Abstract
The Compound I derivative of cytochrome P450 119 (CYP119) was produced by laser flash photolysis of the corresponding Compound II derivative, which, in turn, was prepared by reaction of the resting enzyme with peroxynitrite. The UV-visible spectrum of the Compound I species contains an asymmetric Soret band that can be resolved into overlapping transitions centered at ca. 367 and 416 nm and a Q-band with λmax ≈ 650 nm. Reactions of the Compound I derivative with organic substrates gave epoxideized (alkene oxidations) and hydroxylated (C-H oxidations) products as demonstrated by product studies and oxygen-18 labeling studies. The kinetics of oxidations by CYP119 Compound I were measured directly; the reactions included hydroxylations of benzyl alcohol, ethylbenzene, Tris buffer, lauric acid, and methyl laurate, and epoxidations of styrene and 10-undecenoic acid. Apparent second-order rate constants, equal to the product of the equilibrium binding constant (Kbind) times the first-order oxidation rate constant (kox), were obtained for all substrates. The oxidations of lauric acid and methyl laurate displayed saturation kinetic behavior, which permitted solution of both Kbind and kox for these substrates. The unactivated C-H positions of lauric acid reacted with a rate constant of kox = 0.8 s−1 at room temperature. The CYP119 Compound I derivative is more reactive than model Compound I species, iron(IV)-oxo porphyrin radical cations, and similar in reactivity to the Compound I derivative of the heme-thiolate enzyme chloroperoxidase. Kinetic isotope effects (kH/kD) for oxidations of benzyl alcohol and ethylbenzene were small, reflecting the increased reactivity of the Compound I derivative in comparison to models. Nonetheless, CYP119 Compound I apparently is much less reactive than the oxidizing species formed in the P450cam reaction cycle. Competition kinetic studies employing CYP119 activated by hydrogen peroxide indicate that the same oxidizing transient is formed in the photochemical reaction and in the hydrogen peroxide shunt reaction.
The ubiquitous cytochrome P450 (CYP or P450) enzymes are heme-containing enzymes with thiolate from protein cysteine as the fifth ligand to iron.1 P450s serve in several catalytic roles in nature, but the major function is to catalyze oxidation reactions, typically via two-electron, oxo-transfer processes. In Man, the P450s perform both highly specific reactions, such as oxidation of androgens to estrogens, and broad-spectrum oxidations of drugs, pro-drugs, and xenobiotics in the liver.2 Much of the interest in P450s derives from the pharmaceutical impact of these enzymes and their relationships to disease states, including cancers and liver disease, that result from over-expression of P450s.3–5
Exceptionally high reactivity is displayed by some P450s in their ability to oxidize unactivated C-H bonds in substrates, and the nature of the P450 transient involved in the oxidation steps has been a subject of study since soon after the initial discoveries of P450s in the 1960s. Other heme-containing enzymes, such as chloroperoxidase and horseradish peroxidase react with hydrogen peroxide to convert the ferric forms of the enzymes to iron(IV)-oxo porphyrin radical cations that are termed “Compound I” derivatives,6,7 and it has long been assumed that the activated transient in a P450 enzyme that effects oxidation reactions is a Compound I species.1
The oxygen-containing transients in P450s typically are formed by a reaction sequence unlike that in the peroxidases (Figure 1).8–10 Following substrate binding, the ferric enzyme is reduced, molecular oxygen binds reversibly, and a second reduction occurs to give the peroxoiron species. Protonation of this species on the distal oxygen gives the hydroperoxyiron intermediate, which is the last well characterized transient in the P450 reaction sequence, observed by EPR and ENDOR spectroscopies when cryogenic conditions were employed.11,12 A second protonation on the distal oxygen and loss of water by heterolytic fragmentation of the O-O bond would give an iron-oxo species with the iron atom in the formal +5 oxidation state, either a transient perferryl-oxo species that can relax to a Compound I derivative or a Compound I derivative directly. The perferryl-oxo species or the Compound I derivative can react in two-electron, oxo transfer reactions to return the ferric enzyme.
Figure 1.
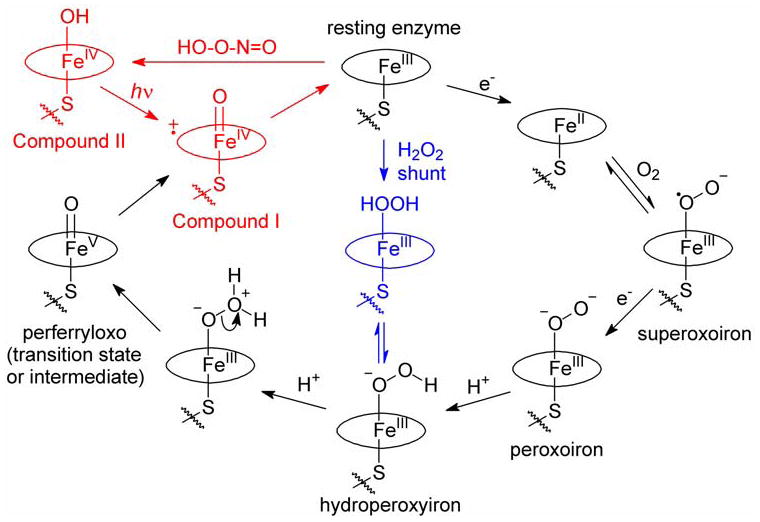
Reaction cycles for P450 enzymes. The normal reaction cycle is shown in black, and the shunt reaction pathway is shown in blue. The sequence of reactions used to produce Compound I in this work is the red pathway. Note that the Compound II derivative is formulated as a ferryl-hydroxy species based on recent XAS studies of the CYP119 Compound II species (ref 34).
Peroxidase enzymes form Compound I species by reactions with hydrogen peroxide,7 and P450s also can react with hydrogen peroxide or other hydroperoxy compounds to give an active oxidant in what is termed a “shunt” reaction (Figure 1). Fast mixing, stopped-flow mixing, and freeze-quench mixing experiments with P450s and peroxy species have been attempted for many years13 with limited success. A short-lived transient with a calculated spectrum resembling that of chloroperoxidase Compound I was detected in reactions of P450cam (CYP101) with m-chloroperoxybenzoic acid (mCPBA),14,15 but freeze-quench studies of the same enzyme with either mCPBA or peroxyacetic acid oxidation employing EPR, ENDOR and Mössbauer spectroscopic analyses indicated that a Compound I derivative did not accumulate to a detectable level.16–19 Moreover, production of various iron-oxo transients under “cryo-reduction” conditions suggested that the active oxidant in P450cam reacts with substrate faster than its rate of formation, which likely precludes its detection in mixing studies.11,12 Despite the outcome of attempted P450cam oxidations with mCPBA, reactions of another P450 enzyme, CYP119, with mCPBA gave promising results in that a short-lived transient with a calculated spectrum similar to that of CPO Compound I was formed;20 these results are discussed in detail below.
The difficulties in observing a Compound I derivative of a P450 enzyme in fast mixing studies prompted us to explore an alternative entry to these intermediates that has much shorter temporal resolution than mixing. In principle, photolyses of iron(IV)-oxo neutral porphyrin complexes, so-called Compound II derivatives, could give Compound I derivatives by photo-ejection of an electron from the porphyrin macrocycle, and laser flash photolysis (LFP) methods with photo-multiplier detection would permit sub-microsecond temporal resolution. In practice, we found that photolyses of Compound II derivatives of a model iron-porphyrin complex, horseradish peroxidase, and myoglobin gave Compound I derivatives.21 Extension of the photo-oxidation method for production of a Compound I derivative of the CYP119 enzyme was subsequently reported in a communication.22 In the present report, we detail the spectrum and kinetic studies of the CYP119 Compound I derivative.
Results
The enzyme used in this study was cytochrome P450 119 (CYP119), which was expressed in E. coli and purified as previously described.22–24 The samples of CYP119 employed were high purity as determined by the R/Z values of 1.5 or greater, where R/Z is the ratio of absorbances at 416 nm and 280 nm. CYP119 was originally thought to derive from the thermophile Sulfolobus solfataricus, but a recent study indicates that it is from the closely related organism S. acidocaldarius that contaminated an S. solfataricus culture.25
Formation of a Compound II derivative by peroxynitrite oxidation of CYP119
The photo-oxidation method for production of a Compound I derivative requires that a Compound II derivative first be produced. The only report of formation of a true Compound II derivative of a P450 enzyme prior to our work was the claim that peroxynitrite (PN) reacted with cytochrome P450BM3 (CYP102) to give the corresponding Compound II derivative as a transient and eventually deactivated the enzyme by nitration of a tyrosine residue.26 Compound II derivatives of other heme-containing enzymes were known to be formed by treatment of the resting ferric enzymes with PN,27–31 and chloroperoxidase (CPO), which is a heme-thiolate enzyme closely related to the P450s, also gives a transient Compound II derivative upon reaction with PN.22,26,32
In a communication, we reported that CYP119 was oxidized to the corresponding Compound II derivative by PN.22 Subsequent to that publication, Green and co-workers reported that CYP102 did not give the Compound II derivative upon reaction with PN but instead gave a nitrosyl complex.33 The implications in the recent paper33 were that the UV-visible spectral changes were incorrectly interpreted in the original CYP102 study26 and that PN does not oxidize P450 enzymes to Compound II derivatives. The dramatically different interpretations26,33 for the outcome of the PN reaction with CYP102 suggest that further study of this system is in order, especially since a short-lived transient was detected in both works (life-times of a few seconds or less), whereas nitrosyl complexes of heme proteins typically are quite stable.
For the CYP119 enzyme, the formation of the Compound II derivative by reaction with PN was further demonstrated with X-ray absorption spectroscopy (XAS) studies of the Compound II species that gave a detailed picture of the bonding to iron in this derivative.34 The edge and near-edge features in the XANES spectrum supported an iron(IV) oxidation state, and the EXAFS spectrum gave bond lengths to iron similar to those found for the CPO Compound II species,35 including a long Fe-O bond (1.82 Å) implicating a protonated ferryl oxygen species, Fe(IV)-OH.34 In the XAS project,34 an authentic sample of the nitrosyl complex of CYP119 was prepared and studied for comparison to the Compound II derivative. A long-lived transient (lifetime of hours) was formed either by reaction of the resting enzyme with NO gas or with the NO donor diethylamine NONOate (diethylamine diazeniumdiolate), and XAS spectra of the NO complex differed from those of Compound II. In the X-ray beam at < 140 K, photoreduction of the CYP119-NO complex was observed with multi-minute lifetimes, whereas the CYP119 Compound II derivative showed no apparent photoreduction after hours of irradiation under the same conditions, and we concluded that the Compound II derivative contained essentially no nitrosyl adduct as a contaminant.34
In the kinetic studies reported in this work, CYP119 was treated with 20–25 equivalents of PN. Excess PN is required for efficient conversion of the enzyme to the Compound II derivative because PN, which is prepared and stored in basic solutions, is protonated in buffer solutions to give peroxynitrous acid that rapidly decomposes in an acid-catalyzed process to give nitrite and nitrate.36–39 The CYP119 enzyme also catalyzes the decay of PN, similar to other heme-containing enzymes.22,28,31,32 Figure S1A in Supporting Information shows the spectra of CYP119 resting enzyme and the Compound II derivative, and Figure S1B in Supporting Information shows typical results from monitoring the signal at 429 nm (λmax for the Compound II derivative) with time in two types of detection conditions. The Compound II derivative formed in less than 2 seconds, and PN decayed fully in less than 10 seconds.
The CYP119 that was present after decay of the Compound II species was spectroscopically indistinguishable from the original enzyme, but it was possible that the enzyme was altered by the PN treatment. For example, PN treatment results in nitration of a tyrosine residue in CYP102.26 As a control reaction for our work, we exposed CYP119 to PN at a concentration typical for the LFP studies, allowed the PN to decay, and then conducted a shunt reaction (oxidation of styrene using hydrogen peroxide as a sacrificial oxidant). The yield of styrene oxide in this reaction was the same as when untreated CYP119 was used as discussed later, and, thus, the CYP119 behaved the same before and after PN treatment. This study does not prove that CYP119 was unaltered by PN, but it does show that the reactivity of the enzyme was not affected by the PN treatment at the concentrations used in our studies.
In summary for this section, the reaction of CYP119 with PN at ambient temperature rapidly formed the Compound II derivative. This species persisted when excess PN was present and decayed with a rate constant of ca. 0.5 s−1 at ambient temperature after the PN was depleted. After the PN treatment and decay of the Compound II species back to ferric enzyme, the UV-visible spectrum of enzyme was indistinguishable from that of the original enzyme, and the catalytic chemistry of the CYP119 enzyme when activated in a hydrogen peroxide shunt reaction was unaffected.
Laser flash photolysis generation of CYP119 Compound I
The apparatus used for the LFP studies was a commercial kinetic unit affixed with a stopped-flow mixing unit that permitted mixing on the ms time scale. Samples were delivered to a sample cell that could be irradiated with laser light from the bottom and analyzed by an analyzing beam from one side. The third harmonic of a Nd-YAG laser (355 nm) was used with nominal power at the reaction cell of 4 mJ delivered in 7 ns. Spectra were acquired with either a diode array detector with 1.2 ms minimum integration time or photo-multiplier (PM) tubes with ca. 2 ns rise times. The analysis beam was produced by a 150 W xenon-lamp under constant power for long detection times or pulsed to high power for short (<0.1 ms) detection times.
The CYP119 Compound II derivative is photo-labile, and its decay under constant irradiation from the analysis beam was noticeably faster when it was irradiated with a full spectrum of UV-visible light as opposed to low intensity monochromatic light. Nonetheless, some diode array experiments were conducted with a full range of light in order to characterize the spectra of the transients. In all studies using PM tubes, the monochromator was placed before the sample cell to reduce the exposure of the sample to light.
Figure 2 shows the difference spectrum (dashed line) obtained from irradiating samples containing CYP119 Compound II with 355 nm laser light. This spectrum, which was formed in the 7 ns time span of the laser pulse, contains a bleached region where the absorbance of the Compound II derivative was stronger than the absorbance of the product, and a growth region where the product of the photochemical reaction absorbed more strongly than the Compound II derivative. The difference spectrum is quite similar to those observed when the Compound II derivatives of a model porphyrin-iron complex, horseradish peroxidase, and myoglobin were irradiated with 355 nm light to give the corresponding Compound I derivatives.21 Specifically, in each of those cases, negative peaks were red-shifted from positive peaks because the absorbance maximum for the Soret band of the Compound II species was at longer wavelength than that of the Compound I species.
Figure 2.
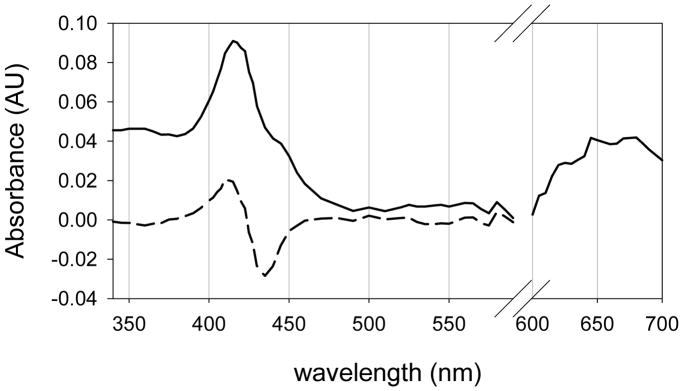
UV-visible spectrum of CYP119 Compound I. The dashed line is the observed difference spectrum from LFP irradiation of the CYP119 Compound II species. A scaled spectrum of CYP119 Compound II was added to the difference spectrum to produce the spectrum for the Compound I derivative. The Q-band region from 600 to 700 nm is an amplification of an observed difference spectrum with no correction for bleaching.
Addition of a normalized spectrum of the CYP119 Compound II derivative to the difference spectrum in Figure 2 compensated for the destroyed Compound II species and gave a spectrum of the Compound I derivative of CYP119 (solid line). The spectrum consists of a broad Soret band comprised of two overlapping peaks, the smaller one at ca. 360–370 nm, and the larger one at ca. 416 nm, and a broad Q-band in the range 600–700 nm that is highly characteristic of a porphyrin radical cation.40
The position of the Q-band absorbance for the CYP119 Compound I species was expected,40 but that for the absorbance of the Soret band is surprising. P450 Compound I derivatives are predicted to have a Soret band absorbance with λmax = 367 nm based on the UV-visible spectral results for the heme-thiolate enzyme chloroperoxidase (CPO). The UV-visible spectrum of CPO-I, first reported in 1980,41 has a highly asymmetric Soret band. Egawa and coworkers later demonstrated that this band can be resolved into two overlapping symmetric peaks.42
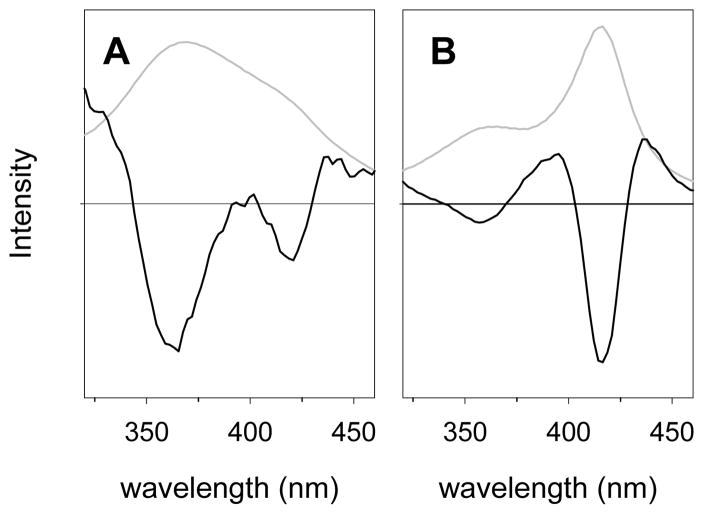
The second derivative of the Soret band spectrum provides another demonstration that the “peak” of the Soret band of CPO-I is actually comprised of overlapping peaks. Figure 3A shows a spectrum of CPO-I from a previous study in our laboratory43 and its second derivative spectrum, which clearly demonstrates that two peaks are present by the two minima observed. The minima of the negative peaks in the second derivative spectrum are slightly displaced from the maxima of the individual peaks (short wavelength band to shorter wavelength and long wavelength band to longer wavelength) by about 2 nm from the values that were used by Egawa and co-workers for their simulation,42 which is an artifact of the derivitization. The CYP119 Compound I Soret band also contains two peaks, which are apparent in the spectrum and even more obvious in the second derivative spectrum (Figure 3B). The two peaks in the Soret bands for the Compound I derivatives in CPO and CYP119-I have similar λmax values, but the relative intensities are reversed in the two spectra. From this type of analysis, the Soret bands of CPO Compound I and CYP119 Compound I are seen to be quite similar.
Figure 3.
Absorbance spectra (grey lines) and second derivative spectra (black lines) for the Soret bands in the Compound I derivative of (A) CPO and (B) CYP119. The scales are arbitrarily adjusted to show the signals. The horizontal lines are at zero intensity.
No P450 Compound I spectrum was measured previously, but P450 Compound I spectra have been calculated from deconvolution of spectral data obtained in rapid mixing studies. Egawa and co-workers reported results from rapid mixing of m-chloroperoxybenzoic acid (mCPBA) and P450cam in 1994,14 and similar studies were conducted with CYP119 by Sligar and co-workers in 2002.20 In both studies, the spectra for the Soret band of the Compound I derivatives calculated from the composite data using global analysis methods were highly asymmetric peaks with λmax ≈ 367 nm, similar to that of CPO-I. The deconvolution methods often cannot give unique simulations, but instead give variable answers as a function of the initial guesses for the rate constants. In such a case, one must select the “correct” spectrum from the possibilities, which, in this case, involves evaluation of the results using the known spectrum of CPO-I as a guide.40
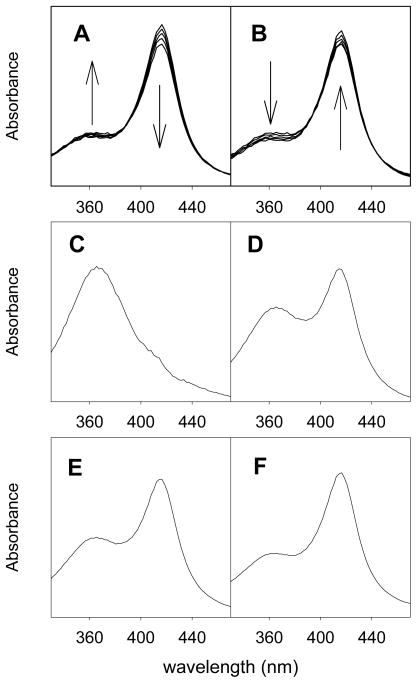
We repeated the stopped-flow mixing study20 of CYP119 with mCPBA at 4 °C and obtained the results shown in Figure 4. For the first 65 ms of the reaction, the signal centered at λ ≈ 367 nm grew slightly and the signal centered at λ ≈ 416 nm decreased slightly (Figure 4A). After 65 ms, the signals returned to the original absorbances of the resting enzyme (Figure 4B). The spectral file (800 spectra) was deconvoluted by global analysis using the model of A + B → C with second-order rate constant kAB and C → A with first-order rate constant kC, where A is the CYP119 enzyme, B is mCPBA, and C is the Compound I derivative of CYP119. We used the same software and kinetic model as used in the previous study.20 Examples of simulated spectra of CYP119 Compound I resulting from four deconvolutions with different initial guesses for the kinetic values are shown in Figure 4, and more spectra are reported in the Supporting Information where we show 12 sets of spectra and their residuals. We emphasize that no constraints were employed in these deconvolutions.
Figure 4.
Results from stopped-flow mixing of 14 μM CYP119 with 8.6 μM mCPBA at 4 °C. (A) Observed spectra at 1.0, 7.0, 13, 26, and 65 ms after mixing. (B) Observed spectra at 65, 180, 290, 390, and 700 ms after mixing. (C–F) Simulated spectra of the intermediate as found from unconstrained minimizations with different initial guesses for the rate constants. The program-derived final values for kC in the deconvolutions are 17.4 s−1 (C), 9.2 s−1 (D), 6.7 s−1 (E), and 2.3 s−1 (F). The Supporting Information has more information.
As noted earlier, the Soret band of CYP119 Compound I can be resolved into two peaks, one centered at 367 nm and the other at 416 nm. The deconvolutions weight these peaks differently depending on the values for the rate constants. A simulated spectrum associated with kC = 17.4 s−1 (Figure 4C) is essentially the same as the simulated spectrum for CYP119 Compound I reported previously20 and is similar to the CPO Compound I spectrum.41 As the value of kC decreases, the simulated spectra display an increasing component of the 416 nm peak and a decreasing component of the 367 nm peak. Eventually, when kC = 2.3 s−1 (Figure 4F), the simulated spectrum closely matches the experimental spectrum for the CYP119 Compound I species (cf. Figure 2). Importantly, a kC value of 2 s−1 for decay of the transient at 4 °C is consistent with the measured rate constant for decomposition of the CYP119 Compound I derivative in the absence of substrate, whereas a decay rate constant of 17 s−1 is too large (see below).
In summary for this section, laser flash photolysis of the CYP119 Compound II derivative gave the CYP119 Compound I derivative. The experimental spectrum of this species was obtained from the LFP experiments using the difference spectrum from before and immediately after the flash. Stopped-flow mixing results for oxidation of CYP119 with mCPBA gave the same calculated UV-visible spectrum for the Soret band of CYP119 Compound I as obtained in the LFP experiments when an appropriate rate constant for decay of this transient was found in the spectral deconvolution. The Soret band absorbance of CYP119 Compound I contains two overlapping transitions, much like the Soret band of CPO Compound I,42 but with a different ratio of the component peaks. The Q-band displays a broad, weak absorbance between 600 and 700 nm as expected for a porphyrin radical cation structure.40
Kinetics of CYP119 Compound I reactions
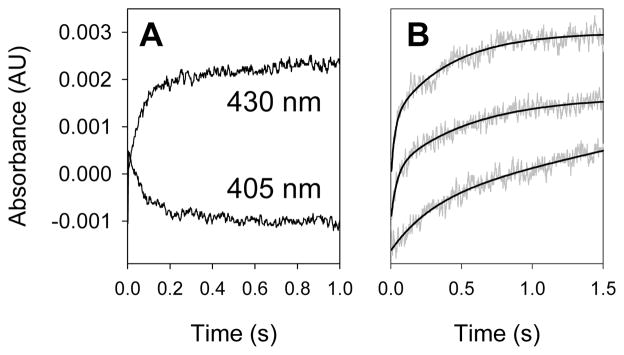
No direct kinetic studies of reactions of a P450 Compound I derivative with substrate were reported previously, but such studies are now possible. Using 2 ns rise time PM tubes, first-order rate constants as large as 2 × 108 s−1 can be measured, and diffusion-controlled bimolecular reactions are readily monitored. Decay of CYP119 Compound I to the ferric enzyme resting state resulted in decreasing absorbance in the range 390–420 nm and increasing absorbance at wavelengths in the range 425–450 nm. Kinetics could be followed in either range, and the results were the same (Figure 5A). In practice, we generally followed signal growth at ca. 430 nm.
Figure 5.
Kinetic traces. A. Growth at 430 nm and decay at 405 nm for reaction of CYP119 Compound I with 1.5 mM styrene; the rate constants for the two traces are the same. B. Kinetic traces (grey lines) and fits (black lines) at 430 nm for reactions of CYP119 Compound I with benzyl alcohol at (from the bottom) 0.0, 0.6 and 2.2 mM substrate concentrations.
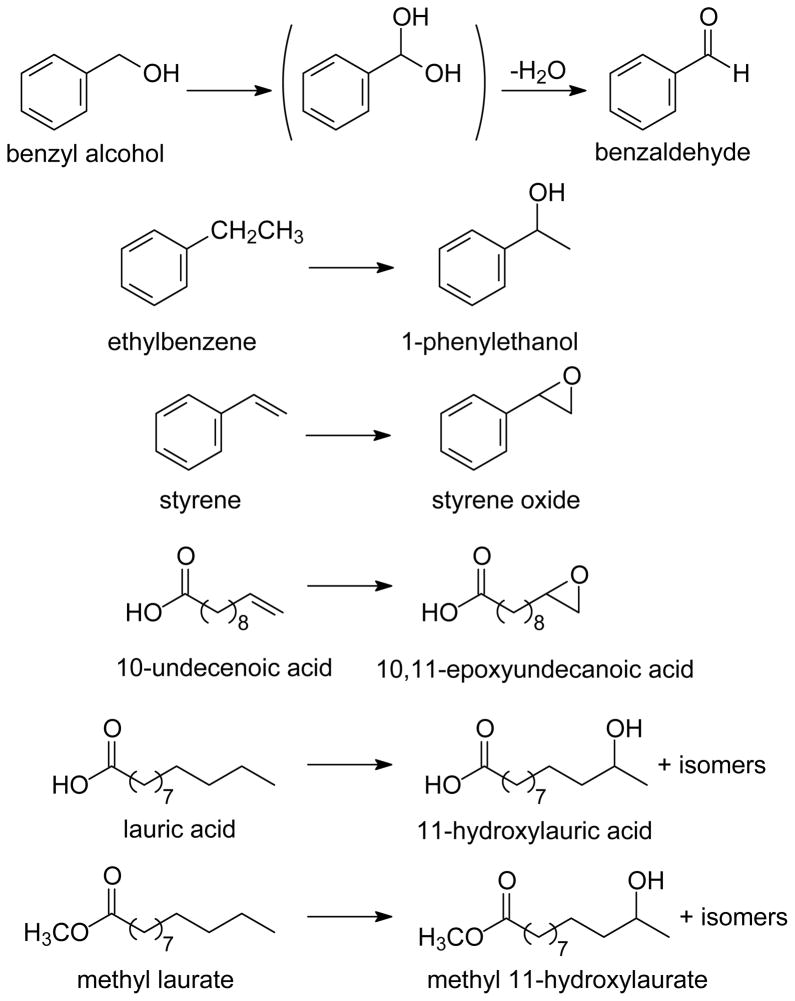
The kinetic studies involved the oxidation reactions shown in Chart 1. Characterizations of the products from these oxidation reactions are discussed below. All kinetic studies were performed with large excesses of substrates such that secondary oxidations were not important.
Chart 1.
For reactions with substrates, the substrate of choice was present in the enzyme solution prior to mixing that solution with the PN solution. A series of reactions was conducted with varying concentrations of substrate such that pseudo-first-order reaction conditions were maintained. Figure 5B shows typical results obtained with benzyl alcohol. Each trace is fit by a double exponential function where the major process (80–90%) varies with the concentration of substrate, and the minor process is constant in successive experiments. Because the CYP119 Compound I derivative reacts with excess PN, the laser flash was delayed until approximately 5 seconds after mixing to permit PN decay. In all cases for a series of studies with a given substrate, the reaction conditions were maintained such that the background rate remained constant. For example, a series of studies with a given substrate employed one solution of enzyme, one solution of PN, and a constant delay time after mixing before the laser flash.
First-order rate constants for reaction of CYP119 Compound I without added substrate varied in the range of 2–8 s−1 at 20 °C depending on the amount of excess peroxynitrite present in the solution with larger rate constants when the concentration of PN was greater. PN is stored in basic solutions and decays rapidly in an acid-catalyzed reaction when mixed with the buffered solutions of enzyme. By delaying the laser irradiation for 5 seconds after mixing, most of the PN decayed as determined by UV-visible spectroscopy, and relatively consistent small rate constants for decay of CYP119 Compound I were achieved. Details of the background decay reaction for the Compound I derivative should be revealed with further study, and this “reaction” might actually be a sum of several reactions including, in addition to reaction with PN, reaction of the Compound I derivative with nitrite, which is a major product of PN decay,36,38 and self reaction of the Compound I moiety with an oxidizable portion of the protein. In any event, the first-order decay reaction of Compound I introduced a constant kinetic effect that was subtracted from the observed rate constants.
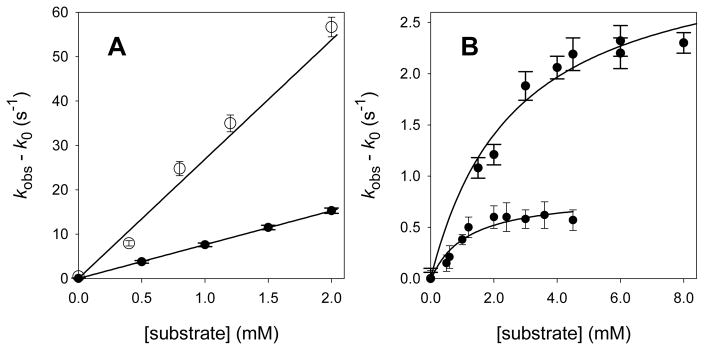
For reactions of CYP119 Compound I with most of the substrates studied, we observed linear relationships between the pseudo-first-order rate constants for reaction of Compound I and the concentrations of substrate.44 Figure 6A shows typical results for this group of substrates. The rate constants are described by Eq 1 where kobs is the observed pseudo-first-order rate constant, k0 is the background first-order rate constant, kApp is an apparent second-order rate constant for reaction with the substrate, and [subs] is the concentration of substrate. Plots of (kobs – k0) versus [subs] had slopes of kApp, as shown in Figure 6A, and values of kApp determined for the reactive substrates are listed in Table 1. We note that the apparent second-order rate constants for reaction in an enzyme’s active site are composite values comprised of two terms, an equilibrium constant for binding substrate (Kbind) which has the units of M−1, and a first-order rate constant (units of s−1) for the oxidation reaction of the bound substrate (kox) (Eq 2).
Figure 6.
Kinetic results for reactions of CYP119 Compound I in 25 mM phosphate buffer (pH 7.4) at 22 °C. (A) Benzyl alcohol (top) and styrene (bottom). (B) Methyl laurate (top) and lauric acid (bottom). The lines are regression solutions for equations 1 or 3. The error bars on the pseudo-first-order rate constants are one standard deviation.
Table 1.
Apparent second-order rate constants for reactions of CYP119 Compound I with organic substrates.a
| Substrate | kApp (M−1 s−1) | CPO-I kApp (M−1 s−1)b |
|---|---|---|
| benzyl alcohol | (2.70 ± 0.07) × 104 | |
| benzyl alcohol (pH 6.4)c | (1.97 ± 0.08) × 104 | |
| benzyl alcohol-d7 | (1.17 ± 0.06) × 104 | |
| ethylbenzene | (2.54 ± 0.12) × 103 | 9.6 × 102 |
| ethylbenzene-d10 | (1.6 ± 0.4) × 103 | 3.7 × 102 |
| styrene | (7.64 ± 0.18) × 103 | 6.1 × 104 |
| 10-undecenoic acid | (9.5 ± 0.3) × 103 | 6.4 × 103 |
| Tris buffer (pH 7.4)d | (2.5 ± 0.2) × 102 | |
| lauric acide | 7.2 × 102 | |
| methyl lauratee | 1.2 × 103 |
Reactions at 22 ± 1 °C in 25 mM phosphate buffer (pH 7.4) unless noted. Errors are at 1σ.
Apparent second-order rate constants for reactions of CPO Compound I derivative from ref 43.
This reaction was run in 25 mM phosphate buffer (pH ~ 6.4).
The reaction was conducted in 25 mM phosphate buffer (pH 7.4) using Tris buffer as a substrate; Tris is the abbreviation for tris-(hydroxymethyl)aminomethane, (HOCH2)3CNH2, which is mainly protonated at pH 7.4.
For lauric acid and methyl laurate, the apparent second-order rate constants were calculated from the measured values of Kbind and kox discussed in the text.
| (1) |
| (2) |
For reactions of lauric acid and methyl laurate with CYP119 Compound I, the kinetic behavior was different than that found with other substrates. We observed saturation kinetics with the rate of reaction approaching a limiting value at high concentrations of substrates (Figure 6B). When saturation kinetics are observed, the rate constants are described by Eq 3, where the terms are the same as defined for Eqs 1 and 2. Non-linear regression analysis of the data according to Eq 3 gave both the binding equilibrium constant and the oxidation rate constant. For reactions at 22 °C in 25 mM phosphate buffer (pH 7.4), the results for lauric acid were Kbind = 900 ± 300 M−1 and kox = 0.8 ± 0.1 s−1, and the results for methyl laurate were Kbind = 370 ± 90 M−1 and kox = 3.3 ± 0.3 s−1.
| (3) |
Because kApp values are the product of the binding constant and the oxidation rate constant, we calculated kApp for lauric acid and methyl laurate and included the values in Table 1 for comparison to the other substrates studied. Table 1 also contains some rate constants measured for reactions of the Compound I derivative of the heme-thiolate enzyme chloroperoxidase.43
Perhaps the most noteworthy kinetic result is the small rate constant for oxidation of unactivated C-H bonds in lauric acid (kox = 0.8 s−1). If the binding constant for lauric acid were not large enough to result in saturation of the activated enzyme, then we would not have been able to measure the kinetics of this reaction. Nonetheless, the measured rate constant is consistent with the observed reactivities of a number of iron(IV)-oxo porphyrin radical cations, which are known to be too low in activity to oxidize unactivated C-H bonds in hydrocarbons efficiently.8,45 P450 enzymes do oxidize unactivated C-H bonds rapidly under natural conditions, of course, and it is commonly assumed that the requisite reactivity arises from the electron-donating effect of the cysteinate ligand.7,10 For CYP119-I, the cysteinate ligand does appear to increase reactivity somewhat (see below), but not enough to match the apparent reactivity for C-H oxidations displayed by some P450s in nature.
A paradox of Compound I reactivities and P450 oxidations is that Compound I derivatives in models are not reactive enough to explain the oxidation strength of P450 enzymes. When P450cam with substrate present in the active site was allowed to react with radiolytically-generated electrons and oxygen under cryogenic conditions, the last intermediate in the sequence observable by EPR and ENDOR spectroscopy was the hydroperoxyiron species (see Figure 1).11,12 Extrapolation of the low temperature results to ambient temperatures suggests that the hydroperoxyiron species would decay at room temperature with a rate constant of about k ≈ 1000 s−1. Because no subsequent iron-oxo transient was observed, the transient that effected the oxidation must have reacted with the unactivated C(5)-H bond of camphor with a rate constant greater than that of its formation. Thus, if the estimate for decay of the hydroperoxyiron species is correct, then the oxidation reaction at ambient temperature would have a rate constant of kox > 1000 s−1, and, if that approximation is correct, then camphor C(5)-H oxidation by activated P450cam is more than 3 orders of magnitude faster than the lauric acid C-H oxidation by CYP119 Compound I. In principle, the large difference in reactivities might be due to very poor “tuning” of the reaction in CYP119, which displays maximal activity at ca. 70 °C.25,46,47 Alternatively, it is possible that P450s can produce a more reactive transient than Compound I, a perferryl-oxo species (see Figure 1) that has finite lifetime and can effect oxidations before it relaxes to a Compound I species by internal electron transfer. This controversial conjecture is supported by recent evidence that perferryl-oxo porphyrins and corroles can be produced that are several orders of magnitude more reactive in oxidation reactions than their iron(IV)-oxo ligand radical cation isomers48,49 and by results of thermodynamic cycles that indicate that a perferryl-oxo intermediate in a P450 enzyme is energetically accessible.50
The rate constants found for oxidations of ethylbenzene, styrene, and 10-undecenoic acid by CYP119-I are in general agreement with those obtained for reactions of the same substrates with the Compound I derivative of chloroperoxidase (CPO). CPO-I has long been available from hydrogen peroxide oxidation of the enzyme, and it has served as a model for P450 Compound I in a variety of spectroscopic studies.35,41,42,51,52 Apparent second-order rate constants for two-electron, oxo-transfer reactions of CPO-I also are available.43,53 The similarity in rate constants for CYP119-I and CPO-I oxidations of common substrates reinforces the conclusion that Compound I derivatives of P450 enzymes are well modeled by CPO-I, but one cautions that the observed rate constants for oxidations by CYP119-I and CPO-I are apparent second-order rate constants that contain both equilibrium binding constants and first-order oxidation rate constants. More meaningful comparisons can be made when these composite rate constants are separated into their constituent parts.
It is possible to estimate crudely the equilibrium binding constants and oxidation rate constants for substrates that gave only apparent second-order rate constants. The binding constant for lauric acid by CYP119-I measured in this work is 900 M−1. For CYP119 resting enzyme, the lauric acid binding constant is nearly 400 times larger than the styrene binding constant.54 If one assumes that the same differential exists for complexation of these two substrates by CYP119-I, then one estimates that Kbind ≈ 2 M−1 for styrene, and the value for the oxidation rate constant would be kox ≈ 4000 s−1. This estimated first-order rate constant for styrene oxidation at ambient temperature gives ΔG‡ = 12.4 kcal/mol, which appears to be in reasonable agreement with ΔG‡ values of ca. 15 kcal/mol for styrene oxidations by simple Compound I models that should be less reactive than P450 Compound I species45 if one factors in an expected accelerating effect for the thiolate ligand in CYP119-I. If similarly small Kbind values are assumed for the other aromatic substrates studied here, benzyl alcohol and ethylbenzene, then the kox values for these substrates are in the 1000 s−1 (ethylbenzene) and 20,000 s−1 (benzyl alcohol) ranges, which again appears to be in line with rate constants for reactions of simple Compound I models.45
Another manifestation of the kinetic acceleration afforded by the thiolate ligand in CYP119-I is seen in the kinetic isotope effect results. For benzyl alcohol, kH/kD = 2.3, and for ethylbenzene, kH/kD = 1.6, where we assume that the substrate binding constants were unaffected by isotopic substitution. These KIE values are relatively small; for example, an H/D KIE value of 4.4 for ethylbenzene-d0 and -d10 was found for oxidation by the Compound I derivative of 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin-iron.45 The small KIE values for CYP119-I suggests a large (negative) ΔG0 for oxidation of these substrates relative to the value in the models, leading to an early transition state and a small difference in ΔG‡ values (and small KIEs) for reactions of the non-deuterated and perdeuterated substrates.
We performed some experiments to evaluate crudely the effect of buffer on the Compound I reaction kinetics. Most of the oxidations in Table 1 were conducted in phosphate buffer at pH 7.4. For benzyl alcohol, the apparent second-order rate constant for oxidation was reduced by about 25% when the reaction was conducted in phosphate buffer at pH ~ 6.4. Until it is possible to separate the constituent terms in the apparent second-order rate constants, we are not able to determine whether the kinetic pH effect is due to reduced binding or to a change in the oxidation rate constant (or both), but the noteworthy point is that one should take care to monitor the pH closely in kinetic studies of P450 Compound I oxidations.
We also performed a cursory study of Tris buffer as a substrate for CYP119-I because the hydroxymethyl groups of Tris buffer, tris(hydroxymethyl)aminomethane, were expected to be reactive, and some oxidations by CYP119 have been conducted with bis-Tris buffer.54,55 We did not attempt to isolate products from the oxidation of Tris because they undoubtedly are highly water soluble, but it is most likely that the oxidation gave a geminal diol at one carbon that could dehydrate to an aldehyde group. The point of the study was to determine if Tris would react rapidly with CYP119 Compound I, and this was accomplished by using a phosphate buffer system and adding Tris in varying amounts as if it was a typical substrate. When studied in this manner, we obtained a reasonably large apparent second-order rate constant for Tris of kApp = 250 M−1 s−1. This result indicates a caution in the use of Tris or bis-Tris buffers for Compound I oxidation studies. For example, the bis-Tris buffer has been used at 50 mM concentration in studies of styrene oxidations by CYP119-I under H2O2 shunt conditions.55 If bis-Tris reacts with the same rate constant as Tris, then reaction of CYP119-I with the buffer had a pseudo-first-order rate constant of k ≈ 12 s−1, which is about 6 times as fast as decay in the presence of phosphate buffer.
Comparisons of CYP119 shunt oxidation reactions to CYP119-I reactions
For most P450 enzymes, the sequence of reactions that produces active oxidant involves reduction of the ferric enzyme, reversible binding of oxygen, a second reduction step and protonation reactions (see Figure 1). Many P450 enzymes can be “shunted” with peroxides such as hydrogen peroxide in a reaction that likely resembles those of the peroxidase enzymes. Although there is limited evidence for the formation of a Compound I derivative in these shunt reactions, P450 enzymes have been known to catalyze oxidizations of substrates under shunt conditions for many years,56 and the presence of a Compound I transient generally has been assumed. In the specific case of CYP119, hydrogen peroxide and alkyl hydroperoxide shunt reactions are known to give oxidized substrates.25,46,47,54,55
We performed CYP119 hydrogen peroxide shunt reactions for comparisons to the LFP kinetic studies. In the shunt reactions, CYP119 in buffer with a mixture of two substrates was treated with aliquots of hydrogen peroxide. Following the reactions, the products were extracted into an organic solvent, a standard was added, and the mixtures were analyzed by GC for quantification and by GC-mass spectrometry for product identification. The relative rate constants for reactions of the two substrates were determined from Eq 4, which is the equation for competition kinetics for reactions of substrates A and B where the competing reactions are second-order processes.57 In Eq 4, [X]0 is the initial concentration of substrate X, and [X]F is the concentration of substrate X at the end of the reaction.
| (4) |
Table 2 lists the results of the competition kinetic studies. The ratio of rate constants for the competing reactions in the shunt experiments is listed as kA/kB in column 3. Column 4 in the table contains the ratio of rate constants for the same substrates obtained in the LFP studies. The ratios of rate constants for the two types of studies typically should not be the same because the shunt reactions studied here involve competitions, and a substrate with a large binding constant might be a competitive inhibitor of a substrate with a small binding constant. Nonetheless, if there is an equality in the equilibrium binding constants for the two substrates, then the ratios of rate constants for the two types of studies should be equal. That condition can be assumed to hold for the kinetic isotope effect studies because the isotopic substitution should have at most only a minor effect on substrate binding. For both benzyl alcohol and ethylbenzene, we found the same KIE values in the competition studies as in the LFP studies, which indicates that the oxidizing species is the Compound I derivative in both types of oxidation experiments.
Table 2.
Results of competition shunt oxidations by CYP119.a
The ratios of rate constants for the competition study with benzyl alcohol and styrene compared to the ratio of LFP rate constants for these substrates also were the same. This suggests either that the two aromatic substrates have similar equilibrium binding constants or that the binding constants for both were too small to saturate the enzyme. In either case, the apparent second-order rate constants found in the LFP studies would control the relative reactivities in the competition studies.
The competition results with lauric acid were much different than those between two aromatic substrates. In the LFP studies, lauric acid reacted with calculated apparent second-order rate constants that were much smaller than the measured kApp values for benzyl alcohol or styrene (see Table 1), but lauric acid was oxidized more efficiently than either of the aromatic substrates in the competition study. Thus, lauric acid was a competitive inhibitor of the aromatic substrates in the competition studies. The binding constant for lauric acid found in the LFP studies, Kbind = 900 M−1, is the equilibrium constant for complexation in the active site of the Compound I derivative. For the competition studies, where the substrate concentrations were 0.2 mM, a binding equilibrium constant of 900 M−1 will not result in saturation of the activated enzyme. For the resting CYP119 enzyme, however, the reported binding constant for lauric acid is about 8 × 105 M−1,54 which would result in saturation of the resting enzyme with 0.2 mM lauric acid. More detailed studies are needed to understand the kinetic effects fully, but the tentative conclusion is that CYP119 saturation by lauric acid before activation of the enzyme by reaction with H2O2 resulted in limited access of the aromatic substrates to the active site of the activated enzyme.
Products from oxidation reactions
For the substrates studied in this work, the products of oxidations by P450 enzymes in general are known to be epoxidation products for alkenes and hydroxylation products for hydrocarbons.10 In the specific case of CYP119, oxidation reactions of lauric acid and styrene have been studied under shunt conditions as well as under a more natural reaction sequence.25,46,47,54,55 The major product from lauric acid oxidation is 11-hydroxylauric acid, and the 12-, 10-, and 9-hydroxylated products also are formed. The major product from styrene oxidation is styrene oxide.
In the present work, shunt reactions similar to the competition kinetic studies were conducted, and the products were analyzed by GC and GC-mass spectrometry. The oxidation products were identified by comparison of the GC retention time and mass spectral fragmentation patterns to those of authentic samples of the products benzaldehyde (from benzyl alcohol), styrene oxide (from styrene), 1-phenylethanol (from ethylbenzene), and 10,11-epoxyundecanoic acid (from 10-undecenoic acid). For lauric acid, the product mixture from reaction with CYP119 is known to contain 11-hydroxylauric acid as the major product, 12-hydroxylauric acid as the second most abundant product, and other hydroxylauric acids as minor products.54 We converted the lauric acid products to the corresponding methyl esters by reaction with diazomethane. The mixture of products from this sequence was compared to the mixture obtained from oxidation of methyl laurate in order to identify the methyl laurate oxidation products. The methyl laurate products were the same as those from oxidation and subsequent methylation of lauric acid, although the ratios of products differed slightly.
The yields of products from some of the shunt reactions were determined by GC by addition of a calibrated internal standard to the product mixture from the oxidations. The yields of oxidation products from styrene and ethylbenzene were 8–10% based on hydrogen peroxide as the limiting reagent. Similar yields for hydrogen peroxide shunt reactions with CYP119 were reported previously.54
In order to evaluate the effect of PN on the CYP119 enzyme, we conducted a shunt reaction that was identical to the shunt reaction with styrene with the exception that the CYP119 enzyme was first treated with 20 equivalents of PN. The yield of styrene oxide formed in this shunt reaction was the same as that found with untreated CYP119. Thus, the PN reaction using 20 equivalents of PN appears to have no effect on the CYP119 enzyme.
The LFP study involves production of sub-nanomole amounts of reactive transients, but we performed bulk photolysis reactions that were designed to simulate the LFP studies on a somewhat larger scale. In these reactions, 200 μM PN was added to a mixture of 10 μM CYP119 and 100 μM substrate (final concentrations are listed) in a vessel held in a 0 °C bath. After 10 seconds, the reaction vessel was removed from the bath, placed in a photochemical reactor at room temperature, and irradiated for ca. 1 min with five 15-W bulbs emitting light centered at 350 nm. The reaction mixture was then worked up and analyzed by GC and GC-mass spectrometry to identify and quantity the products. For styrene and ethylbenzene as substrates, we found styrene oxide in ca. 70% yield and 1-phenylethanol in ca. 65% yield, respectively, based on enzyme. Under the conditions employed for production of the Compound II species in these experiments, we estimate that 80–90% of the enzyme was converted to the Compound II derivative. Thus, the total efficiency for the two processes (photochemical production of Compound I and its reaction with substrate) is very high, in the range of 70–90%, given that “self-decay” of the CYP119 Compound I derivative occurs at a measurable rate on the time-scale of the bulk photolysis studies. In a series of control reactions with styrene as the substrate, we found no oxidization product when the PN, the CYP119, or the light was omitted from experiments otherwise identical to the styrene reaction described above.
The origin of the oxygen atoms in the oxidation products was established by performing two sets of bulk photochemical oxidation reactions with oxygen-18 labeled species. In the first set, we used doubly-labeled peroxynitrite (NaN16O18O2) that was synthesized from H218O2 that in turn was prepared from 18O2. In the second set, we used unlabeled peroxynitrite in a 1:1 mixture of unlabeled and oxygen-18 labeled water (H216O:H218O = 1:1). The reactions were conducted by adding substrate to the enzyme followed by addition of PN and then photolysis. The products were analyzed by GC-mass spectrometry using single ion monitoring (SIM) for the molecular ions. For styrene, the molecular ion of unlabeled styrene oxide product is at m/z = 120; in the study conducted with doubly labeled PN, the ratio of integrals for the m/z = 120 and 122 peaks was 1:2, and the ratio of integrals for these peaks using unlabeled PN in labeled water was >99:1. Similar results were obtained for oxidation of ethylbenzene, where the unlabeled product alcohol has a molecular ion at m/z = 122; for the reaction with doubly labeled PN, the ratio of integrals for m/z = 122 and 124 was 1:2, whereas as the ratio found in the reaction with unlabeled PN in labeled water was >99:1.
The labeling results demonstrate that the Compound I oxidations involved insertion reactions of the oxygen from the iron-oxo species as opposed to electron transfer reactions followed by water capture of the radical cations. The production of oxidation products via radical cations already was judged to be highly unlikely because styrene radical cation reacts largely to give benzaldehyde,58 and electron transfer oxidations of ethylbenzene and other alkylarenes give good yields of phenol products.59 Neither benzaldehyde nor ethylphenols were found in our product mixtures.
Conclusions
Photo-ejection of an electron from the P450 119 Compound II derivative produced the P450 Compound I species (CYP119-I), which is widely regarded as the active transient in P450-catalyzed oxidation reactions. The UV-visible spectrum of CYP119-I was thus directly observed, as opposed to calculated, and it was found to be similar to the UV-visible spectrum of chloroperoxidase Compound I, consisting of two overlapping peaks in the Soret band region and a long wavelength Q-band absorbance.
The LFP method for production of the P450 Compound I derivative permitted direct kinetic studies of reactions of this transient with a variety of organic substrates. The measured rate constants for many substrates were apparent second-order rate constants, which are products of a binding constant times a first-order rate constant for the oxidation reaction, that were similar in magnitude to those for oxidations of the same substrates by the Compound I derivative of chloroperoxidase. For lauric acid and methyl laurate, saturation kinetics were observed, and both the binding constants and first-order rate constants for oxidation were determined. The rate constant for oxidations of unactivated C-H positions in lauric acid was 0.8 s−1 at ambient temperature, which is more than 3 orders of magnitude smaller than the estimated first-order rate constant for oxidation of the unactivated C(5)-H bond in camphor by the oxidizing transient formed in P450cam. Possible explanations for the differences in reactivities are that CYP119, which is from a thermophile, is not optimized for reactions at ambient temperature, or that a different, more highly reactive type of oxidant can be formed in the natural reaction sequence of P450 enzymes.
By comparing relative reactivities in competition studies with CYP119 and hydrogen peroxide with the ratios of rate constants for the same substrates in the LFP studies, we conclude that the same transient is produced in both types of experiments. Furthermore, product studies, including oxygen-18 labeling studies, demonstrated that the known products of P450-catalyzed oxidations were formed from photochemically-generated CYP119-I by insertion of the oxygen atom of the iron-oxo group, the expected reaction pathway for Compound I derivatives.
The methods developed in this work are expected to be useful for studies of Compound I derivatives from other P450 enzymes. Further insights into the nature and reactivity of the active oxidants in P450s should be forthcoming.
Experimental Section
The preparation and isolation of CYP119 followed the methods previously described.22–24 The enzyme used in these studies had an R/Z ratio (A416/A280) of >1.5. All substrates used in oxidation studies were commercial samples of nominal high purity that were checked by NMR spectroscopy or GC for purity. Basic solutions containing peroxynitrite were prepared by the method of Uppu and Pryor.60
Laser flash photolysis (LFP) studies
The spectroscopic and kinetic studies of CYP119 were conducted in a similar manner using a stopped-flow mixing unit (SX-18, Applied Photophysics), affixed to an LKS-60 laser flash photolysis kinetic unit (Applied Photophysics). The laser light used was the third harmonic (355 nm) from a Quantel Brilliant B Nd-YAG laser that delivered ca. 4 mJ to the base of a 2 mm × 10 mm quartz reaction flow cell that was charged by the mixing unit. A 150 W Xenon lamp under continuous irradiation conditions was used for most studies; the lamp was pulsed for the data used for the CYP119 Compound I spectrum. Photomultiplier tubes with monochromatic light or a PDA 1 diode array detector (Applied Photophysics) with broad spectrum light were used for detection. When monochromatic light was used, the monochrometer was placed before the reaction cell such that low intensity light impinged on the sample.
In a typical LFP study, 10 μM CYP119 in 50 mM phosphate buffer pH 7 was placed in one syringe of the mixing unit, and 0.25 mM PN solution was placed in a second syringe. Equal volumes of the two syringes were mixed in the reaction cell, and the resulting mixture had pH 7.4. After a delay of 5 seconds, the laser was fired. For kinetic studies, the substrate at the desired concentration was added to the CYP119 solution.
Competition shunt reactions
A 2 mL solution of phosphate buffer (pH 7.0) containing 20 μM CYP119 and 0.2 mM of the desired substrates was stirred at room temperature as aliquots of H2O2 were added over 0.5 hours (total addition of 0.4 mM). The mixture was extracted with methylene chloride. The organic phase was washed with brine, dried (MgSO4), filtered, and analyzed by GC and/or GC-mass spectrometry on low polarity columns (DB-5 or equivalent). Relative rate constants were determined from Eq 4 in the text.
Product identifications and yields in shunt reactions
Reactions were conducted as described above for shunt reactions with the exception that one substrate was employed. Following the reaction, the products were analyzed by GC and GC/mass spectrometry using low polarity columns (DB-5 or equivalent) for all substrates except lauric acid and methyl laurate. Products were identified by comparison of GC retention times and mass spectral fragmentation patterns to those of authentic samples, most of which were commercial samples. A sample of 10,11-epoxyundecanoic acid was prepared by mCPBA oxidation of 11-undecenoic acid as previously reported.61 Yields were determined relative to an internal standard added after the product work-up. For lauric acid, the product mixture was converted to a mixture of methyl esters by reaction with diazomethane, and the mixture was analyzed on high polarity columns (Carbowax or equivalent). Products from lauric acid oxidation by CYP119 and H2O2 were reported,54 and a commercial sample of 12-hydroxylauric acid was available for comparison. The methyl laurate product mixture also was analyzed on high polarity columns, and the products were identified by comparison to the products from the lauric acid oxidations.
Product yields in bulk photolyses
A solution of CYP119 (10 μM) and substrate (styrene or ethylbenzene) (0.1 mM) in 50 mM phosphate buffer pH 7.0 (1.0 mL) was cooled in a 0 °C bath. To this solution was added 5 μL of 100 mM PN solution. After 10 seconds, the mixture was removed from the ice bath and irradiated in a photochemical reactor containing five 15-W fluorescent bulbs (300–400 nm irradiation band) for 1 min. The reaction mixture was worked up by extraction into CHCl3, an internal standard was added, and the mixture was analyzed by GC for yield as described above. In a series of control reactions for the styrene oxidation, the reaction was repeated with the exception that the CYP119 enzyme, the PN, and the irradiation step were omitted; no styrene oxide was detected in these reactions.
Oxygen-18 labeling studies
Doubly-labeled hydrogen peroxide, H218O2, was prepared from 18O2 as previously described.62,63 The H218O2 was then used for the preparation of doubly-labeled sodium peroxynitrite, NaN16O18O2, from isoamyl nitrite by the method of Uppu and Pryor.60 Experiments were performed with doubly-labeled PN in unlabeled water and with unlabeled PN in labeled water (1:1, H216O:H218O). In these studies, solutions of CYP119 in 50 mM phosphate buffer (pH 7.0) were prepared in a cell placed in a temperature-regulated chamber cooled to 0 °C. Substrate was added followed by PN solution, and the UV-visible spectrum was measured with a fiber-optics diode array UV-visible spectrometer. The final concentrations were 10 μM CYP119, 0.1 mM substrate, and 0.5 mM PN. The samples were irradiated with 15 pulses of 320–490 light (1 J delivered in 0.1 s per pulse). UV-visible spectroscopy confirmed that the Compound II species was depleted by the photolysis. The samples were worked up as above and analyzed by GC-mass spectrometry using SIM mode.
mCPBA mixing study
The experiment followed the procedure reported by Kellner et al.20 Thus, thermostated (4 °C) solutions of CYP119 and mCPBA were mixed in the stopped-flow kinetic unit to give 14 μM CYP119 and 8.6 μM mCPBA. Data acquisition over 2 s gave 800 spectra. Deconvolution was achieved with global analysis software from Applied Photophysics for the model A + B → C and C → A. The results of the global analysis program were functions of the initial guesses of the rate constants.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-48722). We thank Prof. S. G. Sligar for providing the DNA for cloning CYP119.
Footnotes
Supporting Information Available
Results of PN mixing studies and addional examples of deconvolutions of spectra from stopped-flow mixing of CYP119 with mCPBA.
References
- 1.Ortiz de Montellano PR. Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Kluwer; New York: 2005. [Google Scholar]
- 2.Guengerich FP. In: Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Ortiz de Montellano PR, editor. Kluwer; New York: 2005. pp. 377–530. [Google Scholar]
- 3.Scripture CD, Sparreboom A, Figg WD. Lancet Oncol. 2005;6:780–789. doi: 10.1016/S1470-2045(05)70388-0. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Antona C, Ingelman-Sundberg M. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 5.Lieber CS. Drug Metabol Rev. 2004;36:511–529. doi: 10.1081/dmr-200033441. [DOI] [PubMed] [Google Scholar]
- 6.Dawson JH. Science. 1988;240:433–439. doi: 10.1126/science.3358128. [DOI] [PubMed] [Google Scholar]
- 7.Sono M, Roach MP, Coulter ED, Dawson JH. Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 8.Groves JT. In: Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Ortiz de Montellano PR, editor. Kluwer; New York: 2005. pp. 1–43. [Google Scholar]
- 9.Makris TM, Denisov I, Schlichting I, Sligar SG. In: Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Ortiz de Montellano PR, editor. Kluwer; New York: 2005. pp. 149–182. [Google Scholar]
- 10.Ortiz de Montellano PR, De Voss JJ. In: Cytochrome P450 Structure, Mechanism, and Biochemistry. 3. Ortiz de Montellano PR, editor. Kluwer; New York: 2005. pp. 183–245. [Google Scholar]
- 11.Davydov R, Macdonald IDG, Makris TM, Sligar SG, Hoffman BM. J Am Chem Soc. 1999;121:10654–10655. [Google Scholar]
- 12.Davydov R, Makris TM, Kofman V, Werst DE, Sligar SG, Hoffman BM. J Am Chem Soc. 2001;123:1403–1415. doi: 10.1021/ja003583l. [DOI] [PubMed] [Google Scholar]
- 13.Coon MJ, Blake RC, II, Oprian DD, Ballou DP. Acta Biol Med Ger. 1979;38:449–458. [PubMed] [Google Scholar]
- 14.Egawa T, Shimada H, Ishimura Y. Biochem Biophys Res Commun. 1994;201:1464–1469. doi: 10.1006/bbrc.1994.1868. [DOI] [PubMed] [Google Scholar]
- 15.Spolitak T, Dawson JH, Ballou DP. J Biol Chem. 2005;280:20300–20309. doi: 10.1074/jbc.M501761200. [DOI] [PubMed] [Google Scholar]
- 16.Schunemann V, Jung C, Terner J, Trautwein AX, Weiss R. J Inorg Biochem. 2002;91:586–596. doi: 10.1016/s0162-0134(02)00476-2. [DOI] [PubMed] [Google Scholar]
- 17.Schunemann V, Trautwein AX, Jung C, Terner J. Hyperfine Interact. 2002;141:279–284. [Google Scholar]
- 18.Schunemann V, Lendzian F, Jung C, Contzen J, Barra AL, Sligar SG, Trautwein AX. J Biol Chem. 2004;279:10919–10930. doi: 10.1074/jbc.M307884200. [DOI] [PubMed] [Google Scholar]
- 19.Jung C, Schunemann V, Lendzian F, Trautwein AX, Contzen J, Galander M, Bottger LH, Richter M, Barra AL. Biol Chem. 2005;386:1043–1053. doi: 10.1515/BC.2005.120. [DOI] [PubMed] [Google Scholar]
- 20.Kellner DG, Hung SC, Weiss KE, Sligar SG. J Biol Chem. 2002;277:9641–9644. doi: 10.1074/jbc.C100745200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R, Chandrasena REP, Martinez E, II, Horner JH, Newcomb M. Org Lett. 2005;7:1193–1195. doi: 10.1021/ol050296j. [DOI] [PubMed] [Google Scholar]
- 22.Newcomb M, Zhang R, Chandrasena REP, Halgrimson JA, Horner JH, Makris TM, Sligar SG. J Am Chem Soc. 2006;128:4580–4581. doi: 10.1021/ja060048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean MA, Maves SA, Weiss KE, Krepich S, Sligar SG. Biochem Biophys Res Commun. 1998;252:166–172. doi: 10.1006/bbrc.1998.9584. [DOI] [PubMed] [Google Scholar]
- 24.Maves SA, Sligar SG. Protein Sci. 2001;10:161–168. doi: 10.1110/ps.17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabe KS, Kiko K, Niemeyer CM. Chembiochem. 2008;9:420–425. doi: 10.1002/cbic.200700450. [DOI] [PubMed] [Google Scholar]
- 26.Daiber A, Herold S, Schöneich C, Namgaladze D, Peterson JA, Ullrich V. Eur J Biochem. 2000;267:6729–6739. doi: 10.1046/j.1432-1033.2000.01768.x. [DOI] [PubMed] [Google Scholar]
- 27.Floris R, Piersma SR, Yang G, Johnes P, Wever R. Eur J Biochem. 1993;215:767–775. doi: 10.1111/j.1432-1033.1993.tb18091.x. [DOI] [PubMed] [Google Scholar]
- 28.Mehl M, Daiber A, Herold S, Shoun H, Ullrich V. Nitric Oxide Biol Chem. 1999;3:142–152. doi: 10.1006/niox.1999.0217. [DOI] [PubMed] [Google Scholar]
- 29.Exner M, Herold S. Chem Res Toxicol. 2000;13:287–293. doi: 10.1021/tx990201k. [DOI] [PubMed] [Google Scholar]
- 30.Boccini F, Herold S. Biochemistry. 2004;43:16393–16404. doi: 10.1021/bi0482250. [DOI] [PubMed] [Google Scholar]
- 31.Furtmüller PG, Jantschko W, Zederbauer M, Schwanninger M, Jakopitsch C, Herold S, Koppenol WH, Obinger C. Biochem Biophys Res Commun. 2005;337:944–954. doi: 10.1016/j.bbrc.2005.09.138. [DOI] [PubMed] [Google Scholar]
- 32.Gebicka L, Didik J. J Inorg Biochem. 2007;101:159–164. doi: 10.1016/j.jinorgbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Behan RK, Hoffart LM, Stone KL, Krebs C, Green MT. J Am Chem Soc. 2007;129:5855–5859. doi: 10.1021/ja064590y. [DOI] [PubMed] [Google Scholar]
- 34.Newcomb M, Halgrimson JA, Horner JH, Wasinger EC, Chen LX, Sligar SG. Proc Natl Acad Sci USA. 2008;105:0000–0000. doi: 10.1073/pnas.0708299105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone KL, Behan RK, Green MT. Proc Natl Acad Sci USA. 2005;102:16563–16565. doi: 10.1073/pnas.0507069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolzan RM, Cueto R, Squadrito GL, Uppu RM, Pryor WA. Methods Enzymol. 1999;301:178–187. doi: 10.1016/s0076-6879(99)01081-2. [DOI] [PubMed] [Google Scholar]
- 37.Kissner R, Koppenol WH. J Am Chem Soc. 2002;124:234–239. doi: 10.1021/ja010497s. [DOI] [PubMed] [Google Scholar]
- 38.Kirsch M, Korth HG, Wensing A, Sustmann R, de Groot H. Arch Biochem Biophys. 2003;418:133–150. doi: 10.1016/j.abb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein S, Lind J, Merényi G. Chem Rev. 2005;105:2457–2470. doi: 10.1021/cr0307087. [DOI] [PubMed] [Google Scholar]
- 40.Makris TM, von Koenig K, Schlichting I, Sligar SG. J Inorg Biochem. 2006;100:507–518. doi: 10.1016/j.jinorgbio.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 41.Palcic MM, Rutter R, Araiso T, Hager LP, Dunford HB. Biochem Biophys Res Commun. 1980;94:1123–1127. doi: 10.1016/0006-291x(80)90535-5. [DOI] [PubMed] [Google Scholar]
- 42.Egawa T, Proshlyakov DA, Miki H, Makino R, Ogura T, Kitagawa T, Ishimura Y. J Biol Inorg Chem. 2001;6:46–54. doi: 10.1007/s007750000181. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Nagraj N, Lansakara DSP, Hager LP, Newcomb M. Org Lett. 2006;8:2731–2734. doi: 10.1021/ol060762k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.In the initial communication of this work (ref 22), we reported that lauric acid and styrene had no effect on the kinetics of CYP119 Compound I decay. The substrate concentrations used in those studies were too small to give measurable kinetic effects.
- 45.Pan ZZ, Zhang R, Newcomb M. J Inorg Biochem. 2006;100:524–532. doi: 10.1016/j.jinorgbio.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Puchkaev AV, Wakagi T, de Montellano PRO. J Am Chem Soc. 2002;124:12682–12683. doi: 10.1021/ja0282036. [DOI] [PubMed] [Google Scholar]
- 47.Puchkaev AV, Ortiz de Montellano PR. Arch Biochem Biophys. 2005;434:169–177. doi: 10.1016/j.abb.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Harischandra DN, Zhang R, Newcomb M. J Am Chem Soc. 2005;127:13776–13777. doi: 10.1021/ja0542439. [DOI] [PubMed] [Google Scholar]
- 49.Pan ZZ, Zhang R, Fung LWM, Newcomb M. Inorg Chem. 2007;46:1517–1519. doi: 10.1021/ic061972w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koppenol WH. J Am Chem Soc. 2007;129:9686–9690. doi: 10.1021/ja071546p. [DOI] [PubMed] [Google Scholar]
- 51.Rutter R, Hager LP, Dhonau H, Hendrich M, Valentine M, Debrunner P. Biochemistry. 1984;23:6809–6816. doi: 10.1021/bi00321a082. [DOI] [PubMed] [Google Scholar]
- 52.Kim SH, Perera R, Hager LP, Dawson JH, Hoffman BM. J Am Chem Soc. 2006;128:5598–5599. doi: 10.1021/ja060776l. [DOI] [PubMed] [Google Scholar]
- 53.Osborne RL, Coggins MK, Terner J, Dawson JH. J Am Chem Soc. 2007;129:14838–14839. doi: 10.1021/ja0746969. [DOI] [PubMed] [Google Scholar]
- 54.Koo LS, Immoos CE, Cohen MS, Farmer PJ, Ortiz de Montellano PR. J Am Chem Soc. 2002;124:5684–5691. doi: 10.1021/ja017174g. [DOI] [PubMed] [Google Scholar]
- 55.Koo LS, Tschirret-Guth RA, Straub WE, Moenne-Loccoz P, Loehr TM, de Montellano PRO. J Biol Chem. 2000;275:14112–14123. doi: 10.1074/jbc.275.19.14112. [DOI] [PubMed] [Google Scholar]
- 56.Nordblom GD, White RE, Coon MJ. Arch Biochem Biophys. 1976;175:524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- 57.Newcomb M. Tetrahedron. 1993;49:1151–1176. [Google Scholar]
- 58.Ren Y, Che Y, Ma W, Zhang X, Shen T, Zhao J. New J Chem. 2004;28:1464–1469. [Google Scholar]
- 59.Bartoli JF, Lambert F, Morgenstern-Badarau I, Battioni P, Mansuy D. C R Chim. 2002;5:263–266. [Google Scholar]
- 60.Uppu RM, Pryor WA. Anal Biochem. 1996;236:242–249. [PubMed] [Google Scholar]
- 61.Yao ZJ, Wu YL. J Org Chem. 1995;60:1170–1176. [Google Scholar]
- 62.Sawaki Y, Foote CS. J Am Chem Soc. 1979;101:6292–6296. [Google Scholar]
- 63.Groziak MP, Chern JW, Townsend LB. J Org Chem. 1986;51:1065–1069. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.