Abstract
Remote conditioning induced by ischemia in distant organs protects the heart from ischemia/reperfusion injury however, its effect on ischemia-induced ventricular arrhythmias is unknown. Therefore, we tested the hypothesis that partial hindlimb occlusion during coronary artery occlusion increases the ventricular arrhythmia threshold induced by coronary artery occlusion. Rats (n=7) were instrumented with a radio-telemetry device for recording arterial pressure, ECG and body temperature. A Doppler ultrasonic flow probe and vascular occluder were placed around the terminal aorta. Finally, a snare was placed around the left main coronary artery. The ventricular arrhythmia threshold was determined without, and on an alternate day, during partial hindlimb occlusion (remote conditioning) in conscious rats. Without remote conditioning, the ventricular arrhythmia threshold was 4.56 ± 0.15 min. Importantly, remote conditioning significantly increased the ventricular arrhythmia threshold (6.29 ± 0.49 min) suggesting that ischemia in a distant organ may delay the development of ischemia-induced ventricular arrhythmias.
Keywords: cardiovascular risks, arrhythmia, remote conditioning
INTRODUCTION
Coronary artery occlusion induced sudden cardiac death (SCD) is the major cause of mortality in industrially developed countries 1–4. The majority of SCDs are caused by ischemia-induced ventricular tachy-arrhythmias 5, 6. Despite improvements in cardiac care, most victims of cardiac arrest do not survive to leave the hospital 2. Of the survivors, the 1-yr mortality is 10% or higher with SCD accounting for approximately one-third of the deaths in these patients 7. Thus, ischemia-induced SCD represents a major health concern.
In 1986, Murry, Jennings, and Reimer first described the phenomenon of ischemic preconditioning (brief periods of ischemia interspaced with reperfusion reduced the infarct size of the pre-conditioned heart) 8. Specifically, four brief 5-min periods of ischemia, prior to 40 min of coronary artery occlusion and 72 h of reperfusion, produced a marked reduction in myocardial infarct size in the pre-conditioned area. Thus short periods of non-injurious ischemia/reperfusion applied before a prolonged ischemic insult reduced tissue damage in the pre-conditioned area. The ischemic preconditioning concept was extended in 1993 by Przyklenk and colleagues who documented intra-organ ischemic preconditioning 9. Specifically remote ischemic preconditioning of the left circumflex bed protected the left anterior descending coronary artery bed from infarction during sustained ischemia in this region. Thus a brief period of ischemia in a remote area of the heart reduced the infarct size in a non-preconditioned area after prolonged ischemia/reperfusion. This finding stimulated investigators to examine the cardio-protective effects of ischemic pre-conditioning of organs distant from the heart. These investigations documented that remote ischemic preconditioning induced by ischemia in distant organs protects the heart from ischemia/reperfusion injury 10. For example, remote preconditioning induced by brief periods of limb ischemia using a blood pressure cuff or tourniquet, reduced myocardial infarction by 50% in a porcine model 11. Similarly, brief periods of ischemia of the intestine 12, kidney 13, or limb 11 in advance of a prolonged period of ischemia protects the myocardium and other tissues from ischemia/reperfusion injury 14–17.
It is thought that remote ischemic conditioning protocols mainly prevent tissue damage that occurs during early reperfusion rather than during ischemia 18. This is an important consideration because temporary occlusion of the coronary arteries can lead to potentially lethal arrhythmias that are triggered by the ischemic insult directly or during the reperfusion phase. The mechanism mediating the ventricular arrhythmias during ischemia and reperfusion are related but distinct and ischemia is a more common trigger of sudden death than is reperfusion. Accordingly, the effect of remote conditioning on ischemia-induced ventricular arrhythmias is unknown.
Therefore, we tested the hypothesis that partial hindlimb occlusion during coronary artery occlusion increases the ventricular arrhythmia threshold (VAT) induced by coronary artery occlusion. The VAT was defined as the time from coronary occlusion to sustained ventricular tachycardia resulting in a reduction in arterial pressure. Conscious, chronicallyinstrumented rats were studied to negate the confounding effects of anesthetic agents and surgical trauma.
MATERIALS AND METHODS
Ethical Approval
Experimental preparations and protocols were reviewed and approved by the Animal Care and Use Committee of Wayne State University and complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health 19.
Surgical Procedures
All surgical procedures were performed using aseptic procedures. Male Sprague Dawley rats (n=7, pre-surgical body weight 280 ± 12.6g; pre-sacrifice body weight 365 ± 16g) were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and supplemental doses (10 mg/kg, i.p.) were administered if the rat regained the blink reflex or responded during the surgical procedures. Subsequently, a telemetry device (Data Sciences International PhysioTel C50-PXT; pressure, temperature, and electrocardiogram) was implanted as previously described 20–22 and a catheter was placed in the intra-peritoneal (IP) space for the infusion of fluids. The transmitter body, which contains the thermistor, was placed in the IP space. The pressure sensor of the telemetry device, located within the tip of a catheter, was inserted into the descending aorta for continuous, non-tethered, recording of pulsatile arterial blood pressure. The electrical leads from the telemetry device were placed in a modified lead II configuration by placing the negative electrode slightly to the right of the manubrium and the positive electrode at the anterior axillary line along the 5th intercostal space. In addition, a Doppler ultrasonic flow probe and vascular occluder were positioned around the terminal aorta as previously described 23. A minimum of one week was allowed for the animals to regain their pre-surgical body weight. During the recovery period, the rats were handled, weighed, and acclimatized to the laboratory and investigators.
After recovery, the animals were anesthetized as described above and the hearts were approached via a left thoracotomy through the fourth intercostal space. A coronary artery occluder was made from 5.0-gauge atraumatic prolene suture (8720H, Ethicon Inc), which passed through a PE-50 polyethylene guide tubing (Clay Adams). The suture was positioned around the left main coronary artery 2 to 3 mm from the origin by inserting the needle into the LV wall under the overhanging left atrial appendage and bringing it out high on the pulmonary conus 22, 24–28. The guide tubing with the other end of the occluder was then exteriorized at the back of the neck. The tubing was filled with a mixture of Vasoline and mineral oil to prevent a pneumothorax. At least one week was allowed for recovery. During the recovery periods, the rats were handled, weighed, and acclimatized to the laboratory and investigators.
Experimental Procedures
Susceptibility to Ischemia-induced Ventricular Tachycardia
Conscious, unrestrained rats were studied in their home cages (approximately 13,350 cm3) for all experiments. Rats were allowed to adapt to the laboratory environment for approximately one hour to ensure stable hemodynamic conditions. After the stabilization period, beat by beat, steady state pre-occlusion hemodynamic variables were recorded over 10–15 seconds. Subsequently, the left main coronary artery was temporarily occluded by use of the prolene suture. Specifically, acute coronary artery occlusion was performed by pulling up on the suture that was around the left main coronary artery and manually holding it in place (Figure 1). Rapid changes in arterial pressure and the ECG (peaked T wave followed by S-T segment elevation) occur within seconds of pulling on the suture, documenting coronary artery occlusion. The occlusion was maintained until the onset of ventricular tachycardia but no longer than 8.0 minutes to prevent myocardial damage. Ventricular tachycardia (VT) was defined as sustained ventricular rate (absence of P wave, rapid, wide QRS complexes) greater than 1000 beats/min with a reduction in arterial pressure below 40 mmHg. In the control and remote conditions, all of the rats experienced tachycardia before the 8.0 minute occlusion limit. Normal sinus rhythm appeared upon termination of the occlusion by gently compressing the thorax. Without compressing the thorax, the sustained ventricular tachycardia progresses to ventricular fibrillation. Ventricular fibrillation was defined as a ventricular rhythm without a recognizable QRS complex, in which signal morphology changed from cycle to cycle, and for which it was impossible to estimate heart rate. In the event when the animal did not resume normal sinus rhythm, cardioversion was achieved (after the rat lost consciousness) with the use of 1 shock (10 Joules) of DC current. All rats were successfully resuscitated following the ventricular arrhythmias. The ventricular arrhythmia threshold was defined as the time from coronary artery occlusion to sustained ventricular tachycardia resulting in a reduction in arterial pressure below 40 mmHg (Figure 1).
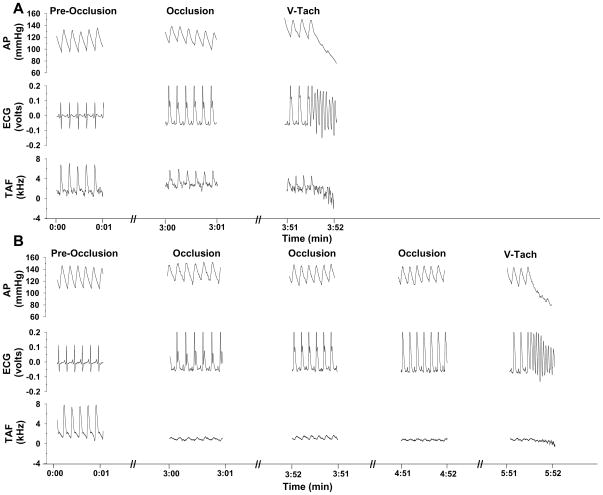
Figure 1.
One second of data at each time period for arterial pressure, ECG, and terminal aortic blood flow velocity before occlusion of the left main coronary artery (pre-occlusion), at 3.5 minutes of coronary artery occlusion and at the onset of ventricular tachycardia in an intact conscious male rat under the control (panel A) and remote (panel B) experimental conditions. Coronary artery occlusion resulted in S-T segment elevation followed by sustained ventricular tachycardia. Coronary artery occlusion in the remote conditioning group was associated with a lower ST segment elevation and a delayed onset of ventricular tachycardia compared to the control group. In this example, the ST segment elevation in the control condition was 0.17 vs 0.08 volts in the remote condition. Furthermore, the ventricular arrhythmia threshold was significantly longer in the control versus the remote conditioning group.
On an alternate day (at least one week later) the protocol was repeated with partial hindlimb occlusion 23. Briefly, coincident with the coronary artery occlusion, the vascular occluder around the terminal aorta was partially occluded reducing flow by 62±9 % and maintained until the induction of sustained ventricular tachycardia. The order of the protocols, control and partial terminal aorta occlusion were randomized.
Determination of Ischemic Zone
After the experiments, the rats were euthanized with an overdose of sodium pentobarbital. To determine the size of the ischemic zone, the heart was excised with the occluder intact and perfused via the aorta with 30 ml of 0.9% saline to wash out the blood. Subsequently, the left main coronary artery was occluded by tying the suture. Evans Blue dye (100μl, 0.5%) was perfused via the aorta, allowing the dye to infuse into the non-ischemic area of the heart, leaving the ischemic regions unstained. The heart was trimmed leaving only the right and left ventricles, rinsed to remove the excess blue dye, and weighed. The heart was trimmed again leaving only the ischemic region. The weight of the ischemic zone was expressed as the percentage of total ventricular weight 22.
To determine if the occlusion produced a myocardial infarction, the heart was sliced transversally into approximately 1.0 mm sections and incubated in a 1% solution TTC (2,3,5-triphenyltetrazolium chloride, Sigma) at 37°C for 20 minutes. The heart sections were placed between two glass slides and immersed in 10% formalin overnight to enhance the contrast of the stain. TTC staining differentiates viable tissue by reacting with myocardial dehydrogenase enzymes to form a red brick stain. Necrotic tissue which has lost its dehydrogenase enzymes does not form a red stain and shows up as pale yellow. This stain has been shown to be a reliable indicator of myocardial infarction 29.
Data Analysis
All recordings were sampled at 2 kHz and the data were expressed as means ± SE. A paired t-test was used to compare the VAT between the control and remote conditioning conditions. A two-factor ANOVA with post hoc Tukey Test was used to compare mean arterial blood pressure, heart rate and terminal aortic blood flow velocity between the two experimental conditions (control and remote conditioning) before occlusion (pre-occlusion) and at 3.5 minutes of the occlusion. This standardized time point was selected to compare identical time point between conditions. This was necessary because the VAT was different between conditions. Pre-occlusion and 3.5 minutes of the occlusion data were the average of every beat during the last 10–15 seconds of the period. Finally, paired t-tests were used to compare ST segment elevation and rate-pressure product in the 2 experimental conditions. The ECGs were analyzed off-line to measure the ST segment elevation (voltage difference between the baseline and J point) using the ECG analysis software for Chart (ADInstruments) 30. The rate-pressure product, an index of myocardial oxygen demand, was calculated as systolic blood pressure × heart rate/100031.
RESULTS
Figure 1 presents one second of data at each time period for arterial pressure, the electrocardiogram, and terminal aortic blood flow velocity before occlusion of the left main coronary artery (pre-occlusion), at 3.5 minutes of coronary artery occlusion and at the onset of ventricular tachycardia in an intact conscious male rat under the control (top panel) and remote (bottom panel) experimental conditions. Coronary artery occlusion resulted in S-T segment elevation followed by sustained ventricular tachycardia (absence of P wave, rapid, wide QRS complexes, zero terminal aortic blood flow velocity and decrease in arterial pressure). Importantly however, coronary artery occlusion during remote conditioning was associated with a lower ST segment elevation and a delayed onset of ventricular tachycardia compared to control. In this example, the ST segment elevation in the control condition was 0.17 versus 0.08 volts in the remote condition. Furthermore, the ventricular arrhythmia threshold was significantly longer in the control versus the remote conditioning condition.
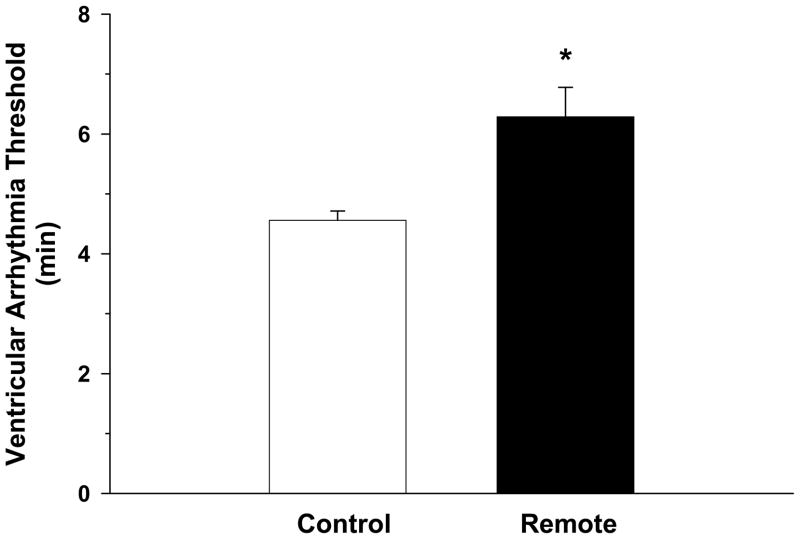
Figure 2 presents the VAT in the control and remote conditioning conditions. The VAT was significantly longer in the remote versus control condition (6.29±0.49 min vs 4.56±0.15 min, respectively). Thus, remote conditioning reduced the susceptibility to ischemia-induced sustained ventricular tachycardia..
Figure 2.
Figure 2 presents the ventricular arrhythmia threshold (VAT) in the control, and remote conditioning conditions. The VAT was significantly longer in the remote versus control condition. Thus, remote conditioning reduced the susceptibility to ischemia-induced sustained ventricular tachycardia.
*P<0.05, Remote vs Control
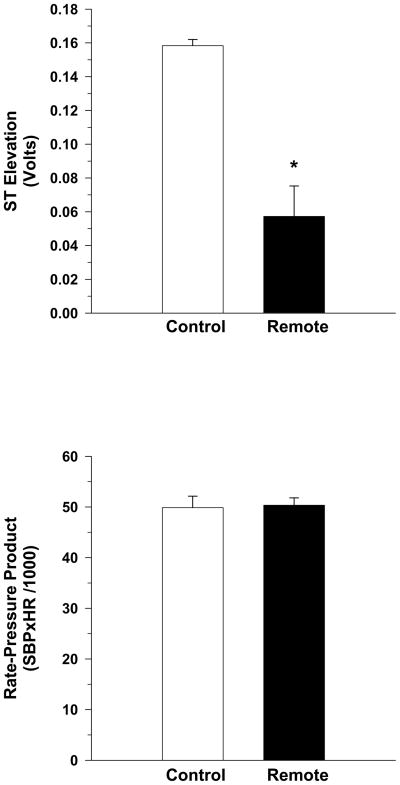
Figure 3 presents the ST-segment elevation (top panel) and rate-pressure product (bottom panel) at 3.5 minutes of occlusion. This standardized time point was selected to compare identical time point between conditions. This was necessary because the VAT was different between the control and remote conditioning conditions. The ST segment elevation was significantly lower in the remote versus control condition (0.06 ± 0.02 vs 0.16 ± 0.004 volts, respectively). There were no differences in RPP between the conditions. Thus, remote conditioning reduced the severity of myocardial ischemia.
Figure 3.
The ST-segment elevation (top panel) and rate-pressure product (bottom panel) at 3.5 minutes of occlusion. The ST segment elevation was significantly lower in the remote versus control group. Thus, remote preconditioning reduced the severity of myocardial ischemia.
*P<0.05, Remote vs Control
Table 1 presents mean arterial pressure, heart rate, and terminal aortic blood flow immediately before the occlusion and at 3.5 minutes of occlusion in the two experimental conditions of control and remote conditioning. There were no differences in mean arterial pressure before the occlusion (pre-occlusion) or at 3.5 minutes of occlusion in the control and remote conditioning experimental conditions (condition effect, P=0.052). In contrast, heart rate was significantly higher at 3.5 minutes of occlusion in both conditions (significant treatment effect with no group by treatment interaction). Furthermore, terminal aortic blood flow was significantly lower at 3.5 minutes of occlusion in both conditions (significant treatment effect) with a significant group by treatment interaction. Post hoc Tukey Test indicated that terminal aortic blood flow was significantly lower at 3.5 minutes of occlusion within the remote conditioning condition. The size of the ischemic zone was 53±3 % and based on the TTC protocol, the occlusion did not cause an infarction in any rat.
TABLE 1.
Mean arterial pressure (MAP), heart rate (HR), and terminal aortic blood flow velocity (TAF) immediately before the occlusion (pre-occlusion) and at 3.5 minutes into the occlusion in conscious rats under two experimental conditions: control and remote conditioning.
| Control | Remote Conditioning | |
|---|---|---|
| Pre-Occlusion | ||
| MAP (mmHg) | 111±2 | 116±3 |
| HR (bpm) | 352±8 | 364±14 |
| TAF (kHz) | 2.3±0.2 | 2.7±0.2 |
| 3.5 Minutes of Occlusion | ||
| MAP (mmHg) | 112±4 | 121±3 |
| HR (bpm) | 412±23* | 377±16* |
| TAF (kHz) | 1.76±0.3* | 1.08±0.3*† |
P<0.05, treatment effect, pre-occlusion vs 3.5 minutes of occlusion
P<0.05, group × treatment interaction, pre-occlusion vs 3.5 minutes of occlusion within Remote Conditioning
DISCUSSION
In this study, we tested the hypothesis that partial hindlimb occlusion (remote conditioning) during coronary artery occlusion increases the ventricular arrhythmia threshold induced by coronary artery occlusion. Specifically, we recorded the VAT induced by myocardial ischemia in conscious rats in the control and partial terminal aortic occlusion (remote conditioning) conditions. The major finding of this study is that the VAT was significantly longer during the remote conditioning versus control (6.29±0.49 min vs 4.56±0.15 min). Thus, remote conditioning reduced the susceptibility to ischemia-induced sustained ventricular tachycardia.
Ischemia induced tachy-arrhythmias that culminate in ventricular fibrillation 6 are the leading cause of death in industrially developed countries 32. Despite the clinical relevance of ischemia-induced arrhythmias, very little information is available regarding remote conditioning protocols to prevent ischemia-induced tachy-arrhythmias. In fact, this is the first study to document the protective effect of remote conditioning [the application of ischemia to a distant vascular area during myocardial ischemia (not reperfusion)] on ventricular tachy-arrhythmias in conscious rats. Although Schmidt and colleagues demonstrated, in anesthetized pigs, that remote conditioning had a tendency to reduce arrhythmias during left anterior artery occlusion it did not reach statistical significance 33. In contrast, there was a significant reduction in the number of episodes of malignant ventricular arrhythmia during reperfusion 33.
It is well documented that remote conditioning reduces myocardial infarct size 12, 13, 34, 35. Gho and colleagues documented that remote preconditioning from the intestine or kidney was abolished by ganglionic blockade with hexamethonium, suggesting the involvement of a neuronal mechanism 34. However, reperfusion of the mesenteric artery was required to achieve protection which also suggests a humoral mechanism for the infarct sparing effects 34. Similarly, mesenteric intra-arterial infusion of bradykinin reduced myocardial infarct size which was abolished by hexamethonium. These data suggested that intestinal ischemia may induce local bradykinin release which activated a neuronal mechanism. However, spillover and transport of bradykinin through the systemic circulation could not be excluded 12. Subsequent studies demonstrated that adenosine and activation of KATP channels are involved in remote preconditioning of the myocardium 13, 36.
Patel and colleagues 15 reported that the opioid antagonist naloxone abolished the infarct sparing effects of remote preconditioning in anesthetized rats. These results extend the well established role of opioids in classic ischemic preconditioning 37 and delayed preconditioning 38. However, it is unclear whether the remote ischemia/reperfusion elicited a local release of opioids which were then transported through the systemic circulation to induce cardioprotection or whether a neuronal mechanism was involved. Thus although adenosine, bradykinin, opioids and activation of KATP channels are involved in remote preconditioning, it is unclear if the protection from one organ to the other is through a neuronal pathway or the systemic circulation.
Changes in the ST-segment shift as well as indirect indices of myocardial oxygen consumption, (tension-time index, double product and triple product) are valid markers of the severity of myocardial ischemia 39–45. We used ST-segment shifts and double product (rate-pressure product, Figure 3) as an index of the severity of myocardial ischemia. Remote conditioning reduced ST segment elevation during coronary artery occlusion. These data document that remote conditioning reduced the severity of myocardial ischemia. However it is important to note that the mechanisms mediating the remote conditioning induced cardio-protection were not examined in this study and remain uncertain. Importantly, Tapuria and colleagues reviewed the underlying mechanisms of remote ischemic conditioning and provided 10 tables of mechanisms with reference for its effects 46. Readers are referred to this complete review for insights on mechanisms 46.
Conclusion
Remote conditioning during coronary occlusion increased the ventricular arrhythmia threshold in conscious rats. It remains to be determined if the protection from one organ to the other is through a neuronal pathway or the systemic circulation. Furthermore, additional research is required to determine if the benefits of remote conditioning on arrhythmias is mediated by the same mechanism as its benefit on infarct size.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute: HL-088615.
Reference List
- 1.Abildstrom S, Køber L, Torp-Pedersen C. Epidemiology of Arrhythmic and Sudden Death in the Chronic Phase of Ischemic Heart Disease. Cardiac Electrophysiology Review. 1999;3:177–9. doi: 10.1023/a:1017935732134. [DOI] [PubMed] [Google Scholar]
- 2.Bigger J., Jr The Predictive Value of RR Variability and Baroreflex Sensitivity in Coronary Heart Disease. Cardiac Electrophysiology Review. 1997;1:198–204. [Google Scholar]
- 3.Gillum RF. Sudden coronary death in the United States: 1980–1985. Circulation. 1989 April;79(4):756–65. doi: 10.1161/01.cir.79.4.756. [DOI] [PubMed] [Google Scholar]
- 4.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998 November 24;98(21):2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 5.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117(1):151–9. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 6.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65(3):457–64. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 7.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999 December 16;341(25):1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 8.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986 November;74(5):1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 9.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993 March;87(3):893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 10.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003 October;83(4):1113–51. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 11.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002 December 3;106(23):2881–3. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 12.Schoemaker RG, van Heijningen CL. Bradykinin mediates cardiac preconditioning at a distance. Am J Physiol Heart Circ Physiol. 2000 May;278(5):H1571–H1576. doi: 10.1152/ajpheart.2000.278.5.H1571. [DOI] [PubMed] [Google Scholar]
- 13.Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998 November;275(5 Pt 2):H1542–H1547. doi: 10.1152/ajpheart.1998.275.5.H1542. [DOI] [PubMed] [Google Scholar]
- 14.Takaoka A, Nakae I, Mitsunami K, et al. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning”. J Am Coll Cardiol. 1999 February;33(2):556–64. doi: 10.1016/s0735-1097(98)00559-2. [DOI] [PubMed] [Google Scholar]
- 15.Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002 October;34(10):1317–23. doi: 10.1006/jmcc.2002.2072. [DOI] [PubMed] [Google Scholar]
- 16.Kharbanda RK, Li J, Konstantinov IE, et al. Remote ischaemic preconditioning protects against cardiopulmonary bypass-induced tissue injury: a preclinical study. Heart. 2006 October;92(10):1506–11. doi: 10.1136/hrt.2004.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005 August 2;46(3):450–6. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Loukogeorgakis SP, Williams R, Panagiotidou AT, et al. Transient limb ischemia induces remote preconditioning and remote postconditioning in humans by a K(ATP)-channel dependent mechanism. Circulation. 2007 September 18;116(12):1386–95. doi: 10.1161/CIRCULATIONAHA.106.653782. [DOI] [PubMed] [Google Scholar]
- 19.Institute of laboratory animals resources Commission of life science. Guide for the care and use of laboratory animals. National Research Council. Washington DC: National Academy Press; 1996. [Google Scholar]
- 20.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol. 2002 October;283(4):1734–9. doi: 10.1152/ajpheart.00253.2002. [DOI] [PubMed] [Google Scholar]
- 21.Rodenbaugh DW, Collins HL, Nowacek DG, DiCarlo SE. Increased susceptibility to ventricular arrhythmias is associated with changes in Ca2+ regulatory proteins in paraplegic rats. Am J Physiol Heart Circ Physiol. 2003 December;285(6):2605–13. doi: 10.1152/ajpheart.00319.2003. [DOI] [PubMed] [Google Scholar]
- 22.Collins HL, DiCarlo SE. Acute Exercise Increases the Ventricular Arrhythmia Threshold via the Intrinsic Adenosine Receptor System in Conscious Hypertensive Rats. Am J Physiol Heart Circ Physiol. 2005;289(3):1020–6. doi: 10.1152/ajpheart.00156.2005. [DOI] [PubMed] [Google Scholar]
- 23.Collins HL, DiCarlo SE. Cardiac afferents attenuate the muscle metaboreflex in the rat. J Appl Physiol. 1993;75:114–20. doi: 10.1152/jappl.1993.75.1.114. [DOI] [PubMed] [Google Scholar]
- 24.Lujan HL, DiCarlo SE. T5 spinal cord transection increases susceptibility to reperfusion-induced ventricular tachycardia by enhancing sympathetic activity in conscious rats. Am J Physiol Heart Circ Physiol. 2007 December;293(6):H3333–H3339. doi: 10.1152/ajpheart.01019.2007. [DOI] [PubMed] [Google Scholar]
- 25.Lujan HL, Kramer VA, DiCarlo SE. Electro-acupuncture Decreases the Susceptibility to Ventricular Tachycardia in Conscious Rats by Reducing Cardiac Metabolic Demand. Am J Physiol Heart Circ Physiol. 2007 January 5;292(5):2550–5. doi: 10.1152/ajpheart.00979.2006. [DOI] [PubMed] [Google Scholar]
- 26.Lujan HL, Kramer VJ, DiCarlo SE. Sex Influences the Susceptibility to Reperfusion-Induced Sustained Ventricular Tachycardia and Beta-adrenergic Receptor Blockade in Conscious Rats. Am J Physiol Heart Circ Physiol. 2007;293(5):2799–808. doi: 10.1152/ajpheart.00596.2007. [DOI] [PubMed] [Google Scholar]
- 27.Lujan HL, Britton SL, Koch LG, DiCarlo SE. Reduced Susceptibility to Ventricular Tachyarrhythmias in Rats Selectively Bred for High Aerobic Capacity. Am J Physiol Heart Circ Physiol. 2006;291:H2933–H2941. doi: 10.1152/ajpheart.00514.2006. [DOI] [PubMed] [Google Scholar]
- 28.Collins HL, Loka AM, DiCarlo SE. Daily exercise-induced cardioprotection is associated with changes in calcium regulatory proteins in hypertensive rats. Am J Physiol Heart Circ Physiol. 2005 February;288(2):532–40. doi: 10.1152/ajpheart.00873.2004. [DOI] [PubMed] [Google Scholar]
- 29.Fishbein MC, Meerbaum S, Rit J, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101(5):595–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 30.Smith SW. ST segment elevation differs depending on the method of measurement. Acad Emerg Med. 2006 April;13(4):406–12. doi: 10.1197/j.aem.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972 April;32(4):516–22. doi: 10.1152/jappl.1972.32.4.516. [DOI] [PubMed] [Google Scholar]
- 32.Murray CJL, Lopez AD. Alternative projection of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt MR, Smerup M, Konstantinov IE, et al. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007 April;292(4):H1883–H1890. doi: 10.1152/ajpheart.00617.2006. [DOI] [PubMed] [Google Scholar]
- 34.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996 November 1;94(9):2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 35.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997 September 2;96(5):1641–6. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 36.Schulz R, Cohen MV, Behrends M, Downey JM, Heusch G. Signal transduction of ischemic preconditioning. Cardiovasc Res. 2001 November;52(2):181–98. doi: 10.1016/s0008-6363(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 37.Fryer RM, Hsu AK, Nagase H, Gross GJ. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J Pharmacol Exp Ther. 2000 August;294(2):451–7. [PubMed] [Google Scholar]
- 38.Fryer RM, Hsu AK, Eells JT, Nagase H, Gross GJ. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ Res. 1999 April 16;84(7):846–51. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- 39.Leesar MA, Stoddard M, Ahmed M, Broadbent J, Bolli R. Preconditioning of human myocardium with adenosine during coronary angioplasty. Circulation. 1997 June 3;95(11):2500–7. doi: 10.1161/01.cir.95.11.2500. [DOI] [PubMed] [Google Scholar]
- 40.Baller D, Bretschneider HJ, Hellige G. A critical look at currently used indirect indices of myocardial oxygen consumption. Basic Res Cardiol. 1981 March;76(2):163–81. doi: 10.1007/BF01907955. [DOI] [PubMed] [Google Scholar]
- 41.Maroko PR, Radvany P, Braunwald E, Hale SL. Reduction of infarct size by oxygen inhalation following acute coronary occlusion. Circulation. 1975 September;52(3):360–8. doi: 10.1161/01.cir.52.3.360. [DOI] [PubMed] [Google Scholar]
- 42.Matsuki T, Shoji T, Yoshida S, et al. Sympathetically induced myocardial ischaemia causes the heart to release plasma kinin. Cardiovasc Res. 1987 June;21(6):428–32. doi: 10.1093/cvr/21.6.428. [DOI] [PubMed] [Google Scholar]
- 43.Hartman JC, Wall TM, Hullinger TG, Shebuski RJ. Reduction of myocardial infarct size in rabbits by ramiprilat: reversal by the bradykinin antagonist HOE 140. J Cardiovasc Pharmacol. 1993 June;21(6):996–1003. doi: 10.1097/00005344-199306000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Martorana PA, Kettenbach B, Breipohl G, Linz W, Scholkens BA. Reduction of infarct size by local angiotensin-converting enzyme inhibition is abolished by a bradykinin antagonist. Eur J Pharmacol. 1990 July 3;182(2):395–6. doi: 10.1016/0014-2999(90)90301-l. [DOI] [PubMed] [Google Scholar]
- 45.Leesar MA, Stoddard MF, Manchikalapudi S, Bolli R. Bradykinin-induced preconditioning in patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999 September;34(3):639–50. doi: 10.1016/s0735-1097(99)00297-1. [DOI] [PubMed] [Google Scholar]
- 46.Tapuria N, Kumar Y, Habib MM, Abu AM, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res. 2008 December;150(2):304–30. doi: 10.1016/j.jss.2007.12.747. [DOI] [PubMed] [Google Scholar]