Abstract
Background
The neurosteroid allopregnanolone has pronounced neuroprotective actions, increases myelination, and enhances neurogenesis. Evidence suggests that allopregnanolone dysregulation may play a role in the pathophysiology of Alzheimer’s disease (AD) and other neurodegenerative disorders. Our prior data demonstrate that allopregnanolone is reduced in prefrontal cortex in male patients with AD compared to male cognitively intact control subjects, and inversely correlated with neuropathological disease stage (Braak and Braak). We therefore determined if allopregnanolone levels are also reduced in AD patients compared to control subjects in temporal cortex, utilizing a larger set of samples from both male and female patients. In addition, we investigated if neurosteroids are altered in subjects who are APOE4 allele carriers.
Methods
Allopregnanolone, dehydroepiandrosterone (DHEA), and pregnenolone levels were determined in temporal cortex postmortem samples by gas chromatography/mass spectrometry, preceded by high performance liquid chromatography (40 subjects with AD/41 cognitively intact control subjects).
Results
Allopregnanolone levels are reduced in temporal cortex in patients with AD (median 2.68 ng/g, n= 40) compared to control subjects (median 5.64 ng/g, n=41), Mann-Whitney p=0.0002, and inversely correlated with Braak and Braak neuropathological disease stage (Spearman r= −0.38, p=0.0004). DHEA and pregnenolone are increased in patients with AD compared to control subjects. Patients carrying an APOE4 allele demonstrate reduced allopregnanolone levels in temporal cortex (Mann-Whitney p=0.04).
Conclusions
Neurosteroids are altered in temporal cortex in patients with AD and related to neuropathological disease stage. The APOE4 allele is associated with reduced allopregnanolone levels. Neurosteroids may be relevant to the neurobiology and therapeutics of AD.
Introduction
Allopregnanolone is a neurosteroid with a number of properties that may be relevant to the pathophysiology and treatment of Alzheimer’s disease (AD) and other neurodegenerative disorders, demonstrating pronounced neuroprotective actions in the setting of excitotoxicity [1, 2], traumatic brain injury (TBI) [3–5], and neurodegeneration [6–8]. It also increases myelination [9–11], enhances neurogenesis [12], decreases inflammation [11, 13, 14], and reduces apoptosis [15–17]. Since excitotoxicity [18–21], neurodegeneration [22, 23], and traumatic brain injury [24, 25], as well as dysregulation in myelination [26, 27], neurogenesis [28], apoptosis [29, 30], and inflammation [31] have been implicated in the pathogenesis and clinical course of AD, deficits in allopregnanolone and/or alterations in its regulation could represent critical components of AD pathophysiology.
Emerging evidence demonstrating allopregnanolone deficits in neurodegenerative disorders is consistent with this hypothesis. For example, allopregnanolone levels are decreased in Niemann-Pick type C mice [7], a neurodegenerative disorder that shares a number of properties with AD. These include cholesterol dysregulation, neurofibrillary tangle formation, β-cleaved amyloid precursor protein accumulation, and myelin breakdown [28, 32–37]. Further, allopregnanolone administration delays neurological symptom onset and doubles lifespan in Niemann-Pick type C mice [6–8]. Also consistent with a role for allopregnanolone in disorders in which neurodegeneration is a salient characteristic, allopregnanolone and other neurosteroids are altered in AD. Our laboratory determined previously that allopregnanolone levels in prefrontal cortex are significantly decreased in male AD patients compared to male cognitively intact control subjects, and that allopregnanolone levels are inversely correlated with neuropathological disease stage (Braak and Braak) [38]. Additional data also support the hypothesis that there may be allopregnanolone deficits in AD; for example, allopregnanolone is reduced in the periphery in serum [39] and plasma [40] in patients with AD compared to control subjects. Importantly, these earlier serum and plasma investigations also raise the possibility that other GABAergic neurosteroids (i.e. 3α-hydroxy-4-pregnen-20-one, [41, 42]) with considerable cross-reactivity with the antibody used in radioimmunoassay procedures [40, 43] may also be altered in AD.
It is possible that the determination of peripheral neurosteroid levels in blood may have proxy or surrogate biomarker potential for central neurosteroid levels in brain. Our prior efforts demonstrating that serum pregnenolone levels are closely correlated with hippocampal pregnenolone levels in rodents support this possibility [44]. Further, human data demonstrating that cerebrospinal fluid (CSF) levels of pregnenolone and dehydroepiandrosterone (DHEA) are correlated with temporal cortex levels of these respective neurosteroids within the same patient cohort are also consistent with proxy or surrogate biomarker potential for neurosteroid levels in more accessible tissues such as blood and CSF [45]. It should be noted, however, that several mechanisms for cerebral uptake of neurosteroids from peripheral blood circulation may influence central concentrations [46]. Given a compelling rationale informed by both preclinical and clinical findings from multiple research groups that implicate allopregnanolone dysregulation as a component in the pathophysiology of neurodegenerative disorders such as AD (and suggest a possible role for allopregnanolone or synthetic analogs in AD therapeutics), we thus investigated allopregnanolone levels in temporal cortex in patients with AD and cognitively intact control subjects. The overarching goal of the current study was to determine if we could replicate our prior neurosteroid findings in prefrontal cortex in male AD and male control patients in a second brain region (temporal cortex) utilizing samples from a larger cohort of subjects that includes both male and female patients
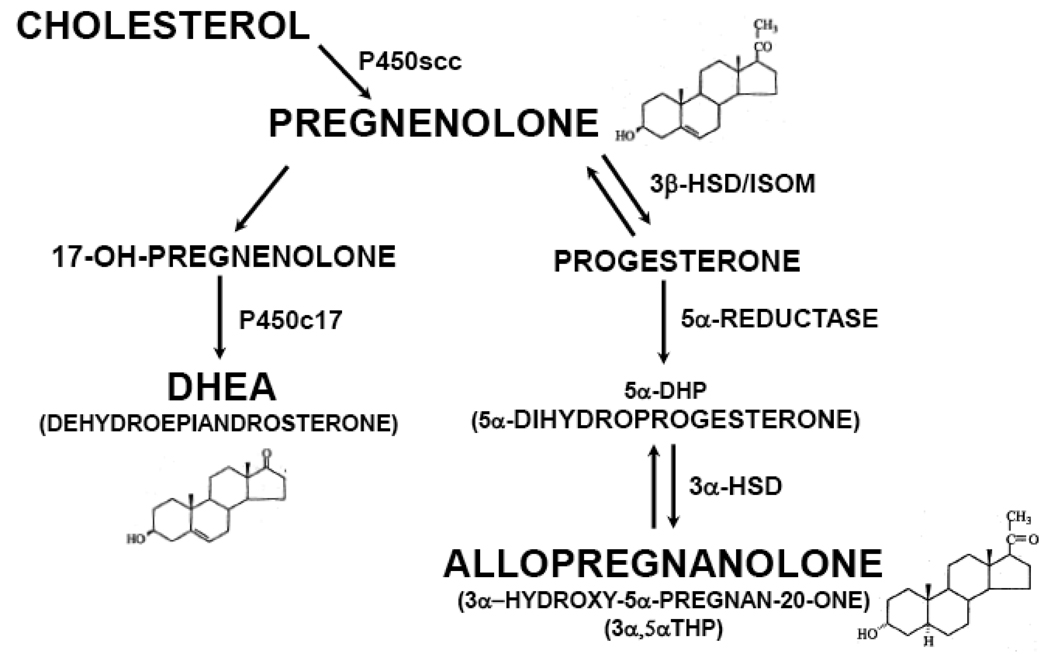
In addition to allopregnanolone, other neurosteroids such as DHEA and pregnenolone may be candidate modulators of AD pathophysiology (see Figure 1 for biosynthetic pathways). For example, DHEA appears to be elevated in postmortem brain tissue [38, 47] and CSF [45, 47, 48] in AD patients compared to control subjects, and positively correlated with Braak and Braak neuropathological disease stage [38]. Like allopregnanolone, DHEA demonstrates a number of neuroprotective effects. For example, DHEA is protective against amyloid β-protein toxicity [49, 50] and a number of other insults involving oxidative stress, including anoxia [51], glucocorticoid-induced toxicity [52, 53], and NMDA-induced excitotoxicity [54]. In addition, DHEA enhances neurogenesis in rodent models and augments cell proliferation of human neural stem cells [52, 55, 56]. Pregnenolone may also play a role in the pathogenesis and clinical course of AD, given its neuroprotective effects against glutamate [57] and amyloid β-protein toxicity [58], and actions on learning and memory in animal models [59, 60]. We therefore determined DHEA and pregnenolone levels in this postmortem brain tissue investigation using temporal cortex samples from patients with AD and cognitively intact control subjects. Since the current study includes temporal cortex postmortem tissue from males and females, this larger collection of samples will also provide data to determine if our prior neurosteroid findings in male subjects are also generalizable to female patients. To our knowledge, this is among the largest postmortem brain tissue investigations focusing on neurosteroids and AD to date.
Figure 1.
Biosynthetic Pathways and Chemical Structures for Selected Neurosteroids.
In addition, this larger cohort of 40 patients with AD and 41 cognitively intact control subjects provides the opportunity to conduct exploratory analyses to determine if the presence of the APOE4 allele (the ε4 allele of apoliprotein E, or ApoE), a known risk factor for the development of late onset AD [61], is associated with alterations in allopregnanolone, DHEA, and/or pregnenolone levels in temporal cortex. The mechanisms by which the APOE4 isoform of ApoE mediates AD risk are not yet completely understood. ApoE is a cholesterol transport protein that is present at high concentrations in the brain [62–64]. Since a major role of ApoE involves the regulation of cholesterol uptake into neurons (an action critical for synaptic function), and since cholesterol is the immediate precursor to pregnenolone (and pregnenolone is a precursor to other neurosteroids such as allopregnanolone), it is possible that AD risk conferred by the APOE4 allele may include a mechanism involving dysregulation in downstream events such as neurosteroid biosynthesis. To begin to test this possibility, we compared neurosteroid levels in temporal cortex in patients who are heterozygous or homozygous for the APOE4 allele, to subjects who do not carry this APOE isoform associated with elevated AD risk.
Methods and Materials
Postmortem Tissue
Frozen right hemisphere temporal cortex samples from a total of 81 patients were utilized in this investigation: samples from 40 subjects with AD (17 males, 23 females) and 41 cognitively intact control subjects (21 males, 20 females) from the Joseph and Kathleen Bryan Alzheimer’s Disease Research Center (ADRC) collection at Duke University were analyzed for the neurosteroids allopregnanolone, DHEA, and pregnenolone by highly sensitive and specific gas chromatography/mass spectrometry (GC/MS) preceded by high performance liquid chromatography (HPLC) purification. A subset of this collection for which CSF was also available within the same cohort (n=41, approximately half of the total collection utilized for the current study) has been analyzed previously for pregnenolone and DHEA levels, and correlations of CSF pregnenolone and DHEA levels to respective temporal cortex levels of these two neurosteroids have been reported in an earlier investigation [45]. Allopregnanolone levels in temporal cortex and APOE findings have not been reported previously (except in poster format, [65]). Temporal lobe boundaries were the superior and middle temporal gyri. Subjects were enrolled in the ADRC autopsy and brain donation program, as described previously [66]. Procedures for enrollment were approved by the Duke University Medical Center Institutional Review Board. Cognitively intact control subjects had no neurological disorders. AD was diagnosed clinically according to National Institute of Neurological and Communicative Disorders/Alzheimer’s Disease and Related Disorders Association (NINCDS/ADRDA) criteria. AD diagnosis was confirmed at autopsy using the National Institute on Aging/Reagan Institute criteria. Neuropathological disease stage was determined using the Braak method (Braak and Braak 1991). Postmortem interval (PMI) was less than 35 hours for all tissue samples tested.
Neurosteroid Analyses
Gas Chromatography/Mass Spectrometry Analyses (GC/MS) preceded by High Performance Liquid Chromatography (HPLC)
Neurosteroid analyses in temporal cortex tissue were performed as previously described [38, 45, 67], with minor modifications. All glassware was silanized. Between 100 and 180 mg of brain tissue was homogenized in 5 volumes of distilled water containing trace quantity (4000 dpm/injection) of tritiated neurosteroid (New England Nuclear Life, Wellesley, MA) to detect the HPLC fraction containing the neurosteroid of interest, and a constant amount of deuterated allopregnanolone (D4-allopregnanolone, 400 pg) and deuterated pregnenolone (D4-pregnenolone, 400 pg) as the internal standards (Cambridge Isotopes, Andover MA). Supernatants were extracted three times with three volumes of ethyl acetate and dried under nitrogen prior to HPLC purification, performed with 900 µL injections per sample on an 1100 Series Agilent HPLC equipped with a Packard 500TR Flow Scintillation Analyzer for radiopeak detection. Each neurosteroid was collected into a separate fraction based upon the retention time of its radioactive analogue, utilizing hexane, tetrahydrofuran, and ethanol as the mobile phase and a Phenomenex LiChrosorb DIOL (5 µm particle size) 250 mm × 4.6 mm column. The HPLC fractions containing allopregnanolone, DHEA, and pregnenolone were transferred to 1 mL Reacti-Vials, evaporated to dryness, and derivatized utilizing heptafluorobutyric acid anhydride (HFBA) [50 µL HFBA added to 450 µL ethyl acetate at room temperature for 2 hours]. Derivatized samples were transferred to autosampler vials equipped with deactivated glass inserts using 2 × 50 µL pesticide-grade heptane and evaporated, then reconstituted and vortexed in pesticide-grade heptane. Standards and samples were injected onto an Agilent 5973 Mass Spectrometer (MS) coupled to an Agilent 6890N Gas Chromatograph (GC) equipped with an Agilent HP-5MS 30 meter × 0.250 mm × 0.25 um capillary column, and analyzed in the negative ion chemical ionization mode (NICI) utilizing methane as the reaction gas and helium as the carrier gas. The derivatized steroids of interest subjected to NICI yield negative ions in the mass range between m/z 100 and m/z 700. In addition to the GC retention time characteristic of each steroid, the structural identification of each neurosteroid assayed was provided by its unique mass fragmentation pattern. Mass spectrometer single ion monitoring mode was utilized to focus on the most abundant ion fragment for each steroid derivative (allopregnanolone = 474.4 and 494.3; DHEA = 464.4 and 444.4; pregnenolone = 492.3 and 472.4).
For neurosteroid quantification, the standard curve for the steroid of interest was prepared by combining varying known quantities of the steroid (Steraloids, Newport, RI) with a constant amount of deuterated internal standard. Identical to the samples, the standard curve was extracted three times in ethyl acetate prior to HPLC purification and GC/MS injection (standard curve r2=0.99 for each neurosteroid). The area under the peak of each known quantity of neurosteroid was divided by the area under the peak of the internal standard. This ratio was then plotted on the y-axis against known quantities of each steroid to generate the standard curve. Only peaks with a signal to noise ratio greater or equal to 5:1 were integrated. The limit of detection with this method was 2 pg for allopregnanolone, 2 pg for DHEA, and 5 pg for pregnenolone.
Statistical Analysis
Non-parametric statistical approaches were utilized. Neurosteroid levels in AD patients and cognitively intact control subjects were analyzed by Mann-Whitney U test statistic. Correlational analyses (neurosteroid levels vs. PMI, and neurosteroid levels vs. Braak and Braak staging) were also assessed non-parametrically and Spearman correlation coefficients were determined. Both AD subjects and cognitively intact control subjects were included in the correlational analyses of neurosteroid levels vs. Braak and Braak neuropathological disease stage, since cognitively intact control subjects without clinical evidence of AD may meet neuropathological criteria for early Braak stages, and since these changes may reflect early stages of AD (or predisposition to developing AD) in the absence of detectable clinical symptomatology. P values < 0.05 were considered to be statistically significant.
Results
Median PMI was 6.33 hours for the Alzheimer’s group and 7.68 hours for the cognitively intact control group (See Table 1 for patient characteristics). There was no significant difference in median PMI in the Alzheimer’s group compared to the cognitively intact control group (Mann-Whitney U test statistic p=0.20). Median age was 81.0 years for the Alzheimer’s group and 82.0 years for the cognitively intact control group. There was no significant difference in median age in the Alzheimer’s group (n=40) compared to the cognitively intact control group (n=41), Mann-Whitney U test statistic p=0.44. None of the three neurosteroids tested (allopregnanolone, DHEA, pregnenolone) demonstrated significant correlations with PMI (Spearman p≥0.40 for each neurosteroid).
Table 1.
Patient Characteristics.
| Age | Post mortem Interval (min) |
Gender | APOE4 Status |
Clinical Diagnosis |
Cause of Death |
|---|---|---|---|---|---|
| 75 | 154 | Male | 3.4 | AD | Unavailable |
| 88 | 150 | Male | 4.4 | AD | Unavailable |
| 81 | 965 | Male | 3.3 | AD | Unavailable |
| 66 | 42 | Male | 4.4 | AD | Acute Bronchopneumonia |
| 82 | 80 | Male | 3.4 | AD | Pneumonia |
| 68 | 425 | Male | 3.3 | AD | Cardia Arrhythmia |
| 81 | 570 | Male | 3.4 | AD | Unavailable |
| 90 | 120 | Male | 3.4 | AD | Secondary to Carcinoma of the Colon |
| 90 | 2040 | Male | 3.4 | AD | Pneumonia |
| 74 | 160 | Male | 4.4 | AD | Acute Aspiration Bronchopneumonia |
| 83 | 120 | Male | 3.3 | AD | Aspiration Pneumonia |
| 80 | 390 | Male | 4.4 | AD | Pneumonia |
| 90 | 1050 | Male | 3.4 | AD | Cardiorespiratory failure |
| 85 | 587 | Male | 3.3 | AD | End Stage Dementia |
| 71 | 390 | Male | 3.4 | AD | Aspiration Pneumonia |
| 69 | 1145 | Male | 3.4 | AD | Aspiration Pneumonia |
| 83 | 769 | Male | 3.3 | AD | Unavailable |
| 85 | 120 | Male | 3.3 | Control | Conqestive Heart Failure |
| 75 | 1133 | Male | 3.3 | Control | Cardiovascular Disease |
| 78 | 1149 | Male | 3.3 | Control | Sepsis |
| 66 | 65 | Male | 3.3 | Control | Adenocarcinoma of the Prostate |
| 74 | 69 | Male | 3.4 | Control | Adenocarcinoma of the Prostate |
| 81 | 630 | Male | 3.3 | Control | Unavailable |
| 82 | 195 | Male | 3.3 | Control | Cardiac Arrest |
| 63 | 420 | Male | 3.3 | Control | Myocardial Infarct |
| 80 | 255 | Male | 3.3 | Control | Unwitnessed Cardiopulmonary Arrest |
| 40 | 345 | Male | Unknown | Control | Myocardial Infarct |
| 81 | 433 | Male | 3.4 | Control | Dehydration; GI Hemorrhage |
| 90 | 461 | Male | 3.3 | Control | Myocardial Infarct |
| 83 | 550 | Male | 3.3 | Control | Prostatic Carcinoma |
| 90 | 720 | Male | 3.3 | Control | Unavailable |
| 83 | 120 | Male | 3.3 | Control | Urinary Bladder Rupture |
| 90 | 330 | Male | 3.3 | Control | Cardiovascular Disease |
| 90 | 444 | Male | 3.3 | Control | Myocardial Infarct |
| 87 | 1110 | Male | 3.3 | Control | Adenocarcinoma of the Lung |
| 79 | 390 | Male | 2.4 | Control | Pneumonia and Urinary Tract Infection |
| 88 | 1040 | Male | 3.3 | Control | Myocardial Infarct |
| 90 | 300 | Male | 3.3 | Control | Unavailable |
| 67 | 369 | Female | 4.4 | AD | Unavailable |
| 90 | 114 | Female | 3.3 | AD | Unavailable |
| 70 | 435 | Female | 3.4 | AD | Unavailable |
| 65 | 283 | Female | 3.4 | AD | Not determined |
| 82 | 969 | Female | 3.4 | AD | Unavailable |
| 56 | 390 | Female | 3.3 | AD | Unavailable |
| 63 | 715 | Female | 3.4 | AD | Heart Disease |
| 67 | 69 | Female | 4.4 | AD | Aspiration Pneumonia |
| 85 | 165 | Female | 3.3 | AD | Acute Pneumonia |
| 79 | 145 | Female | 3.4 | AD | Cerebrovascular Accident |
| 90 | 350 | Female | 3.4 | AD | Unavailable |
| 78 | 120 | Female | 3.4 | AD | Unavailable |
| 81 | 754 | Female | 3.4 | AD | Acute Bronchopneumonia |
| 81 | 195 | Female | 4.4 | AD | Unavailable |
| 85 | 165 | Female | 3.4 | AD | Unavailable |
| 88 | 574 | Female | 3.4 | AD | Aspiration Pneumonia |
| 59 | 840 | Female | 2.3 | AD | Cardiorespiratory Arrest |
| 82 | 112 | Female | 3.4 | AD | Cardiorespiratory Failure |
| 80 | 309 | Female | 3.3 | AD | Respiratory Failure. Aspiration Pneumonitis, Bronchopneumonia |
| 90 | 652 | Female | 3.4 | AD | Advanced Alzheimer's Disease |
| 59 | 255 | Female | 3.3 | AD | Unavailable |
| 90 | 1230 | Female | 3.4 | AD | Unavailable |
| 49 | 1051 | Female | 3.3 | AD | Acute Bronchopneumonia |
| 50 | 1193 | Female | 2.3 | Control | Multiple Myeloma |
| 85 | 945 | Female | 3.3 | Control | Pneumonia. COPD. Heart Disease |
| 61 | 249 | Female | 3.4 | Control | Myocardial Infarct |
| 49 | 698 | Female | 3.3 | Control | Respiratory Failure secondary to Multiple Tumor Emboli within the Lungs |
| 72 | 180 | Female | 3.4 | Control | Bronchopneumonia secondary to Metastatic Breast Cancer |
| 78 | 331 | Female | 3.3 | Control | Bronchopneumonia |
| 80 | 69 | Female | 3.3 | Control | Pancreatic Cancer |
| 57 | 540 | Female | 3.4 | Control | Myocardial Infarct |
| 90 | 1380 | Female | 3.3 | Control | Metastatic Carcinoma |
| 90 | 637 | Female | 3.3 | Control | Pneumonia |
| 90 | 990 | Female | 3.4 | Control | Unavailable |
| 90 | 225 | Female | 2.3 | Control | Unavailable |
| 82 | 930 | Female | 3.3 | Control | Myocardial Infarct |
| 90 | 240 | Female | 3.3 | Control | Unavailable |
| 67 | 480 | Female | 3.3 | Control | Colon Cancer |
| 82 | 487 | Female | 3.3 | Control | Dissecting Aortic Aneurysm |
| 74 | 283 | Female | 2.4 | Control | Cardiorespiratory Failure |
| 88 | 1230 | Female | 2.3 | Control | Lung Cancer |
| 72 | 1800 | Female | 3.3 | Control | Unavailable |
| 90 | 600 | Female | 2.3 | Control | Unavailable |
Allopregnanolone Levels in Temporal Cortex in Patients with AD vs. Control Subjects, and Relationship to Neuropathological Disease Stage (Braak and Braak)
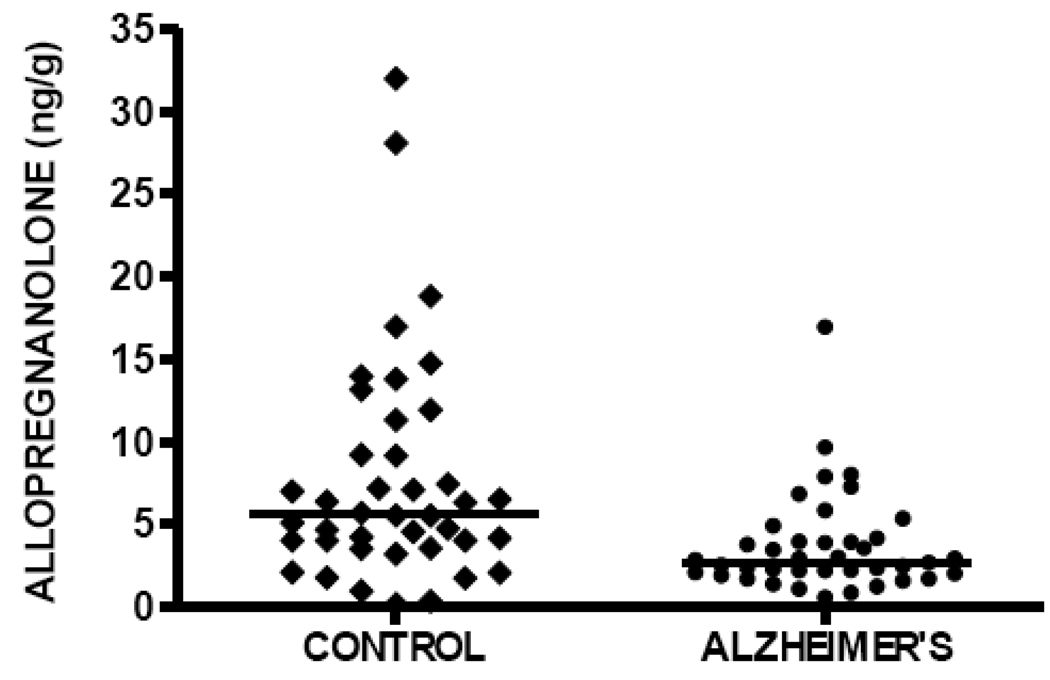
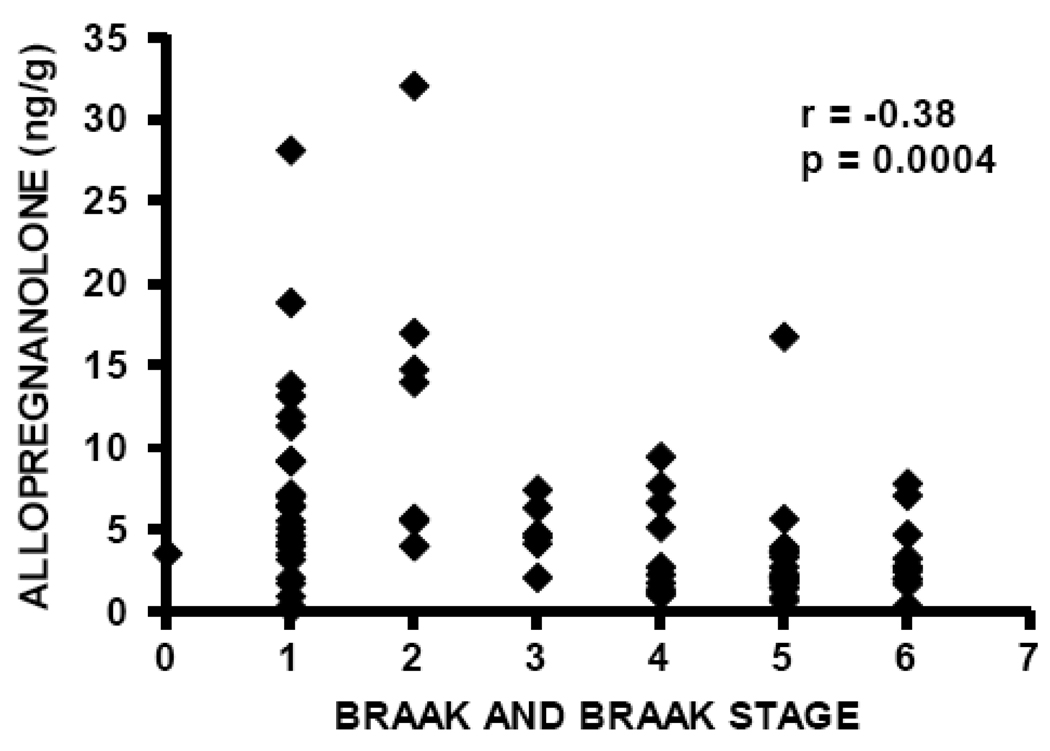
Allopregnanolone levels are significantly decreased in temporal cortex in patients with AD (median 2.68 ng/g, n=40) compared to cognitively intact control subjects (median 5.64 ng/g, n=41), Mann-Whitney p=0.0002 (Figure 2, Table 2). These findings are very similar to those previously reported in prefrontal cortex, in which median allopregnanolone level in male patients with AD was 2.50 ng/g (n=14), and median allopregnanolone level in male cognitively intact control subjects was 5.59 ng/g (n=15), using the same mass spectrometry-based methodology [38]. Also consistent with our prior investigation in prefrontal cortex, allopregnanolone levels are inversely correlated with Braak and Braak neuropathological disease stage in the current study, Spearman r=−0.38, p=0.0004 (Figure 3), thus replicating our previous findings in prefrontal cortex in which an inverse correlation with Braak and Braak stage was also reported (Spearman r=−0.49, p=0.007) [38].
Figure 2.
Allopregnanolone levels in temporal cortex are significantly decreased in subjects with Alzheimer's disease (median 2.68 ng/g, n=40) compared to cognitively intact control subjects (median 5.64 ng/g, n=41), Mann-Whitney p=0.0002.
Table 2.
Neurosteroid Levels (median; ng/g) in Temporal Cortex in Cognitively Intact Control Subjects and Patients with Alzheimer’s Disease.
| n | Min | Q1 | Med | Q3 | Max | P-value | |
|---|---|---|---|---|---|---|---|
| Allopregnanolone | 0.0002 | ||||||
| Alzheimer's disease | 40 | 0.477 | 1.954 | 2.681 | 3.96 | 16.84 | |
| Control | 41 | 0.266 | 3.896 | 5.644 | 10.37 | 32.11 | |
| Pregnenolone | 0.022 | ||||||
| Alzheimer's disease | 40 | 3.184 | 12.32 | 22.24 | 52.49 | 291.2 | |
| Control | 41 | 2.148 | 6.089 | 10.24 | 27.91 | 218.2 | |
| DHEA | 0.0185 | ||||||
| Alzheimer's disease | 40 | 0.87 | 2.332 | 4.751 | 7.523 | 40.31 | |
| Control | 41 | 0.103 | 1.625 | 2.232 | 5.409 | 42.51 |
Min, minimum, Q1, lower quartile; Med; median; Q3, upper quartile; Max, maximum.
p<05, Mann-Whitney U test statistic
Figure 3.
Allopregnanolone levels in temporal cortex are inversely correlated with neuropathological disease stage (Braak and Braak), Spearman r = −0.38, p=0.0004.
DHEA and Pregnenolone Levels in Temporal Cortex in Patients with AD vs. Control Subjects, and Relationship to Neuropathological Disease Stage (Braak and Braak)
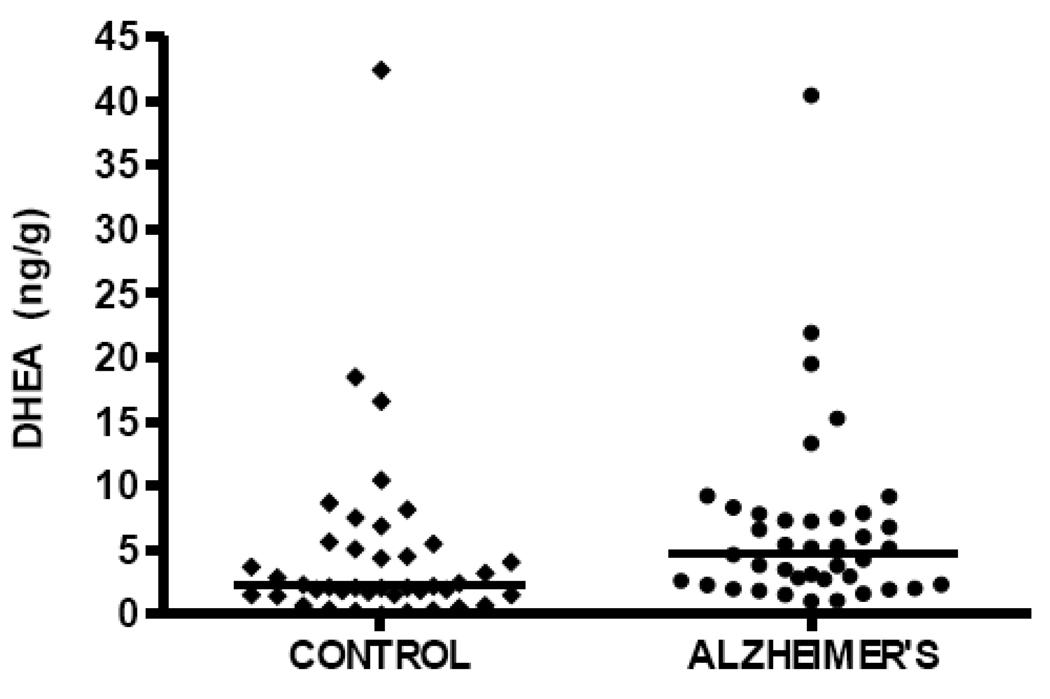
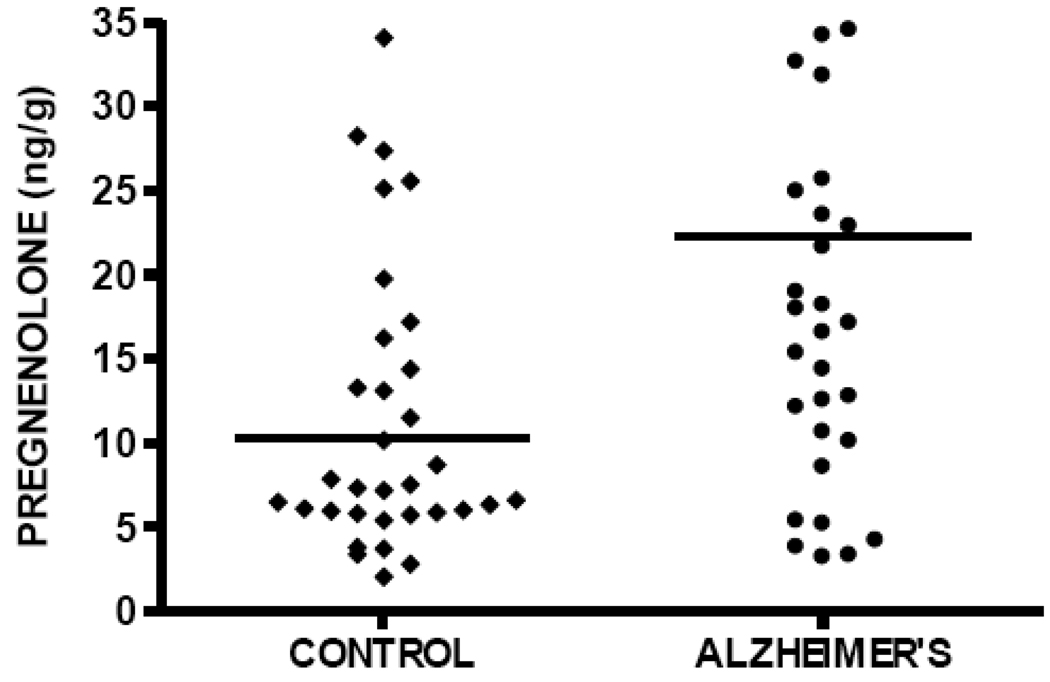
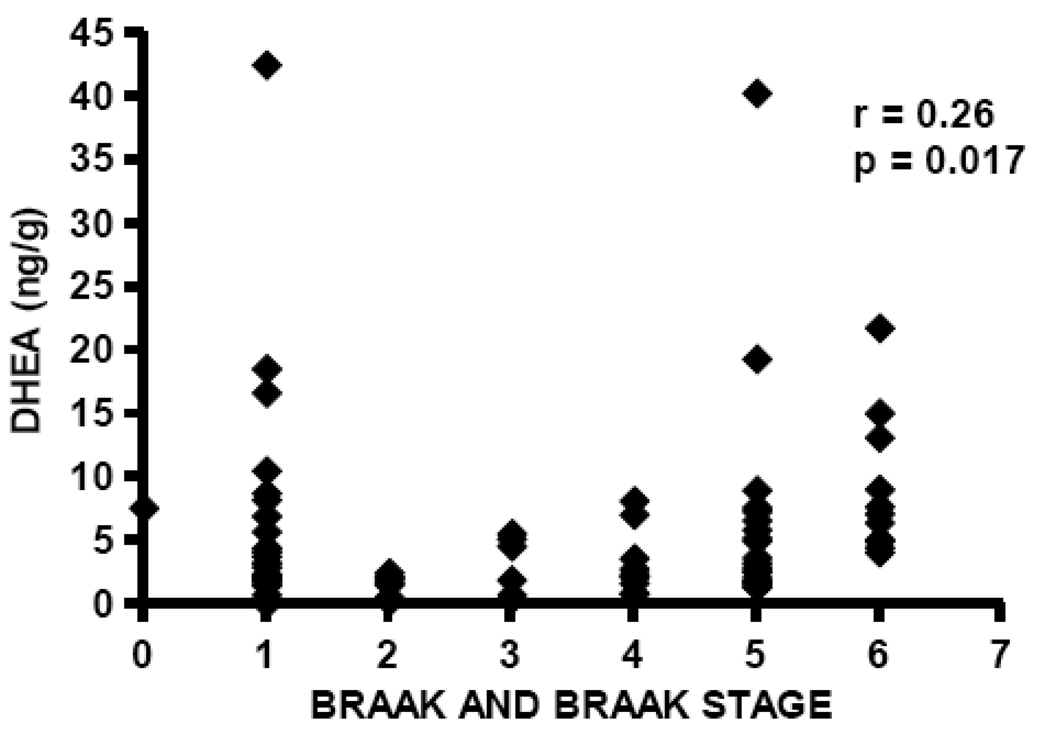
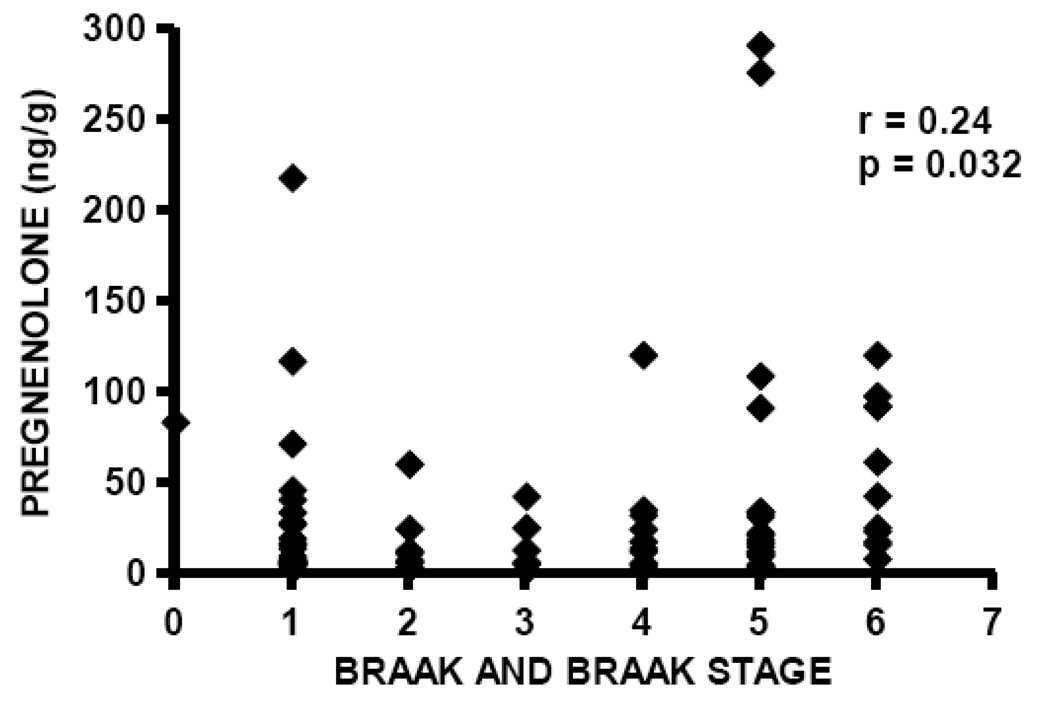
DHEA levels are significantly elevated in temporal cortex in the current study in subjects with AD (median DHEA level 4.75 ng/g, n=40) compared to cognitively intact control subjects (median DHEA level 2.23 ng/g, n=41), Mann-Whitney p=0.0185 (Figure 4, Table 2). Pregnenolone levels are also significantly elevated in patients with AD (median pregnenolone level 22.24 ng/g, n=40) compared to cognitively intact control subjects (median pregnenolone level 10.24 ng/g, n=41), Mann-Whitney p=0.022 (Figure 5, Table 2). Both DHEA levels in temporal cortex (Spearman r= 0.26, p=0.017, n=81; Figure 6) and pregnenolone levels in temporal cortex (Spearman r=0.24, p = 0.032, n=81; Figure 7), are positively correlated with Braak and Braak neuropathological disease stage.
Figure 4.
DHEA levels in temporal cortex are significantly increased in subjects with Alzheimer's disease (median 4.75 ng/g, n=40) compared to cognitively intact control subjects (median 2.23 ng/g, n=41), Mann-Whitney p=0.0185.
Figure 5.
Pregnenolone levels in temporal cortex are significantly increased in subjects with Alzheimer's disease (median 22.24 ng/g) compared to cognitively intact control subjects (median 10.24 ng/g), Mann-Whitney p=0.022.
Figure 6.
DHEA levels are positively correlated with neuropathological disease stage (Braak and Braak) in temporal cortex, Spearman r= 0.26, p=0.017.
Figure 7.
Pregnenolone levels are positively correlated with neuropathological disease stage (Braak and Braak) in temporal cortex, Spearman r=0.24, p=0.032.
DHEA levels are significantly higher in female patients compared to male patients, in both the AD group (6.48 vs. 2.85 ng/g, respectively; p=0.04) and the cognitively intact control group (3.39 vs. 2.04 ng/g, respectively; p=0.036). There are no significant differences in pregnenolone or allopregnanolone levels in female patients compared to male patients (in either the AD group or the cognitively intact control group), data not shown.
APOE4 Allele Status and Neurosteroid Levels
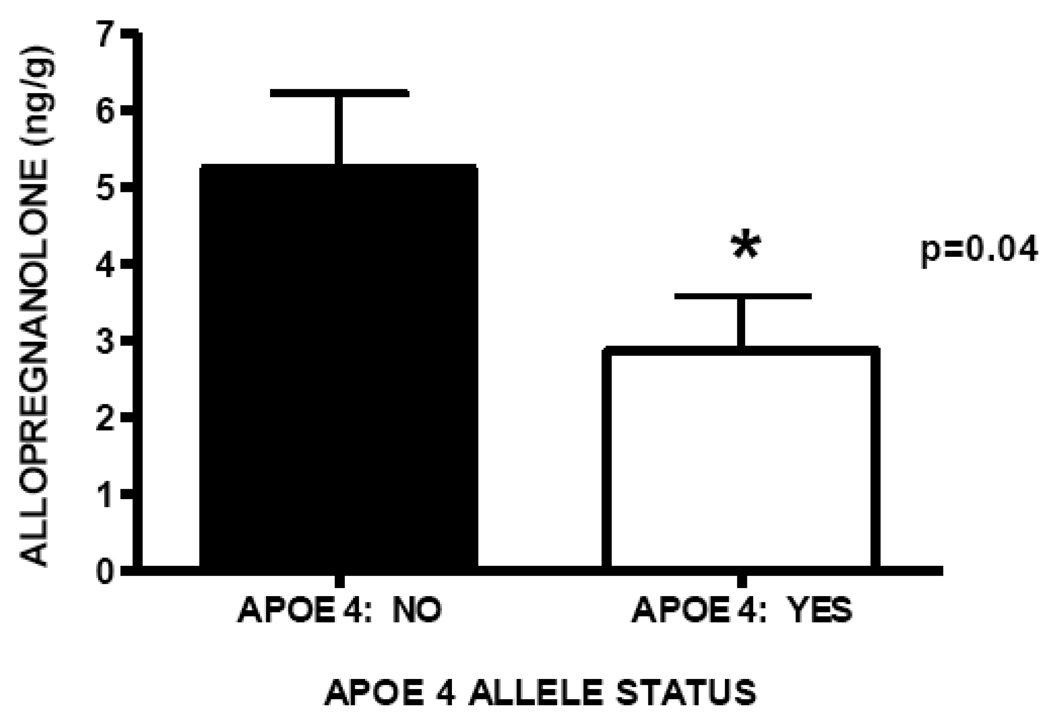
APOE status was available for 80 of 81 subjects. Allopregnanolone median level in temporal cortex is significantly decreased in patients homozygous or heterozygous for the APOE4 allele (2.86 ng/g, n=36) compared to patients not carrying an APOE4 allele (5.23 ng/g, n=44), Mann Whitney p=0.04; Figure 8. Patients homozygous or heterozygous for the APOE4 allele did not demonstrate significantly different median DHEA or pregnenolone levels compared to subjects who are not APOE4 allele carriers (data not shown).
Figure 8.
Allopregnanolone levels in temporal cortex are significantly decreased in subjects homozygous or heterozygous for the APOE4 allele (median 2.86 ng/g, n=36) compared to patients who are not APOE4 allele carriers (5.23 ng/g, n=44), p=0.04.
Discussion
This investigation demonstrates that neurosteroid levels are altered in temporal cortex in patients with AD compared to cognitively intact control subjects using a postmortem brain tissue collection that includes both male and female patient samples (n=81; 40 AD, 41 control). Findings are consistent with our prior study in prefrontal cortex, using a smaller collection of samples from male patients only (n=29; 14 AD, 15 control) [38]. In the current study in temporal cortex, allopregnanolone levels are significantly reduced in AD patients compared to cognitively intact control subjects, and inversely correlated with Braak and Braak neuropathological disease stage (replicating our prior findings in prefrontal cortex). DHEA and pregnenolone levels, conversely, are significantly elevated in patients with AD compared to cognitively intact control subjects, and positively correlated with Braak and Braak neuropathological disease stage (also similar to our earlier findings in prefrontal cortex). In addition, the presence of the APOE4 allele is associated with neurosteroid alterations; specifically, subjects either heterozygous or homozygous for the APOE4 allele demonstrate significantly reduced allopregnanolone levels compared to patients who do not carry this APOE isoform. These findings and their potential ramifications are discussed below.
Allopregnanolone Levels in Temporal Cortex Are Reduced in Alzheimer’s Disease and Inversely Correlated with Neuropathological Disease Stage
Allopregnanolone levels in temporal cortex are significantly decreased in patients with AD, similar to our prior findings in a smaller study in prefrontal cortex that included samples from males only [38]. Allopregnanolone levels are also inversely correlated with neuropathological disease stage (Braak and Braak), suggesting that decreases in this neurosteroid may have functional relevance and reducing the likelihood that the finding of decreased allopregnanolone levels in AD is simply an epiphenomenon. Replication of our prior prefrontal cortex allopregnanolone results in a second brain region (and extension of earlier prefrontal cortex findings to female subjects) further strengthens the rationale for the preliminary hypothesis that a dysequilibrium in neurosteroid pathways may contribute to the pathogenesis of AD. As previously noted, allopregnanolone is a molecule with pleiotropic actions, including pronounced neuroprotective effects against several processes associated with AD risk and/or pathophysiology; deficits in this neurosteroid may thus impact multiple dimensions of AD neurobiology and merit additional investigation.
In addition to the possible role of allopregnanolone reductions in AD pathogenesis, converging preclinical and clinical evidence also support the preliminary hypothesis that restoration of allopregnanolone levels in states of deficiency could be clinically therapeutic. For example, allopregnanolone is significantly decreased in Niemann-Pick type C mice, and one-time administration of this neurosteroid doubles lifespan and markedly delays neurological symptom onset [6–8]. The mechanisms by which allopregnanolone exerts these robust effects may include GABAA receptor modulation and activity at pregnane-X-receptors [8]. Further, allopregnanolone administration is neuroprotective against TBI [3, 4, 12, 13] and stroke [68] in rodent models, to an even greater degree than its precursor, progesterone. Clinically, progesterone reduces death by 50% at 30 days post-injury in patients with moderate to severe TBI [69], a finding that has been replicated in an independent cohort [70] and led to a multi-site randomized controlled trial projected to enroll over 1100 patients (ClinicalTrials.gov Identifier: NCT00822900). Given the superior neuroprotective effects of allopregnanolone compared to progesterone in TBI and stroke rodent models, it is possible that progesterone metabolism to allopregnanolone may represent a mechanistic component to its therapeutic efficacy. This is further supported by prior work demonstrating that the anticonvulsant effects of progesterone are prevented if metabolism to allopregnanolone is blocked with an enzyme inhibitor [71–73]. Data are hence accruing to suggest that allopregnanolone may be a key modulator of neurobiological events in disorders in which neurodegeneration is a prominent component.
DHEA Levels in Temporal Cortex are Elevated in Alzheimer’s Disease and Positively Correlated with Neuropathological Disease Stage
Our current findings suggest that temporal cortex DHEA levels are significantly increased in patients with AD compared to cognitively intact control subjects, and that DHEA levels in this brain region are positively correlated with Braak and Braak neuropathological disease stage (replicating our prior findings in prefrontal cortex [38], and extending this earlier effort by including samples from female subjects). Elevation in median DHEA level in temporal cortex in this investigation is also consistent with prior evidence of DHEA increases in CSF in subjects with AD [45]. As DHEA levels decline precipitously with age to levels approximately 20% of those observed in young adulthood, it has been hypothesized that decreases in DHEA may be linked to AD pathophysiology. Along these lines, evidence that DHEA protects against amyloid β-protein toxicity [49], attenuates β25–35-amyloid peptide-induced memory impairment [74], and inhibits amyloid β-protein-induced calcium increases [75], supports the possibility that DHEA elevations in temporal cortex in AD patients could be compensatory in some manner. Also, the administration of β-amyloid peptide produces elevations in DHEA [47], and it is thus possible that β-amyloid deposition precedes DHEA alterations in AD and potentially plays an etiological role in the DHEA changes observed in temporal cortex in the current study. DHEA enhancement of axonal outgrowth [76] and other neurotrophic and neuroprotective actions previously outlined in the introduction are also consistent with this possibility, as are the positive effects of DHEA on learning and memory in rodent models [59, 60, 77]. Alternatively, DHEA positively modulates excitatory NMDA receptors [78, 79] and negatively modulates inhibitory GABAA receptors [80]; therefore, this neurosteroid could potentially contribute to excitotoxic mechanisms in AD (particularly in the setting of concurrent deficits in allopregnanolone, an inhibitory GABAergic neurosteroid).
Pregnenolone Levels in Temporal Cortex are Elevated in Alzheimer’s Disease and Positively Correlated with Neuropathological Disease Stage
Similar to the DHEA findings described above, pregnenolone levels in temporal cortex are also significantly elevated in patients with AD, and positively correlated with Braak and Braak neuropathological disease stage. Like allopregnanolone and DHEA, pregnenolone demonstrates a number of properties that suggest it may be a logical candidate for future study in AD, such as enhancing learning and memory and increasing long-term potentiation in rodents [59] [81]. Given its diverse effects including microtubule stabilization [82], positive actions on neuritic outgrowth [83], the enhancement of myelination [84], protection against glutamate-induced cellular damage [57], prevention of increases in intracellular calcium induced by β-amyloid protein [75], and protection of mouse hippocampal (HT-22) cells against β-amyloid protein toxicity [58], pregnenolone elevations in AD could result in pleiotropic actions that impact a number of neurobiological processes relevant to AD. However, the precise etiology of these alterations and their functional consequences will require extensive additional study.
APOE4 Allele Status and Neurosteroid Levels in Temporal Cortex
Patients either homozygous or heterozygous for the APOE4 allele also had significantly reduced allopregnanolone levels compared to subjects without this APOE isoform. Since ApoE is a major cholesterol transport protein in the brain, and since cholesterol is the immediate precursor to pregnenolone (and pregnenolone is a precursor for multiple other downstream neurosteroids, including allopregnanolone and DHEA), it is possible that the APOE4 allele may be associated with compromised neurosteroid regulation by impacting upstream events involving cholesterol regulation and metabolism. This may be relevant to pregnenolone alterations in AD, since the conversion of cholesterol to pregnenolone is the rate-limiting step in neurosteroid formation [85, 86]. Recent evidence that mRNA levels of diazepam binding inhibitor (which facilitates cholesterol transport to the inner mitochondrial membrane, an action required for pregnenolone synthesis) are elevated in patients with AD [87] is potentially consistent with our finding of elevated pregnenolone levels in temporal cortex in AD patients compared to cognitively intact control subjects. Conversely, however, the presence of the APOE4 allele is not associated with alterations in pregnenolone (or DHEA) in temporal cortex in this cohort, unlike the association of the APOE4 allele with significantly reduced allopregnanolone levels in this study. Of note, it is possible that the neurosteroid allopregnanolone may also play a role in cholesterol homeostasis, as allopregnanolone administration at postnatal day 7 significantly reduces neuronal and microglial cholesterol accumulation in Niemann-Pick type C mice [8, 11]. Precisely how the presence of the APOE4 allele may influence neurosteroid regulation will require future investigation, but initial evidence that the presence of this isoform is associated with significant reductions in allopregnanolone merit additional study. Since accruing data strongly suggest that alterations in cholesterol metabolism are involved in the pathogenesis of AD [64, 88], the investigation of downstream neurosteroid biosynthesis represents a logical avenue for future examination.
Study Limitations
Limitations of this investigation in temporal cortex postmortem tissue include a relatively small sample size (40 subjects with AD and 41 cognitively intact control subjects). However, the current investigation is more than twice as large as our prior study in prefrontal cortex [38], and also larger than a number of published postmortem brain tissue investigations focusing on neurosteroids and AD [67, 89, 90]. Another limitation that is frequently unavoidable in postmortem brain tissue studies is inability to control for medication status at the time of death, for either AD patients or cognitively intact control subjects. Since some pharmacological agents increase allopregnanolone [51, 91–97] and pregnenolone [44, 95, 98], this could be a confounding element. In addition, smoking status at the time of death is not known for this cohort, and smoking is associated with elevations in DHEA [99] and may also be associated with elevations in allopregnanolone and pregnenolone [100].
Summary
In congregate, the results of this investigation are consistent with a role for neurosteroids in the pathophysiology and therapeutics of AD. In light of recent converging preclinical and clinical evidence linking allopregnanolone deficits to neurodegenerative disorders, restoration of this neurosteroid may represent a logical treatment strategy. Since allopregnanolone levels are inversely related to Braak and Braak neuropathological disease stage, reductions in this neurosteroid may have functional significance and relevance to illness progression. Decreases in allopregnanolone levels in the presence of the APOE4 allele, a well-established risk factor for late-onset AD, introduce additional lines of investigation for future exploration and hypothesis-testing. Findings that DHEA and pregnenolone are elevated in AD compared to cognitively intact control subjects (and positively correlated with Braak and Braak neuropathological disease stage) suggest altered regulation of neurosteroid biosynthetic pathways, potentially involving the accumulation of pregnenolone precursor and blockage in downstream allopregnanolone formation. Additional efforts and replication of these findings will be required to examine these possibilities.
Acknowledgements
This work was supported by the following sources:
VA Advanced Research Career Development Award (CEM), VA Mid-Atlantic Mental Illness, Research, Education, and Clinical Center (MIRECC), and the National Institute of Mental Health (NIH), K23 MH 65080 (CEM), National Institute on Ageing (NIA) P50 AG05128 (CMH), and NIA P30: AG028377(CMH). Dr. Marx discloses that she is a co-applicant on pending U.S. patent applications for the use of neurosteroids and derivatives for the treatment of central nervous system disorders and for lowering cholesterol. The remaining authors have no potential conflicts of interest to disclose. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci Lett. 2002;328:33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- 3.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 4.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- 6.Ahmad I, Lope-Piedrafita S, Bi X, Hicks C, Yao Y, Yu C, Chaitkin E, Howison CM, Weberg L, Trouard TP, Erickson RP. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J Neurosci Res. 2005;82:811–821. doi: 10.1002/jnr.20685. [DOI] [PubMed] [Google Scholar]
- 7.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 8.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–420. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azcoitia I, Leonelli E, Magnaghi V, Veiga S, Garcia-Segura LM, Melcangi RC. Progesterone and its derivatives dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol Aging. 2003;24:853–860. doi: 10.1016/s0197-4580(02)00234-8. [DOI] [PubMed] [Google Scholar]
- 10.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O'Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 11.Liao G, Cheung S, Galeano J, Ji AX, Qin Q, Bi X. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res. 2009;1270:140–151. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189:404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci Lett. 2007;425:94–98. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charalampopoulos I, Alexaki VI, Tsatsanis C, Minas V, Dermitzaki E, Lasaridis I, Vardouli L, Stournaras C, Margioris AN, Castanas E, Gravanis A. Neurosteroids as endogenous inhibitors of neuronal cell apoptosis in aging. Ann N Y Acad Sci. 2006;1088:139–152. doi: 10.1196/annals.1366.003. [DOI] [PubMed] [Google Scholar]
- 16.Charalampopoulos I, Tsatsanis C, Dermitzaki E, Alexaki VI, Castanas E, Margioris AN, Gravanis A. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc Natl Acad Sci U S A. 2004;101:8209–8214. doi: 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xilouri M, Papazafiri P. Anti-apoptotic effects of allopregnanolone on P19 neurons. Eur J Neurosci. 2006;23:43–54. doi: 10.1111/j.1460-9568.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 18.Francis PT. The interplay of neurotransmitters in Alzheimer's disease. CNS Spectr. 2005;10:6–9. doi: 10.1017/s1092852900014164. [DOI] [PubMed] [Google Scholar]
- 19.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer's disease. Neurochem Int. 2004;45:583–595. doi: 10.1016/j.neuint.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Lipton SA. The molecular basis of memantine action in Alzheimer's disease and other neurologic disorders: low-affinity, uncompetitive antagonism. Curr Alzheimer Res. 2005;2:155–165. doi: 10.2174/1567205053585846. [DOI] [PubMed] [Google Scholar]
- 21.Wenk GL, Parsons CG, Danysz W. Potential role of N-methyl-D-aspartate receptors as executors of neurodegeneration resulting from diverse insults: focus on memantine. Behav Pharmacol. 2006;17:411–424. doi: 10.1097/00008877-200609000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 23.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 24.Ariza M, Pueyo R, Matarin Mdel M, Junque C, Mataro M, Clemente I, Moral P, Poca MA, Garnacho A, Sahuquillo J. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:1191–1193. doi: 10.1136/jnnp.2005.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellinger KA. Traumatic brain injury as a risk factor for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2004;75:511–512. [PMC free article] [PubMed] [Google Scholar]
- 26.Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- 27.Benes FM. A disturbance of late myelination as a trigger for Alzheimer's disease. Neurobiol Aging. 2004;25:41–43. doi: 10.1016/j.neurobiolaging.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Jin LW, Shie FS, Maezawa I, Vincent I, Bird T. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004;164:975–985. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calissano P, Matrone C, Amadoro G. Apoptosis and in vitro Alzheimer disease neuronal models. Commun Integr Biol. 2009;2:163–169. doi: 10.4161/cib.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohn T. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities. Apoptosis. 2010 doi: 10.1007/s10495-010-0463-2. Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 31.Zotova E, Nicoll JA, Kalaria R, Holmes C, Boche D. Inflammation in Alzheimer's disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2:1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papassotiropoulos A, Wollmer MA, Tsolaki M, Brunner F, Molyva D, Lutjohann D, Nitsch RM, Hock C. A cluster of cholesterol-related genes confers susceptibility for Alzheimer's disease. J Clin Psychiatry. 2005;66:940–947. [PubMed] [Google Scholar]
- 33.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer's disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- 34.Shobab LA, Hsiung GY, Feldman HH. Cholesterol in Alzheimer's disease. Lancet Neurol. 2005;4:841–852. doi: 10.1016/S1474-4422(05)70248-9. [DOI] [PubMed] [Google Scholar]
- 35.Treiber-Held S, Distl R, Meske V, Albert F, Ohm TG. Spatial and temporal distribution of intracellular free cholesterol in brains of a Niemann-Pick type C mouse model showing hyperphosphorylated tau protein. Implications for Alzheimer's disease. J Pathol. 2003;200:95–103. doi: 10.1002/path.1345. [DOI] [PubMed] [Google Scholar]
- 36.Wolozin B. Cholesterol and the biology of Alzheimer's disease. Neuron. 2004;41:7–10. doi: 10.1016/s0896-6273(03)00840-7. [DOI] [PubMed] [Google Scholar]
- 37.Yu W, Ko M, Yanagisawa K, Michikawa M. Neurodegeneration in heterozygous Niemann-Pick type C1 (NPC1) mouse: implication of heterozygous NPC1 mutations being a risk for tauopathy. J Biol Chem. 2005;280:27296–27302. doi: 10.1074/jbc.M503922200. [DOI] [PubMed] [Google Scholar]
- 38.Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer's disease. Biol Psychiatry. 2006;60:1287–1294. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Bernardi F, Lanzone A, Cento RM, Spada RS, Pezzani I, Genazzani AD, Luisi S, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone and dehydroepiandrosterone response to corticotropin-releasing factor in patients suffering from Alzheimer's disease and vascular dementia. Eur J Endocrinol. 2000;142:466–471. doi: 10.1530/eje.0.1420466. [DOI] [PubMed] [Google Scholar]
- 40.Smith CD, Wekstein DR, Markesbery WR, Frye CA. 3alpha,5alpha-THP: a potential plasma neurosteroid biomarker in Alzheimer's disease and perhaps non-Alzheimer's dementia. Psychopharmacology (Berl) 2006;186:481–485. doi: 10.1007/s00213-005-0186-1. [DOI] [PubMed] [Google Scholar]
- 41.Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with gamma-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- 42.Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3 alpha-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. J Med Chem. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- 43.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 44.Marx CE, Shampine LJ, Duncan GE, VanDoren MJ, Grobin AC, Massing MW, Madison RD, Bradford DW, Butterfield MI, Lieberman JA, Morrow AL. Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: candidate mechanism for superior efficacy? Pharmacol Biochem Behav. 2006;84:598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 45.Naylor JC, Hulette CM, Steffens DC, Shampine LJ, Ervin JF, Payne VM, Massing MW, Kilts JD, Strauss JL, Calhoun PS, Calnaido RP, Blazer DG, Lieberman JA, Madison RD, Marx CE. Cerebrospinal fluid dehydroepiandrosterone levels are correlated with brain dehydroepiandrosterone levels, elevated in Alzheimer's disease, and related to neuropathological disease stage. J Clin Endocrinol Metab. 2008;93:3173–3178. doi: 10.1210/jc.2007-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–10795. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 47.Brown RC, Han Z, Cascio C, Papadopoulos V. Oxidative stress-mediated DHEA formation in Alzheimer's disease pathology. Neurobiol Aging. 2003;24:57–65. doi: 10.1016/s0197-4580(02)00048-9. [DOI] [PubMed] [Google Scholar]
- 48.Kim SB, Hill M, Kwak YT, Hampl R, Jo DH, Morfin R. Neurosteroids: Cerebrospinal fluid levels for Alzheimer's disease and vascular dementia diagnostics. J Clin Endocrinol Metab. 2003;88:5199–5206. doi: 10.1210/jc.2003-030646. [DOI] [PubMed] [Google Scholar]
- 49.Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone protects hippocampal neurons against neurotoxin-induced cell death: mechanism of action. Proc Soc Exp Biol Med. 1999;222:145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 50.Tamagno E, Guglielmotto M, Bardini P, Santoro G, Davit A, Di Simone D, Danni O, Tabaton M. Dehydroepiandrosterone reduces expression and activity of BACE in NT2 neurons exposed to oxidative stress. Neurobiol Dis. 2003;14:291–301. doi: 10.1016/s0969-9961(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 51.Marx CE, Jarskog LF, Lauder JM, Gilmore JH, Lieberman JA, Morrow AL. Neurosteroid modulation of embryonic neuronal survival in vitro following anoxia. Brain Res. 2000;871:104–112. doi: 10.1016/s0006-8993(00)02452-5. [DOI] [PubMed] [Google Scholar]
- 52.Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- 53.Kimonides VG, Spillantini MG, Sofroniew MV, Fawcett JW, Herbert J. Dehydroepiandrosterone antagonizes the neurotoxic effects of corticosterone and translocation of stress-activated protein kinase 3 in hippocampal primary cultures. Neuroscience. 1999;89:429–436. doi: 10.1016/s0306-4522(98)00347-9. [DOI] [PubMed] [Google Scholar]
- 54.Kurata K, Takebayashi M, Morinobu S, Yamawaki S. beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J Pharmacol Exp Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- 55.Azizi H, Mehrjardi NZ, Shahbazi E, Hemmesi K, Bahmani MK, Baharvand H. Dehydroepiandrosterone Stimulates Neurogenesis in Mouse Embryonal Carcinoma Cell- and Human Embryonic Stem Cell-Derived Neural Progenitors and Induces Dopaminergic Neurons. Stem Cells Dev. 2009 doi: 10.1089/scd.2009.0261. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leskiewicz M, Jantas D, Budziszewska B, Lason W. Excitatory neurosteroids attenuate apoptotic and excitotoxic cell death in primary cortical neurons. J Physiol Pharmacol. 2008;59:457–475. [PubMed] [Google Scholar]
- 58.Gursoy E, Cardounel A, Kalimi M. Pregnenolone protects mouse hippocampal (HT-22) cells against glutamate and amyloid beta protein toxicity. Neurochem Res. 2001;26:15–21. doi: 10.1023/a:1007668213330. [DOI] [PubMed] [Google Scholar]
- 59.Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci U S A. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vallee M, Mayo W, Le Moal M, Role of pregnenolone. dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Brain Res Rev. 2001;37:301–312. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- 61.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 63.Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martins IJ, Berger T, Sharman MJ, Verdile G, Fuller SJ, Martins RN. Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;111:1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 65.Marx C, Steffens D, Blazer D, Ervin J, Hulette C, Massing M, Butterfield M, Lieberman J, Shampine L. Deficits in the GABAergic neuroactive steroid allopregnanolone in Alzheimer's disease and relevance to neuropathological disease stage: investigations in temporal cortex. Proc 44th Annual Meeting of the American College of Neuropsychopharmacology. 2005:S79. (Abstract 7) [Google Scholar]
- 66.Hulette CM, Welsh-Bohmer KA, Crain B, Szymanski MH, Sinclaire NO, Roses AD. Rapid brain autopsy. The Joseph and Kathleen Bryan Alzheimer's Disease Research Center experience. Arch Pathol Lab Med. 1997;121:615–618. [PubMed] [Google Scholar]
- 67.Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- 68.Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. 402 e1-2. [DOI] [PubMed] [Google Scholar]
- 70.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3alpha,5alpha-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- 72.Kaufman KR, Tanchuck MA, Strong MN, Finn DA. Replacement with GABAergic steroid precursors restores the acute ethanol withdrawal profile in adrenalectomy/gonadectomy mice. Neuroscience. 166:5–14. doi: 10.1016/j.neuroscience.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kokate TG, Banks MK, Magee T, Yamaguchi S, Rogawski MA. Finasteride, a 5alpha-reductase inhibitor, blocks the anticonvulsant activity of progesterone in mice. J Pharmacol Exp Ther. 1999;288:679–684. [PubMed] [Google Scholar]
- 74.Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- 75.Kato-Negishi M, Kawahara M. Neurosteroids block the increase in intracellular calcium level induced by Alzheimer's beta-amyloid protein in long-term cultured rat hippocampal neurons. Neuropsychiatr Dis Treat. 2008;4:209–218. doi: 10.2147/ndt.s2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signalling molecule for neocortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meunier J, Maurice T. Beneficial effects of the sigma1 receptor agonists igmesine and dehydroepiandrosterone against learning impairments in rats prenatally exposed to cocaine. Neurotoxicol Teratol. 2004;26:783–797. doi: 10.1016/j.ntt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Debonnel G, Bergeron R, de Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. J Endocrinol. 1996;150 Suppl:S33–S42. [PubMed] [Google Scholar]
- 80.Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- 81.Sliwinski A, Monnet FP, Schumacher M, Morin-Surun MP. Pregnenolone sulfate enhances long-term potentiation in CA1 in rat hippocampus slices through the modulation of N-methyl-D-aspartate receptors. J Neurosci Res. 2004;78:691–701. doi: 10.1002/jnr.20332. [DOI] [PubMed] [Google Scholar]
- 82.Hsu HJ, Liang MR, Chen CT, Chung BC. Pregnenolone stabilizes microtubules and promotes zebrafish embryonic cell movement. Nature. 2006;439:480–483. doi: 10.1038/nature04436. [DOI] [PubMed] [Google Scholar]
- 83.Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu EE, Robel P. Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor. Proc Natl Acad Sci U S A. 2006;103:4711–4716. doi: 10.1073/pnas.0600113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koenig HL, Schumacher M, Ferzaz B, Thi AN, Ressouches A, Guennoun R, Jung-Testas I, Robel P, Akwa Y, Baulieu EE. Progesterone synthesis and myelin formation by Schwann cells. Science. 1995;268:1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 85.Costa E, Auta J, Guidotti A, Korneyev A, Romeo E. The pharmacology of neurosteroidogenesis. J Steroid Biochem Mol Biol. 1994;49:385–389. doi: 10.1016/0960-0760(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 86.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, Hardwick M, Li H, Vidic B, Brown AS, Reversa JL, Bernassau JM, Drieu K. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/s0039-128x(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 87.Luchetti S, Bossers K, Van de Bilt S, Agrapart V, Morales RR, Frajese GV, Swaab DF. Neurosteroid biosynthetic pathways changes in prefrontal cortex in Alzheimer's disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 88.Bales KR. Brain lipid metabolism, apolipoprotein E and the pathophysiology of Alzheimer's disease. Neuropharmacology. doi: 10.1016/j.neuropharm.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Brown RC, Cascio C, Papadopoulos V. Pathways of neurosteroid biosynthesis in cell lines from human brain: regulation of dehydroepiandrosterone formation by oxidative stress and beta-amyloid peptide. J Neurochem. 2000;74:847–859. doi: 10.1046/j.1471-4159.2000.740847.x. [DOI] [PubMed] [Google Scholar]
- 90.Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- 91.Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 92.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically facilitate pentobarbital sedation by increasing neurosteroids. Proc Natl Acad Sci U S A. 2004;101:6222–6225. doi: 10.1073/pnas.0401479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Romeo E, Strohle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 95.Serra M, Pisu MG, Muggironi M, Parodo V, Papi G, Sari R, Dazzi L, Spiga F, Purdy RH, Biggio G. Opposite effects of short- versus long-term administration of fluoxetine on the concentrations of neuroactive steroids in rat plasma and brain. Psychopharmacology (Berl) 2001;158:48–54. doi: 10.1007/s002130100853. [DOI] [PubMed] [Google Scholar]
- 96.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci U S A. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, Massing MW, Madison RD, Butterfield MI, Lieberman JA, Morrow AL. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006;84:609–617. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 99.Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK. Effects of low-and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology. 2005;30:1751–1763. doi: 10.1038/sj.npp.1300753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marx CE, Trost WT, Shampine L, Behm FM, Giordano LA, Massing MW, Rose JE. Neuroactive steroids, negative affect, and nicotine dependence severity in male smokers. Psychopharmacology (Berl) 2006;186:462–472. doi: 10.1007/s00213-005-0226-x. [DOI] [PubMed] [Google Scholar]