Abstract
Introduction
The objective was to determine the effects of growth factor treatment on dental pulp cell sensitivity to toxicity of two composite restoration materials, Flow Line and Durafill VS, and a calcium hydroxide pulp capping material, Dycal.
Methods
Toxicity of the dental materials to cultures of primary dental pulp cells was determined by the MTT metabolism assay. The ability of six different growth factors to influence the toxicity was tested.
Results
A 24 hour exposure to either Flow Line or Durafill VS caused approximately 40% cell death, while Dycal exposure caused approximately 80% cell death. The toxicity of Flow Line and Durafill VS was mediated by oxidative stress. Four of the growth factors tested (BMP-2, BMP-7, EGF, and TGF-β) decreased the basal MTT values while making the cells resistant to Flow Line and Durafill VS toxicity, except BMP-2 which made the cells more sensitive to Flow Line. Treatment with FGF-2 caused no change in basal MTT metabolism, prevented the toxicity of Durafill VS, but increased the toxicity of Flow Line. Treatment with IGF-I increased basal MTT metabolism and made the cells resistant to Flow Line and Durafill VS toxicity. None of the growth factors made the cells resistant to Dycal toxicity.
Conclusions
The results indicate that growth factors can be used to alter the sensitivity of dental pulp cells to commonly used restoration materials. The growth factors BMP-7, EGF, TGF-β, and IGF-I provided the best profile of effects, making the cells resistant to both Flow Line and Durafill VS toxicity.
Keywords: Pulp capping, dental pulp, toxicity, IGF-I, BMP-2, BMP-7, EGF, FGF-2, TGF-β
Introduction
Direct pulp capping involves covering the exposed pulp with the intent of preserving its viability. The goal is to not only provide a protective coat, but to also stimulate dentin bridge formation. Calcium hydroxide has traditionally been the most commonly used pulp capping material because it can stimulate dentin bridge formation (1) and appears to stimulate healing and repair (2). However, the success rate of pulp capping has been highly variable (3), and its effectiveness has been questioned (4). The concerns regarding the use of calcium hydroxide involve: a high rate of tunnel defects in dentin bridges under calcium hydroxide, it may dissolve, it may degrade upon tooth flexure, and it has been shown to pull away from the cavity surface leaving a gap between the calcium hydroxide and the dentin (2,5). A further complication of treatment involving calcium hydroxide is that if this therapy fails, subsequent endodontic therapy is difficult due to calcification in the root canal (6). A potential alternative to the use of calcium hydroxide is the use of growth factors. Growth factors are naturally occurring proteins that cause cellular proliferation, differentiation and maturation. Specifically, they have been shown to stimulate odontoblast differentiation and dentin formation (7,8). An advantage of growth factors over calcium hydroxide is that they stimulate the formation of reparative dentin that is mainly superficial to the pulp tissue, while the effects of calcium hydroxide are often at the expense of the pulp tissue (9). However, before growth factor treatments can be fully implemented, further studies need to be conducted to understand in detail their effects on pulp cells.
In the current study we tested the effects of the growth factors, bone morphogenic peptide-2 and 7 (BMP-2 and 7), fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and insulin-like growth factor-I (IGF-I), on dental pulp cells to determine if they alter the cells sensitivity to toxicity of composite restoration materials and a calcium hydroxide pulp capping material. Each of the growth factors was chosen because it has been shown to alter pulp cell growth or differentiation. BMP-2 can induce differentiation of odontoblasts (10,11) is produced in developing teeth (12), and can induce dentin formation in vivo (13). BMP-7 is expressed in developing teeth (14) and can induce the formation of reparative dentin in an in vivo model (15). BMP-7 has also been shown to cause increased extracellular matrix secretion in vitro (16). FGF-2 is expressed during tooth development (17), it has been associated with tooth morphogenesis (18), and it promotes the differentiation of dental pulp cells into odontoblasts in culture (19). EGF is a mitogenic protein that stimulates proliferation and differentiation of a wide variety of cell types. The receptor for EGF has been shown to be present in differentiating odontoblasts (20) and EGF is expressed in developing teeth (21). TGF-β can stimulate primary odontoblasts (10), is expressed in developing teeth (22), and increases alkaline phosphatase activity and formation of mineralization nodules (23). IGF-I is produced in response to growth hormone and induces subsequent cellular alterations. Specifically, It has been shown to increase alkaline phosphatase activity in cultured dental pulp cells (24) and can enhance reparative dentinogenesis in vivo (25). Some of the growth factors being tested are already in clinical use for other applications; IGF-1 is used to treat IGF-1 deficiency, BMP-2 and 7 are used enhance bone growth (26,27), and EGF has been used to enhance wound healing (28).
The aim of the present study was to test whether growth factors can alter the sensitivity of pulp cells to the toxicity of dental materials. We studied the effects of growth factor treatment on the toxicity of two commonly used restoration materials (Flow Line and Durafill VS), and a commonly used calcium hydroxide based pulp capping material (Dycal). An emphasis was placed on the toxicity of restoration materials because a goal is to eventually replace traditional pulp capping materials with treatment involving growth factors.
Materials and methods
Materials
Serum was obtained from Life Technologies (Gaithersburg, MD, USA). Flow Line, Durafill VS, and Dycal were obtained from Henry Schein Inc. (Melville, NY, USA) Growth factors were obtained from ProSpec-Tany Technogene (Rehorot, Israel). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Subjects and human dental pulp cell cultures
Normal human impacted third molars were collected from adults at the Marquette University School of Dentistry Surgical Services Department with informed consent under a protocol approved by the Institutional Review Board at Marquette University. Tooth surfaces were cleaned and cut around the cementum-enamel junction by using sterilized diamond stones to access the pulp chamber. The pulp tissue was separated from the tooth and digested in a solution of 3 mg/ml collagenase type I and 4 mg/ml dispase for 1 hour at 37°C (29,30). The cells were then plated onto 24-well plates coated with poly-D-lysine and laminin in Eagle’s medium supplemented with 20% fetal calf serum/100 μM L-ascorbic acid 2-phosphate/2 mM L-glutamine/100 units/ml penicillin/100 μg/ml streptomycin, and then incubated at 37°C with 5% CO2 Growth factors (100 ng/ml) were added at the time of plating on 24 well plates for experiments. Toxicity experiments were performed on cultures 6–8 days in vitro. Growth factors were added at plating to achieve their maximal effect of the differentiation of the cultures. The effects of growth factors on cell viability was assessed using the MTT assay at the time of the experiments.
Preparation of dental materials and exposure to cultures
Flow Line, Durafill VS, and Dycal were prepared according to the manufacturer’s instructions. The freshly prepared materials were placed into the wells containing cultured cells for 24 hours in media similar to plating media except lacking serum. The weights of the materials used were: Flow Line: 6.4 ± .6 mg; Durafill VS: 6.6 ± .4 mg; Dycal: 2.2 ± .1 mg. The dental materials are light and caused no obvious physical damage to the cells. In experiments where trolox or ZVAD were tested, they were present during the 24 hour exposure to the dental materials.
Cell viability assessment
Cell injury was quantified by the measurement of the reduction of 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide (MTT) to produce a dark blue formazan product (31). MTT was added to each well 24 hours after the beginning of the insult to the cells. After a 30 minute incubation, the media was removed, and cells dissolved in DMSO. The formation of formazan was measured as the amount of reaction product by absorbance change at a wavelength of 590 nm using a VersaMax tunable microplate reader (Molecular Devices, Sunnyvale, CA). Levels of formazan formation from cultures exposed to 10 μM of the calcium ionophore A23187 (100% cell death) were subtracted from insult formazan levels, and results were normalized to a sham wash.
Statistical analysis
Statistical calculations were performed using one-way ANOVA followed by the Bonferroni t-test. P-values <0.05 were considered to indicate significant differences. Each individual well is considered an n = 1. Data is presented as mean + SEM.
Results
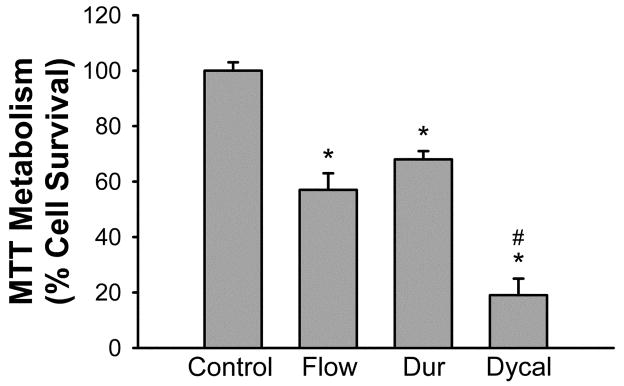
We first tested whether 24 hour exposure to Flow Line, Durafill VS, or Dycal was toxic to dental pulp cells as measured by the MTT metabolism assay. Each of the compounds was toxic, with Flow Line and Durafill VS inducing similar toxicity, and Dycal causing significantly greater toxicity (Fig 1).
Figure 1.
Flow Line, Durafill VS, and Dycal each cause significant toxicity to dental pulp cells, with Dycal causing significantly more toxicity. Flow: Flow Line; Dur: Durafill VS. Cultures were exposed to the dental materials for 24 hours. Bars show % cell survival (mean ± SEM, n=15–24) quantified by inhibition of MTT reduction. Control represents MTT levels in untreated cultures and is defined as 100% cell survival. * indicates significant difference from control (P < 0.05). # indicates significant difference from Flow Line and Durafill VS (P < 0.05).
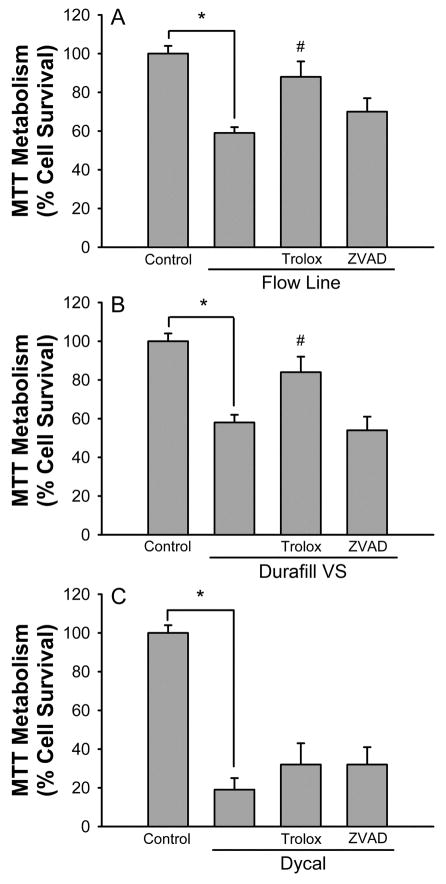
To obtain an indication of the mechanism of toxicity induced by each of the compounds we tested the protective effects of trolox and Z-Val-Ala-Asp-fluoromethylketone (ZVAD). Trolox is a free radical scavenger and if it had protective effects that would indicate an oxidative stress mediated cell death, while ZVAD is a general caspase inhibitor and if it had protective effects that would indicate an apoptotic cell death. Trolox was protective against both Flow Line and Durafill VS toxicity, but had no effect on Dycal toxicity (Fig 2), suggesting that oxidative stress played a role in the death process induced by Flow Line and Durafill VS. ZVAD was not protective against the toxicity induced by any of the compounds (Fig 2), suggesting that the death did not occur through caspase mediated apoptosis.
Figure 2.
The toxicity of Flow Line and Durafill VS is attenuated by the free radical scavenger trolox (100 μM), but not by the caspase inhibitor ZVAD (100 μM). The toxicity of Dycal is not attenuated by either compound. The trolox and ZVAD were present during the 24 hour exposure to the dental materials. Control represents MTT levels in untreated cultures and is defined as 100% cell survival. Bars show % cell survival (mean ± SEM, n=12–16) quantified by inhibition of MTT reduction. * indicates significant difference from control (P < 0.05).
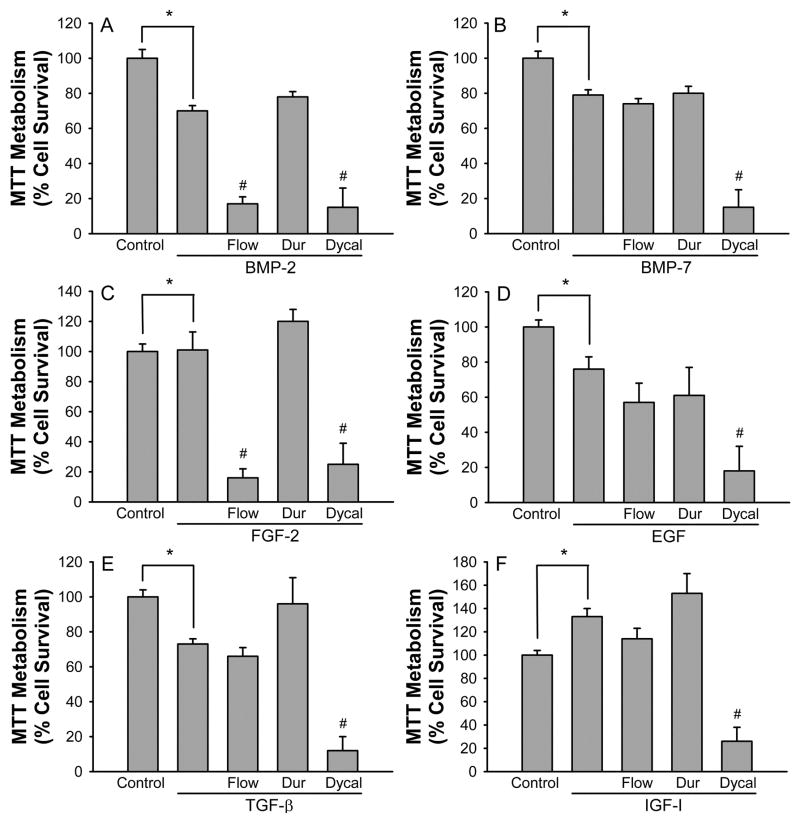
We next tested the effects of six different growth factors that have been implicated in pulp cell differentiation on their ability to induce resistance to toxicity. Treatment with BMP-2 caused decreased basal MTT metabolism values and prevented Durafill VS toxicity. However, Flow Line remained toxic and Dycal killed even more cells (Fig 3A). Treatment with BMP-7, EGF, and TGF-β all had similar effects; they caused decreased basal MTT metabolism, increased cell survival in the presence of Flow Line and Durafill VS, but did not prevent Dycal toxicity (Fig 3B, D, and E). Treatment with FGF-2 caused no change in basal MTT metabolism, increased cell survival in the presence of Durafill VS, increased Flow Line toxicity, and had no effect on Dycal toxicity (Fig 3C). Finally, IGF-I actually increased the basal levels of MTT metabolism and increased the cell survival in the presence of Flow Line and Durafill VS toxicity, while it had no effect on Dycal toxicity (Fig 3F).
Figure 3.
Growth factor treatment can alter the sensitivity of pulp cells to the toxicity of Flow Line and Durafill VS. A). BMP-2 decreases basal MTT metabolism and makes the cells resistant to Durafill VS toxicity. B). BMP-7 decreases basal MTT metabolism and makes the cells resistant to Durafill VS and Flow Line toxicity. C). FGF-2 has no effect on basal MTT metabolism but makes the cells resistant to Durafill VS toxicity. D). EGF decreases basal MTT metabolism and makes the cells resistant to Durafill VS and Flow Line toxicity. E). TGF-β decreases basal MTT metabolism and makes the cells resistant to Durafill VS and Flow Line toxicity. F). IGF-I increases basal MTT metabolism and makes the cells resistant to Durafill VS and Flow Line toxicity. Growth factors (100 ng/ml) were added to the cultures at the time of plating. Control represents MTT levels in untreated cultures and is defined as 100% cell survival. Bars show % cell survival (mean ± SEM, n=12–24) quantified by inhibition of MTT reduction. * indicates significant difference from control (P < 0.05). # indicates significant difference from growth factor treated (P < 0.05).
Discussion
There are two main findings from this study. First, the calcium hydroxide pulp capping material Dycal is highly toxic to pulp cells and growth factors are not able to alter that toxicity. Second, specific growth factors can make pulp cells resistant to composite restoration material toxicity.
Dycal has been shown previously to be toxic (32). This is not surprising in that it is a bioactive compound used to stimulate pulp cells, bioactive substances are more likely to be toxic. Still the toxicity is a concern. Direct comparison of the relative toxicity of the different materials was not the purpose of these studies. The objective was to determine the mechanism of toxicity and how growth factors alter that toxicity, therefore rigorous matching of the weight or size of the different materials was not attempted. Altering the size, shape, or weight of the dental materials, as well as washing the materials before adding them to the cultures could change the results. The use of other pulp capping materials is being explored. Mineral trioxide aggregate (MTA) has low toxicity (32,33) and has been shown to be an effective pulp capping material (34). However, whether it is effective in the long-term, and with general use, is yet to be fully determined.
Following pulp capping, a restoration must be applied. Therefore, toxicity of not only the pulp capping material, but also the restoration material must be considered. This is particularly true when calcium hydroxide is used as the pulp capping agent since it has been shown to be mechanically weak and soluble over time, allowing for possible leakage from the restoration to the dental pulp (2). Restoration material toxicity would also be of concern if growth factors were used instead of a traditional pulp capping material. While the potential toxicity of composite restoration materials has received less attention than amalgam, they contain compounds such as methacrylates, that can cause toxicity (35).
We tested the effects of two pharmacological agents to gain some understanding of the mechanism of cell death induced by the dental materials. Flow Line and Durafill VS were similar in that the free radical scavenger, trolox, but not the general caspase inhibitor, ZVAD, was protective. The protective effect of trolox suggests that Flow Line and Durafill VS induce free radical mediated cell death. The lack of protection by ZVAD suggests that caspase mediated apoptosis is not occurring in any of these conditions. It will be of interest to determine whether the protective effects of the growth factors are specific for free radical mediated cell death, and by what mechanism that protection is provided.
While each of the growth factors tested have been shown to have effects on dental pulp cell growth or differentiation, they had different effects in these studies. We have shown previously that EGF, FGF-2, TGF-β, or BMP-2, but not BMP-7 or IGF-I, induced a visible change in the appearance of the cells (30). In the current studies, we found that four of the growth factors tested (BMP-2, BMP-7, EGF, and TGF-β) decreased the basal MTT values, suggesting altered differentiation or growth of the cells. In each case the cells became resistant to Flow Line and Durafill VS toxicity, except for treatment with BMP-2, which made the cells more sensitive to Flow Line toxicity. Decreased basal MTT metabolism was not the determining factor regarding resistance to toxicity as treatment with IGF-I increased basal MTT metabolism and still made the cells resistant to Flow Line and Durafill VS toxicity. None of the growth factors made the cells resistant to Dycal toxicity.
In conclusion, the finding that Dycal was highly toxic, and the toxicity could not be attenuated by pharmacological agents or growth factors, raises concerns regarding its use in applications involving direct exposure to dental pulp cells. The results also show that growth factors can be used to alter the sensitivity of dental pulp cells to commonly used restoration materials, but that this effect is both growth factor and restoration material dependent. The growth factors BMP-7, EGF, TGF-β, and IGF-I provided the best profile of effects, making the cells resistant to both Flow Line and Durafill VS toxicity. The mechanism by which this alteration in sensitivity to toxicity occurs requires further study.
Acknowledgments
This investigation was supported by NIH/NIDCR grant DE018250.
Footnotes
The authors deny any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stanley HR. Pulp capping: conserving the dental pulp--can it be done? Is it worth it? Oral Surg Oral Med Oral Pathol. 1989;68:628–39. doi: 10.1016/0030-4220(89)90252-1. [DOI] [PubMed] [Google Scholar]
- 2.Cox CF, Suzuki S. Re-evaluating pulp protection: calcium hydroxide liners vs. cohesive hybridization. J Am Dent Assoc. 1994;125:823–31. doi: 10.14219/jada.archive.1994.0205. [DOI] [PubMed] [Google Scholar]
- 3.Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26:525–28. doi: 10.1097/00004770-200009000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Ward J. Vital pulp therapy in cariously exposed permanent teeth and its limitations. Aust Endod J. 2002;28:29–37. doi: 10.1111/j.1747-4477.2002.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 5.Goracci G, Mori G. Scanning electron microscopic evaluation of resin-dentin and calcium hydroxide-dentin interface with resin composite restorations. Quintessence Int. 1996;27:129–35. [PubMed] [Google Scholar]
- 6.Weine FS. Alternatives to routine endodontic treatment. In: Weine FS, editor. Endodontic Therapy. St. Louis: Mosby; 2004. pp. 513–45. [Google Scholar]
- 7.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. J Dent. 2000;28:77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 8.Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38:314–20. doi: 10.1159/000077771. [DOI] [PubMed] [Google Scholar]
- 9.Rutherford B, Fitzgerald M. A new biological approach to vital pulp therapy. Crit Rev Oral Biol Med. 1995;6:218–29. doi: 10.1177/10454411950060030401. [DOI] [PubMed] [Google Scholar]
- 10.Bègue-Kirn C, Smith AJ, Loriot M, Kupferle C, Ruch JV, Lesot H. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol. 1994;38:405–20. [PubMed] [Google Scholar]
- 11.Saito T, Ogawa M, Hata Y, Bessho K. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod. 2004;30:205–08. doi: 10.1097/00004770-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Nadiri A, Kuchler-Bopp S, Haikel Y, Lesot H. Immunolocalization of BMP-2/-4, FGF-4, and WNT10b in the developing mouse first lower molar. J Histochem Cytochem. 2004;52:103–12. doi: 10.1177/002215540405200110. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M. Tissue engineering in endodontics. Aust Endod J. 2005;31:111–13. doi: 10.1111/j.1747-4477.2005.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 14.Helder MN, Karg H, Bervoets TJ, Vukicevic S, Burger EH, D’Souza RN, Wöltgens JH, Karsenty G, Bronckers AL. Bone morphogenetic protein-7 (osteogenic protein-1, OP-1) and tooth development. J Dent Res. 1998;77:545–54. doi: 10.1177/00220345980770040701. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg M, Six N, Decup F, Buch D, Soheili Majd E, Lasfargues JJ, Salih E, Stanislawski L. Application of bioactive molecules in pulp-capping situations. Adv Dent Res. 2001;15:91–5. doi: 10.1177/08959374010150012401. [DOI] [PubMed] [Google Scholar]
- 16.Sloan AJ, Rutherford RB, Smith AJ. Stimulation of the rat dentine-pulp complex by bone morphogenetic protein-7 in vitro. Arch Oral Biol. 2000;45:173–7. doi: 10.1016/s0003-9969(99)00131-4. [DOI] [PubMed] [Google Scholar]
- 17.Russo LG, Maharajan P, Maharajan V. Basic fibroblast growth factor (FGF-2) in mouse tooth morphogenesis. Growth Factors. 1998;5:125–33. doi: 10.3109/08977199809117188. [DOI] [PubMed] [Google Scholar]
- 18.Nakao K, Itoh M, Tomita Y, Tomooka Y, Tsuji T. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochem Biophys Res Commun. 2004;325:1052–9. doi: 10.1016/j.bbrc.2004.10.136. [DOI] [PubMed] [Google Scholar]
- 19.Hao J, Wang P, Shi J. The effects of basic fibroblast growth factor on proliferation and differentiation of human dental pulp cells in vitro. Zhonghua kouqiang yixue zazhi (Chinese Journal of Stomatology) 1999;34:239–41. [PubMed] [Google Scholar]
- 20.Davideau JL, Sahlberg C, Blin C, Papagerakis P, Thesleff I, Berdal A. Differential expression of the full-length and secreted truncated forms of EGF receptor during formation of dental tissues. Int J Dev Biol. 1995;39:39605–15. [PubMed] [Google Scholar]
- 21.Cobo J, Hernández LC, del Valle ME, Vijande M, Vega JA. Immunohistochemical localization of epidermal growth factor and its receptor during odontogenesis in the rat. Eur J Orthod. 1992;14:333–8. doi: 10.1093/ejo/14.5.333. [DOI] [PubMed] [Google Scholar]
- 22.D’Souza RN, Happonen RP, Ritter NM, Butler WT. Temporal and spatial paterns of transforming growth factor-beta 1 expression in developing rat molars. Arch Oral Biol. 1990;35:957–65. doi: 10.1016/0003-9969(90)90015-3. [DOI] [PubMed] [Google Scholar]
- 23.Nie X, Tian W, Zhang Y, Chen X, Dong R, Jiang M, Chen F, Jin Y. Induction of transforming growth factor-beta 1 on dentine pulp cells in different culture patterns. Cell Biol Int. 2006;30:295–300. doi: 10.1016/j.cellbi.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Onishi T, Kinoshita S, Shintani S, Sobue S, Ooshima T. Stimulation of proliferation and differentiation of dog dental pulp cells in serum-free culture medium by insulin-like growth factor. Arch Oral Biol. 1999;44:361–71. doi: 10.1016/s0003-9969(99)00007-2. [DOI] [PubMed] [Google Scholar]
- 25.Lovschall H, Fejerskov O, Flyvbjerg A. Pulp-capping with recombinant human insulin-like growth factor I (rhIGF-I) in rat molars. Adv Dent Res. 2001;15:108–12. doi: 10.1177/08959374010150010301. [DOI] [PubMed] [Google Scholar]
- 26.Rittenberg B, Moghadam HG, Sandor GKB, Clokie CML. Mandibular reconstruction with BMP-7. A prospective clinical study. Br J Oral Maxillofac Surg. 2003;61:92–93. [Google Scholar]
- 27.Khan SN, Lane JM. The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in orthopaedic applications. Expert Opin Biol Ther. 2004;4:741–48. doi: 10.1517/14712598.4.5.741. [DOI] [PubMed] [Google Scholar]
- 28.Berlanga-Acosta J, Gavilondo-Cowley J, López-Saura P, González-López T, Castro-Santana MD, López-Mola E, Guillén-Nieto G, Herrera-Martinez L. Epidermal growth factor in clinical practice - a review of its biological actions, clinical indications and safety implications. Int Wound J. 2009;6:331–46. doi: 10.1111/j.1742-481X.2009.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabrera S, Barden D, Wolf M, Lobner D. Effects of growth factors on dental pulp cell sensitivity to amalgam toxicity. Dent Mater. 2007;23:1205–10. doi: 10.1016/j.dental.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Lobner D. Comparison of the LDH and MTT assays for quantifying cell death: validity for neuronal apoptosis? J Neurosci Methods. 2000;96:147–52. doi: 10.1016/s0165-0270(99)00193-4. [DOI] [PubMed] [Google Scholar]
- 32.Yasuda Y, Ogawa M, Arakawa T, Kadowaki T, Saito T. The effect of mineral trioxide aggregate on the mineralization ability of rat dental pulp cells: an in vitro study. J Endod. 2008;34:1057–60. doi: 10.1016/j.joen.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Asrari M, Lobner D. In vitro neurotoxic evaluation of root-end-filling materials. J Endod. 2003;29:743–46. doi: 10.1097/00004770-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: a review of the literature. Dent Mater. 2008;24:149–64. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Mainwaring G, Foster JR, Lund V, Green T. Methyl methacrylate toxicity in rat nasal epithelium: studies of the mechanism of action and comparisons between species. Toxicology. 2001;158:109–18. doi: 10.1016/s0300-483x(00)00332-2. [DOI] [PubMed] [Google Scholar]