Abstract
The prefrontal cortex (PFC)—the most evolved brain region—subserves our highest-order cognitive abilities. However, it is also the brain region that is most sensitive to the detrimental effects of stress exposure. Even quite mild acute uncontrollable stress can cause a rapid and dramatic loss of prefrontal cognitive abilities, and more prolonged stress exposure causes architectural changes in prefrontal dendrites. Recent research has begun to reveal the intracellular signalling pathways that mediate the effects of stress on the PFC. This research has provided clues as to why genetic or environmental insults that disinhibit stress signalling pathways can lead to symptoms of profound prefrontal cortical dysfunction in mental illness.
The prefrontal cortex (PFC) intelligently regulates our thoughts, actions and emotions through extensive connections with other brain regions (BOX 1). It creates a “mental sketch pad” (to use a phrase coined by Alan Baddeley) through networks of neurons that can maintain information in the absence of environmental stimulation1. Neuroscientists such as Patricia Goldman-Rakic referred to this process as working memory: the ability to keep in mind an event that has just occurred, or bring to mind information from long-term storage, and use this representational knowledge to regulate behaviour, thought and emotion2. The PFC is able to protect these fragile representations from the interference of external or internal distractions, and is key for inhibiting inappropriate actions and promoting task-relevant operations (so-called ‘top-down’ regulation)3–6. PFC operations allow the flexible regulation of behaviour to enable us to properly respond to a changing environment — for example, the ability to shift attentional set to new dimensions and to alter decision making as reward contingencies shift7,8. The PFC also monitors errors, giving us the insight that we are incorrect and need to shift strategies9. All of these abilities depend on proper PFC neuronal network connections, which are highly sensitive to their neurochemical environment.
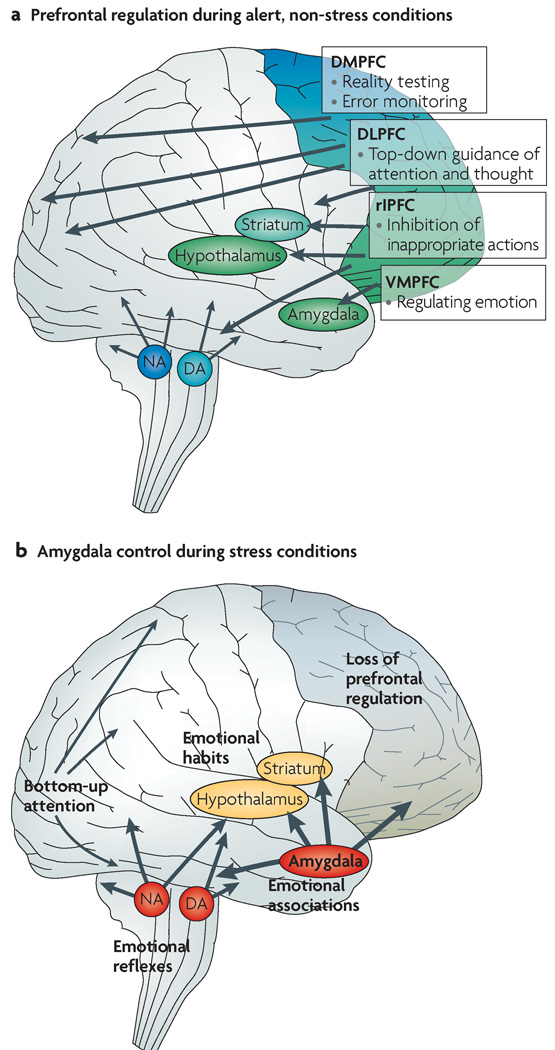
Box 1| Prefrontal cortical versus amygdala circuits: the switch from non-stress to stress conditions
The prefrontal cortex (PFC) has extensive connections with other cortical and subcortical regions that are organized in a topographical manner, such that regions that regulate emotion are situated ventrally and medially (green area in part a of the figure) and regions that regulate thought and action are situated more dorsally and laterally (blue and blue–green areas in part a). The dorsolateral PFC (DLPFC) has extensive connections with sensory and motor cortices and is key for regulating attention, thought and action163. In humans, the right inferior PFC (rIPFC) seems to be specialized for inhibiting inappropriate motor responses4. By contrast, the ventromedial PFC (VMPFC) has extensive connections with subcortical structures (such as the amygdala, the nucleus accumbens and the hypothalamus) that generate emotional responses and habits164–166 and is thus able to regulate emotional responses. Finally, the dorsomedial PFC (DMPFC) has been associated with error monitoring9 and, in human functional MRI studies, reality testing167. These PFC regions extensively interconnect to regulate higher-order decision making and to plan and organize for the future. Under non-stress conditions (see part a of the figure), the extensive connections of the PFC orchestrate the brain’s activity for intelligent regulation of behaviour, thought and emotion. The PFC also has direct and indirect connections to monoamine cell bodies in the brainstem, such as the locus coeruleus (LC) (where noradrenaline projections arise) and the substantia nigra (SN) and ventral tegmental area (VTA) (where the major dopamine projections originate), and thus can regulate its own catecholamine inputs. Optimal levels of catecholamine release in turn enhance PFC regulation, thus creating a ‘delicious cycle’. Under conditions of psychological stress (see part b of the figure) the amygdala activates stress pathways in the hypothalamus and brainstem, which evokes high levels of noradrenaline (NA) and dopamine (DA) release. This impairs PFC regulation but strengthens amygdala function, thus setting up a ‘vicious cycle’. For example, high levels of catecholamines, such as occur during stress, strengthen fear conditioning mediated by the amygdala168. By contrast, stress impairs higher-order PFC abilities such as working memory and attention regulation. Thus, attention regulation switches from thoughtful ‘top-down’ control by the PFC that is based on what is most relevant to the task at hand to ‘bottom-up’ control by the sensory cortices, whereby the salience of the stimulus (for example, whether it is brightly coloured, loud or moving) captures our attention5. The amygdala also biases us towards habitual motor responding rather than flexible, spatial navigation14. Thus, during stress, orchestration of the brain’s response patterns switches from slow, thoughtful PFC regulation to the reflexive and rapid emotional responses of the amygdala and related subcortical structures.
This Review discusses how neuromodulatory changes that occur during stress rapidly disrupt PFC network connections and markedly impair PFC function. It focuses on the spatial working memory functions of the PFC because the circuitry, physiology and modulation of the dorsolateral PFC neurons that mediate working memory are the best characterized of this brain region. The Review first describes how exposure to even mild uncontrollable stress can rapidly impair PFC functions in humans and animals. It then describes the extracellular and intracellular mechanisms that contribute to PFC deficits, and how chronic stress exposure leads to structural changes in the PFC. Finally, it highlights how genetic and environmental changes in stress signalling pathways are associated with mental illness, and how an understanding of these pathways might lead to better treatments for neuropsychiatric disorders.
Acute stress impairs PFC function
Human studies
Some of the first studies on the effects of stress on cognition began after the Second World War, based on observations that pilots who were highly skilled during peacetime often crashed their planes in the stress of battle owing to mental errors10. Research was initiated to experimentally manipulate stress levels to see how this altered performance and cognitive abilities11. Many of these early studies showed that stress exposure impaired the performance of tasks that required complex, flexible thinking, but that it could actually improve the performance of simpler and/or well-rehearsed tasks10,12. We now understand that the types of tasks that were impaired by stress were those that required PFC operations13, whereas engrained habits that rely on basal ganglia circuits were spared or enhanced14.
These early studies also pointed to the essential role of the subject’s sense of control over the stressor. Subjects who felt in control of the situation (even if this was an illusion) were often not impaired by stress exposure, whereas those who felt out of control were impaired15. Animal studies have confirmed the key role of control16. The control factor poses a particular problem for modern stress research in human subjects, as ethically all subjects must be given control of their situation and be able to leave the experiment at any time. Nonetheless, paradigms have emerged that are effective in creating a sense of uncontrollable stress in an experimental setting. For example, one functional imaging study had female subjects watch emotionally upsetting movies and found evidence of reduced activation in PFC regions17. emotional distractors can similarly diminish dorsolateral PFC activity in subjects performing a working memory task18. Many studies of hormonal responses to stress have used a public speaking task as an effective stressor (the Trier social stress test). This social stressor has been found to impair cognitive flexibility19 and working memory20, both of which require PFC function. Interestingly, this same social stressor actually improved classical conditioning for negative stimuli, as well as hippocampal spatial memory20. These findings in humans are highly consistent with animal studies showing that acute mild stress impairs prefrontal function but actually improves the functioning of the amygdala and hippocampus (see below). other studies have found impaired PFC function in subjects using mental imagery of their own overwhelming experiences21,22 and in medical students as they studied for the Boards23.
The reduction in PFC functioning that occurs during stress is highly relevant to understanding human mental and physical health. loss of self-control during stress exposure can lead to relapse of a number of maladaptive behaviours, such as drug addiction, smoking, drinking alcohol and overeating22. Prolonged stress is a major risk factor for depression24,25, and exposure to traumatic stress can cause post-traumatic stress disorder (PTSD)26. Stress can also exacerbate the symptoms of schizophrenia27,28 and bipolar disorder by, for example, switching patients from a period of normalcy (euthymia) into a manic state29. Given these powerful effects on human health, it is important to have animal models of the stress response to help us understand the mechanisms that render us vulnerable.
Animal studies
Early studies of the effects of stress on performance in rodents used the so-called learned helplessness paradigm. These studies were among the first to show that uncontrollable stress (that is, inescapable shock) but not controllable stress impaired performance on a Y-maze task owing to deficits in selective attention16. More recent studies using this paradigm have shown that when the animal perceives itself as being in control the PFC is key for suppressing stress responses triggered in the brainstem30. Indeed, the PFC and the hippocampus are the two brain structures that are positioned to inhibit the glucocorticoid stress response31.
Several studies have used tasks that explicitly rely on PFC function to examine the effects of mild stress on cognitive performance. These studies used spatial working memory tasks in rats and monkeys and found that quite mild acute stress impaired the accuracy of responding and often produced a perseverative pattern of response that is consistent with PFC dysfunction32–34. For example, a white-noise stress that impairs cognitive abilities in humans was found to also impair spatial working memory in monkeys33. Conversely, performance of control tasks with similar motor and motivational demands but no need for PFC regulation was not altered by mild stress exposure. Similarly, rats exposed to acute stressors were impaired on a spatial delayed alternation task that requires medial PFC function, but were not impaired on a non-PFC-reliant spatial discrimination task in the same maze32.
By contrast, acute mild stress exposure has no effect on or can actually improve the memory consolidation functions of the hippocampus and the amygdala35,36. (Note that the ventromedial PFC regulates the fear responses mediated by the amygdala; see REF. 37 for a recent review.) More severe acute stressors impair hippocampal functions38 but continue to strengthen the emotional associations and motor habits carried out by the amygdala and striatum, respectively14,39. Thus, acute uncontrollable stress impairs PFC-mediated cognitive functions in humans and animals and switches the control of behaviour and emotion to more primitive brain circuits.
Catecholamines mediate acute-stress effects
How do neurochemical changes evoked by exposure to acute stress impair PFC function? PFC working memory abilities crucially depend on the precise activity of interconnected neuronal networks. The microcircuitry that subserves spatial working memory in primates has been an attractive model for study, as the anatomy, physiology and neuromodulation of these networks are well characterized owing to the pioneering work of Goldman-Rakic and colleagues40. This Review focuses on catecholamine modulation of spatial working memory circuits, as this is currently the arena where we best understand how stress-like alterations in the chemical environment alter neuronal response patterns in primate prefrontal networks that are actively engaged in a cognitive operation. It is hoped that future studies will extend this approach to other cortical regions, additional cognitive operations and other key neuromodulators.
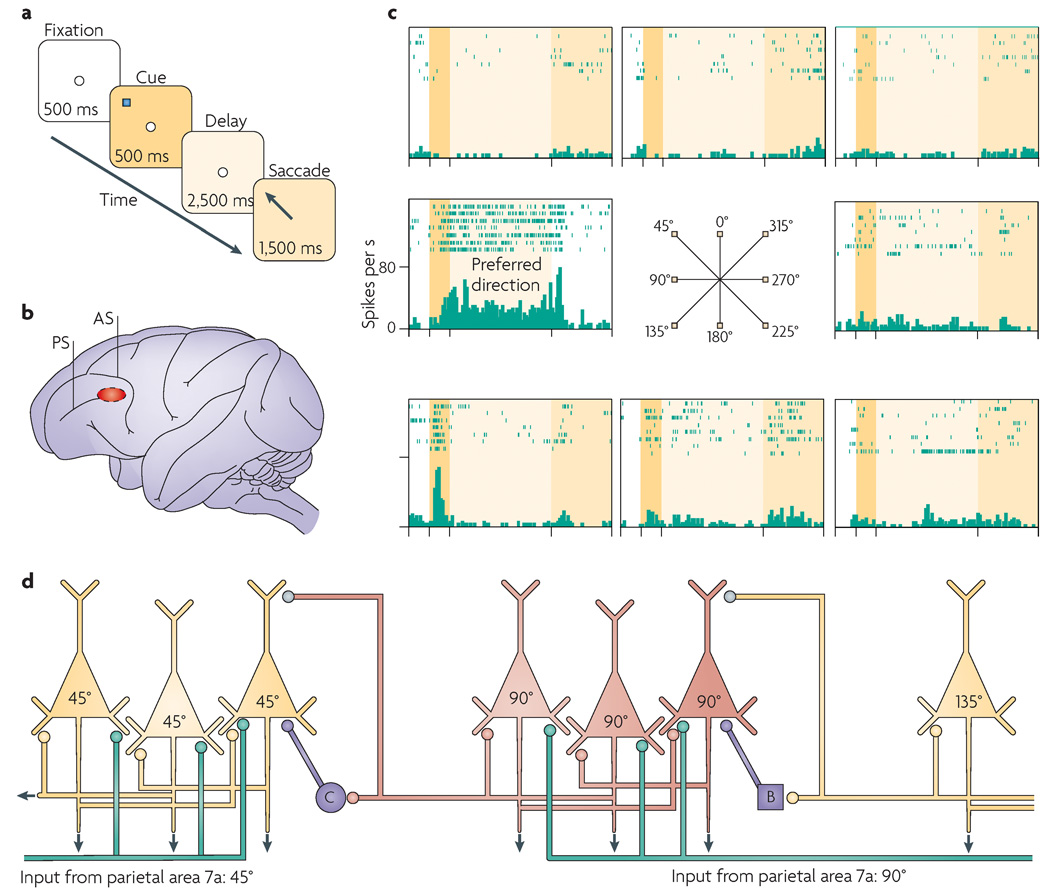
The circuits that underlie spatial working memory have been mapped in great detail. In the primate cortex, highly processed visual spatial information is fed forward from the parietal association cortices to the principal sulcal cortex in the dorsolateral PFC40. This area is crucial to spatial working memory, as lesions in this area but not in the surrounding tissue produce profound and permanent deficits on spatial working memory tasks40. Microcircuits in the PFC can maintain representations of visuospatial information in the absence of environmental stimulation — for example, during the delay period in a working memory task. Funahashi et al.41 developed a task to probe the spatial working memory circuitry, called the oculomotor delayed response (ODR) task (FIG. 1). In this task, the monkey has to remember a precise spatial location over a delay period before moving its eyes to the remembered location to receive a juice reward (FIG. 1a). each session consists of hundreds of trials, with the correct spatial position changing between trials. Thus, the contents of working memory must be constantly updated. Single units are recorded from the PFC (FIG. 1b) while the monkey performs the ODR task. In this region many neurons show highly tuned, persistent activity during the delay period; this activity represents spatial position in the absence of environmental stimulation (for example, see FIG. 1c). The persistence of neuronal activity during the delay period is thought to arise from recurrent excitation between PFC pyramidal cells with shared inputs from the parietal cortex — that is, between pyramidal cells with similar spatial characteristics (FIG. 1d). These excitatory recurrent connections probably involve NMDA (N-methyl-d-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptor synapses onto dendritic spines. By contrast, the high degree of spatial tuning arises from the activity of local inhibitory interneurons (for example, GABA (γ-aminobutyric acid)-ergic basket cells and chandelier cells (FIG. 1d)), which provide lateral inhibition. Thus, when pyramidal cells representing, for example, spatial positions ∼90° from a central reference point are active, they excite each other to create persistent activity and at the same time activate GABAergic interneurons that suppress the activity of pyramidal cells representing other directions (such as the cells dedicated to 45°). In this example, 90° would be called the ‘preferred direction’ of the excited neurons and other spatial positions would be referred to as the ‘non-preferred directions’ for those neurons. Although glutamate and GABA are the foundation of this spatially tuned, mnemonic firing, the strength of the persistent firing and the degree of spatial tuning are also powerfully influenced by the catecholamines noradrenaline and dopamine.
Figure 1. Spatial working memory networks in the dorsolateral prefrontal cortex.
a | The oculomotor delayed response (ODR) task is a spatial working memory task that is used to probe the physiological profiles of prefrontal cortex (PFC) neurons. The subject must remember the spatial position of the most recent cue over a delay period of several seconds and then indicate that position with a saccade to the memorized location. b | The region of the monkey dorsolateral PFC (Walker’s area 46) where neurons show persistent, spatially tuned firing during the delay period in the ODR task. c | An example of a PFC neuron that showed persistent firing during the delay period if the cue had occurred at 90° — the ‘preferred direction’ for this neuron (left middle plot) — but not if the cue appeared in a ‘non-preferred’ direction (other plots). The rasters show the firing of the neuron over seven trials at each spatial position. d | A schematic drawing of the PFC microcircuits that underlie spatial working memory as described by Goldman-Rakic40. Layer III pyramidal cells receive highly processed spatial information (represented by the green lines) from parietal association cortices. Pyramidal cells with similar spatial characteristics engage in recurrent excitation to maintain persistent activity over the delay period (note that the subcellular localization of these excitatory connections is not currently known; they could be on the apical and/or basal dendrites). GABA (γ-aminobutyric acid)-ergic interneurons, such as basket cells (B) and chandelier cells (C) help to spatially tune neurons through lateral inhibition. Inputs from cross-directional microcircuits (neurons with different tuning characteristics) are shown in grey. AS, arcuate sulcus; PS, principal sulcus. Parts a–c are modified, with permission, from REF. 60 ©(2007) Cell Press.
Noradrenaline and dopamine neurons in the brainstem change their firing rate according to our arousal state and according to the relevance of events in the environment. Noradrenaline neurons in the locus coeruleus are silent during rapid eye movement sleep and have low tonic firing during slow wave sleep, moderate tonic firing and pronounced phasic firing to relevant stimuli during non-stressed waking, and high tonic firing with dysregulated phasic firing during stress42. They fire to relevant stimuli during alert waking but can fire to irrelevant stimuli (distractors) during fatigue or stress42. Dopamine neurons have not been followed as methodically with regard to states of arousal, but in general increased phasic firing is related to prediction of reward43. However, recent studies suggest that a subset of midbrain dopamine neurons increase their firing to aversive stimuli44, and it is possible that these underlie the changes in PFC function that occur during stress. Importantly, both dopamine and noradrenaline release are increased in the PFC during exposure to acute stress45,46. Indeed, even very mild stress increases dopamine release in the PFC47.
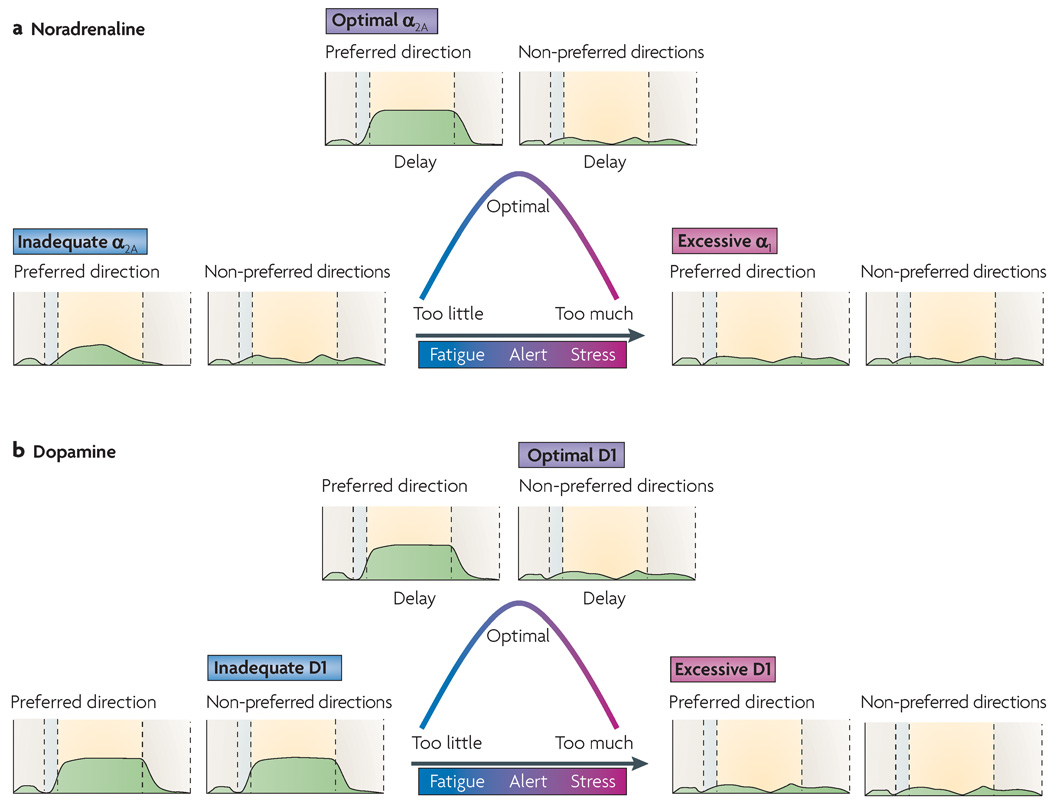
Although the noradrenaline and dopamine innervation of the PFC is quite delicate48, it is extraordinarily powerful. Noradrenaline and dopamine each have an ‘inverted U’-shaped influence on working memory whereby either too little or too much noradrenaline and/or dopamine impairs PFC function (FIG. 2). Noradrenaline and dopamine each provide essential excitatory influences that put the cortex into an awake state in which neurons can process and exchange information49,50. However, they also have additional modulatory influences that powerfully influence the strength of PFC connections as networks engage in working memory operations.
Figure 2. Catecholamine influences on prefrontal cortex physiology and function.
Both noradrenaline (NA, part a) and dopamine (DA, part b) have ‘inverted U-shaped’ influences on prefrontal cortex (PFC) physiology and cognition, whereby either too little or too much of the neurotransmitter impairs PFC function. In an oculomotor delayed response task, with optimal levels of NA or DA release under alert, non-stressed conditions (top of the curves), PFC neurons fire (as shown in the green traces) during the delay period following cues for preferred but not non-preferred directions. NA enhances delay-related firing in response to cues in preferred directions by stimulating α2A-receptors (increasing the ‘signal’), whereas DA weakens delay-related firing in response to cues in non-preferred directions by stimulating D1 receptors (decreasing the ‘noise’). Administration of appropriate concentrations of the α2A-receptor agonist guanfacine or the D1 receptor agonist SKF81297 also has this effect. With high levels of NA release during stress (right side of the curve), NA engages the lower-affinity α1-receptors and so reduces neuronal firing. Similarly, excessive D1 receptor stimulation during stress suppresses cell firing. Administration of the α1-receptor agonist phenylephrine or a high concentration of SKF81297 can mimick the effects of high NA and DA levels, respectively. In each graph, the dotted lines indicate the transition between (from left to right) the fixation period, the cue period, the delay period and the oculomotor response period.
The inverted U-shaped relationship between noradrenaline levels and working memory is especially interesting, as noradrenaline engages different types of receptors with varying levels of release. Noradrenaline has highest affinity for α2-adrenergic receptors, and lower affinity for α1- and β-adrenergic receptors51. As shown in FIG. 2, the levels of noradrenaline that are released during alert, non-stressed waking optimize working memory by engaging α2A-receptors52,53, whereas the high levels of noradrenaline that are released during stress impair PFC function by stimulating lower-affinity α1-receptors54 and β1-receptors55. Thus, either depletion of noradrenaline52 or blockade of α2A-receptors in the PFC53 impairs working memory, whereas stimulation of postsynaptic α2A-receptors in the PFC improves working memory performance52,56–58. These effects can be seen at the cellular level too: inadequate α2A-receptor stimulation reduces PFC firing, whereas α2A-receptor stimulation in the PFC enhances neuronal firing during the delay period for the neurons’ preferred direction in the ODR task59,60 (FIG. 2a). By contrast, high levels of α1-receptor stimulation in the PFC rapidly suppress neuronal firing and impair spatial working memory performance61,62. Similarly, stress-induced cognitive deficits can be prevented by administration of α1-receptor antagonists in the PFC or systemically54. These findings are consistent with clinical investigations showing that the α1-receptor antagonist prazosin is useful in treating PTSD63,64. Studies in humans have also found that a β-receptor antagonist can prevent stress-induced impairment of cognitive flexibility19. Thus, there is good agreement between the animal and human studies.
Dopamine exerts its inverted U-shaped influence on working memory abilities through actions at the D1 receptor family (as current drugs cannot dissociate D1 and D5 receptors, their respective contributions are not understood). Both blockade and excessive stimulation of D1 receptors impair spatial working memory65–67. Working memory deficits during stress exposure involve excessive D1 receptor stimulation, as they are prevented by D1 antagonist administration32. The inverted U-shaped relationship can also be observed at the cellular level (FIG. 2b), where an optimal level of D1 receptor stimulation enhances spatial tuning by suppressing delay-related firing to the neurons’ non-preferred directions in the ODR task68. Thus, dopamine and noradrenaline have complementary roles: α2A-receptor stimulation enhances network firing for shared inputs (that is, it increases the ‘signal’ (REF. 60)), whereas D1 receptor stimulation sculpts neuronal firing by decreasing firing to non-preferred inputs (that is, it decreases ‘noise’ (REF. 68)). However, at higher levels of D1 receptor stimulation, firing is suppressed for all directions and the neuron loses both its spatial tuning and its level of responsiveness68 (FIG. 2b). Thus, high levels of catecholamines during stress reduce both the persistent firing and the tuning of PFC neurons. It is likely that the D2 family of dopamine receptors contributes to the stress response as well. excessive D2 receptor stimulation impairs PFC working memory function in animals69 and humans70 and is associated with increased response-related firing in monkeys71 and increased response-related blood oxygen level-dependent signals in human imaging studies70. The inverted u-shaped relationship between dopamine levels and PFC functioning has been observed in humans both in pharmacological studies72 and with regard to catechol-O-methyltransferase (COMT) genotype73: the methionine substitution in the COMT protein that results from one of the COMT polymorphisms reduces the ability of COMT to catabolize dopamine and increases vulnerability to stress- or stimulant-induced working memory impairments in human subjects74 and in mice75.
It is noteworthy that stress-induced impairments in working memory can be rescued by blocking either D1 or α1-receptors in the PFC. These findings are consistent with synergistic interactions between dopamine and noradrenaline effects. The catecholamine actions might also synergize with glucocorticoid effects in the PFC. Stress increases the release of glucocorticoids as well as that of catecholamines, and studies in both animals76 and humans77 have shown that high levels of glucocorticoids by themselves can impair working memory. These effects of glucocorticoids are rapid and presumably do not occur through traditional genomic mechanisms. It is possible that glucocorticoids exaggerate catecholamine actions in the PFC by blocking the extraneuronal catecholamine transporters on glia that clear the extrasynaptic space of catecholamines78. Such a synergistic relationship between glucocorticoids and cat-echolamines has already been observed in the amyg dala, where glucocorticoids and α1- and β-receptor actions reinforce memory consolidation79,80.
The amygdala plays a key part in changing the neurochemical environment of the PFC during stress. lesions to the amygdala prevent the increase in dopamine and noradrenaline release that occurs in the PFC in response to a psychological stressor81. As shown in BOX 1, under stress conditions the amygdala projections to the brain-stem and to the hypothalamus stimulate the release of catecholamines and glucocorticoids throughout the neuraxis. These high levels of catecholamines and glucocorticoids strengthen amygdala functions, such as fear conditioning and consolidation of emotionally relevant information79,80, but weaken PFC functioning; thus they shift the orchestration of brain responses from the PFC to the amygdala. High levels of catecholamines also strengthen the functioning of other subcortical brain regions and posterior cortical areas. For example, high levels of noradrenaline acting at α1- and β-receptors enhance memory consolidation processes in the hippo-campus82,83 and increase the signal/noise ratio in primary sensory cortices84–86. Higher levels of dopamine release also promote habit formation in the basal ganglia87. It is possible that high levels of dopamine release in corticobasal ganglia circuits during stress serve to capture whatever successful behaviour has just rescued subjects from danger and engrain this pattern as a habit. But this same evolutionary solution could make humans vulnerable to maladaptive behaviours (for example, motor tics, drug addiction and auditory hallucinations) when an inappropriate behavioural pattern is ‘captured’ by high dopamine levels. Taken together, the evidence indicates that high levels of catecholamine release initiated by the amyg dala during stress switch the brain from thoughtful, reflective regulation by the PFC to more rapid reflexive regulation by the amygdala and other subcortical structures (BOX 1). These mechanisms might save our life when we are in danger and need to react rapidly, but they can be detrimental when we need to make choices that require thoughtful analysis and inhibitory control.
Intracellular signalling pathways
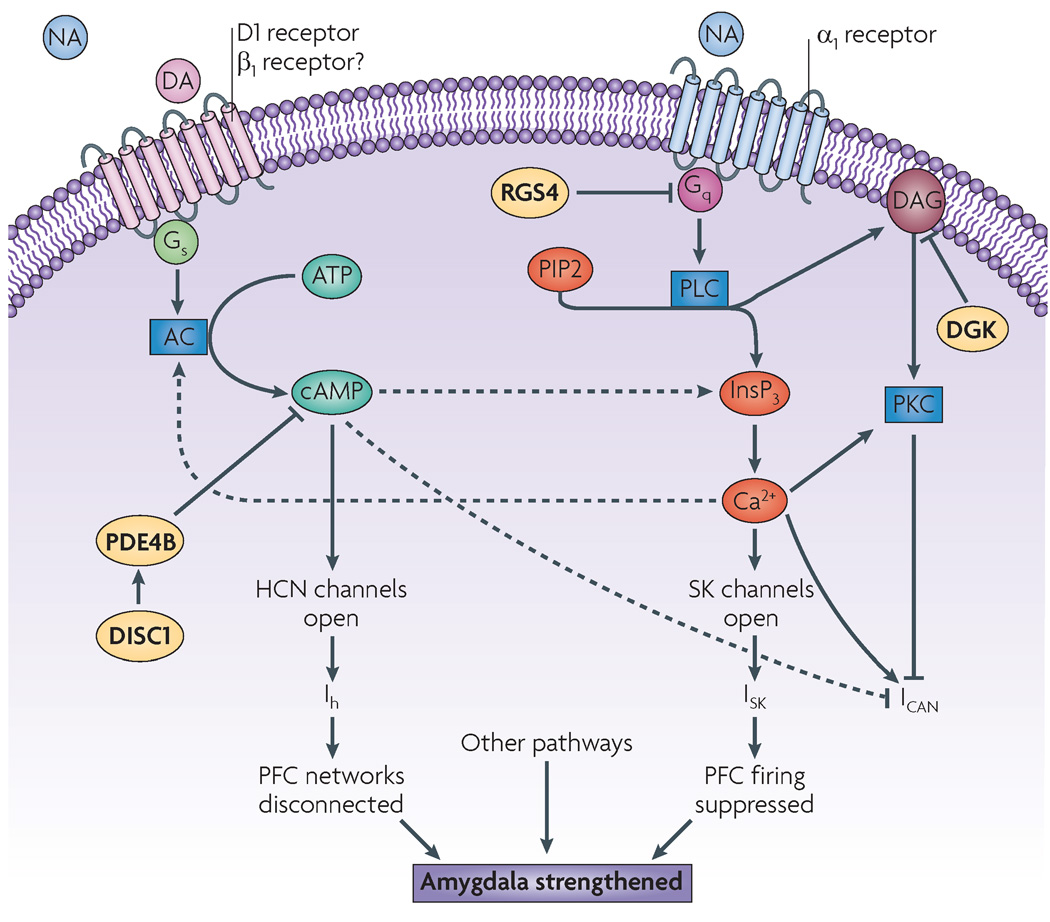
The intracellular mechanisms that cause the loss of PFC working memory function during acute stress are now beginning to be understood, and they provide important clues for understanding the genetic basis and treatment of mental illness. A summary of these signalling pathways and their interactions can be seen in FIG. 3.
Figure 3. Intracellular signalling pathways that impair prefrontal cortex working memory functions during stress.
Intracellular signalling pathways activated by stress exposure have feedforward interactions that rapidly impair prefrontal cortex (PFC) cognitive function. High levels of dopamine (DA) D1 receptor stimulation (and probably noradrenaline (NA) β1-receptor stimulation as well) activate adenylyl cyclases (ACs) to produce cyclic AMP. cAMP opens hyperpolarization-activated cyclic nucleotide-gated cation channels (HCN channels) on dendritic spines to produce the h current (Ih), which weakens network inputs and decreases delay-related firing. High levels of NA also stimulate α1-receptors, which activate phosphatidylinositol biphosphate (PIP2)–protein kinase C (PKC) signalling. Subsequent inositol-1,4,5-trisphosphate (InsP3)-mediated Ca2+ release has been shown to reduce PFC cell firing by opening SK channels, leading to a current (ISK), and has been shown to maintain firing through the depolarizing current ICAN. ICAN can be reduced by PKC and cAMP, such that the suppressive effects of ISK probably predominate under conditions of stress. The two signalling pathways interact to potentiate each other’s actions (dotted arrows); for example, cAMP can potentiate InsP3-medicated Ca2+ release through protein kinase A-mediated phosphorylation of InsP3 receptors. Conversely, Ca2+ can activate many isoforms of AC to generate more cAMP. Glucocorticoids might also activate these pathways (see main text). Enzymes that normally provide the molecular brakes on these stress signalling pathways (disrupted in schizophrenia 1 (DISC1), regulator of G-protein signalling 4 (RGS4) and DAG kinase (DGK)) are often genetically altered in families with serious mental illness, thus increasing susceptibility to stress exposure. Note that the cAMP and PKC signalling pathways have also been shown to be activated in the amygdala during stress, where they strengthen long-term memory consolidation and fear conditioning. DAG, diacyglycerol; PDE4B, phosphodiesterase 4B.
High levels of α1-adrenergic receptor stimulation during stress impair PFC working memory function by activating phosphatidylinositol–protein kinase C (PKC) intracellular signalling pathways. In rodents the working memory deficits that are caused by pharmacological stress, cold water stress or administration of α1-agonists can be reversed by infusing PKC inhibitors directly into the PFC61,88. Conversely, stress-induced working memory impairments can be mimicked by infusing a PKC activator into the PFC61. Similar effects are observed at the cellular level: the suppression of PFC neuron firing that is induced by α1-receptor stimulation can be reversed by iontophoresis of a PKC inhibitor61. In vitro recordings from PFC slices have shown that the inositol-1,4,5-tris-phosphate (InsP3)-Ca2+ arm of phosphatidylinositol signalling is also important89. As shown in FIG. 4g, activation of phosphatidylinositol signalling liberates InsP3, which can release Ca2+ from intracellular stores in dendrites. With adequate InsP3 generation, waves of Ca2+ release are triggered, which can travel towards the soma and suppress neuronal firing by opening small-conductance, Ca2+-activated/K+ channels (SK channels) on the soma, leading to a current (ISK)89. In addition, Ca2+ liberation facilitates PKC activity by causing it to translocate from the cytosol to the membrane, where it is activated by diacylglycerol (DAG) (FIG. 3). Intracellular Ca2+ release also activates a depolarizing current, ICAN, which restores neuronal firing89 (FIG. 3). However, ICAN is suppressed by high levels of PKC and cyclic AMP activity, and thus might not restore normal firing under conditions of stress, when PKC and cAMP activity are high.
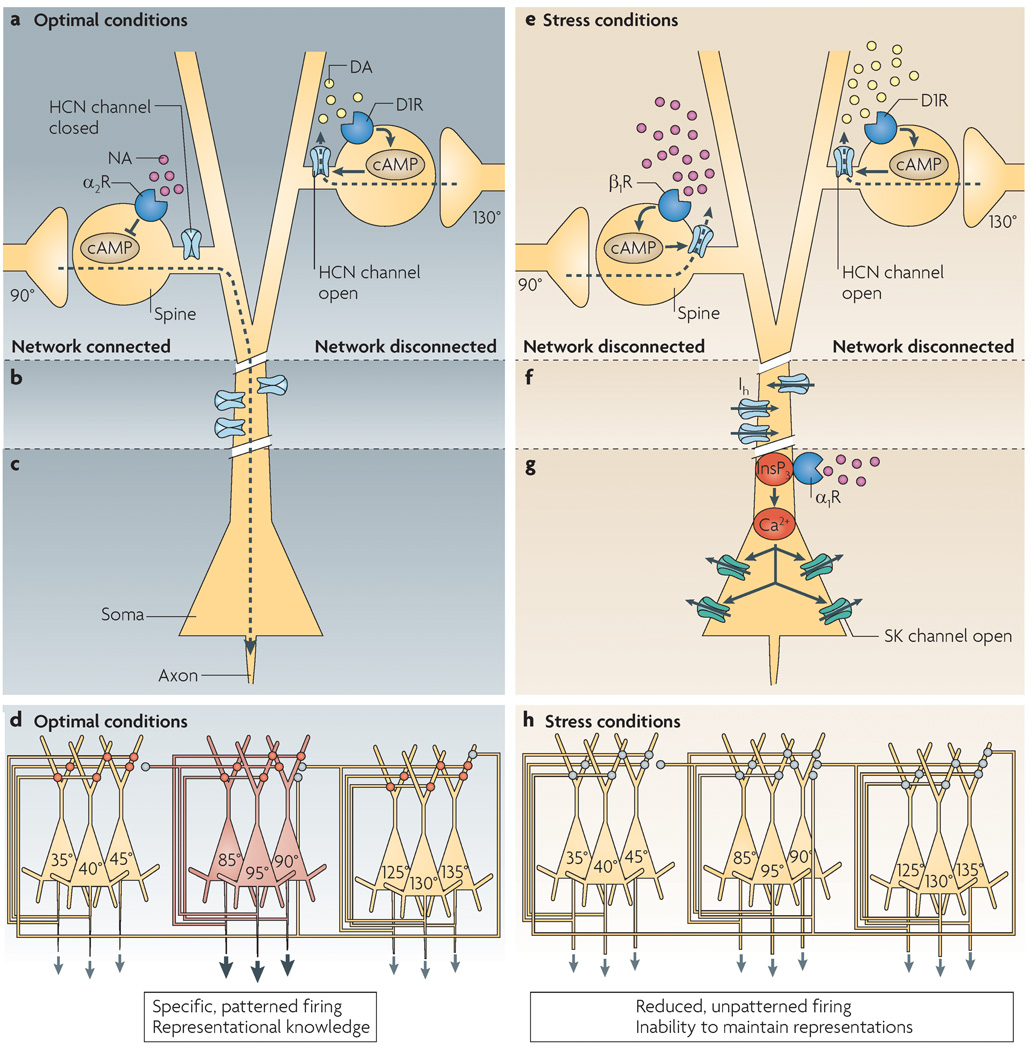
Figure 4. A working model showing how prefrontal cortex pyramidal cell activity might be regulated under optimal versus stress conditions.
a | The efficacy of network inputs onto dendritic spines of the apical and/or basal dendrites is dynamically regulated by cyclic AMP–hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN channel) signalling. Under optimal conditions, appropriate network connections are strengthened by α2A-receptor inhibition of cAMP–HCN channel signalling, whereas inappropriate network connections are weakened by dopamine D1 receptor activation of cAMP–HCN channel signalling. The small volume of the spine compartment probably allows very localized regulation of nearby HCN channels. The resulting specific pattern of network connectivity allows accurate representation of the cue’s spatial position in the oculomotor delayed response task, and the breadth of connectivity can be dynamically regulated based on current cognitive demands. b | HCN channels on the plasma membrane of dendritic shafts probably regulate excitability. They might not be open when the dendrite is depolarized by large numbers of excitatory network connections. c | Under optimal conditions, the cell body probably receives network signals and fires accordingly. d | Under optimal conditions the PFC shows highly patterned activity, whereby specific subsets of neurons are active to represent the precise spatial position of a stimulus. This is denoted in the figure, in which only the microcircuit representing 90° is active. Note that interneurons are not shown in this diagram for the sake of clarity. Network inputs are shown on the apical dendrites, but they might actually be on basal dendrites. Red circles represent α2A-receptor strengthening of network inputs; grey circles represent D1 receptor–cAMP weakening of a network connection. e | Under conditions of stress, high levels of cAMP open HCN channels throughout the dendrite, weakening all network connections. f | With loss of network excitatory inputs the dendrite might hyperpolarize, and HCN channels on dendritic shafts might open to help maintain dendritic excitability. HCN channel opening would also reduce the effectiveness of inputs onto the distal portions of the dendrite. g | High levels of phosphatidylinositol signalling during stress would increase inositol-1,4,5-trisphosphate (InsP3)-mediated waves of intracellular Ca2+ release. When the Ca2+ waves invade the soma, they open small-conductance, Ca2+-activated K+ channels (SK channels) and reduce cell firing. h | Under stressful conditions, network connections are weakened (grey circles) and, as a result, network firing is reduced and unpatterned. Thus, prefrontal cortex neurons are unable to accurately represent information in working memory. Unpatterned, generalized activity might serve another function — for example, stimulating stress pathways in the brainstem.
High levels of catecholamines during stress can also impair PFC functioning through excessive cAMP signalling. The cAMP intracellular signalling cascade has power ful effects on working memory network activity and cognitive function. cAMP frequently acts through protein kinase A (PKA) signalling, and it is likely that many plastic processes in the PFC require PKA signalling, just as plastic processes in other brain regions do90. For example, memory consolidation over long delays (∼30 min or more) requires prefrontal–hippocampal interactions that benefit from cAMP activation of PKA. However, cAMP can also act at two other targets, namely exchange protein activated by cAMP (EPAC; also known as RAPGEF3)91 and hyperpolarization-activated cyclic nucleotide-gated cation channels (HCN channels)92. Physiological data indicate that cAMP might have particularly powerful effects on working memory networks through actions on HCN channels. cAMP increases the probability that HCN channels will open93 and allow passage of both Na+ and K+ (this is also referred to as the h current or Ih). HCN channels are localized on both the dendritic shafts and the dendritic spines of PFC pyramidal cells60 (FIG. 4). HCN channels on dendritic shafts probably have the traditional role of helping to depolarize the neuron when the cell has become hyperpolarized. Indeed, extensive blockade of HCN channels with high doses of ZD7288 induces a loss of cell firing, consistent with loss of this depolarizing influence60. However, HCN channels on spines seem to have a more exquisite role, gating network inputs onto spines. HCN channels are localized next to α2A-adrenergic receptors60 or dopamine D1 receptors (C. Paspalas and A.F.T.A., unpublished observations) on spines near asymmetric (presumably excitatory) synapses. Thus, the catecholamine receptors can regulate cAMP levels in a confined cellular compartment, and so determine the state of HCN channels (open or closed) near synaptic inputs on the spine (FIG. 4a). When HCN channels are opened in the presence of cAMP they shunt nearby inputs. Recent data suggest that this shunting arises from opening of K+ channels (Kv7 channels), giving rise to a hyperpolarizing M current94. High levels of cAMP might potentiate these actions through PKA modulation of Kv7 channels95, thus producing a coordinated cAMP shunting of network inputs.
Under optimal arousal conditions (alert but not stressed), HCN and Kv7 channel opening would be tightly regulated. Physiological and behavioural data indicate that under such conditions α2A-receptor stimulation strengthens network inputs from neurons with shared spatial characteristics by inhibiting cAMP–HCN (and possibly cAMP–PKA–Kv7) signalling60. For example, blocking HCN channels with low doses of ZD7288 reverses the effects of α2A-receptor blockade and restores normal network activity60. Similarly, infusion of low doses of ZD7288 or knockdown of HCN1 channels in the rat PFC improves working memory performance60. Conversely, we propose that in an ODR task, D1 receptors suppress neuronal responses to non-preferred directions by increasing cAMP production68 and opening HCN channels on spines that receive network inputs from cells that are tuned to these other directions (FIG. 4a). Thus, under optimal conditions cAMP production would be tightly controlled and confined to a specific subset of spines receiving input from neurons that are irrelevant to current task demands. under these conditions, HCN channels on dendrites would be closed and PFC cell firing would be highly patterned (FIG. 4d).
By contrast, under conditions of uncontrollable stress there is excessive production of cAMP and impairment in working memory. The impairment in working memory that is induced by stress exposure or excessive D1 receptor stimulation can be prevented by blocking cAMP activity or HCN channels in the PFC60,68 (A.F.T.A., unpublished observations) or can be mimicked by administering the cAMP analogue Sp-cAMPS96. Similar results are observed at the cellular level, whereby Sp-cAMPS, high doses of D1 agonist or a phosphodiesterase inhibitor all suppress neuronal firing in monkeys performing a working memory task, whereas inhibition of cAMP or gentle blockade of HCN channels restores normal firing patterns60,68. These findings are schematically illustrated in FIG. 4e,f, which show that high levels of cAMP during stress open HCN channels throughout the dendrite, weakening network inputs from all directions (FIG. 4e). loss of excitatory network inputs on spines would hyperpolarize the cell, which could open HCN channels on dendritic shafts to help to maintain some cell firing (FIG. 4f). under these conditions, the cell would show greatly reduced and unpatterned activity, as schematically diagrammed in FIGS 2,4h.
Stress signalling pathways have several feedforward interactions that could further contribute to the rapid and dramatic changes in brain responses to an acute stressor (FIG. 3). For example, cAMP–PKA signalling can stimulate the phosphorylation of InsP3 receptors, which promotes InsP3-mediated Ca2+ release97. Conversely, intracellular Ca2+ release from increased phosphatidylinositol signalling can activate Ca2+-sensitive adenyl cyclases to increase cAMP signalling98 (FIG. 3). Both cAMP99 and PKC100 signalling reduce the depolarizing current ICAN, which would further weaken persistent firing89,101,102. Glucocorticoid release during stress could further potentiate these pathways through non-genomic activation of PKC signalling103 (although it is not specifically known whether glucocorticoids have this effect in the PFC). These synergistic interactions would be needed to rapidly switch the control of behaviour from the PFC to more reflexive subcortical pathways in the presence of acute danger.
Chronic-stress effects on the PFC
Compared with exposure to acute stress, exposure to chronic stress leads to more extensive alterations in the rat prelimbic PFC, including architectural changes. The layer II/III neurons that form PFC networks lose dendritic material — that is, dendrite length, branching and spine density are reduced by chronic stress104,105. Spine loss is particularly evident at distal sites (∼200 µm from the soma), and the mushroom-shaped spines that are probably involved in established synaptic connections seem to be particularly vulnerable106. These dendritic changes in the rat PFC are associated with marked PFC dysfunction in attentional set shifting107 and working memory (A.B. Hains and A.F.T.A., unpublished observations). Chronic stress also disrupts the plastic relationship between the PFC and the hippocampus that is needed for flexible memory consolidation108. The dendritic changes in the PFC gradually reverse when the stress abates109.
As with acute stress, the PFC seems to be particularly sensitive to architectural changes induced by chronic stress compared with other brain regions. Whereas structural changes in the hippocampus require several weeks of stress exposure35, dendrites in the PFC begin to change after only one week of stress110 or possibly even a single exposure111. By contrast to the PFC and hippocampus, dendrites in the amygdala expand in response to chronic stress exposure112. Thus, chronic stress weakens the structures that provide negative feedback on the stress response and strengthens the structures that promote the stress response. Interestingly, recent data indicate that neurons in the rat infralimbic PFC that project to the amygdala do not lose their dendrites in response to stress, highlighting the distinct response of amygdala circuits113.
The signalling mechanisms that underlie dendritic changes in the PFC are just beginning to be studied. Chronic stress alters catecholamine pathways, increasing noradrenergic innervation of the PFC114 (although dopamine becomes depleted with severe chronic stress115). Increased noradrenaline might lead to higher levels of PKC and cAMP signalling. We have found that increased PKC signalling contributes to spine loss, as daily treatment with a PKC inhibitor protects PFC spine density and working memory from the detrimental effects of chronic stress (A.B. Hains and A.F.T.A., unpublished observations). Stress-induced changes in dendritic morphology can be mimicked by chronic administration of high doses of glucocorticoids116,117, which may involve excitotoxicity or oxidative stress118. Chronic glucocorticoid exposure also reduces brain-derived neurotrophic factor levels in the PFC119, as does chronic stress120, but it is not yet known whether this contributes to the observed dendritic changes.
Chronic stress during brain development or in childhood may have a particularly large effect on PFC structure and function in adulthood. Dendritic changes can occur in utero when the mother is exposed to stress121. Chronic, uncontrollable stress in childhood might also have enduring effects on the adult PFC. For example, rat pups exposed to extensive maternal deprivation have enduring dendritic retraction in the PFC and increased anxiety-like behaviours122. By contrast, exposure to a mild stress, for example when a juvenile monkey learns that the mother will return after a short period of separation, seems to encourage resilient responses to stress in adulthood123 and reduces glucocorticoid receptor expression in the dorsolateral PFC124. Similar effects have been shown to occur in rats, in which very mild stress early in life promotes resilience whereas more severe stressors exacerbate the stress response later in life125. Changes in utero and during childhood probably contribute to the susceptibility to formal mental illnesses that emerge during adolescence and adulthood126.
Genetic and environmental factors
Both genetic and environmental factors can dysregulate stress signalling pathways in the PFC. They can weaken PFC abilities and, in some cases, lead to symptoms of mental illness.
Genetic insults
Some genetic variations in genes that encode enzymes which catabolize catecholamines that lead to relatively subtle effects on PFC function. For example, methionine substitutions in COMT increase the stress response in mice75 and make humans more susceptible to cognitive impairment following stress and psychostimulant medication74. Similarly, a genetic variation in the promoter region for the monoamine oxidase A gene (MAOA) increases antisocial behaviours in boys exposed to the stress of child abuse127.
Alterations in genes that encode molecules which serve as the intracellular brakes on the stress signalling pathways are associated with serious mental illness (FIG. 3). For example, disrupted in schizophrenia 1 (DISC1) normally provides negative feedback on cAMP signalling, but a mutation in DISC1 decreased the activity of the phosphodiesterase PDE4B in the presence of high concentrations of cAMP128. loss-of-function translocations in DISC1 are associated with high rates of mental illness129, and with reduced PFC grey matter and impaired working memory in patients with schizophrenia130. DISC1 is important for the development of the PFC131, but it is also likely that loss of function of DISC1 leads to increased cAMP–HCN signalling in spines132 and thus to PFC network disconnection. Another important molecular brake on stress signalling pathways is regulator of G-protein signalling 4 (RGS4), which inhibits Gq signalling. RGS4 is normally concentrated in the dendritic stem, where Ca2+ waves that shut off PFC cell firing are generated133. RGS4 levels are greatly reduced in the PFC of patients with schizophrenia134,135, and there is evidence of genetic alterations in RGS4 in some families with schizophrenia or bipolar disorder136,137. loss of RGS4 function would disinhibit phosphatidylinositol–PKC signalling, leading to reduced PFC neuronal firing and susceptibility to spine loss. Finally, DAG kinase-η (DGKH) has been identified as the gene that is most strongly associated with bipolar disorder138. DGK is the enzyme that hydrolyses DAG and stops the activation of PKC. Importantly, medications for these disorders indirectly inhibit the PKC signalling pathway (for example, lithium suppresses phosphatidylinositol signalling), and the atypical antipsychotic medications block α1-adrenergic and serotonin (5-hydroxytryptamine, 5-HT) type 2 (5-HT2) receptors, which are coupled to Gq signalling139,140. There is recent evidence that lithium can restore PFC activity and grey matter levels in patients with bipolar disorder141–143. Thus, medications that correct the consequences of genetic insults could restore normal structure and function.
Environmental insults
Stressors sometimes take the form of environmental insults that threaten our safety. exposure to traumatic stressors can exaggerate subsequent stress responses and lead to PTSD. PTSD is associated with reduced medial PFC activity and grey matter volume144 and with heightened noradrenaline activity145. Imaging studies have shown that experiencing trauma-related distractor stimuli disrupts working memory networks in patients with PTSD146. Similar effects have been seen in patients with depression147. Interestingly, post-pubertal, pre-menopausal women are at much higher risk for PTSD and depression148, and intact female rats (that is, rats with normal oestrogen levels) are more sensitive to stress-induced PFC dysfunction than are male rats or ovariectomized females34,149. This suggests that oestrogen might amplify the PFC response to environmental insults.
Various toxins can impair PFC function by exaggerating the activity of stress signalling pathways. For example, drugs of abuse such as cocaine produce excessive dopamine signalling in the PFC, which weakens PFC function150, including the ability to resist taking drugs. This triggers a vicious cycle because the subsequent drug taking leads to increasingly weaker PFC regulation of behaviour. Drug taking is especially prevalent during stress, when PFC function is further weakened by stress signalling pathways22.
Another important environmental toxin is lead. lead is a divalent cation that mimics Ca2+ and potently activates PKC signalling151. Administration of low levels of lead to rats produces deficits in PFC attentional regulation that mimic the symptoms of attention-deficit hyper-activity disorder (ADHD)152. In humans, lead poisoning is associated with reductions in PFC grey matter153 and with various behavioural changes that arise from PFC dysfunction, including ADHD-like attentional and impulse-control problems, and even sociopathic, irresponsible and criminal behaviours154,155. lead poisoning highly correlates with crime rates and out-of-wedlock pregnancy in modern North America154,155. Interestingly, lead poisoning has also been proposed to have been the cause of the fall of the Roman empire, as the wealthy class ate foods preserved in lead syrup and ate from lead utensils156. The poor decision making and impulsive behaviours of the wealthy class — consistent with PFC dysfunction — led to the downfall of a great empire156. Thus, factors that dysregulate stress signalling pathways and weaken PFC structure and function can erode the civilized behaviour of entire cultures.
Conclusions and future directions
Tremendous advances have been made in understanding neurochemical influences on prefrontal function since the first landmark finding by Brozoski and colleagues 30 years ago157, which showed that dopamine is essential for dorsolateral prefrontal working memory abilities. However, an immense amount of work remains to be done, including studies of additional neuromodulators, different prefrontal regions and other PFC cognitive operations. For example, important work is underway to reveal the complex and powerful effects on prefrontal functions of serotonin, another modulator that is released in the PFC during exposure to uncontrollable stress158 and that is key to orbital PFC function159. Serotonin is particularly relevant to the aetiology and treatment of depression, and genetic alterations in serotonin receptors or the serotonin transporter can influence PFC limbic connections160. However, little is known about how serotonin alters PFC cell firing in animals performing tasks that involve the PFC. one study has shown that high levels of 5-HT2A receptor stimulation can suppress dorsolateral PFC neuronal firing in monkeys performing a working memory task161. These results are similar to those observed with high levels of stimulation of the α1-adrenergic receptor which, like the 5-HT2A receptor, is coupled to phosphatidylinositol–PKC signalling. A recent study in rats suggests that serotonin stimulation of 5-HT2C receptors impairs reward reversal162, a function that is highly dependent on the orbital PFC. It would be fascinating to investigate whether 5-HT2C receptor stimulation would suppress the firing of orbital PFC neurons in monkeys performing a reward reversal task, and whether these effects could be blocked by agents that inhibit phosphatidylinositol–PKC signalling.
The detrimental effects of stress on PFC networks are particularly problematic in the ‘information age’, when PFC-mediated cognitive abilities are increasingly needed for success. understanding the molecular mechanisms that alter PFC structure and impair PFC function during stress exposure will help to reveal how genetic and environmental insults can increase an individual’s susceptibility to PFC deficits. Identifying the molecular mechanisms that alter PFC function will provide the foundation for a new era in psychiatry, when we will understand how genetic changes affect intracellular signalling, neural development and neurophysiology in the circuits that underlie neuropsychiatric symptoms. It is hoped that this information will provide the framework for therapies aimed at rectifying molecular errors and ameliorating the symptoms of mental illness.
figure.
Acknowledgements
Much of the research cited in this Review has been supported by MERIT Award AG06036, and by P50MH068789, PO1AG030004 and RL1AA017536 as part of U54RR024350, as well as by the Kavli Neuroscience Institute at Yale and a National Alliance for Research on Schizophrenia and Depression Distinguished Investigator Award to A.F.T.A.
Glossary
- Attentional set
A predisposition to attend to one dimension of a stimulus while inhibiting other dimensions — for example, attending to colour rather than shape.
- Trier social stress test
A test in which subjects have to give a speech and perform calculations in front of a panel of people they do not know. Blood or saliva samples and blood pressure measurements can be taken before, during and after the test to determine the physical response to the stressor.
- Learned helplessness paradigm
A paradigm developed more than 30 years ago in which rats were exposed to inescapable shock, and supposedly learned that they were helpless to respond. Research has debunked this interpretation and instead determined that uncontrollable stress can cause cognitive deficits.
- Y-maze task
A task in which rats must learn to escape from a Y-shaped maze by making the correct decision. Exposure to uncontrollable stress and the presence of task-irrelevant cues in the maze has been found to impair performance.
- Spatial delayed alternation task
A test of spatial working memory for primates or rodents in which the subject is required to make alternate responses on successive trials, with a delay period interposed between trials.
- Tuned persistent firing of neurons
The neuronal representation of a specific stimulus — for example, a cue in a specific spatial location. Owing to network connections, a cell can sustain firing without stimulation from the environment, but the sustained firing (in this example) occurs only following a cue to a specific location in space; thus the neuron is ‘tuned’ to that direction.
- Phosphodiesterase
An enzyme that hydrolizes cAMP. Inhibition of phosphodiesterase leads to a build-up in cAMP concentrations.
Footnotes
Competing interests statement
The author declares competing financial interests: see web version for details.
DATABASES
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
FURTHER INFORMATION
Amy f. t. Arnsten’s homepage: http://info.med.yale.edu/neurobio/arnsten/Index.html
References
- 1. Fuster JM. The Prefrontal Cortex. Academic Press; 2008. This is the most recent edition of a classic and eloquent book on the PFC.
- 2.Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 3.Thompson-Schill SL, et al. Effects of frontal lobe damage on interference effects in working memory. Cogn. Affect. Behav. Neurosci. 2002;2:109–120. doi: 10.3758/cabn.2.2.109. [DOI] [PubMed] [Google Scholar]
- 4.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 6.Gazzaley A, et al. Functional interactions between prefrontal and visual association cortex contribute to top-down modulation of visual processing. Cereb. Cortex. 2007;17(Suppl. 1):i125–i135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbins TW. From arousal to cognition: the integrative position of the prefrontal cortex. Prog. Brain Res. 2000;126:469–483. doi: 10.1016/S0079-6123(00)26030-5. This review brings together important information on prefrontal circuits and how they are modulated.
- 8.Lee D, Seo H. Mechanisms of reinforcement learning and decision making in the primate dorsolateral prefrontal cortex. Ann. NY Acad. Sci. 2007;1104:108–122. doi: 10.1196/annals.1390.007. [DOI] [PubMed] [Google Scholar]
- 9.Modirrousta M, Fellows LK. Dorsal medial prefrontal cortex plays a necessary role in rapid error prediction in humans. J. Neurosci. 2008;28:14000–14005. doi: 10.1523/JNEUROSCI.4450-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broadbent D. Decision and Stress. London: Academic; 1971. This is a classic book on the effects of stress on cognitive function.
- 11.Hockey GRJ. Effect of loud noise on attentional selectivity. Q. J. exp. Psychol. 1970;22:28–36. [Google Scholar]
- 12.Hartley LR, Adams RG. Effect of noise on the Stroop test. J. exp. Psychol. 1974;102:62–66. doi: 10.1037/h0035695. [DOI] [PubMed] [Google Scholar]
- 13.Arnsten AFT. The biology of feeling frazzled. Science. 1998;280:1711–1712. doi: 10.1126/science.280.5370.1711. [DOI] [PubMed] [Google Scholar]
- 14.Elliott AE, Packard MG. Intra-amygdala anxiogenic drug infusion prior to retrieval biases rats towards the use of habit memory. Neurobiol. Learn. Mem. 2008;90:616–623. doi: 10.1016/j.nlm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Glass DC, Reim B, Singer JE. Behavioral consequences of adaptation to controllable and uncontrollable noise. J. exp. Social Psychol. 1971;7:244–257. [Google Scholar]
- 16.Minor TR, Jackson RL, Maier SF. Effects of task-irrelevant cues and reinforcement delay on choice-escape learning following inescapable shock: evidence for a deficit in selective attention. J. exp. Psychol. Anim. Behav. Process. 1984;10:543–556. [PubMed] [Google Scholar]
- 17.Qin S, Hermans EJ, van Marle HJF, Lou J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry. doi: 10.1016/j.biopsych.2009.03.006. (in the press). [DOI] [PubMed] [Google Scholar]
- 18.Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J. Cogn. Neurosci. 2007;19:468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- 20.Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front. Behav. Neurosci. 2009 Jan 15; doi: 10.3389/neuro.08.005.2008. (doi:10.3389/neuro.08.005.2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha R, Lacadie CM, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann. NY Acad. Sci. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- 22. Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci. Biobehav. Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. This paper relates prefrontal dysfunction during stress to substance abuse.
- 23. Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl Acad. Sci. USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. This paper includes data on how chronic stress weakens prefrontal connectivity in both human subjects and rats exposed to chronic stress.
- 24. Mazure CM, editor. Does Stress Cause Psychiatric illness? Washington DC: American Psychiatric Press; 1995. This book gives many examples of how stress worsens mental illness.
- 25.Mazure CM, Maciejewski PK. A model of risk for major depression: effects of life stress and cognitive style vary by age. Depress. Anxiety. 2003;17:26–33. doi: 10.1002/da.10081. [DOI] [PubMed] [Google Scholar]
- 26.Southwick S, Rasmusson A, Barron X, Arnsten AFT. In: Neuropsychology of PTSD: Biological, Cognitive and Clinical Perspectives. Vasterling JJ, Brewin CR, editors. New York: Guilford Publications; 2005. pp. 27–58. [Google Scholar]
- 27.Breier A, Wolkowitz O, Pickar D. In: Schizophrenia Research. Tamminga C, Schult S, editors. Vol. 1. New York: Raven; 1991. [Google Scholar]
- 28.Dohrenwend BP, Shrout PE, Link BG, Skodol AE, Stueve A. In: Does Stress Cause Psychiatric illness? Mazure CM, editor. Washington DC: American Psychiatric Press; 1995. pp. 43–65. [Google Scholar]
- 29.Hammen C, Gitlin M. Stress reactivity in bipolar patients and its relation to prior history of disorder. Am. J. Psychiatry. 1997;154:856–857. doi: 10.1176/ajp.154.6.856. [DOI] [PubMed] [Google Scholar]
- 30. Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. This study showed that it is the PFC that ascertains (rightly or wrongly) whether we are in control over a stressor. The PFC suppresses the brainstem stress response even when the perceived control is only an illusion.
- 31.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy BL, Arnsten AFT, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc. Natl Acad. Sci. USA. 1996;93:1325–1329. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnsten AFT, Goldman-Rakic PS. Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch. Gen. Psychiatry. 1998;55:362–369. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 34.Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav. Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. NY Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- 36.Cahill L, McGaugh JL. Modulation of memory storage. Curr. Opin. Neurobiol. 1996;6:237–242. doi: 10.1016/s0959-4388(96)80078-x. [DOI] [PubMed] [Google Scholar]
- 37.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JJ, Yoon KS. Stress: metaplastic effects in the hippocampus. Trends Neurosci. 1998;21:505–509. doi: 10.1016/s0166-2236(98)01322-8. [DOI] [PubMed] [Google Scholar]
- 39.Packard MG, Teather LA. Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. Neurobiol. Learn. Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 40. Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. This paper describes the microcircuits that underlie spatial working memory, summarizing both their anatomy and their physiology.
- 41.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J. Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 42.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 43.Schultz W. The phasic reward signal of primate dopamine neurons. Adv. Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M, Hikosaka O. Excitatory and inhibitory responses of midbrain dopamine neurons to cues predicting aversive stimuli. Soc. Neurosci. Abstr. 2008;691.24 [Google Scholar]
- 45.Roth RH, Tam S-Y, Ida Y, Yang J-X, Deutch AY. Stress and the mesocorticolimbic dopamine systems. Ann. NY Acad. Sci. 1988;537:138–147. doi: 10.1111/j.1749-6632.1988.tb42102.x. [DOI] [PubMed] [Google Scholar]
- 46.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience. 1995;64:619–628. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 47. Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog. Brain Res. 1990;85:367–403. doi: 10.1016/s0079-6123(08)62691-6. This paper summarizes the numerous biochemical studies of dopamine release in the PFC during stress exposure.
- 48.Lewis DA, Cambell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J. Neurosci. 1987;282:317–330. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormick DA, Pape HC, Williamson A. Actions of norepinephrine in the cerebral cortex and thalamus: implications for function of the central noradrenergic system. Prog. Brain Res. 1991;88:293–305. doi: 10.1016/s0079-6123(08)63817-0. [DOI] [PubMed] [Google Scholar]
- 50.Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ. Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc. Natl Acad. Sci. USA. 2001;98:301–306. doi: 10.1073/pnas.011518798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnsten AFT. Through the looking glass: differential noradrenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- 53.Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- 54.Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: α-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- 55.Ramos B, et al. The beta-1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol. Psychiatry. 2005;58:894–900. doi: 10.1016/j.biopsych.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Cai JX, Ma Y, Xu L, Hu X. Reserpine impairs spatial working memory performance in monkeys: reversal by the alpha-2 adrenergic agonist clonidine. Brain Res. 1993;614:191–196. doi: 10.1016/0006-8993(93)91034-p. [DOI] [PubMed] [Google Scholar]
- 57.Mao Z-M, Arnsten AFT, Li B-M. Local infusion of alpha-1 adrenergic agonist into the prefrontal cortex impairs spatial working memory performance in monkeys. Biol. Psychiatry. 1999;46:1259–1265. doi: 10.1016/s0006-3223(99)00139-0. [DOI] [PubMed] [Google Scholar]
- 58.Ramos B, Stark D, Verduzco L, van Dyck CH, Arnsten AFT. Alpha-2A-adrenoceptor stimulation improves prefrontal cortical regulation of behavior through inhibition of cAMP signaling in aging animals. Learn. Mem. 2006;13:770–776. doi: 10.1101/lm.298006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B-M, Mao Z-M, Wang M, Mei Z-T. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21:601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 60.Wang M, et al. α2A-adrenoceptor stimulation strengthens working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Birnbaum SB, et al. Protein kinase C overactivity impairs prefrontal cortical regulation of working memory. Science. 2004;306:882–884. doi: 10.1126/science.1100021. [DOI] [PubMed] [Google Scholar]
- 62.Arnsten AFT, Mathew R, Ubriani R, Taylor JR, Li B-M. α-1 noradrenergic receptor stimulation impairs prefrontal cortical cognitive function. Biol. Psychiatry. 1999;45:26–31. doi: 10.1016/s0006-3223(98)00296-0. [DOI] [PubMed] [Google Scholar]
- 63.Taylor F, Raskind MA. The α1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J. Clin. Psychopharmacol. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Raskind MA, et al. Prazosin reduces nightmares and other PTSD symptoms in combat veterans: a placebo-controlled study. Am. J. Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 65.Arnsten AFT, Goldman-Rakic PS. Stress impairs prefrontal cortex cognitive function in monkeys: role of dopamine. Soc. Neurosci. Abstr. 1990;16:164. [Google Scholar]
- 66.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 67.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J. Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vijayraghavan S, et al. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 69.Druzin MY, Kurzina NP, Malinina EP, Kozlov AP. The effects of local application of D2 selective dopaminergic drugs into the medial prefrontal cortex of rats in a delayed spatial choice task. Behav. Brain Res. 2000;109:99–111. doi: 10.1016/s0166-4328(99)00166-7. [DOI] [PubMed] [Google Scholar]
- 70.Gibbs SE, D’Esposito M. A functional MRI study of the effects of bromocriptine, a dopamine receptor agonist, on component processes of working memory. Psychopharmacology. 2005;180:644–653. doi: 10.1007/s00213-005-0077-5. [DOI] [PubMed] [Google Scholar]
- 71.Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- 72.Kimberg DY, D’Esposito M, Farah MJ. Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport. 1997;8:3581–3585. doi: 10.1097/00001756-199711100-00032. [DOI] [PubMed] [Google Scholar]
- 73.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl Acad. Sci. USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mattay VS, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl Acad. Sci. USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Papaleo F, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J. Neurosci. 2008;28:8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J. Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav. Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- 78.Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the cortisone-sensitive extraneuronal catecholamine transporter. Nature Neurosci. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 79.Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between β- and α-1-adrenoceptors. J. Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor–cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 81.Goldstein LE, Rasmusson AM, Bunney SB, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine and prefrontal cortical monoamine responses to psychological stress in the rat. J. Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hopkins WF, Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophys. 1988;59:667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- 83.Hu H, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 84.Foote SL, Freedman FE, Oliver AP. Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res. 1975;86:229–242. doi: 10.1016/0006-8993(75)90699-x. [DOI] [PubMed] [Google Scholar]
- 85.Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative transmitters. Exp. Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- 86.Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- 87.Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J. Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Runyan JD, Moore AN, Dash PK. A role for prefrontal calcium-sensitive protein phosphatase and kinase activities in working memory. Learn. Mem. 2005;12:103–110. doi: 10.1101/lm.89405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hagenston AM, Fitzpatrick JS, Yeckel MF. mGluR-mediated calcium waves that invade the soma regulate firing in layer V medial prefrontal cortical pyramidal neurons. Cereb. Cortex. 2008;18:407–423. doi: 10.1093/cercor/bhm075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Runyan JD, Dash PK. Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes. Learn. Mem. 2005;12:232–238. doi: 10.1101/lm.92405. This important study demonstrated that distinct memory processes can be modulated in very different ways, even in the same brain region.
- 91.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem. Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell. Mol. Life Sci. 2009;66:470–494. doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. George MS, Abbott LF, Siegelbaum SA. Hyperpolarization-activated HCN channels inhibit subthreshold EPSPs through voltage-dependent interactions with M-type K+ channels. Nature Neurosci. doi: 10.1038/nn.2307. (in the press). This important new study used both computational modelling and physiology to explain how HCN channels can shunt incoming synaptic inputs.
- 95.Delmas P, Brown DA. Pathways modulating neural KCNG/M (Kv7) potassium channels. Nature Rev. Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 96.Taylor JR, Birnbaum SG, Ubriani R, Arnsten AFT. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soulsby MD, Wojcikiewicz RJ. The type III inositol 1,4,5-trisphosphate receptor is phosphorylated by cAMP-dependent protein kinase at three sites. Biochem. J. 2005;392:493–497. doi: 10.1042/BJ20051325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferguson GD, Storm DR. Why calciumstimulated adenylyl cyclases? Physiology. 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- 99.Partridge LD, Swandulla D, Muller TH. Modulation of calcium-activated non-specific cation currents by cyclic AMP-dependent phosphorylation in neurons of Helix. J. Physiol. 1990;429:131–145. doi: 10.1113/jphysiol.1990.sp018248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soboloff J, et al. TRPC channels: integrators of multiple cellular signals. Handb. exp. Pharmacol. 2007;179:575–591. doi: 10.1007/978-3-540-34891-7_34. [DOI] [PubMed] [Google Scholar]
- 101.Haj-Dahmane S, Andrade R. Ionic mechanism of the slow afterdepolarization induced by muscarinic receptor activation in rat prefrontal cortex. J. Neurophysiol. 1998;80:1197–1210. doi: 10.1152/jn.1998.80.3.1197. [DOI] [PubMed] [Google Scholar]
- 102.Tegner J, Compte A, Wang XJ. The dynamical stability of reverberatory neural circuits. Biol. Cybern. 2002;87:471–481. doi: 10.1007/s00422-002-0363-9. [DOI] [PubMed] [Google Scholar]
- 103.Han JZ, Lin W, Lou SJ, Qiu J, Chen YZ. A rapid, nongenomic action of glucocorticoids in rat B103 neuroblastoma cells. Biochim. Biophys. Acta. 2002;1591:21–27. doi: 10.1016/s0167-4889(02)00242-2. [DOI] [PubMed] [Google Scholar]
- 104.Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2008 Dec 6; doi: 10.1016/j.neubiorev.2008.11.005. (doi:10.1016/j. neubiorev.2008.11.005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 106.Michelsen KA, et al. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J. Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Radley JJ, et al. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp. Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb. Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- 111.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb. Cortex. 2009 Feb 4; doi: 10.1093/cercor/bhp003. (doi:10.1093/cercor/bhp003). This study shows that not all prefrontal neurons respond similarly to stress, and that there are distinct changes based on the circuits involved.
- 114.Miner LH, et al. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J. Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mizoguchi K, et al. Chronic stress induces impairment of spatial working memory due to prefrontal dopaminergic dysfunction. J. Neurosci. 2000;20:1568–1575. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 117.Liu RJ, Aghajanian GK. Stress blunts serotonin-and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl Acad. Sci. USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu MY, Wang WP, Huang J, Regunathan S. Chronic treatment with glucocorticoids alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. J. Neurochem. 2007;103:1811–1820. doi: 10.1111/j.1471-4159.2007.04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin Y, et al. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb. Cortex. 2008 Dec 10; doi: 10.1093/cercor/bhn225. (doi:10.1093/cercor/bhn225). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murmu MS, et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur. J. Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 122.Pascual R, Zamora-León SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiol. exp. (Wars.) 2007;67:471–479. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- 123.Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, Lyons DM. Mild early life stress enhances prefrontal-dependent response inhibition in monkeys. Biol. Psychiatry. 2005;57:848–855. doi: 10.1016/j.biopsych.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 124.Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Meaney MJ, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev. Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 126.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on brain, behaviour and cognition. Nature Rev. Neurosci. 2009 Apr 29; doi: 10.1038/nrn2639. (doi:10.1038/nrn2639). [DOI] [PubMed] [Google Scholar]
- 127.Kim-Cohen J, et al. MAOA, maltreatment, and geneenvironment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol. Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 128.Millar JK, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 129.Millar JK, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 130.Cannon TD, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch. Gen. Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 131.Ishizuka K, Paek M, Kamiya A, Sawa A. A review of Disrupted-In-Schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 132.Kirkpatrick B, et al. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J. Comp. Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 133.Paspalas CD, Selemon LD, Arnsten AF. Mapping the regulator of G protein signaling 4 (RGS4): presynaptic and postsynaptic substrates for neuroregulation in prefrontal cortex. Cereb. Cortex. 2009 Jan 19; doi: 10.1093/cercor/bhn235. (doi:10.1093/cercor/bhn235). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol. Psychiatry. 2001;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- 135.Erdely HA, Tamminga CA, Roberts RC, Vogel MW. Regional alterations in RGS4 protein in schizophrenia. Synapse. 2006;59:472–479. doi: 10.1002/syn.20265. [DOI] [PubMed] [Google Scholar]
- 136.Chowdari KV, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum. Mol. Genet. 2002;11:1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- 137.Morris DW, et al. Confirming RGS4 as a susceptibility gene for schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;125:150–153. doi: 10.1002/ajmg.b.20109. [DOI] [PubMed] [Google Scholar]
- 138.Baum AE, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol. Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Manji HK, Lenox RH. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol. Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- 140.Arnsten AFT, Manji HK. Mania: a rational neurobiology. Future Neurol. 2008;3:125–131. [Google Scholar]
- 141.Blumberg HP, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: a cross-sectional study. Biol. Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 142.Bearden CE, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol. Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain gray matter. The Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- 144.Bremner JD. Neuroimaging studies in post-traumatic stress disorder. Curr. Psychiatry Rep. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. [DOI] [PubMed] [Google Scholar]
- 145.Southwick SM, et al. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol. Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 146.Morey RA, et al. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J. Psychiatr. Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J. Adolesc. Health. 2002;30:49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- 149.Shansky RM, et al. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol. Psychiatry. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- 150.Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog. Brain Res. 2000;126:433–453. doi: 10.1016/S0079-6123(00)26028-7. [DOI] [PubMed] [Google Scholar]
- 151.Markovac J, Goldstein GW. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- 152.Morgan RE, et al. Early lead exposure produces lasting changes in sustained attention, response initiation, and reactivity to errors. Neurotoxicol. Teratol. 2001;23:519–531. doi: 10.1016/s0892-0362(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 153.Cecil KM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nevin R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. environ. Res. 2000;83:1–22. doi: 10.1006/enrs.1999.4045. [DOI] [PubMed] [Google Scholar]
- 155.Wright JP, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Woolley DE. A perspective of lead poisoning in antiquity and the present. Neurotoxicology. 1984;5:353–361. This paper describes how the fall of the Roman Empire probably involved lead poisoning, a topic of immediate relevance to crime and inappropriate social behaviours in today’s society.
- 157. Brozoski T, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–931. doi: 10.1126/science.112679. This is the landmark paper that first showed that catecholamines are essential to dorsolateral prefrontal working memory abilities. Although the paper focused on dopamine, it is now known that noradrenaline also has an essential role.
- 158.Bland ST, et al. Stressor controllability modulates stress-induced dopamine and serotonin efflux and morphine-induced serotonin efflux in the medial prefrontal cortex. Neuropsychopharmacology. 2003;28:1589–1596. doi: 10.1038/sj.npp.1300206. [DOI] [PubMed] [Google Scholar]
- 159.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb. Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 160.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci. Biobehav. Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J. Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- 163. Goldman-Rakic PS. In: Handbook of Physiology, The Nervous System, Higher Functions of the Brain. Plum F, editor. Vol. V. Bethesda: American Physiological Society; 1987. pp. 373–417. This classic paper describes the parallel anatomical circuits that underlie representational knowledge.
- 164.Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? Prog. Brain Res. 1996;107:523–536. doi: 10.1016/s0079-6123(08)61885-3. [DOI] [PubMed] [Google Scholar]
- 166.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 167.Simons JS, Henson RN, Gilbert SJ, Fletcher PC. Separate forms of realty monitoring by the anterior prefrontal cortex. J. Cogn. Neurosci. 2008;20:447–457. doi: 10.1162/jocn.2008.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann. NY Acad. Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]