Abstract
Members of the nuclear hormone receptor superfamily, including the peroxisome proliferator-activated receptor (PPAR) and liver X receptor (LXR) subfamilies, orchestrate transcriptional networks involved in the control of metabolism and the development of vascular disease. In addition to these well characterized ligand-activated transcription factors, the nuclear receptor superfamily comprises a large number of orphan receptors, whose ligands and physiological functions remain unknown. Among this group of orphan receptors is the NR4A subfamily including the three members Nur77 (NR4A1), Nurr1 (NR4A2) and NOR1 (NR4A3). These orphan nuclear receptors constitute an evolutionary ancient and highly conserved group of transcription factors. In contrast to other members of the superfamily, NR4A receptors function as ligand-independent transcription factors and immediate/early response genes, which are rapidly induced by a pleiotropy of environmental cues. Early functional studies have pointed to a critical role of NR4A receptors in regulating differentiation, proliferation and apoptosis. More recent research has characterized NR4A receptors as key transcriptional regulators of glucose and lipid homeostasis, adipogenesis, inflammation, and vascular remodeling. In this review, we will summarize recent advances in understanding the molecular biology and physiological functions of NR4A receptors and discuss their role in the transcriptional control of metabolism and vascular remodeling.
Keywords: nuclear receptor, gene expression, metabolism, vascular biology
Introduction
Members of the nuclear hormone receptor superfamily have emerged as a potentially large class of therapeutic targets for the treatment of obesity, diabetes, and atherosclerotic disease 1. Most signaling pathways in these complex diseases ultimately converge to control networks of gene expression through signal-regulated transcription factors, including nuclear receptors (NR). The understanding of their ability to sense environmental cues and translate endocrine and metabolic signals into specific gene expression programs in metabolism and vascular biology has considerably expanded our knowledge on the pathophysiology of these most prevalent diseases. For example, the adopted orphan NR of the peroxisome proliferator-activated receptors (PPAR) and liver X receptors (LXR) subfamilies have been characterized to orchestrate gene expression programs involved in the control of glucose homeostasis, lipid metabolism, as well as inflammation and proliferation in the vascular wall 2, 3. Pharmacological ligands for these two NR have been developed and their ligand-induced activation improves glucose metabolism and prevents atherosclerosis in murine models, attesting to the importance of these receptors and the approach to develop pharmacologic ligands 4–7. The human genome contains 48 members of the NR superfamily 8. In addition to the classical endocrine receptors and the adopted orphan receptors, the NR superfamily comprises an even larger group of orphan receptors 9. Although the ligands for these orphan receptors remain unknown, considerable progress has been made to identify their regulated target genes and characterize their physiological functions 10. Among these orphan receptors is the NR4A subfamily including the three members Nur77 (NR4A1) 11, Nurr1 (NR4A2) 12, and NOR1 (NR4A3) 13. Members of this subfamily function as ligand-independent NR and early-response genes regulating key cellular processes, including inflammation, proliferation, differentiation, and survival 14–16. In this review will summarize recent progress in understanding the physiological function of NR4A receptors and discuss their role as transcriptional regulators of gene expression in metabolism and vascular biology.
Molecular Biology of NR4A Orphan Nuclear Receptors
Nuclear receptors share a common structure consisting of a ligand-independent AF-1 transactivation domain in the N-terminal region, a highly conserved DNA-binding domain (DBD) composed of two zinc fingers recognizing specific DNA sequences, and a ligand-binding domain (LBD) that contains a ligand-dependent AF-2 transactivation domain in its C-terminal portion 17. NR4A receptors share this common NR structure, and the three members reveal a high degree of homology in their genomic structure and conservation of their DNA binding domain (degree of conservation > 90%) 18. However, several lines of evidence indicate that NR4A receptors may represent a distinct group of transcription factors that do not function in a classical manner. Mutational analysis indicated that NR4A receptors function as constitutively-active receptors whose transcriptional activity is independent of the LBD 19, 20. Instead, their transcriptional activity and coactivator recruitment appear to be dependent on the N-terminal AF-1 domain 20–22, which constitutes a common distinction of ligand-independent transcriptional activation by NR 23,24. This initial observation was supported by the finding that the LBD of NR4A contains hydrophilic surfaces instead of the classical hydrophobic cleft that mediates coactivator recruitment of other NR 22. Finally, this unusual structure of the NR4A LBD has been recently confirmed by X-ray crystallography demonstrating that the Nurr1 LBD contains no cavity as a result of hydrophobic residues in the region normally occupied by ligands 25. Considering these observations, NR4A receptors are currently thought to function as constitutively-active and ligand-independent receptors, whose transcriptional activity is primarily dependent on the expression of the receptor and its posttranslational modification.
NR4A receptors are early immediate response genes, which are induced by a pleiotropy of stimuli including growth factors, inflammatory stimuli, cytokines, peptide hormones, and cellular stress 16. Once their expression is induced, NR4A receptors activate transcription by binding as monomers or homodimers to canonical DNA target sites, the NGFI-B-responsive element (NBRE) consisting of an octanucleotide AAAGGTCA motif 26, 27. NR4A homodimers preferentially bind to the Nur-responsive element (NurRE), which constitutes an everted repeat of the NBRE-related sequence (AAAT(G/A)(C/T)CA) found in the pro-opiomelanocortin (POMC) gene promoter 28. In addition, Nur77 and Nurr1 (but not NOR1) heterodimerize with RXR and activate transcription through a DR-5 element in a 9-cis retinoic acid-dependent manner 29, 30. This heterodimerization of Nurr1 with RXR is isotype-specific since Nurr1 interacts only with RXRα and RXRγ but not with RXRβ31. Furthermore, different NR4A receptors can form heterodimers to synergistically activate transcription 32. While it was initially thought that NR4A receptors only activate genes, a recent study has provided first evidence that Nurr1 can also repress inflammatory gene promoters by recruiting corepressor complexes 33.
In addition to the rapid expression as early response genes, the transcriptional activity of NR4A receptors is regulated by posttranslational modification. All three NR4A receptors are phosphorylated at serine residues in response to growth factor-dependent activation of various kinases, including MAPK, PI3K, Akt, JNK, and RSK 31, 34–37. For example, Nur77 is phosphorylated at Ser-350 and Ser-354 within the DNA binding domain, which inhibits the transactivation activity 38, 39. Furthermore, phosphorylation of Nur77 at Ser-105 induces nuclear export of the Nur77/RXR heterodimer complex 40, providing an additional mechanism by which phosphorylation may inhibit the transcriptional activity of Nur77. In addition to phosphorylation, all NR4A receptors contain sumoylation consensus sites, and sumoylation of Nurr1 induces or inhibits the transcriptional activity in a sumoylation site-specific manner 41. Although still in its infancy, these postranslational modifications regulate the transcriptional activity and may represent a major mode of the control of gene expression by NR4A receptors.
NR4A Receptors in Metabolism and Energy Balance
Carbohydrate Metabolism
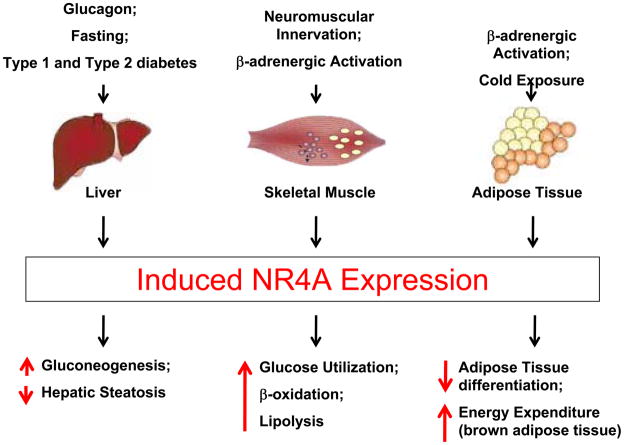
All three NR4A receptors are potently induced in the liver in response to physiological stimuli, including fasting and glucagon stimulation (Figure 1) 42, 43. Furthermore, hepatic NR4A receptor expression is increased in diabetic mice as a model of pathologic gluconeogenesis 42. Functional studies further demonstrated that adenoviral overexpression of Nur77 increases the expression of genes involved in gluconeogenesis and stimulates hepatic glucose production in mice 42. Interestingly, Nur77 overexpression induces several gluconeogenic genes including G6pc, Fbp1 and Fbp2, and enolase 3, which all contain NBRE consensus sites in their promoters 42. Therefore, this study has provided the first experimental evidence that NR4A receptors regulate gluconeogenesis and may serve to link hormonal stimulation to downstream metabolic gene expression.
Figure 1.
NR4A Receptor Function in Metabolism and Energy Balance. NR4A orphan nuclear receptors are potently induced by physiological and pathological stimuli in liver, muscle and adipose tissue. In these tissues, NR4A orphan receptors function as transcriptional regulators of gene expression programs involved in the control of glucose homeostasis, lipid metabolism, and energy expenditure (see text for details).
In skeletal muscle, NR4A receptors are induced by growth factors, β-adrenergic signalling, and endurance exercise 44–47. Maxwell et al. first demonstrated that knock-down of Nur77 in muscle cells results in decreased lipolysis and expression of genes regulating energy expenditure and lipid homeostasis, including AMP-activated protein kinase, UCP3, Glut4, CD36, adiponectin receptor 2, and caveolin-345. Conversely, Cao et al. reported that overexpression of Nur77 in C2C12 muscle cells increases the expression of genes involved in glucose and glycogen metabolism while Nur77 deficiency in mice reduces the expression of genes involved in skeletal muscle glucose utilization in vivo 48. Consistent with this role of Nur77 to promote glucose utilization was the observation that Nur77-deficient mice develop skeletal muscle insulin resistance when fed a high fat diet due to altered insulin signaling and reduced GLUT4 expression 49. Although glucose metabolism has not been studied in NOR1-deficient mice, knock-down of NOR1 in skeletal muscle cells attenuates the expression of genes that control fatty acid oxidation and pyruvate use (i.e. PGC-1α, PGC-1β, lipin-1α, PDP1r and PDP1c) indicating that NOR1 may be necessary for oxidative metabolism 50. Finally, NOR1 has recently been demonstrated to also promote insulin-stimulated glucose uptake in adipocytes by augmenting insulin signaling and Glut4 translocation 51. In concert, these intriguing observations point to a key role of NR4A receptors in the transcriptional control of glucose homeostasis and oxidative metabolism.
Lipid Metabolism
Accumulating evidence indicates that NR4A receptors regulate various aspects of lipid metabolism. As noted earlier, initial experiments by Maxwell et al. demonstrated that Nur77 promotes lipolysis in muscle 45. Subsequently, Pols et al. revealed that Nur77 modulates plasma lipoprotein profiles and hepatic lipid metabolism in mice 52. In this study, adenoviral-mediated overexpression of Nur77 increased plasma LDL cholesterol and decreased HDL cholesterol while reducing hepatic triglyceride levels, which was thought to be due to a repression of the lipogenic transcription factor SREBP1c 52. Consistent with these data, Chao et al. noted hepatic steatosis and increased SREBP1c expression in Nur77-deficient mice fed a high fat diet 49. However, since Nur77 did not directly affect SREBP1c activity in reporter assays, the authors concluded that the hepatic steatosis in Nur77-deficient mice was likely secondary to the lipogenic effect of hyperinsulinemia 49.
In 3T3-L1 preadipocytes, NR4A receptors expression is induced during adipogenesis and initiating of the differentiation program 53, 54. Initial studies using siRNA approaches and overexpression of a Nur77 mutant lacking the N-terminal AF-1 transactivation domain indicated that Nur77 is not required for adipocyte differentiation 55. However, a functional role for NR4A receptors in adipogenesis was suggested by two recent in vitro studies, which have demonstrated that constitutive NR4A receptor expression in 3T3-L1 preadipocytes inhibits adipocyte differentiation 56, 57. One of the mechanisms proposed for this negative regulation of adipogenesis by NR4A receptors has been the inhibition of the mitotic clonal expansion of preadipocytes, 56. However, considering that the initial mitotic expansion step is primarily a prerequisite for 3T3-L1 preadipocyte differentiation, further studies seem required and there are likely additional mechanisms involved by which NR4A receptors inhibit adipogenesis. These may include a direct regulation of target genes affecting adipogenesis, including extracellular matrix genes 56. In addition, NR4A receptors may cross-talk with adipogenic signalling and transcriptional programs, particularly since Nurr1 and Nur77 have been reported to interact with Wnt signaling pathways or the glucocorticoid receptor, which both play important roles in adipogenesis 58–60.
Energy Homeostasis
Brown adipose tissue plays a key role in energy balance and is the primary organ involved in thermogenesis through uncoupling of mitochondrial respiration by the action of uncoupling proteins (UCP). Early studies have demonstrated that Nur77 expression is highly induced in response to β-adrenergic stimulation of brown adipocytes while transcript levels of all three NR4A receptors are induced during cold-exposure 61, 62. Kanzleiter et al. demonstrated a repressive effect of Nur77 on the UCP-1 promoter in brown adipocytes, which was likely indirect since Nur77 did not directly interact with the UCP-1 promoter 61. Despite this repression of UCP-1, nonshivering thermogenesis was not affected by Nurr77 deficiency in mice 61. In contrast, Kumar et al. observed that NOR1 transcriptionally up-regulates UCP-1 expression by binding to an NBRE site on the UCP-1 promoter 62. Furthermore, overexpression of a Nur77 mutant lacking the N-terminal AF-1 transactivation domain prevented UCP-1 transcription induced by β-adrenergic signaling 62. The reasons underlying these seemingly conflicting two studies remain unclear but are likely due to a differential regulation of UCP-1 by Nur77 and NOR1. Moreover, NR4A receptors may affect the central regulation of energy homeostasis since injection of NOR1 siRNA into the third cerebral ventricle significantly suppresses food intake and body weight in mice 63. In concert, these intriguing studies characterize NR4A receptors as important regulators of energy balance and food intake, although the underlying mechanisms remain elusive and warrant further studies in gene-targeted mice.
NR4A Receptors in Vascular Biology
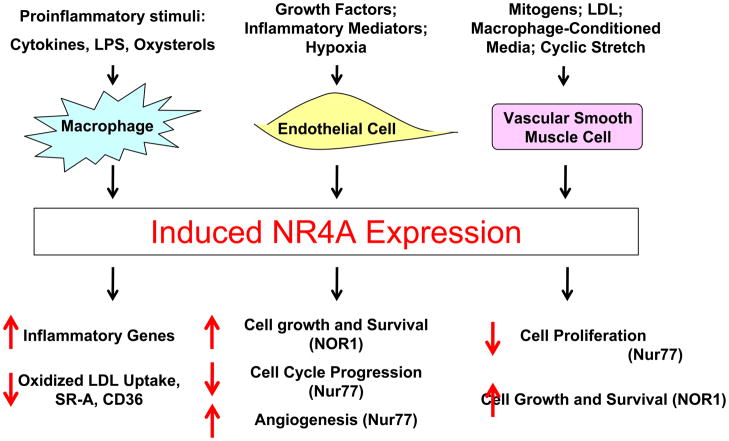
An increasing number of studies have demonstrated that all three members of the NR4A subfamily are expressed in the developing neointima and in advanced atherosclerotic lesions 64–70. Moreover, accumulating evidence indicates that NR4A receptors constitute important transcription factors in the control of vascular gene expression and play critical roles in essentially all aspects of vascular remodeling, including cell viability, proliferation, and inflammation (Figure 2). In the following section, we will briefly summarize these studies pointing to a previously unrecognized function of NR4A receptors in vascular biology.
Figure 2.
Expression and Function of NR4A Receptors in Vascular Cells. NR4A orphan nuclear receptors are induced in vascular cells by a variety of stimuli, including inflammatory mediators, cytokines, hypoxia, and growth factors. In response to these pathophysiological environmental cues, NR4A receptors modulate gene expression leading to cell-specific processes (see text for details).
Cell Viability and Proliferation
Endothelial cell injury followed by the expression of adhesion molecules and the subsequent recruitment of circulating monocytes constitute critical events for the initiation of atherosclerosis 71. All three NR4A receptors are potently induced by a variety of pro-atherogenic stimuli in endothelial cells, including atherogenic lipoproteins, inflammatory cytokines, growth factors, and hypoxia (Figure 2) 65, 72–78. The transcriptional mechanisms governing this inducible expression have been primarily studied in the context of growth factor and hypoxia-induced NOR1 expression. While the former mechanisms involve a cAMP response element binding protein (CREB)-dependent activation of the NOR1 promoter 74, 78, NOR1 expression in response to hypoxia is dependent on hypoxia-inducible factor 1 (HIF-1) binding to a hypoxia response element in the promoter 76. Arkenbout et al. performed the first functional experiments in endothelial cells and demonstrated that adenoviral overexpression of Nur77 inhibits proliferation of this cell type by upregulating p27Kip1 and downregulating cyclin A 65. However, the role of Nur77 for endothelial cell proliferation remains controversial since Zeng et al. reported that Nur77 induces proliferation and cell cycle gene expression 75. Moreover, this report noted that angiogenesis is induced by overexpression of Nur77 and decreased in Nur77−/− mice 75. With respect to the sibling NOR1, Rius et al. identified a mitogenic role for this receptor by demonstrating that antisense oligonucleotides against NOR1 inhibit endothelial cell growth and wound repair after injury 74. Consistent with these observations, NOR1 has recently been characterized as a pro-survival gene in endothelial cells exposed to hypoxia by inducing the expression of cellular inhibitor of apoptosis protein 276. Collectively, these studies establish a role for Nur77 and NOR1 in regulating endothelial cell survival and proliferation; however, little is known about the transcriptional target genes and molecular mechanisms. At present, only two direct NR4A target genes have been identified in endothelial cells. Gruber et al. characterized PAI-1 as a Nur77 target gene, which is activated by the receptor through direct binding to an NBRE site in the promoter 72. In addition, You et al. demonstrated that Nur77 overexpression prevents NF-κB nuclear translocation in endothelial cells by enhancing the expression of IκBα, which is mediated through a direct transactivation of a NBRE site in the IκBα promoter 77. Interestingly, the functional relevance of Nur77-dependent IκBα expression was confirmed by the finding that Nur77 inhibited the expression of VCAM-1 and ICAM-1 in endothelial cells 77.
Similarly as in endothelial cells, NR4A receptor expression is rapidly induced in response to atherogenic stimulation of smooth muscle cells (SMC), including lipoproteins, cyclic stretch, and mitogenic stimuli 64, 66–68, 79. The transcriptional induction of NOR1 in SMC is mediated through mitogen-induced CREB binding to CRE sites in the NOR1 promoter and can be pharmacologically inhibited by simvastatin 66, 67, 79, 80. Consistent with the earlier described growth-inhibitory function of Nur77 in endothelial cells, Nur77 overexpression inhibits SMC proliferation in vitro by stabilizing of p27Kip1 64, 81 and reduces neointima formation in vivo 64. Interestingly, data from the same group has further recently suggested that SMC-specific overexpression of Nur77 inhibits pathological outward remodeling in response to carotid artery ligation, which was associated with decreased macrophage accumulation and MMP expression 82. While these studies clearly indicate that Nur77 prevents SMC proliferation, NOR1 has been reported to act mitogenic suggesting a function that is distinct from that of Nur77. A proliferative role of NOR1 was first reported by Martínez-González et al. using antisense NOR1 oligonucleotides 66. Consistent with these initial observations, data from our group has demonstrated a proliferative defect and an increased propensity for apoptosis in SMC isolated from NOR1-deficient mice 67, 68. In vivo, the proliferative response and neointima formation following endovascular femoral artery guide wire injry was decreased in NOR1-deficient mice 68. At a molecular level, this mitogenic activity of NOR1 was at least in part mediated by a transactivation of a canonical NBRE site in the cyclin D1 promoter, characterizing cyclin D1 as a bona fide NOR1 target gene in SMC 68. Furthermore, DNA microarray profiling revealed a lower expression of NOR1 in elongated SMC while NOR1 knock-down suppressed DNA synthesis, further supporting the mitogenic function of NOR1 and pointing to a potential role of NOR1 in regulating cell shape 83. In concert, these studies establish not only an important but also distinct role for Nur77 and NOR1 in the control of vascular cell proliferation and remodeling. Continued investigation will be required to define the transcriptional target genes and the molecular basis underlying the differential function of NOR1 and Nur77 in SMC biology.
Inflammation
The first evidence linking NR4A expression with inflammatory signaling was reported by Woronicz et al. and Liu et al., who noted that Nur77 is induced in apoptotic T-cells and that inhibition of Nur77 function prevented apoptosis 84, 85. However, mice deficient in Nur77 exhibit unimpaired T-cell apoptosis, and functional redundancy of Nur77 and NOR1 in T-cell apoptosis has been suggested 86, 87. Similarly to T-cells, Nur77 expression is increased in apoptotic macrophages and, in contrast to the experiments performed in T-cells, peritoneal macrophages isolated from Nur77-deficient mice reveal a phenotype of reduced cell death 88. In response to inflammatory activation, all three NR4A receptors are potently induced in macrophages 69, 70. This inducible expression of NR4A receptors in macrophages depends on the activation of NF-κB signaling, as exemplified by the recruitment of NF-κB to response elements in the Nur77 promoter 69. Functional studies have indicated that NR4A receptors both activate and repress inflammatory genes in macrophages 33, 70, 89. An initial microarray analysis by Pei et al. discovered that NR4A overexpression in macrophages induces proinflammatory gene expression 89. Interestingly, among the identified direct Nur77 target genes was the inducible kinase IKKi/IKKepsilon, which functions as a NF-κB activating kinase, providing a potential mechanism for the activation of inflammatory gene expression by Nur77 in macrophages 89. In contrast to these studies, Bonta et al. revealed that lentiviral overexpression of each NR4A member reduces certain inflammatory genes (i.e. IL-1β, IL-6, IL-8, MIP1α and 1β and MCP-1) and the uptake of oxidized LDL 70. Finally, a recent study by Saijo et al. identified that Nurr1 transcriptionally represses the inflammatory genes TNFα, iNOS, and IL-1β in microglia and the murine RAW264.7 cell line 33. This transrepression was mediated through a Nurr1-dependent recruitment of the corepressor for element-1-silencing transcription factor (CoREST) complex to the target promoter and the subsequent clearance of NF-κB 33. While these studies indicate that NR4A receptors function as important transcriptional regulators of inflammatory gene expression, further in vivo evidence using animal models deficient for either of the NR4A receptors seems required, particularly with respect to the development of atherosclerosis.
Concluding Remarks
In conclusion, the ligand-independent NR4A orphan nuclear receptors are immediate early response genes, whose protein products are rapidly induced in metabolic and vascular tissues in response to a pleiotropy of stimuli. Emerging evidence indicates that NR4A receptors regulate the transcription of genes involved in glucose homeostasis, lipid metabolism, and energy balance. Moreover, the initial characterization of their function in vascular biology has implicated these transcription factors in the control of inflammation, proliferation, apoptosis, thrombosis, and angiogenesis. Despite recent advances in understanding the role of NR4A receptor function in physiological and pathological processes, important questions remain for future research. For example, future effort will require further validation of NR4A receptor function in murine models and rely on various gene-targeting approaches. In particular, it seems essential to determine whether the three different NR4A receptors exhibit similar or distinct functions in various tissues. Furthermore, at present, few NR4A receptor-regulated genes have been identified, and it will not only be important to characterize target genes but also to define the detailed transcriptional mechanisms underlying this regulation. Finally, a possibility to modulate the expression and/or transcriptional activity of NR4A receptors may provide pharmacological applications. Considering the lack of a classical ligand-binding pocket, such approach might involve the modulation of cofactor recruitment and/or posttranslational modifications. Continued investigation of these questions and identification of NR4A-regulated target genes will provide new insights into how these orphan nuclear receptors participate in the development of physiology and disease.
Acknowledgments
Dennis Bruemmer is supported by the National Institutes of Health (RO1 HL084611). Yue Zhao is recipient of a Predoctoral Fellowship Grants from the American Heart Association (0815514D).
Footnotes
Disclosures
None.
References
- 1.Olefsky JM. Nuclear receptor minireview series. J Biol Chem. 2001;276:36863–36864. doi: 10.1074/jbc.R100047200. [DOI] [PubMed] [Google Scholar]
- 2.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–614. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefebvre P, Chinetti G, Fruchart JC, Staels B. Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara T, Yoshioka S, Yoshioka T, Ushiyama I, Horikoshi H. Characterization of new oral antidiabetic agent CS-045. Studies in KK and ob/ob mice and Zucker fatty rats. Diabetes. 1988;37:1549–1558. doi: 10.2337/diab.37.11.1549. [DOI] [PubMed] [Google Scholar]
- 6.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2001;2:29. doi: 10.1186/gb-2001-2-8-research0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germain P, Staels B, Dacquet C, Spedding M, Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58:685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 10.Hummasti S, Tontonoz P. Adopting new orphans into the family of metabolic regulators. Mol Endocrinol. 2008;22:1743–1753. doi: 10.1210/me.2007-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 12.Law SW, Conneely OM, DeMayo FJ, O’Malley BW. Identification of a new brain-specific transcription factor, NURR1. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- 13.Ohkura N, Ito M, Tsukada T, Sasaki K, Yamaguchi K, Miki K. Structure, mapping and expression of a human NOR-1 gene, the third member of the Nur77/NGFI-B family. Biochim Biophys Acta. 1996;1308:205–214. doi: 10.1016/0167-4781(96)00101-7. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005;65:609–618. doi: 10.1016/j.cardiores.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Pols TW, Bonta PI, de Vries CJ. NR4A nuclear orphan receptors: protective in vascular disease? Curr Opin Lipidol. 2007;18:515–520. doi: 10.1097/MOL.0b013e3282ef77d1. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 18.Saucedo-Cardenas O, Kardon R, Ediger TR, Lydon JP, Conneely OM. Cloning and structural organization of the gene encoding the murine nuclear receptor transcription factor, NURR1. Gene. 1997;187:135–139. doi: 10.1016/s0378-1119(96)00736-6. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen RE, Weaver CA, Fahrner TJ, Milbrandt J. Domains regulating transcriptional activity of the inducible orphan receptor NGFI-B. J Biol Chem. 1992;267:16491–16496. [PubMed] [Google Scholar]
- 20.Wansa KD, Harris JM, Yan G, Ordentlich P, Muscat GE. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278:24776–24790. doi: 10.1074/jbc.M300088200. [DOI] [PubMed] [Google Scholar]
- 21.Maira M, Martens C, Batsche E, Gauthier Y, Drouin J. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23:763–776. doi: 10.1128/MCB.23.3.763-776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wansa KD, Harris JM, Muscat GE. The activation function-1 domain of Nur77/NR4A1 mediates trans-activation, cell specificity, and coactivator recruitment. J Biol Chem. 2002;277:33001–33011. doi: 10.1074/jbc.M203572200. [DOI] [PubMed] [Google Scholar]
- 23.Tremblay A, Tremblay GB, Labrie F, Giguere V. Ligand-independent recruitment of SRC-1 to estrogen receptor beta through phosphorylation of activation function AF-1. Mol Cell. 1999;3:513–519. doi: 10.1016/s1097-2765(00)80479-7. [DOI] [PubMed] [Google Scholar]
- 24.Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell. 1999;3:521–526. doi: 10.1016/s1097-2765(00)80480-3. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Benoit G, Liu J, Prasad S, Aarnisalo P, Liu X, Xu H, Walker NP, Perlmann T. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423:555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 26.Wilson TE, Fahrner TJ, Johnston M, Milbrandt J. Identification of the DNA binding site for NGFI-B by genetic selection in yeast. Science. 1991;252:1296–1300. doi: 10.1126/science.1925541. [DOI] [PubMed] [Google Scholar]
- 27.Wilson TE, Padgett KA, Johnston M, Milbrandt J. A genetic method for defining DNA-binding domains: application to the nuclear receptor NGFI-B. Proc Natl Acad Sci U S A. 1993;90:9186–9190. doi: 10.1073/pnas.90.19.9186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Philips A, Lesage S, Gingras R, Maira MH, Gauthier Y, Hugo P, Drouin J. Novel dimeric Nur77 signaling mechanism in endocrine and lymphoid cells. Mol Cell Biol. 1997;17:5946–5951. doi: 10.1128/mcb.17.10.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perlmann T, Jansson L. A novel pathway for vitamin A signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9:769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- 30.Zetterstrom RH, Solomin L, Mitsiadis T, Olson L, Perlmann T. Retinoid X receptor heterodimerization and developmental expression distinguish the orphan nuclear receptors NGFI-B, Nurr1, and Nor1. Mol Endocrinol. 1996;10:1656–1666. doi: 10.1210/mend.10.12.8961274. [DOI] [PubMed] [Google Scholar]
- 31.Sacchetti P, Dwornik H, Formstecher P, Rachez C, Lefebvre P. Requirements for heterodimerization between the orphan nuclear receptor Nurr1 and retinoid X receptors. J Biol Chem. 2002;277:35088–35096. doi: 10.1074/jbc.M205816200. [DOI] [PubMed] [Google Scholar]
- 32.Maira M, Martens C, Philips A, Drouin J. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19:7549–7557. doi: 10.1128/mcb.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis IJ, Hazel TG, Chen RH, Blenis J, Lau LF. Functional domains and phosphorylation of the orphan receptor Nur77. Mol Endocrinol. 1993;7:953–964. doi: 10.1210/mend.7.8.8232315. [DOI] [PubMed] [Google Scholar]
- 35.Wingate AD, Campbell DG, Peggie M, Arthur JS. Nur77 is phosphorylated in cells by RSK in response to mitogenic stimulation. Biochem J. 2006;393:715–724. doi: 10.1042/BJ20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, Reed JC, Dawson MI, Zhang XK. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 37.Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of NUR77/NURR1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;16:1638–1651. doi: 10.1210/mend.16.7.0863. [DOI] [PubMed] [Google Scholar]
- 38.Hirata Y, Kiuchi K, Chen HC, Milbrandt J, Guroff G. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J Biol Chem. 1993;268:24808–24812. [PubMed] [Google Scholar]
- 39.Li Y, Lau LF. Adrenocorticotropic hormone regulates the activities of the orphan nuclear receptor Nur77 through modulation of phosphorylation. Endocrinology. 1997;138:4138–4146. doi: 10.1210/endo.138.10.5464. [DOI] [PubMed] [Google Scholar]
- 40.Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol. 2000;2:435–440. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 41.Galleguillos D, Vecchiola A, Fuentealba JA, Ojeda V, Alvarez K, Gomez A, Andres ME. PIASgamma represses the transcriptional activation induced by the nuclear receptor Nurr1. J Biol Chem. 2004;279:2005–2011. doi: 10.1074/jbc.M308113200. [DOI] [PubMed] [Google Scholar]
- 42.Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med. 2006;12:1048–1055. doi: 10.1038/nm1471. [DOI] [PubMed] [Google Scholar]
- 43.Oita RC, Mazzatti DJ, Lim FL, Powell JR, Merry BJ. Whole-genome microarray analysis identifies up-regulation of Nr4a nuclear receptors in muscle and liver from diet-restricted rats. Mech Ageing Dev. 2009;130:240–247. doi: 10.1016/j.mad.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Lim RW, Zhu CY, Stringer B. Differential regulation of primary response gene expression in skeletal muscle cells through multiple signal transduction pathways. Biochim Biophys Acta. 1995;1266:91–100. doi: 10.1016/0167-4889(94)00226-5. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell MA, Cleasby ME, Harding A, Stark A, Cooney GJ, Muscat GE. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J Biol Chem. 2005;280:12573–12584. doi: 10.1074/jbc.M409580200. [DOI] [PubMed] [Google Scholar]
- 46.Pearen MA, Ryall JG, Maxwell MA, Ohkura N, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, is a target of beta-adrenergic signaling in skeletal muscle. Endocrinology. 2006;147:5217–5227. doi: 10.1210/en.2006-0447. [DOI] [PubMed] [Google Scholar]
- 47.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 48.Chao LC, Zhang Z, Pei L, Saito T, Tontonoz P, Pilch PF. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol Endocrinol. 2007;21:2152–2163. doi: 10.1210/me.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chao LC, Wroblewski K, Zhang Z, Pei L, Vergnes L, Ilkayeva OR, Ding SY, Reue K, Watt MJ, Newgard CB, Pilch PF, Hevener AL, Tontonoz P. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58:2788–2796. doi: 10.2337/db09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 2008;149:2853–2865. doi: 10.1210/en.2007-1202. [DOI] [PubMed] [Google Scholar]
- 51.Fu Y, Luo L, Luo N, Zhu X, Garvey WT. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem. 2007;282:31525–31533. doi: 10.1074/jbc.M701132200. [DOI] [PubMed] [Google Scholar]
- 52.Pols TW, Ottenhoff R, Vos M, Levels JH, Quax PH, Meijers JC, Pannekoek H, Groen AK, de Vries CJ. Nur77 modulates hepatic lipid metabolism through suppression of SREBP1c activity. Biochem Biophys Res Commun. 2008;366:910–916. doi: 10.1016/j.bbrc.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 53.Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. A Nuclear Receptor Atlas: 3T3-L1 adipogenesis. Mol Endocrinol. 2005;19:2437–2450. doi: 10.1210/me.2004-0539. [DOI] [PubMed] [Google Scholar]
- 54.Soukas A, Socci ND, Saatkamp BD, Novelli S, Friedman JM. Distinct transcriptional profiles of adipogenesis in vivo and in vitro. J Biol Chem. 2001;276:34167–34174. doi: 10.1074/jbc.M104421200. [DOI] [PubMed] [Google Scholar]
- 55.Au WS, Payne VA, O’Rahilly S, Rochford JJ. The NR4A family of orphan nuclear receptors are not required for adipogenesis. Int J Obes (Lond) 2008;32:388–392. doi: 10.1038/sj.ijo.0803763. [DOI] [PubMed] [Google Scholar]
- 56.Chao LC, Bensinger SJ, Villanueva CJ, Wroblewski K, Tontonoz P. Inhibition of adipocyte differentiation by Nur77, Nurr1, and Nor1. Mol Endocrinol. 2008;22:2596–2608. doi: 10.1210/me.2008-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fumoto T, Yamaguchi T, Hirose F, Osumi T. Orphan nuclear receptor Nur77 accelerates the initial phase of adipocyte differentiation in 3T3-L1 cells by promoting mitotic clonal expansion. J Biochem. 2007;141:181–192. doi: 10.1093/jb/mvm018. [DOI] [PubMed] [Google Scholar]
- 58.Philips A, Maira M, Mullick A, Chamberland M, Lesage S, Hugo P, Drouin J. Antagonism between Nur77 and glucocorticoid receptor for control of transcription. Mol Cell Biol. 1997;17:5952–5959. doi: 10.1128/mcb.17.10.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitagawa H, Ray WJ, Glantschnig H, Nantermet PV, Yu Y, Leu CT, Harada S, Kato S, Freedman LP. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signaling. Mol Cell Biol. 2007;27:7486–7496. doi: 10.1128/MCB.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Carpentier R, Sacchetti P, Segard P, Staels B, Lefebvre P. The glucocorticoid receptor is a co-regulator of the orphan nuclear receptor Nurr1. J Neurochem. 2008;104:777–789. doi: 10.1111/j.1471-4159.2007.05055.x. [DOI] [PubMed] [Google Scholar]
- 61.Kanzleiter T, Schneider T, Walter I, Bolze F, Eickhorst C, Heldmaier G, Klaus S, Klingenspor M. Evidence for Nr4a1 as a cold-induced effector of brown fat thermogenesis. Physiol Genomics. 2005;24:37–44. doi: 10.1152/physiolgenomics.00204.2005. [DOI] [PubMed] [Google Scholar]
- 62.Kumar N, Liu D, Wang H, Robidoux J, Collins S. Orphan nuclear receptor NOR-1 enhances 3′,5′-cyclic adenosine 5′-monophosphate-dependent uncoupling protein-1 gene transcription. Mol Endocrinol. 2008;22:1057–1064. doi: 10.1210/me.2007-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nonogaki K, Ohba Y, Sumii M, Wakameda M, Tamari T. Novel modulators for body weight changes induced by fasting and re-feeding in mice. Biochem Biophys Res Commun. 2009;378:249–254. doi: 10.1016/j.bbrc.2008.11.031. [DOI] [PubMed] [Google Scholar]
- 64.Arkenbout EK, de Waard V, van Bragt M, van Achterberg TA, Grimbergen JM, Pichon B, Pannekoek H, de Vries CJ. Protective function of transcription factor TR3 orphan receptor in atherogenesis: decreased lesion formation in carotid artery ligation model in TR3 transgenic mice. Circulation. 2002;106:1530–1535. doi: 10.1161/01.cir.0000028811.03056.bf. [DOI] [PubMed] [Google Scholar]
- 65.Arkenbout EK, van Bragt M, Eldering E, van Bree C, Grimbergen JM, Quax PH, Pannekoek H, de Vries CJ. TR3 orphan receptor is expressed in vascular endothelial cells and mediates cell cycle arrest. Arterioscler Thromb Vasc Biol. 2003;23:1535–1540. doi: 10.1161/01.ATV.0000084639.16462.7A. [DOI] [PubMed] [Google Scholar]
- 66.Martinez-Gonzalez J, Rius J, Castello A, Cases-Langhoff C, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003;92:96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 67.Nomiyama T, Nakamachi T, Gizard F, Heywood EB, Jones KL, Ohkura N, Kawamori R, Conneely OM, Bruemmer D. The NR4A orphan nuclear receptor NOR1 is induced by platelet-derived growth factor and mediates vascular smooth muscle cell proliferation. J Biol Chem. 2006;281:33467–33476. doi: 10.1074/jbc.M603436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nomiyama T, Zhao Y, Gizard F, Findeisen HM, Heywood EB, Jones KL, Conneely OM, Bruemmer D. Deficiency of the NR4A neuron-derived orphan receptor-1 attenuates neointima formation after vascular injury. Circulation. 2009;119:577–586. doi: 10.1161/CIRCULATIONAHA.108.822056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005;280:29256–29262. doi: 10.1074/jbc.M502606200. [DOI] [PubMed] [Google Scholar]
- 70.Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26:2288–2294. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 71.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gruber F, Hufnagl P, Hofer-Warbinek R, Schmid JA, Breuss JM, Huber-Beckmann R, Lucerna M, Papac N, Harant H, Lindley I, de Martin R, Binder BR. Direct binding of Nur77/NAK-1 to the plasminogen activator inhibitor 1 (PAI-1) promoter regulates TNF alpha-induced PAI-1 expression. Blood. 2003;101:3042–3048. doi: 10.1182/blood-2002-07-2331. [DOI] [PubMed] [Google Scholar]
- 73.Liu D, Jia H, Holmes DI, Stannard A, Zachary I. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler Thromb Vasc Biol. 2003;23:2002–2007. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 74.Rius J, Martinez-Gonzalez J, Crespo J, Badimon L. NOR-1 is involved in VEGF-induced endothelial cell growth. Atherosclerosis. 2006;184:276–282. doi: 10.1016/j.atherosclerosis.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martorell L, Gentile M, Rius J, Rodriguez C, Crespo J, Badimon L, Martinez-Gonzalez J. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol Cell Biol. 2009;29:5828–5842. doi: 10.1128/MCB.00945-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.You B, Jiang YY, Chen S, Yan G, Sun J. The orphan nuclear receptor Nur77 suppresses endothelial cell activation through induction of IkappaBalpha expression. Circ Res. 2009;104:742–749. doi: 10.1161/CIRCRESAHA.108.192286. [DOI] [PubMed] [Google Scholar]
- 78.Martorell L, Martinez-Gonzalez J, Crespo J, Calvayrac O, Badimon L. Neuron-derived orphan receptor-1 (NOR-1) is induced by thrombin and mediates vascular endothelial cell growth. J Thromb Haemost. 2007;5:1766–1773. doi: 10.1111/j.1538-7836.2007.02627.x. [DOI] [PubMed] [Google Scholar]
- 79.Rius J, Martinez-Gonzalez J, Crespo J, Badimon L. Involvement of neuron-derived orphan receptor-1 (NOR-1) in LDL-induced mitogenic stimulus in vascular smooth muscle cells: role of CREB. Arterioscler Thromb Vasc Biol. 2004;24:697–702. doi: 10.1161/01.ATV.0000121570.00515.dc. [DOI] [PubMed] [Google Scholar]
- 80.Crespo J, Martinez-Gonzalez J, Rius J, Badimon L. Simvastatin inhibits NOR-1 expression induced by hyperlipemia by interfering with CREB activation. Cardiovasc Res. 2005;67:333–341. doi: 10.1016/j.cardiores.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 81.de Waard V, Arkenbout EK, Vos M, Mocking AI, Niessen HW, Stooker W, de Mol BA, Quax PH, Bakker EN, VanBavel E, Pannekoek H, de Vries CJ. TR3 nuclear orphan receptor prevents cyclic stretch-induced proliferation of venous smooth muscle cells. Am J Pathol. 2006;168:2027–2035. doi: 10.2353/ajpath.2006.050932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bonta PI, Matlung HL, Vos M, Peters SL, Pannekoek H, Bakker EN, de Vries CJ. Nuclear Receptor Nur77 inhibits vascular outward remodeling and reduces macrophage accumulation and matrix metalloproteinase levels. Cardiovasc Res. doi: 10.1093/cvr/cvq064. [DOI] [PubMed] [Google Scholar]
- 83.Thakar RG, Cheng Q, Patel S, Chu J, Nasir M, Liepmann D, Komvopoulos K, Li S. Cell-shape regulation of smooth muscle cell proliferation. Biophys J. 2009;96:3423–3432. doi: 10.1016/j.bpj.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 85.Liu ZG, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- 86.Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269:532–535. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 87.Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. EMBO J. 1997;16:1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim SO, Ono K, Tobias PS, Han J. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med. 2003;197:1441–1452. doi: 10.1084/jem.20021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pei L, Castrillo A, Tontonoz P. Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol. 2006;20:786–794. doi: 10.1210/me.2005-0331. [DOI] [PubMed] [Google Scholar]