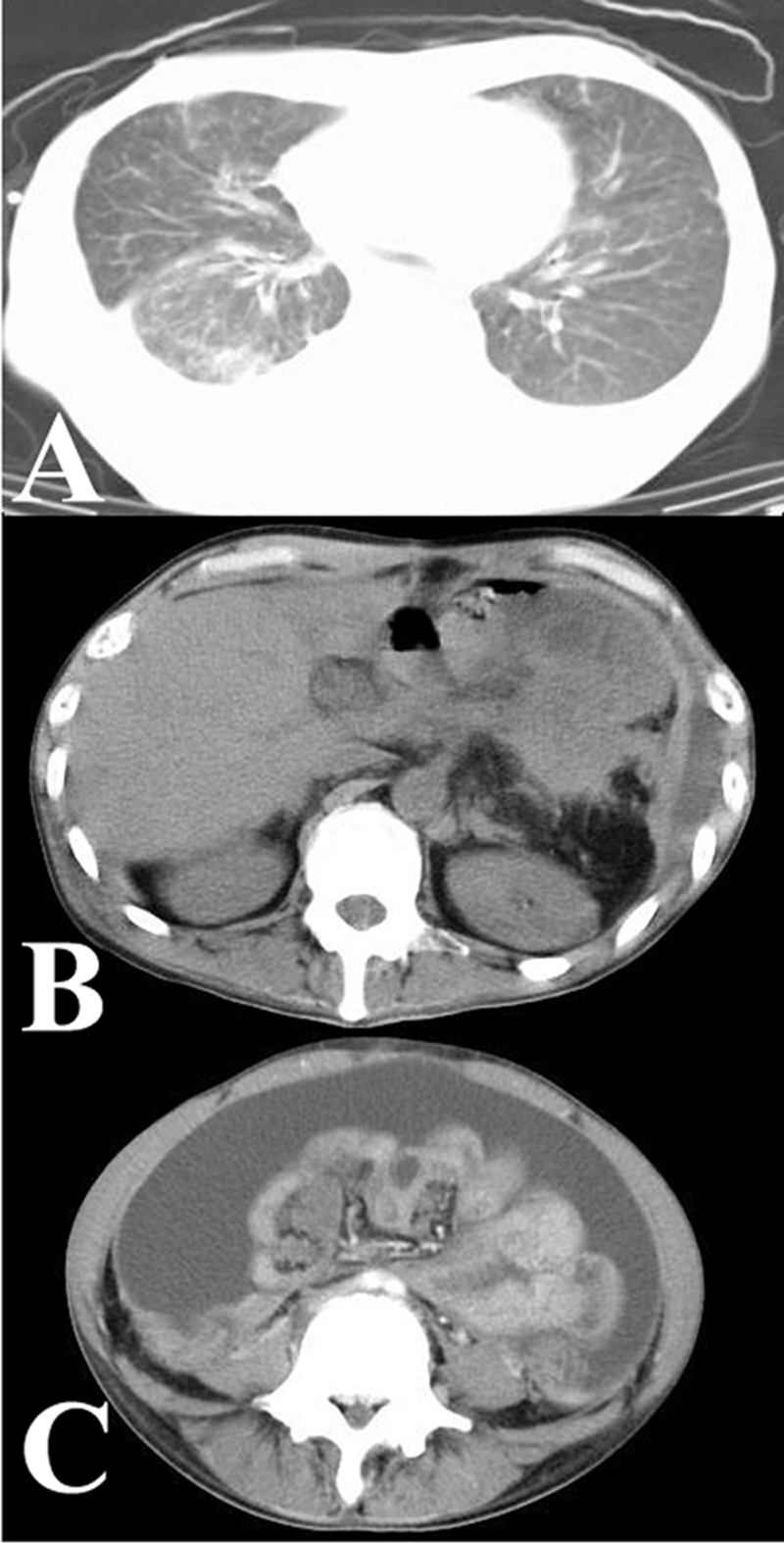
Chest (A) and abdominal (B and C) computerized tomographic (CT) images showing pleural and peritoneal thickening, respectively. Note the massive ascites (C).
Autopsy examination
At the time of the autopsy, the peritoneum and pleura—both parietal and visceral—showed marked thickening, thereby mimicking a mesothelioma. Moreover, the mesentery was also thickened by marked fibrosis. The angiosarcoma also metastasized to the liver, bone marrow, and systemic lymph nodes.
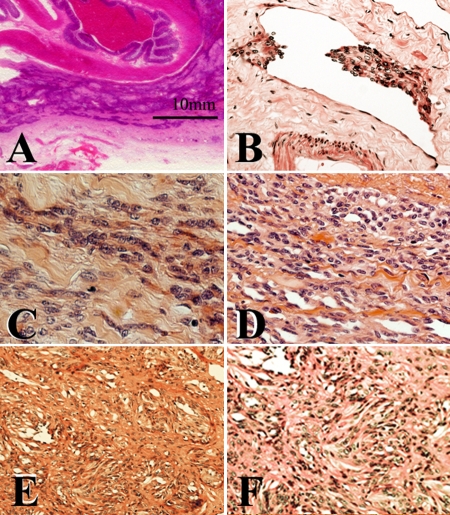
The representative histopathological findings including that of primary splenic focus are shown in Figure 2. The spindle-shaped tumor cells diffusely expanded along the serous membrane with densely collagenized stroma, thereby mimicking the desmoplastic mesothelioma. Lymphatic invasion was frequently found in both the peritoneum and pleura. Notably, some tumor cells exhibited rudimentary slit-like vascular spaces (Figure 2D). The tumor cells also expanded along the sinusoid space in the liver. In addition, the tumor cells exhibited focal growth with the fibrous tissues in the bone marrow.
Figure 2.
Visceral peritoneum was markedly thickened with dense collagenized stroma (A). Lymphatic invasion in the peritoneum is shown (B). Spindle-shaped tumor cells grew in the fibrous stroma (C). The slit-like anastomosing vascular spaces lined by the spindle-shaped tumor cells were partially observed (D). Primary splenic tumor is shown (E and F).
Immunohistochemical staining
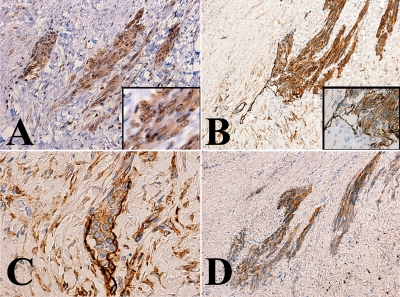
Immunohistochemical staining was performed as previously reported [11]. In brief, staining was performed using an automated immunostainer (Ventana, AZ, USA). The tumor cells including that in primary splenic foci were immunohistochemically stained for various endothelial cell markers, such as CD31 (Dako, Carpenteria, CA), vascular endothelial growth factor receptor 3 (VEGFR3) (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) and podoplanin (D2-40) (DAKO). However, the tumor cells were not stained with anti-CD34 (Becton Dickinson, San Jose, CA), cytokeratin (AE1/AE3 (Boehringer-Mannheim, Indianapolis, IND,), CAM5.2 (Becton Dickinson), cytokeratin5/6 (DAKO)), S-100 (DAKO), WT-1 (Santa Cruz Biotechnology), calretinin (Novacastra, Newcastle upon Tyne, UK), or HBME-1 (DAKO). Representative result of the immunohistochemical staining is shown in Figure 3.
Figure 3.
Representative immunohistochemical staining. Tumor cells were stained with an antibody specific to the vascular endothelial growth factor receptor 3 (VEGFR3) (A), podoplanin (B and C), and CD31 (D). Immunoreactivity with the anti-VEGFR3 antibody was observed in the cytoplasm and nuclei (A, inset). Note the tumor cell clusters in the lymphatic vessels (B and C). The walls of the affected lymphatic vessels were focally obscure; this suggested that the tumor invading to the stroma.
Malignant endothelial tumors often have an overlapping immunophenotype of vascular and lymphatic endothelial cells; therefore, the term angiosarcoma generally encompasses cases accepted as angiosarcomas and lymphangiosarcomas [12, 13]. The present tumor strongly expressed podoplanin (D2-40), which is physiologically expressed in the lymphatic endothelial cells rather than the vascular endothelial cells. Notably, the tumor cell clusters were frequently found in the non-cancerous lymphatic vessels (Figures 3B and C). In the lymphatic vessels, the line of immunoreactivity with the anti-podoplanin antibody was mostly disrupted near the tumor cell clusters.
Taken together with the patient's clinical course, we speculate that the primary splenic angiosarcoma systemically metastasized to the peritoneum and pleura, possibly via the lymphatic route, spread to the serous membrane, and subsequently grew with the fibrous stroma in the form of a “pseudomesotheliomatous angiosarcoma."
Unique expression of MMP-1 in the angiosarcoma cells
Recent studies show that MMP-1 plays an important role in the pathogenesis of mesothelioma by degrading the interstitial collagen and remodeling the tumor stroma [8-10]. We also examined whether the tumor in the present case expressed MMP-1. As demonstrated in Figure 4, immunoreactivity with the antibody specific to MMP-1 (Thermo Fisher Scientific Anatomic Pathology, Fremont, CA) was found in the angiosarcoma cells of this patient (Figure 4A and C); this was similar to the immunoreactivity in the pleural mesothelioma tissues, which were stained in parallel (Figure 4B).
Figure 4.
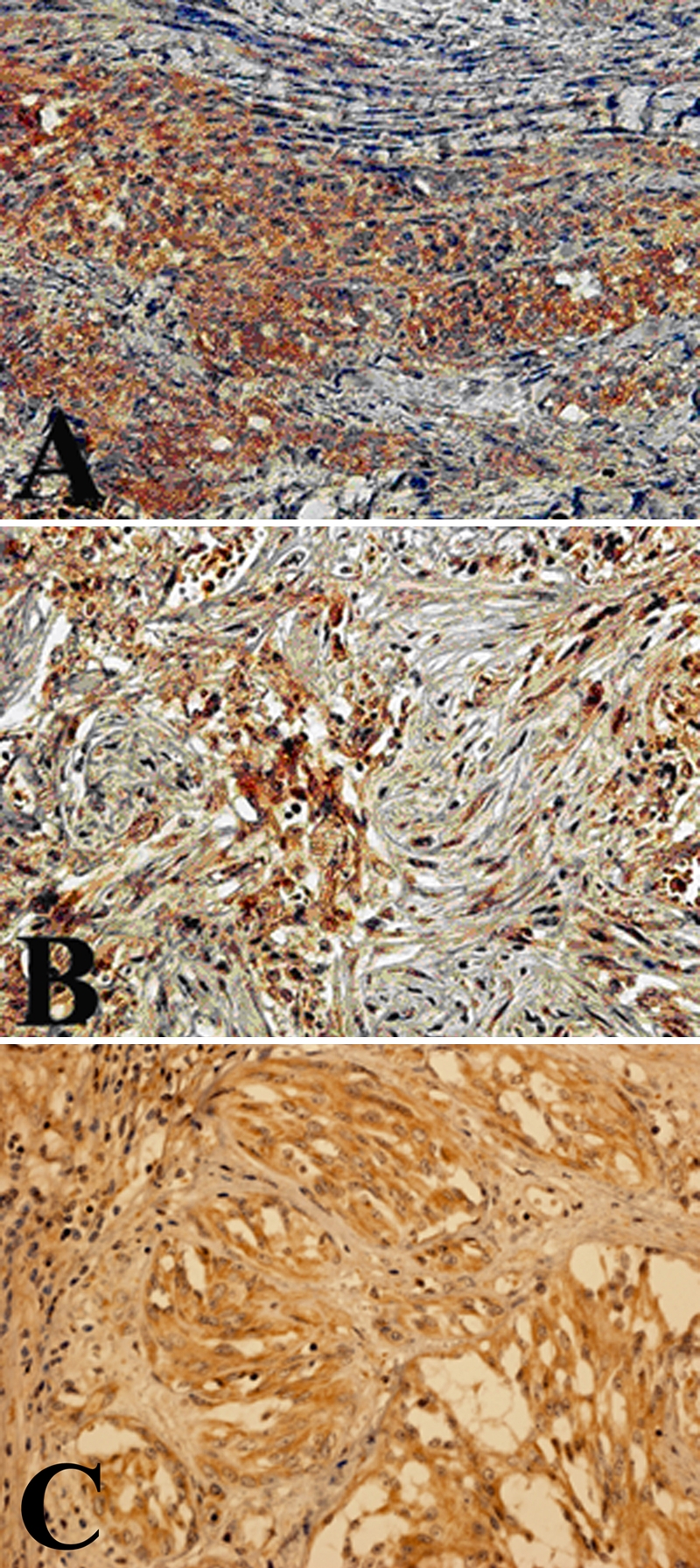
Representative immunohistochemical staining with the anti-MMP-1 antibody. The tumor cells in the peritoneum (A) as well as the mesothelioma tissue specimens (B) were stained with the anti-MMP-1 antibody. Notably, immunoreactivity was also found in primary splenic tumor (C).
Since no report, which described the MMP-1 expression in angiosarcoma, was available, we further examined whether MMP-1 was expressed in angiosarcomas, which did not exhibit metastasis to the serous membrane. Notably, significant immunoreactivity with the anti-MMP-1 antibody was not found in any of the 7 archival pathological angiosarcoma tissue specimens.
We think that the present angiosarcoma is unique with respect to its MMP-1 expression.
Discussion
Here, we report a case of splenic angiosarcoma with a peculiar metastasis pattern, which mimics that of a mesothelioma. Pseudomesotheliomatous angiosarcoma is rare, but it has been increasingly reported in the literature or mentioned in reference textbooks [3, 14-16]. However, the pathogenesis of pseudomesotheliomatous angiosarcoma is largely unclear. In particular, the correlation of mesothelioma and pseudomesotheliomatous angiosarcoma is an important medico-legal issue.
Some investigators have speculated that serosal angiosarcoma may represent a peculiar malignant mesothelioma differentiating along an abnormal angioblastic pathway [6]. This hypothesis is not justifiable, at least in the present case. The clinical course apparently indicated that the angiosarcoma in the present case originated in the spleen and subsequently metastasized to the serous membrane.
Pseudomesotheliomatous cancer of the lung, a well-characterized entity, is believed to be a small subpleural lung cancer that is widely disseminated via the subpleural lymphatic vessels. In the present case, tumor invasion in the lymphatic vessels was frequently found in the serous membrane (Figures 2B, 3B, and 3C). The tumor may have widely disseminated through the lymphatic route and may have invaded the serous membrane causing the remodeling of the fibrous stroma.
To elucidate the molecular mechanism underlying the generation of marked fibrous stroma, we examined MMP-1 expression in the angiosarcoma. Invasion and construction of the stroma of malignant tumor cells requires the destruction of the basement membrane and proteolysis of the extracellular matrix. MMP-1 is the only enzyme able to initiate the breakdown of the interstitial collagens, such as collagen type 1, collagen type 2, and collagen type 3 [18]. MMP-1 expression is also linked to the invasiveness of the mesothelioma cells [8-10]. As shown in Figure 4, the angiosarcoma cells in the present case expressed MMP-1 that is expressed by mesotheliomas and not by other angiosarcomas. We speculate that pseudomesotheliomatous angiosarcoma may be an angiosarcoma that has strong dissemination capability and invades the serous membrane like pseudomesotheliomatous cancer in the lung.
Acknowledgments
This work was supported by grants from the Ministry of Education of Japan (KAKEN 21590371), the Head of Kochi Medical School Hospital's Discretionary Grant, and the Medical Research Fund of Kochi Medical School.
References
- 1.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48:1907–1921. doi: 10.1002/1097-0142(19811015)48:8<1907::aid-cncr2820480832>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 2.Falk S, Krishnan J, Meis JM. Primary angiosarcoma of the spleen: A clinicopathologic study of 40 cases. Am J Surg Pathol. 1995;19:119–120. doi: 10.1097/00000478-199310000-00001. [DOI] [PubMed] [Google Scholar]
- 3.McCaughey WT, Dardick I, Barr JR. Angiosarcoma of serous membranes. Arch Pathol Lab Med. 1983;107:304–307. [PubMed] [Google Scholar]
- 4.Lin BT, Colby T, Gown AM, Hammar SP, Mertens RB, Churg A, Battifora H. Malignant vascular tumors of the serous membranes mimicking mesothelioma. A report of 14 cases. Am J Surg Pathol. 1996;20:1431–1439. doi: 10.1097/00000478-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Falconieri G, Bussani R, Mirra M, Zanella M. Pseudomesotheliomatous angiosarcoma: a pleuropulmonary lesion simulating malignant pleural mesothelioma. Histopathology. 1997;30:419–424. doi: 10.1046/j.1365-2559.1997.5370782.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoeller H, Schmidt P, Tscheliessnigg KH. Malignes angioblastisches mesothelioma des Perikards. Zbl Allg. Pathol. Anat. 1979;123:344–350. [PubMed] [Google Scholar]
- 7.Terada T, Nakanuma Y, Matsubara T, Suematsu T. An autopsy case of primary angiosarcoma of the pericardium mimicking malignant mesothelioma. Acta Path Jpn. 1988;38:1345–1351. doi: 10.1111/j.1440-1827.1988.tb02285.x. [DOI] [PubMed] [Google Scholar]
- 8.Harvey P, Clark IM, Jaurand MC, Warn RM, Edwards DR. Hepatocyte growth factor/scatter factor enhances the invasion of mesothelioma cell lines and the expression of matrix metallo-proteinases. Br J Cancer. 2000;83:1147–1153. doi: 10.1054/bjoc.2000.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Ivanoff A, Klominek J. Expression and activity of matrix metalloproteases in human malignant mesothelioma cell lines. Int J Cancer. 2001;91:638–643. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1102>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 10.Kroczynska B, Cutrone R, Bocchetta M, Yang H, Elmishad AG, Vacek P, Ramos-Nino M, Mossman BT, Pass HI, Carbone M. Crocidolite asbestos and SV40 are cocarcinogens in human mesothelial cells and in causing mesothelioma in hamsters. Proc Natl Acad Sci USA. 2006;103:14128–14133. doi: 10.1073/pnas.0604544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y. Expression of T-cadherin (CDH13, H-Cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- 12.Enzinger FM, Weiss SW. Malignant vascular tumors. In: Enzinger FM, Weiss SW, editors. Soft tissue tumors. 3rd ed. New York: Mosby; 1994. pp. 641–655. [Google Scholar]
- 13.Kindblom LG, Stenman G, Angervall L. Morphological and cytogenetic studies of angiosarcoma in Stewart-Treves syndrome. Virchows Arch A Pathol Anat Histopathol. 1991;419:439–445. doi: 10.1007/BF01605079. [DOI] [PubMed] [Google Scholar]
- 14.Yousem SA, Hochholzer L. Unusual thoracic manifestations of epithelioid hemangioendo-thelioma Archives of Pathology and Laboratory Medicine. 1987;111:459–463. [PubMed] [Google Scholar]
- 15.Battifora H. Epithelioid hemangioendothelioma imitating mesothelioma. Appl. Immunohistochem. 1993;1:220–222. [Google Scholar]
- 16.Rosai J. St Louis: Mosby; 1995. Ackerman's Surgical Pathology. [Google Scholar]
- 17.Harwood TR, Gracey DR, Yokoo H. Pseudomesotheliomatous carcinoma of the lung. A variant of peripheral lung cancer. Am J Clin Pathol. 1976;65:159–167. doi: 10.1093/ajcp/65.2.159. [DOI] [PubMed] [Google Scholar]
- 18.Saffarian S, Collier IE, Marmer BL, Elson EL, Goldberg G. Interstitial collagenase is a Brownian ratchet driven by proteolysis of collagen. Science. 2004;306:108–111. doi: 10.1126/science.1099179. [DOI] [PubMed] [Google Scholar]