Abstract
Objective
To investigate differences in visual function between the healthy eyes of people of African (AD) and European descent (ED).
Methods
Visual function was assessed in 393 AD and 367 ED participants selected from the African Descent and Glaucoma Evaluation Study and the Diagnostic Innovations in Glaucoma Study. Participants had normal appearance of the optic disc and intraocular pressure of less than 22 mm Hg. Each participant had 2 reliable 24-2 standard automated perimetry tests, and most had short-wavelength automated perimetry and frequency-doubling technology tests. The generalized estimating equation was used to adjust for intereye correlations. Results were adjusted for age, vertical cup-disc ratio, disc size, central corneal thickness, and presence of high blood pressure.
Results
The AD participants were younger (mean [SD] age, 46.2 [13.2] years) than the ED participants (age, 49.5 [16.6] years) (P=.003). The AD participants had worse mean deviation and pattern standard deviation and more points triggered as abnormal on the total and pattern deviation plots compared with ED participants on all tests (P<.05). A larger percentage of AD participants had confirmed abnormal glaucoma hemifield test results on standard automated perimetry only.
Conclusions
People of AD have significantly worse performance than people of ED on all tests of visual function. Additional research using longitudinal data is needed to determine the cause of these small but significant ancestry differences in visual function.
Trial Registration
clinicaltrials.gov Identifier: NCT00221923
Glaucoma is one of the leading causes of blindness worldwide.1 Primary open-angle glaucoma (POAG) is the most prevalent form of glaucoma and is characterized by retinal ganglion cell death that results in optic nerve damage and visual field loss.2 Ancestry differences in the prevalence3-8 and incidence9,10 rates of POAG are well documented11 and show that individuals of African descent (AD) are at higher risk of POAG than those of European descent (ED).12-14 Primary open-angle glaucoma is the leading cause of blindness in AD individuals in the United States,5 and those who have developed POAG are more likely than ED individuals to develop visual impairment15 and blindness.16-18 Primary open-angle glaucoma progresses more rapidly18,19 and appears approximately 10 years earlier in AD compared with ED individuals.5,7,8,16,18,20,21
Ancestry differences in visual function have not been studied extensively. One report suggests a stronger association between age and visual field defects in AD compared with ED individuals.22 Compared with ED individuals, a greater proportion of AD individuals may show progressive visual field loss,18,23,24 and the rate of progression may be higher.22 Another study, however, showed a similar rate of progression in visual field loss.25 In the Ocular Hypertension Treatment Study, the mean deviation (MD) of normal and reliable standard automated perimetry (SAP) tests at baseline was significantly worse in AD compared with ED individuals.26 The Glaucoma Laser Trial Research Group reported worse mean thresholds, greater mean defects, and a larger number of abnormal test locations in AD compared with ED individuals at baseline.20 In a small cohort of AD and ED participants with healthy eyes tested by our group, the only significant ancestry difference observed in visual function was worse MD on frequency-doubling technology (FDT N-30) perimetry in the AD participants.27 The goal of this study was to investigate differences in visual function between the healthy eyes of AD and ED individuals in the large National Eye Institute–sponsored African Descent and Glaucoma Evaluation Study (ADAGES).28
METHODS
PARTICIPANTS
Seven hundred sixty participants (393 AD and 367 ED) enrolled in ADAGES (n=582) or in the Diagnostic Innovations in Glaucoma Study (DIGS) (n=178) were included in this study. Ancestry classification in these studies is based on self-report. When eligible (DIGS/ADAGES inclusion/exclusion criteria and the specific criteria required to be included in this study are explained in this section), both eyes of each participant were included (1478 eyes, including 764 AD and 714 ED eyes). DIGS is conducted at the Hamilton Glaucoma Center at the University of California, San Diego (UCSD), whereas ADAGES is a multicenter study conducted at UCSD, the University of Alabama at Birmingham (UAB), and the New York Eye and Ear Infirmary (NYEE). Protocols were in place to ensure that testing procedures were comparable at all sites. These ongoing studies are prospectively designed to assess structure and function in glaucoma. Enrollment of participants is based on the inclusion/exclusion criteria specified below. Patient participants are followed up annually, whereas only baseline data are obtained from most healthy participants (a small subset of healthy participants will have follow-up data). All ADAGES and DIGS participants with healthy eyes were included in the study reported herein. Healthy eyes were defined as having a normal appearance of the optic disc on stereoscopic photographs, intraocular pressure (IOP) of less than 22 mm Hg (measured once), no history of elevated IOP, and no use of glaucoma medication or surgery in either eye. Visual field data were not used for classification purposes. Healthy control participants for ADAGES and DIGS were recruited to join the study by advertisement, from family members of patients, and from primary eye care clinics. Informed consent was obtained from all participants, and the local institutional review board at each site approved all methods. Participants were minimally compensated for their involvement in the study. This study adhered to the tenets of the Declaration of Helsinki for research involving human subjects and conformed to the Health Insurance Portability and Accountability Act.
INCLUSION CRITERIA FOR DIGS AND ADAGES
Simultaneous stereoscopic photographs were obtained for all participants and were of adequate quality for the participant to be included. All participants had open angles, best-corrected visual acuity of 20/40 or better, spherical refraction within 5.0 diopters (D), and cylinder correction within 3.0 D. All participants had reliable visual field results on all tests, defined as 33% or fewer false-negative errors, false-positive errors, and fixation losses. Participants with a family history of glaucoma were included.
EXCLUSION CRITERIA FOR DIGS AND ADAGES
Participants were excluded if they had a history of intraocular surgery (except for uncomplicated cataract surgery), as were participants with diseases affecting the visual field (eg, pituitary lesions, demyelinating diseases, human immunodeficiency virus seropositivity, AIDS, or diabetes mellitus), those using medications known to affect visual field sensitivity, or those with problems affecting color vision other than glaucoma. Each participant underwent a complete ophthalmological examination to rule out the presence of other ocular diseases. This examination included slitlamp biomicroscopy, IOP measurement, and dilated stereoscopic fundus examination.
All DIGS and ADAGES participants were familiar with perimetry or were given practice tests before the baseline data collection. Eyes included in this analysis had 2 reliable SAP test results. The only exception was the inclusion of 50 eyes (22 AD and 28 ED eyes) with only 1 SAP test when the result of that test was normal. These eyes belonged to 35 participants (17 AD and 18 ED). In addition, most participants had 22 short-wavelength automated perimetry (SWAP) and FDT tests on each eye. All tests were performed within a 3-month period, and the order of the tests was randomized across participants. Stereoscopic photographs were taken within 6 months of the visual field tests.
Color simultaneous stereoscopic photographs of the optic disc were obtained using the commercially available Nidek Stereo Camera Model 3-DX (Nidek Inc, Palo Alto, California) after maximal pupillary dilation. Photographs were independently assessed by 2 trained graders who were masked to the identity and diagnosis of the participant, to the evaluation of the other grader, and to the ancestry of the participants. In cases where the 2 graders disagreed, a third experienced grader served as an adjudicator. Stereoscopic photographs were evaluated using a stereoscopic viewer (Asahi Pentax Stereo Viewer II; Asahi Optical Co, Tokyo, Japan) illuminated with color-corrected fluorescent lighting. Glaucomatous optic neuropathy was defined by evidence of any of the following: excavation, neuroretinal rim thinning or notching, nerve fiber layer defects, or an asymmetry of the vertical cup-disc ratio of at least 0.2 between the 2 eyes.
TESTS OF VISUAL FUNCTION
We evaluated visual function using SAP, SWAP, and FDT. Ancestry differences in visual function were first assessed by comparing the AD and ED groups on each of the following variables: MD, pattern standard deviation (PSD), the percentage of participants with abnormal (“outside normal limits”) results on the glaucoma hemifield test (GHT), and the number of points triggered at 5% or worse (<5%, <2%, <1%, or <0.5%) and at 1% or worse (<1% or <0.5%) on the total deviation and pattern deviation plots. To be included in the study, all participants had 2 SAP tests in at least 1 eye (with the exception of the 50 eyes described in the “Exclusion Criteria for DIGS and ADAGES” subsection); whenever possible, they also had 2 SWAP and FDT tests on each eye. The average of the 2 test results (for each test type and for each eye) was used to assess the ancestry differences for each of these variables. Using the average of the 2 visual field test results allowed us to use all the data available for each participant in addition to reducing measurement variability. When only 1 test was available, the results of that test were used. For GHT, we required that the outcome be outside normal limits on both visual field tests for the result to be considered abnormal. When the numbers of abnormal total deviation and pattern deviation points were averaged, the level at which the point was triggered and the location of the points were not considered. In a second analysis, we compared the percentage of abnormal visual field test results in the AD and ED groups. For this analysis, abnormality was defined in the following 2 ways: (1) a PSD abnormal at 5% or worse, a GHT result outside normal limits, or an MD abnormal at 5% or worse, and (2) a PSD abnormal at 5% or worse or a GHT result outside normal limits. The results had to be abnormal on both visual field tests of a given test type for a given eye for the results to be considered abnormal because confirmation of visual field defects has been shown previously to improve the specificity of the results.29-31
The UCSD Visual Field Assessment Center is a visual field reading center overseeing ADAGES, DIGS, and other studies. Reading centers are important to standardize procedures, train and certify technicians, and perform quality control.32 The UCSD Visual Field Assessment Center certified all visual field technicians involved in ADAGES and DIGS after they successfully completed a certification examination and submitted 2 sets of reliable visual fields taken from nonstudy participants. Visual field technicians involved in DIGS and ADAGES are required to be in the room with the participants throughout the visual field testing; technicians adhered to this protocol at each study site. Trained visual field graders at the UCSD Visual Field Assessment Center ensured that each visual field test included in ADAGES and DIGS had been performed using the accurate date of birth and refraction for each participant. Tests performed with a pupil diameter smaller than 3 mm were excluded. Visual fields with more than 33% fixation losses, false-negative errors, and false-positive errors were excluded. The only exception was the inclusion of visual fields with false-negative errors of more than 33% when the field showed advanced disease. Visual field tests with several threshold values higher than what can be expected of healthy participants were excluded. Visual fields exhibiting a learning effect were also excluded. The first visual field available to us from each participant was excluded because of a learning effect if the difference in the score between the first and second tests was at least the 99.5th percentile of difference in scores of a large subset of our population on the MD or the PSD. Visual fields were further reviewed for the following artifacts: lid and rim artifacts, fatigue effects, inappropriate fixation, evidence that the visual field results were due to a disease other than glaucoma (such as homonymous hemianopia), and inattention. The UCSD Visual Field Assessment Center requested repeats of unreliable visual field test results, and these were obtained whenever possible. One trained grader, masked to glaucoma status (healthy vs glaucomatous eye) and ancestry, reviewed the visual fields for artifacts. In cases where this grader was unsure whether an artifact existed, a second experienced grader (L.R. or P.A.S.) adjudicated.
Standard Automated Perimetry
Standard automated perimetry is a nonselective test, in that all types of retinal ganglion cells are able to detect the target. Each participant underwent SAP using the 24-2 program on the Humphrey Field Analyzer II, with the Swedish Interactive Thresholding Algorithm (SITA),33 version 4.1 (Carl Zeiss Meditec, Inc, Dublin, California). The target used in this achromatic test is a small (0.43°) flash of white light presented on a dim background (31.5 apostilbs) for 200 milliseconds.
Short-Wavelength Automated Perimetry
Short-wavelength automated perimetry targets the short-wavelength–sensitive cones and pathway.34 At the ganglion cell level, the response is most likely mediated by the small bi-stratified blue-yellow ganglion cells, which constitute approximately 9% of the total population of retinal ganglion cells.35 Short-wavelength automated perimetry uses a bluish (440-nm wavelength) narrow band target of 1.8° for 200 milliseconds on a bright (100 candelas/m2) yellow background.36 Participants underwent testing with the 24-2 test pattern and with the SITA37 and/or full-threshold (FT) testing strategies (version 4.1), depending on date of enrollment in the study.
Frequency-Doubling Technology Perimetry
Frequency-doubling technology perimetry measures contrast sensitivity. The test is based on the frequency-doubling illusion, which was first described by Kelly38 and later proposed as a sensitive measure of glaucomatous visual field loss.39,40 This illusion occurs when a sinusoidal grating of low spatial frequency undergoes counter-phase flickering at a high temporal frequency. The FDT stimulus targets the magnocellular pathway and is likely detected through flicker-sensitive mechanisms.41,42 Frequency-doubling technology was measured with a visual field instrument (Humphrey Matrix FDT visual field instrument; Carl Zeiss Meditec Inc) using the 24-2 test pattern and the FDT (Welch-Allyn, Skaneateles Falls, New York) and the zippy estimation by sequential testing thresholding algorithm.43 The details of the test have been described elsewhere.44
STATISTICAL ANALYSIS
Patient-specific categorical variables were compared using the Fisher exact test, and continuous variables were compared using the 2-tailed, unpaired t test. The generalized estimating equation45 approach was used to adjust for the possible correlation in measurements between eyes from the same participant when comparing results from diagnostic tests. The generalized estimating equation assumes that variables are normally distributed but is robust to distributional assumptions, particularly given a large sample size. An exchangeable working correlation structure modeled the correlation between eyes. To test the hypothesis that there were differences in visual field test results between AD and ED participants, a contrast for the means from the 2 ancestry groups was computed (1) univariately (without adjustment of any covariate), (2) with a model in which age alone was included as a covariate, and (3) with a model in which age, vertical cup-disc ratio based on stereoscopic photographs, disc size based on Heidelberg retina tomography, central corneal thickness, and presence of high blood pressure were included as covariates. P<.05 was considered statistically significant. Multiple testing corrections were not applied. Statistical analyses were performed in SAS statistical software (version 9.1; SAS Institute Inc, Cary, North Carolina) and R software (version 2.6.2; http://www.r-project.org/).
RESULTS
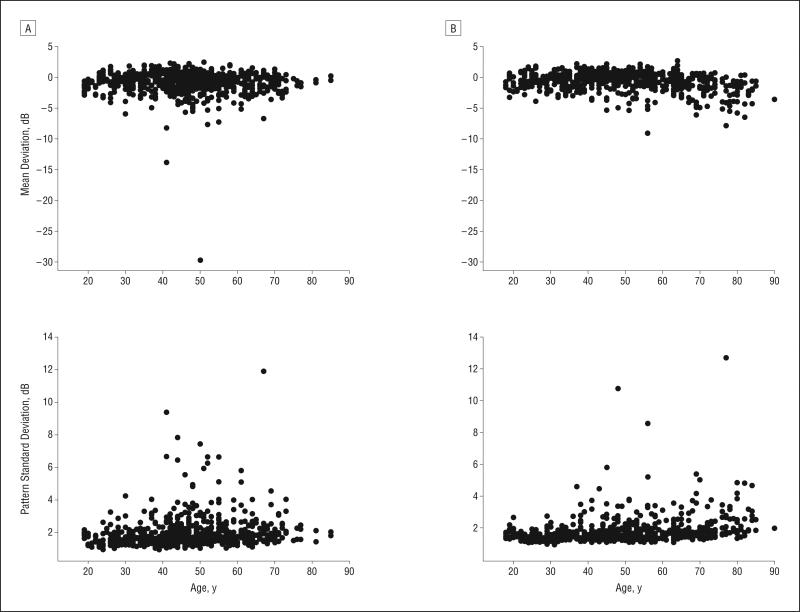
Table 1 shows the demographics of the 2 ancestry groups as well as the ocular measurements and general health conditions. The participants in the AD group (mean [SD] age, 46.2 [13.2] years) were significantly younger than those in the ED group (49.5 [16.6] years) (P=.003). The Figure shows the relationship between age and the MD and PSD within each ancestry group. The relationship between age and MD was more pronounced in the ED group (slope, −0.023) than in the AD group (slope, −0.008) (P=.03). The relationship between age and PSD was similar between the 2 ancestry groups (P=.47). Although the visual field variables included in this study are compared with an age-matched normative database, we applied a statistical correction for age to all results. This was because of (1) the presence of a significant age difference between the groups, (2) significant differences in the relationship between age and MD between the 2 ancestry groups, and (3) the possible association of age with other factors such as media opacities.
Table 1.
Demographics and Ocular and General Health Measurements in Each Ancestry Groupa
| Characteristic | AD Group | ED Group | P Value |
|---|---|---|---|
| Age, y | 46.2 (13.2) | 49.5 (16.6) | .003 |
| Sex, % female | 64.9 | 62.1 | .80 |
| Family history of glaucoma, % | 28.5 | 19.6 | .10 |
| IOP, mm Hg | 15.23 (2.71) | 14.97 (2.61) | .16 |
| Central corneal thickness, μm | 533.83 (33.74) | 551.90 (36.81) | <.001 |
| Vertical cup-disc ratio | 0.45 (0.15) | 0.41 (0.16) | <.001 |
| HRT disc area, mm2 | 2.02 (0.46) | 1.76 (0.39) | <.001 |
| Presence of high blood pressure, % | 30.8 | 20.7 | .004 |
| Presence of diabetes mellitus, % | 5.6 | 2.7 | .14 |
| Presence of heart disease, % | 4.1 | 6.3 | .25 |
Abbreviations: AD, African descent; ED, European descent; HRT, Heidelberg retinal tomography; IOP, intraocular pressure.
Unless otherwise indicated, data are expressed as mean (SD).
Figure.
The relationship between age and mean deviation and pattern standard deviation for the participants of African (A) and European (B) descent.
Only healthy eyes (based on the appearance of the optic disc on stereoscopic photographs) were included in this study, and visual field results were not used for classification purposes. Participants with repeatable visual field defects on SAP were not excluded from the study to avoid biasing the results against function-specific tests. Of the 760 participants included in the study, 158 participants (20.8%) (213 eyes) had repeatable abnormal SAP results (PSD triggered at 5% or worse or GHT results outside normal limits) in at least 1 eye. One hundred twenty-six of these eyes (59.2%) with repeatable abnormal SAP results were AD eyes.
Table 2 shows the results of the comparisons between the AD and ED groups for all visual field variables for each test. The results include all 1478 eyes. The AD group showed significantly worse performance on several measures of visual function on all test types. The results given in Table 2 are derived from the average of 2 visual field tests for each test type. Analyses were also performed on each of the 2 visual fields independently, and similar results were obtained. Finally, analyses were performed independently on the subset of participants with and without repeatable SAP defects to ensure that those participants with SAP defects did not drive the ancestry differences observed. These results are provided in Table 3 and show that several differences exist in those participants without repeatable SAP defects. In this subset, statistically significant differences were observed on PSD for all tests. For SWAP-FT and FDT, the AD group showed worse performance on all variables except for the proportion of abnormal GHT.
Table 2.
Comparison of the AD and ED Groups for Each Visual Field Variable for Each Test
| AD Groupa |
ED Groupa |
P Value |
|||||
|---|---|---|---|---|---|---|---|
| Finding | Median (25th to 75th Percentile) | Finding | Median (25th to 75th Percentile) | 1b | 2c | 3d | |
| SAP-SITA | (n=764) | (n=714) | |||||
| MD, dB | –0.83 (1.86) | –0.59 (–1.57 to 0.24) | –0.65 (1.49) | –0.42 (–1.43 to 0.35) | .13 | .05 | .004 |
| PSD, dB | 1.91 (0.93) | 1.68 (1.44 to 2.02) | 1.79 (0.87) | 1.59 (1.38 to 1.88) | .04 | .003 | .001 |
| Abnormal GHT, % | 6.9 | NA | 4.8 | NA | .15 | .01 | <.001 |
| No. TD <5% | 7.18 (8.85) | 3.50 (1.00 to 10.25) | 6.31 (8.67) | 3.00 (0.50 to 8.50) | .21 | .05 | .001 |
| No. TD <1% | 2.26 (4.98) | 0.50 (0.00 to 2.00) | 1.88 (4.40) | 0.00 (0.00 to 1.50) | .24 | .06 | .004 |
| No. PD <5% | 6.27 (4.94) | 5.00 (3.00 to 8.50) | 5.49 (4.49) | 4.50 (2.00 to 7.50) | .02 | .0003 | <.001 |
| No. PD <1% | 1.90 (3.18) | 1.00 (0.00 to 2.00) | 1.53 (2.58) | 0.50 (0.00 to 1.50) | .06 | .01 | .002 |
| SWAP-FT | (n=391) | (n=360) | |||||
| MD, dB | –4.40 (3.25) | –4.29 (–6.51 to –1.93) | –3.73 (3.32) | –3.50 (–5.90 to –1.39) | .004 | .001 | .02 |
| PSD, dB | 2.98 (0.84) | 2.85 (2.39 to 3.44) | 2.87 (0.79) | 2.77 (2.28 to 3.27) | .06 | .001 | .08 |
| Abnormal GHT, % | 6.6 | NA | 5.8 | NA | .61 | .10 | NA |
| No. TD <5% | 12.20 (12.79) | 8.00 (1.50 to 19.50) | 10.28 (12.33) | 5.00 (0.50 to 16.00) | .02 | .004 | .09 |
| No. TD <1% | 3.76 (6.54) | 1.00 (0.00 to 5.00) | 3.03 (5.56) | 0.50 (0.00 to 3.00) | .09 | .01 | .004 |
| No. PD <5% | 3.04 (3.62) | 2.00 (0.50 to 4.00) | 2.61 (3.07) | 1.50 (0.50 to 4.00) | .08 | .002 | .02 |
| No. PD <1% | 0.78 (1.60) | 0.00 (0.00 to 1.00) | 0.64 (1.20) | 0.00 (0.00 to 1.00) | .16 | .02 | .04 |
| SWAP-SITA | (n=384) | (n=483) | |||||
| MD, dB | –3.90 (3.27) | –3.88 (–5.71 to –1.67) | –3.48 (3.35) | –3.14 (–5.69 to –1.19) | .17 | .02 | .005 |
| PSD, dB | 3.06 (0.96) | 2.91 (2.40 to 3.51) | 2.89 (0.83) | 2.71 (2.28 to 3.35) | .03 | .0001 | .001 |
| Abnormal GHT, % | 6.0 | NA | 5.6 | NA | .84 | .14 | .09 |
| No. TD <5% | 14.60 (13.23) | 12.00 (3.00 to 22.50) | 13.30 (14.17) | 8.00 (1.50 to 22.50) | .32 | .03 | .007 |
| No. TD <1% | 4.74 (7.93) | 1.50 (0.00 to 6.00) | 4.34 (7.57) | 1.00 (0.00 to 5.00) | .57 | .06 | .01 |
| No. PD <5% | 6.48 (5.27) | 5.00 (2.50 to 9.00) | 5.64 (4.49) | 4.50 (2.00 to 8.00) | .07 | .001 | .002 |
| No. PD <1% | 2.10 (3.08) | 1.00 (0.00 to 3.00) | 1.78 (2.50) | 1.00 (0.00 to 2.50) | .24 | .01 | .02 |
| FDT 24-2 | (n=763) | (n=697) | |||||
| MD, dB | –1.69 (2.73) | –1.50 (–3.38 to 0.22) | –0.73 (2.64) | –0.48 (–2.34 to 1.13) | <.001 | <.001 | <.001 |
| PSD, dB | 3.00 (0.78) | 2.81 (2.49 to 3.33) | 2.86 (0.69) | 2.73 (2.43 to 3.12) | .003 | <.001 | <.001 |
| Abnormal GHT, % | 8.8 | NA | 8.9 | NA | .91 | .70 | .30 |
| No. TD <5% | 6.21 (8.12) | 3.00 (0.50 to 8.50) | 4.36 (6.66) | 1.50 (0.00 to 5.50) | <.001 | <.001 | <.001 |
| No. TD <1% | 2.12 (4.03) | 0.50 (0.00 to 2.50) | 1.27 (2.68) | 0.00 (0.00 to 1.00) | <.001 | <.001 | <.001 |
| No. PD <5% | 4.48 (3.98) | 3.50 (1.50 to 6.00) | 3.92 (3.32) | 3.00 (1.50 to 5.50) | .02 | .002 | <.001 |
| No. PD <1% | 1.69 (2.49) | 1.00 (0.00 to 2.00) | 1.30 (1.92) | 0.50 (0.00 to 2.00) | .01 | .001 | <.001 |
Abbreviations: AD, African descent; ED, European descent; FDT, frequency-doubling technology; FT, full-threshold; GHT, glaucoma hemifield test; MD, mean deviation; NA, not applicable; PD, pattern deviation; PSD, pattern standard deviation; SAP, standard automated perimetry; SITA, Swedish Interactive Thresholding Algorithm; SWAP, short-wavelength automated perimetry; TD, total deviation.
The average of the 2 visual fields for each test was used. The number of eyes included for each ancestry group for each test is provided. Unless otherwise indicated, findings are expressed as mean (SD).
Indicates unadjusted.
Indicates adjusted for age.
Indicates adjusted for age, vertical cup-disc ratio, disc size, central corneal thickness, and presence of high blood pressure.
Table 3.
Comparison of the AD and ED Groups for Each Visual Field Variable for Each Test for Participants With and Without Repeatable SAP Defects
| No Repeatable SAP Defectsa |
Repeatable SAP Defectsa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
P Value |
P Value |
|||||||||
| AD Group | ED Group | 1b | 2c | 3d | AD Group | ED Group | 2c | 3d | 1b | |
| SAP-SITA | (n=638) | (n=627) | (n=126) | (n=87) | ||||||
| MD, dB | –0.44 (1.16) | –0.35 (1.14) | .46 | .36 | .11 | –2.78 (3.11) | –2.86 (1.82) | .90 | .90 | .97 |
| PSD, dB | 1.64 (0.34) | 1.59 (0.34) | .05 | .006 | .006 | 3.29 (1.53) | 3.28 (1.69) | .92 | .90 | .96 |
| Abnormal GHT, % | 0 | 0 | NA | NA | NA | 42.1 | 39.1 | .87 | .43 | .06 |
| No. TD <5% | 4.84 (6.04) | 4.38 (6.09) | .55 | .37 | .07 | 19.07 (11.01) | 20.20 (11.44) | .56 | .85 | .87 |
| No. TD <1% | 0.96 (2.10) | 0.86 (2.15) | .69 | .45 | .07 | 8.82 (8.78) | 9.27 (8.02) | .70 | >.99 | .65 |
| No. PD <5% | 4.74 (3.02) | 4.34 (2.95) | .06 | .01 | .01 | 13.98 (5.53) | 13.82 (4.90) | .99 | .53 | .71 |
| No. PD <1% | 0.96 (1.14) | 0.86 (1.08) | .25 | .08 | .07 | 6.68 (5.25) | 6.36 (4.44) | .89 | .44 | .79 |
| SWAP-FT | (n=334) | (n=314) | (n=57) | (n=46) | ||||||
| MD, dB | –4.07 (3.18) | –3.41 (3.13) | .01 | .003 | .005 | –6.33 (3.01) | –5.92 (3.73) | .54 | .98 | .61 |
| PSD, dB | 2.86 (0.74) | 2.75 (0.68) | .04 | .002 | .007 | 3.68 (1.03) | 3.66 (1.03) | .88 | .75 | .61 |
| Abnormal GHT, % | 5.1 | 3.8 | .45 | .13 | .12 | 15.8 | 19.6 | .63 | .94 | .99 |
| No. TD <5% | 10.76 (12.26) | 8.80 (11.27) | .02 | .01 | NA | 20.61 (12.72) | 20.41 (14.48) | .94 | .78 | .96 |
| No. TD <1% | 3.13 (6.19) | 2.37 (4.84) | .07 | .02 | .01 | 7.48 (7.35) | 7.49 (7.76) | .98 | .96 | .78 |
| No. PD <5% | 2.54 (3.17) | 2.23 (2.70) | .18 | .02 | .07 | 5.91 (4.62) | 5.14 (4.13) | .34 | .22 | .95 |
| No. PD <1% | 0.59 (1.33) | 0.47 (0.90) | .17 | .05 | .06 | 1.88 (2.43) | 1.78 (2.10) | .80 | .47 | .89 |
| SWAP-SITA | (n=322) | (n=414) | (n=62) | (n=69) | ||||||
| MD, dB | –3.35 (2.82) | –3.07 (2.98) | .30 | .07 | .03 | –6.74 (3.92) | –5.93 (4.33) | .22 | .12 | .12 |
| PSD, dB | 2.90 (0.77) | 2.77 (0.71) | .09 | .003 | .01 | 3.90 (1.33) | 3.57 (1.10) | .15 | .02 | .05 |
| Abnormal GHT, % | 3.1 | 3.9 | .56 | .73 | .66 | 21.0 | 15.9 | .60 | .31 | .59 |
| No. TD <5% | 12.60 (12.10) | 11.40 (12.75) | .35 | .05 | .02 | 24.94 (14.11) | 24.70 (16.82) | .76 | .58 | .34 |
| No. TD <1% | 3.41 (5.77) | 3.20 (5.84) | .69 | .13 | .06 | 11.61 (12.75) | 11.15 (12.02) | .68 | .26 | .21 |
| No. PD <5% | 5.69 (4.71) | 5.06 (4.02) | .15 | .01 | .02 | 10.62 (6.08) | 9.07 (5.53) | .15 | .02 | .06 |
| No. PD <1% | 1.63 (2.59) | 1.45 (2.11) | .48 | .06 | .13 | 4.56 (4.15) | 3.71 (3.56) | .31 | .05 | .12 |
| FDT 24-2 | (n=638) | (n=613) | (n=125) | (n=84) | ||||||
| MD, dB | –1.45 (2.63) | –0.49 (2.56) | <.001 | <.001 | <.001 | –2.92 (2.92) | –2.52 (2.52) | .14 | .17 | .32 |
| PSD, dB | 2.89 (0.63) | 2.79 (0.57) | .01 | .001 | .002 | 3.54 (1.16) | 3.37 (1.11) | .41 | .11 | .28 |
| Abnormal GHT, % | 5.0 | 7.8 | .06 | .13 | .29 | 28.0 | 16.7 | .09 | .11 | .03 |
| No. TD <5% | 5.48 (7.67) | 3.79 (6.29) | <.001 | <.001 | <.001 | 9.90 (9.31) | 8.49 (7.82) | .13 | .11 | .16 |
| No. TD <1% | 1.74 (3.56) | 1.05 (2.35) | <.001 | <.001 | <.001 | 4.07 (5.47) | 2.92 (4.03) | .07 | .07 | .10 |
| No. PD <5% | 3.91 (3.24) | 3.61 (3.07) | .12 | .04 | .02 | 7.38 (5.76) | 6.14 (4.19) | .12 | .04 | .04 |
| No. PD <1% | 1.35 (1.90) | 1.11 (1.62) | .03 | .01 | .007 | 3.46 (3.96) | 2.71 (3.04) | .21 | .06 | .12 |
Abbreviations: See Table 2.
The average of the 2 visual fields for each test was used. The number of eyes included for each ancestry group for each test is provided. Unless otherwise indicated, findings are expressed as mean (SD).
Indicates unadjusted.
Indicates adjusted for age.
Indicates adjusted for age, vertical cup-disc ratio, disc size, central corneal thickness, and presence of high blood pressure.
Tables 2 and 3 give the results for several visual field variables separately. We also compared the AD and ED groups based on 2 global definitions of visual field abnormality. In 1 case, visual field abnormality was defined as a PSD that was abnormal at 5% or worse, a GHT result outside normal limits, or an MD that was abnormal at 5% or worse. In the other case, visual field abnormality was defined as a PSD that was abnormal at 5% or worse or a GHT result outside normal limits. These results are given in Table 4 for all 1478 eyes. After adjusting for all covariates, regardless of how abnormality was defined, a significantly larger percentage of AD participants had abnormal SAP and SWAP-SITA results compared with ED participants.
Table 4.
Comparison of the Percentage of Abnormal Visual Field Test Results Between the AD and ED Groups
| % of Abnormal Resultsa |
P Value |
||||
|---|---|---|---|---|---|
| Visual Field Test | AD Group | ED Group | 1b | 2c | 3d |
| Based on MD, PSD, or GHT | |||||
| SAP-SITA | 20.4 | 15.5 | .06 | .004 | <.001 |
| SWAP-FT | 30.7 | 26.7 | .18 | .05 | NA |
| SWAP-SITA | 39.8 | 33.7 | .16 | .005 | .003 |
| FDT 24-2 | 19.8 | 18.1 | .50 | .27 | .08 |
| Based on PSD or GHT | |||||
| SAP-SITA | 16.5 | 12.2 | .06 | .003 | <.001 |
| SWAP-FT | 8.4 | 7.5 | .60 | .09 | NA |
| SWAP-SITA | 19.5 | 13.3 | .04 | <.001 | <.001 |
| FDT 24-2 | 14.7 | 14.5 | .98 | .61 | .35 |
Abbreviations: See Table 2.
Visual field abnormality is based on 2 different definitions. The average of the 2 visual fields for each test was used. The numbers of eyes included are identical to those reported in Table 2 (N=1478).
Indicates unadjusted.
Indicates adjusted for age.
Indicates adjusted for age, vertical cup-disc ratio, disc size, central corneal thickness, and presence of high blood pressure.
Site-specific differences in the percentage of abnormal visual field results were investigated within the AD and ED groups. The results are given in Table 5. After adjusting for the effect of all covariates, the only statistically significant site-specific difference was observed for SAP in the AD group. The AD participants with healthy eyes undergoing testing at UAB had a lower percentage of abnormal SAP test results compared with AD participants undergoing testing at UCSD.
Table 5.
Site-Specific Differences in the Percentage of Abnormal Visual Field Results Within the AD and ED Groups for Each Test Type
| AD Group, % of Abnormal Resultsa |
ED Group, % of Abnormal Resultsa |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
P Value |
P Value |
|||||||||||
| Visual Field Test | UCSD (n=159) | UAB (n=311) | NYEE (n=294) | 1b | 2c | 3d | UCSD (n=344) | UAB (n=234) | NYEE (n=136) | 1b | 2c | 3d |
| Based on abnormal MD, PSD or GHT result | ||||||||||||
| SAP-SITA | 30.2 | 13.2 | 22.8 | .001e | .01e | .01f | 23.0 | 6.0 | 13.2 | <.001e | .05 | .20 |
| SWAP-FT | 35.7 | 25.5 | 33.3 | .41 | NA | .16 | 36.3 | 17.4 | 19.1 | .001g | .40 | .55 |
| SWAP-SITA | 43.4 | 33.1 | 43.6 | .16 | .36 | .41 | 41.1 | 17.4 | 26.1 | <.001f | .36 | .64 |
| FDT 24-2 | 22.8 | 17.7 | 20.4 | .50 | .68 | .66 | 22.6 | 12.8 | 16.2 | .02f | .43 | .72 |
| Based on abnormal PSD or GHT result | ||||||||||||
| SAP-SITA | 22.6 | 12.2 | 17.7 | .04f | .34 | .38 | 16.9 | 5.6 | 11.8 | <.001f | .26 | .34 |
| SWAP-FT | 13.1 | 8.3 | 6.0 | .40 | NA | .07 | 10.5 | 3.3 | 7.4 | .04f | .54 | .52 |
| SWAP-SITA | 18.2 | 16.9 | 22.8 | .53 | .85 | .93 | 14.9 | 9.9 | 10.9 | .040 | .44 | .26 |
| FDT 24-2 | 16.5 | 10.6 | 18.0 | .06h | .18 | .40 | 18.3 | 10.3 | 12.5 | .03f | .42 | .68 |
Abbreviations: NYEE, New York Eye and Ear Infirmary; UCSD, University of California, San Diego; UAB, University of Alabama. For other abbreviations, see Table 2.
The average of the 2 visual fields for each test was used. The number of eyes used for SAP at each site is reported.
Indicates unadjusted.
Indicates adjusted for age.
Indicates adjusted for age, vertical cup-disc ratio, disc size, central corneal thickness, and presence of high blood pressure.
Significant difference (P<.05) between UAB and NYEE and between UAB and UCSD.
Significant difference (P<.05) between UAB and UCSD.
Significant difference (P<.05) between UAB and UCSD and between NYEE and UCSD.
Significant difference (P<.05) between UAB and NYEE.
COMMENT
The goal of this study was to investigate differences in visual function between the healthy eyes of AD and ED participants in ADAGES. Significantly worse performance was observed for the AD group compared with the ED group on several visual field variables for each of the tests included in this study. These results suggest that differences in visual function exist between AD and ED participants in the absence of any detectable structural damage to the optic disc based on stereoscopic photography.
In this study, after correcting for all covariates, we found worse performance for AD compared with ED participants for all of the SAP variables evaluated. This is in contrast to the results we previously reported in a small number of healthy eyes27 and is likely due to the greater statistical power achieved in this study. The results of the study reported herein are consistent with those of previous studies that have shown worse performance for AD compared with ED individuals on SAP. The population-based Salisbury Eye Evaluation Project reported that AD individuals missed significantly more points when the central 60° of the visual field was assessed compared with ED individuals.46 This ancestry difference was consistent across all age groups. The Glaucoma Laser Trial reported worse thresholds per test location and a greater number of abnormal test locations in AD compared with ED individuals.20 The Glaucoma Laser Trial also reported worse MD in AD compared with ED individuals.20 In the Ocular Hypertension Treatment Study, worse MD was also reported for AD individuals compared with those of other descents despite an inclusion criterion that required normal and reliable visual field results at baseline.26
This study is, to our knowledge, the first adequately powered study to investigate differences between the healthy eyes of AD and ED individuals on function-specific SWAP and FDT tests. After correcting for all covariates, worse performance was observed in the AD group compared with the ED group for most variables for both SWAP-FT and SWAP-SITA. No ancestry difference was observed in the percentage of abnormal GHT results. A similar trend of ancestry differences was observed for SWAP-FT and SWAP-SITA for each of the visual field variables included in this study. This is consistent with a previous study conducted in our laboratory in which both SWAP algorithms yielded similar sensitivity at set specificity.47 The ancestry differences observed for FDT existed with and without adjustment for the covariates, and the magnitude of these differences tended to be greater than that observed for SAP and SWAP. In a previously published pilot study, our group investigated the ancestry differences in visual function between the healthy eyes of AD and ED individuals.27 That study included SAP, SWAP, and FDT. The only statistically significant difference found was for FDT MD. Frequency-doubling technology may be more sensitive to glaucoma-related ancestry differences than SAP and SWAP. Although statistically significant differences were observed for all tests, these differences are small and arguably not relevant clinically. Their presence in healthy participants is nonetheless of interest for understanding the impact of ancestry on visual function.
The ancestry differences observed in this study may have several causes. First, the tests of visual function used in this study may be detecting very early signs of visual field loss. Follow-up data are needed to determine whether glaucoma will develop in a greater number of AD compared with ED participants. ADAGES is prospectively designed, and follow-up data are being collected on a subset of the healthy participants enrolled in the study. Second, the ancestry differences could be due to the ancestry makeup of the normative databases used for each test of visual function. These databases are composed predominantly of ED participants. It may be that the normal range is different in each ancestry group, resulting in artificially worse performance for AD participants. Third, the ancestry differences reported herein could also be due to different levels of experience in taking tests of visual function between the 2 groups. The AD individuals may have less experience with visual field tests than ED individuals. We believe that experience is unlikely to account for the results we report herein because each participant in the study underwent approximately 6 visual field tests (2 SAP, SWAP, and FDT tests) on each eye, for a total of 12 tests. In addition, participants unfamiliar with taking tests of visual function were given practice tests before data collection. Furthermore, although the results reported herein give the average of 2 tests for each visual field type, we also analyzed the results using the first and second tests independently for each visual field type. If ancestry-based differences in experience played a role in the results we obtained, we would expect to find larger ancestry differences when analyzing the results of the first test for each test type compared with the results of the second test. We found no such difference. In summary, it may not be possible to determine the cause of the differences we observed at this time. These differences may be due to environmental factors, such as socioeconomic status, education level, and mastery of the English language, which was used in the clinical setting; alternatively, the differences could be due to genetic factors such as genetic drift. ADAGES plans to collect environmental and biogeographical data on a subset of its cohort. These data will help determine the cause of the differences observed between the 2 ancestry groups.
The lack of an independent criterion standard for glaucoma is a limitation common to all glaucoma studies. To minimize bias toward any 1 of the tests included in this study, we based our definition of healthy eyes on IOP and on the appearance of the optic disc on stereoscopic photographs but not on visual field results. Consequently, a subset of participants included in this study had visual field defects. The results obtained in 2 separate clinical trials show that, in some patients, visual field defects are detected before the appearance of glaucomatous optic neuropathy. In the Ocular Hypertension Treatment Study, 35% of the patients who reached the study end point showed visual field defects and no optic disc abnormality.48 A similar finding was observed in the European Glaucoma Prevention Study, in which 60% of the patients who reached the study end point showed visual field loss only.49 It is therefore possible that the participants with confirmed visual field defects in our study had glaucoma. To ensure that this group did not drive the ancestry differences we report, we analyzed the results for participants with and without visual field defects separately. Table 3 shows that very few significant differences were observed in the group of participants with visual field defects, whereas the ancestry differences were found in the subset of participants without visual field defects. This supports our finding that small but significant ancestry differences in visual function occur in healthy eyes.
Few site-specific differences in visual function were observed in this study. The only difference was a significantly smaller percentage of abnormal test results in the AD group at the UAB site compared with the UCSD site. This may be due to differences in study population, protocol and procedure adherence, or technicians. Although this is possible, ADAGES guarded against such site-specific differences by using the same study protocol, training, and certification procedures to ensure uniformity across study sites. The recruitment procedures and/or the source population, however, may have differed across sites. It is therefore possible that this site-specific difference in visual function reflects a true difference in the populations tested at these 2 sites. A study by Kosoko-Lasaki et al50 showed that there are statistically significant differences in the prevalence of POAG among populations of the same ancestry. This was true among AD and ED populations. The range of prevalence rates among populations of the same ancestry may be due to mechanisms such as genetic diversity, genetic drift, environmental exposure, and population admixture. Ancestry classification in ADAGES is based on self-report. Although self-report of ancestry is imperfect, several clinical studies have relied on it, and it has been shown to correlate well with genetic admixture techniques.51 Finally, it is possible that the observed site-specific difference was spurious, and it may not be found in longitudinal follow-up data.
In conclusion, after correcting for relevant covariates, several differences in visual function were observed between the AD and ED participants. These ancestry differences were statistically significant but small. Their existence in healthy eyes highlights the importance of carefully monitoring AD individuals, particularly those older than 40 years and with a family history of glaucoma. These small differences should be interpreted in conjunction with clinical data and an assessment of the structural integrity of the optic disc. The differences we observed may be early signs of visual field loss or may be attributed to the lack of ancestry-specific normative databases for each of the visual field tests included in this study. The longitudinal data obtained in ADAGES will be important in determining the nature of these ancestry differences in visual function.
Acknowledgments
Funding/Support: This study was supported by grants U10 EY14267 (Dr Sample), EY08208 (Dr Sample), EY11008 (Dr Zangwill), and EY13959 (Dr Girkin) from the National Eye Institute; by the Eyesight Foundation of Alabama (Dr Girkin); and by grants from Alcon Laboratories, Inc, Allergan, Pfizer, Inc, Merck, Inc, and SANTEN, Inc (for participants’ glaucoma medications).
Footnotes
Group Information: A list of the ADAGES Group investigators was published in Arch Ophthalmol. 2009;127(9):1144.
Financial Disclosure: Dr Liebmann received research support from Carl Zeiss Meditec, Inc, Heidelberg Engineering, Optovue, Inc, and Topcon, Inc, and is a consultant for Alcon, Allergan, and Pfizer; Dr Girkin received research support from Carl Zeiss Meditec, Inc, Heidelberg Engineering, and Optovue, Inc, and is a consultant for Alcon and Allergan; Dr Zangwill received research support from Carl Zeiss Meditec, Inc, Heidelberg Engineering, Optovue, Inc, and Topcon Medical Systems; Dr Medeiros received research support from Alcon, Allergan, Carl Zeiss Meditec, Inc, Pfizer, and Reichert and is a consultant for Alcon, Allergan, Carl Zeiss Meditec, Inc, and Pfizer; Dr Bowd received research support from Lace Elettronica; Dr Weinreb received research support from Carl Zeiss Meditec, Inc, Heidelberg Engineering, Paradigm, and Topcon, Inc, and is a consultant for Carl Zeiss Meditec, Inc, Optovue, Inc, and Topcon, Inc; and Dr Sample received research support from Carl Zeiss Meditec, Inc, Haag-Streit, and Welch-Allyn.
Disclaimer: The sponsors and funding organizations had no role in the design or conduct of this research.
REFERENCES
- 1.Thylefors B, Negrel AD, Pararajasegaram R. Epidemiologic aspects of global blindness prevention. Curr Opin Ophthalmol. 1992;3(6):824–834. doi: 10.1097/00055735-199212000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, Wolfs RC, O'Colmain BJ, et al. Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DS, Jampel HD, Munoz B, West SK. The prevalence of open-angle glaucoma among blacks and whites 73 years and older: the Salisbury Eye Evaluation Glaucoma Study. Arch Ophthalmol. 2006;124(11):1625–1630. doi: 10.1001/archopht.124.11.1625. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325(20):1412–1417. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 6.Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266(3):369–374. [PubMed] [Google Scholar]
- 7.Wilensky JT, Gandhi N, Pan T. Racial influences in open-angle glaucoma. Ann Ophthalmol. 1978;10(10):1398–1402. [PubMed] [Google Scholar]
- 8.Martin MJ, Sommer A, Gold EB, Diamond EL. Race and primary open-angle glaucoma. Am J Ophthalmol. 1985;99(4):383–387. doi: 10.1016/0002-9394(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Honkanen R, et al. Barbados Eye Studies Group. Nine-year incidence of open-angle glaucoma in the Barbados Eye Studies. Ophthalmology. 2007;114(6):1058–1064. doi: 10.1016/j.ophtha.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 10.Leske MC, Wu SY, Hyman L, Nemesure B, Hennis A. Schachat AP; Barbados Eye Studies Group. Four-year incidence of visual impairment: Barbados Incidence Study of Eye Diseases. Ophthalmology. 2004;111(1):118–124. doi: 10.1016/j.ophtha.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Cedrone C, Mancino R, Cerulli A, Cesareo M, Nucci C. Epidemiology of primary glaucoma: prevalence, incidence, and blinding effects. Prog Brain Res. 2008;173:3–14. doi: 10.1016/S0079-6123(08)01101-1. [DOI] [PubMed] [Google Scholar]
- 12.Racette L, Wilson MR, Zangwill LM, Weinreb RN, Sample PA. Primary open-angle glaucoma in blacks: a review. Surv Ophthalmol. 2003;48(3):295–313. doi: 10.1016/s0039-6257(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 13.Girkin CA. Primary open-angle glaucoma in African Americans. Int Ophthalmol Clin. 2004;44(2):43–60. doi: 10.1097/00004397-200404420-00006. [DOI] [PubMed] [Google Scholar]
- 14.Kosoko-Lasaki O, Olivier MM. African American health disparities: glaucoma as a case study. Int Ophthalmol Clin. 2003;43(4):123–131. doi: 10.1097/00004397-200343040-00012. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000;118(6):819–825. doi: 10.1001/archopht.118.6.819. [DOI] [PubMed] [Google Scholar]
- 16.Grant WM, Burke JF., Jr Why do some people go blind from glaucoma? Ophthalmology. 1982;89(9):991–998. doi: 10.1016/s0161-6420(82)34675-8. [DOI] [PubMed] [Google Scholar]
- 17.Hiller R, Kahn HA. Blindness from glaucoma. Am J Ophthalmol. 1975;80(1):62–69. doi: 10.1016/0002-9394(75)90870-3. [DOI] [PubMed] [Google Scholar]
- 18.Wilson R, Richardson TM, Hertzmark E, Grant WM. Race as a risk factor for progressive glaucomatous damage. Ann Ophthalmol. 1985;17(10):653–659. [PubMed] [Google Scholar]
- 19.Wilson MR, Kosoko O, Cowan CL, Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St Lucia, West Indies. Am J Ophthalmol. 2002;134(3):399–405. doi: 10.1016/s0002-9394(02)01585-4. [DOI] [PubMed] [Google Scholar]
- 20.Glaucoma Laser Trial Research Group The Glaucoma Laser Trial (GLT), V: subgroup differences at enrollment. Ophthalmic Surg. 1993;24(4):232–240. [PubMed] [Google Scholar]
- 21.Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106(4):653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA, Tielsch JM, Katz J, Sommer A. Rate of progression in open-angle glaucoma estimated from cross-sectional prevalence of visual field damage. Am J Ophthalmol. 1996;122(3):355–363. doi: 10.1016/s0002-9394(14)72062-8. [DOI] [PubMed] [Google Scholar]
- 23.Smith SD, Katz J, Quigley HA. Analysis of progressive change in automated visual fields in glaucoma. Invest Ophthalmol Vis Sci. 1996;37(7):1419–1428. [PubMed] [Google Scholar]
- 24.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108(11):1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 25.Katz J, Gilbert D, Quigley HA, Sommer A. Estimating progression of visual field loss in glaucoma. Ophthalmology. 1997;104(6):1017–1025. doi: 10.1016/s0161-6420(97)30192-4. [DOI] [PubMed] [Google Scholar]
- 26.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117(5):573–583. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 27.Racette L, Boden C, Kleinhandler SL, et al. Differences in visual function and optic nerve structure between healthy eyes of blacks and whites. Arch Ophthalmol. 2005;123(11):1547–1553. doi: 10.1001/archopht.123.11.1547. [DOI] [PubMed] [Google Scholar]
- 28.Sample PA, Girkin CA, Zangwill LM, et al. African Descent and Glaucoma Evaluation Study Group. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol. 2009;127(9):1136–1145. doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keltner JL, Johnson CA, Levine RA, et al. Normal visual field test results following glaucomatous visual field end points in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2005;123(9):1201–1206. doi: 10.1001/archopht.123.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keltner JL, Johnson CA, Quigg JM, Cello KE, Kass MA, Gordon MO, Ocular Hypertension Treatment Study Group Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2000;118(9):1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 31.Tafreshi A, Sample PA, Liebmann JM, et al. Visual function specific perimetry to identify glaucomatous visual loss using three different definitions of visual field abnormality. Invest Ophthalmol Vis Sci. 2009;50(3):1234–1240. doi: 10.1167/iovs.08-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keltner JL, Johnson CA, Cello KE, et al. Ocular Hypertension Treatment Study Group. Visual field quality control in the Ocular Hypertension Treatment Study (OHTS). J Glaucoma. 2007;16(8):665–669. doi: 10.1097/IJG.0b013e318057526d. [DOI] [PubMed] [Google Scholar]
- 33.Bengtsson B, Olsson J, Heijl A, Rootzen H. A new generation of algorithms for computerized threshold perimetry, SITA. Acta Ophthalmol Scand. 1997;75(4):368–375. doi: 10.1111/j.1600-0420.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 34.Racette L, Sample PA. Short-wavelength automated perimetry. Ophthalmol Clin North Am. 2003;16(2):227–236. vi–vii. doi: 10.1016/s0896-1549(03)00010-5. [DOI] [PubMed] [Google Scholar]
- 35.Dacey DM, Lee BB. The “blue-on” opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367(6465):731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 36.Sample PA, Johnson CA, Haegerstrom-Portnoy G, Adams AJ. Optimum parameters for short-wavelength automated perimetry. J Glaucoma. 1996;5(6):375–383. [PubMed] [Google Scholar]
- 37.Bengtsson B. A new rapid threshold algorithm for short-wavelength automated perimetry. Invest Ophthalmol Vis Sci. 2003;44(3):1388–1394. doi: 10.1167/iovs.02-0169. [DOI] [PubMed] [Google Scholar]
- 38.Kelly DH. Frequency doubling in visual responses. J Opt Soc Am. 1966;56:1628–1633. [Google Scholar]
- 39.Johnson CA, Samuels SJ. Screening for glaucomatous visual field loss with frequency-doubling perimetry. Invest Ophthalmol Vis Sci. 1997;38(2):413–425. [PubMed] [Google Scholar]
- 40.Maddess T. Performance of nonlinear visual units in ocular hypertension and glaucoma. Clin Vis Sci. 1992;7(5):371–383. [Google Scholar]
- 41.Anderson AJ, Johnson CA. Mechanisms isolated by frequency-doubling technology perimetry. Invest Ophthalmol Vis Sci. 2002;43(2):398–401. [PubMed] [Google Scholar]
- 42.White AJ, Sun H, Swanson WH, Lee BB. An examination of physiological mechanisms underlying the frequency-doubling illusion. Invest Ophthalmol Vis Sci. 2002;43(11):3590–3599. [PubMed] [Google Scholar]
- 43.Turpin A, McKendrick AM, Johnson CA, Vingrys AJ. Performance of efficient test procedures for frequency-doubling technology perimetry in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43(3):709–715. [PubMed] [Google Scholar]
- 44.Racette L, Medeiros FA, Zangwill LM, Ng D, Weinreb RN, Sample PA. Diagnostic accuracy of the Matrix 24-2 and original N-30 frequency-doubling technology tests compared with standard automated perimetry. Invest Ophthalmol Vis Sci. 2008;49(3):954–960. doi: 10.1167/iovs.07-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang KY, Zeger SL. Longitudinal data-analysis using generalized linear-models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 46.Rubin GS, West SK, Muñoz B, et al. SEE Project Tea A comprehensive assessment of visual impairment in a population of older Americans: the SEE Study. Invest Ophthalmol Vis Sci. 1997;38(3):557–568. [PubMed] [Google Scholar]
- 47.Ng M, Racette L, Pascual JP, et al. Comparing the full threshold and Swedish interactive thresholding algorithms for short-wavelength automated perimetry. Invest Ophthalmol Vis Sci. 2009;50(4):1726–1733. doi: 10.1167/iovs.08-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713, 829-830. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 49.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I, European Glaucoma Prevention Study (EGPS) Group Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112(3):366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Kosoko-Lasaki O, Gong G, Haynatzki G, Wilson MR. Race, ethnicity and prevalence of primary open-angle glaucoma. J Natl Med Assoc. 2006;98(10):1626–1629. [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg NA, Pritchard JK, Weber JL, et al. Genetic structure of human populations. Science. 2002;298(5602):2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]