Abstract
Increasing renal pelvic pressure results in PGE2-mediated release of substance P leading to increases in afferent renal nerve activity (ARNA) and natriuresis: renorenal reflex response. The renorenal reflexes are impaired in congestive heart failure (CHF). Impairment of the renorenal reflexes may contribute to the increased renal sympathetic nerve activity and sodium retention in CHF. Endothelin (ET)-1contributes to the pathological changes in cardiac and renal function in CHF. Therefore, we examined whether an ETA receptor antagonist BQ123 altered the responsiveness of renal mechanosensory nerves in CHF. The ARNA responses to increasing renal pelvic pressure were suppressed in CHF vs. Sham-CHF rats. In CHF, increasing renal pelvic pressure 7.5 mm Hg before and during renal pelvic perfusion with BQ123 increased ARNA 12±3% and 21±3% (P<0.05 vs vehicle). In isolated renal pelvises from CHF, PGE2 increased substance P release from 5±0 to 7±1 pg/min without and from 4±1 to 9±1 pg/min with BQ123 in the bath(P<0.01 vs vehicle). BQ123 had no effect on the ARNA responses or substance P release in Sham-CHF. Conclusion, activation of ETA receptors contributes to the impaired responsiveness of renal mechanosensory nerves in CHF rats by a mechanism(s) at the renal sensory nerve endings.
Keywords: renal nerves, kidney, prostaglandins, substance P, renal pelvic pressure, sodium retention, sodium excretion
Introduction
The majority of the afferent renal sensory nerves are located in the renal pelvic wall (Liu and Barajas, 1992; Kopp et al., 2000a, 2004, 2007b). These nerves are activated by stretching the renal pelvic wall by increasing renal pelvic pressure. In normotensive rats fed normal sodium diet, stretching the renal pelvic wall leads to induction of cyclooxygenase-2 resulting in increased renal pelvic synthesis of PGE2. PGE2 stimulates EP4 receptors on or close to the renal sensory nerves leading to activation of the cAMP - protein kinase A transduction pathway and a Ca-dependent release of the neuropeptide substance P which in turn activates the afferent renal sensory nerves, (Kopp et al. 2000a, 2000b, 2002a, 2004).
The activation threshold of the renal pelvic mechanosensory nerves is < 5mmHg (Kopp et al., 2002b), i.e., a level of renal pelvic pressure that is less than that required for sensation of pain. Rather the accumulating evidence indicate that activation of the renal mechanosensory nerves plays a role in the renal control of body fluid balance and blood pressure control. In normotensive rats, increases in afferent renal nerve activity (ARNA) produced by the increased renal pelvic pressure lead to a reflex decrease in efferent renal sympathetic nerve activity (ERSNA) and a natriuresis, i.e., a renorenal reflex response (Kopp et al. 1984). Together with other cardiovascular reflexes, e.g., the aortic and carotid baroreceptor reflexes, activation of the inhibitory renorenal reflexes contributes to the maintenance of low basal ERSNA (DiBona and Kopp, 1997). The important contributory role of the renorenal reflexes in the control of water and sodium homeostasis is suggested from studies showing that the responsiveness of the renal mechanosensory nerves is enhanced in rats fed high sodium diet and suppressed in rats fed low sodium diet (Kopp et al., 2002b). Furthermore, rats lacking intact afferent renal innervation are characterized by salt sensitive hypertension in association with increased ERSNA (Kopp et al., 2008).
Suppression of the renorenal reflexes in physiological conditions of sodium retention produced by, e.g. low sodium diet, is an appropriate response. However, in pathological conditions of sodium retention, an impairment of the renorenal reflexes would aggravate the sodium retention. Our previous studies have shown that the renorenal reflexes are impaired in congestive heart failure (CHF) (Kopp et al., 2002c). CHF is a condition characterized by increased ERSNA and sodium retention, the increased ERSNA contributing significantly to the sodium retention (DiBona and Kopp, 1997).
Endothelin-1 (ET-1) contributes to the increased systemic vascular resistance, myocardial ischemia and renal failure in CHF (e.g., Agapitov and Haynes, 2002). A role for ET-1 in suppressing the baroreflex control of ERSNA in CHF has been suggested by studies showing that an ET receptor antagonist enhanced the baroreflex control of ERSNA in CHF rabbits (Liu et al., 2001). ET-1 has also been shown to contribute to the activation of renal mechanosensory nerves in normal rats(Kopp et al., 2006). Both ETA and ETB receptors have been localized to the renal pelvic wall where the majority of the renal sensory nerves are located (Kopp et al., 2009). Interestingly, the effects of ET-1 are dependent on dietary sodium. Whereas an ETB receptor antagonist suppresses the responsiveness of renal mechanosensory nerves in rats fed high sodium diet, an ETA receptor antagonist enhances the responsiveness of renal mechanosensory nerves in rats fed low sodium diet (Kopp et al., 2006). Because these studies suggested that activation of ETA receptors contributes to the impaired responsiveness of renal mechanosensory nerves in physiological conditions of increased ERSNA and sodium retention, we hypothesized that increased activation of ETA receptors may play a role in the impaired responsiveness of renal mechanosensory nerves in CHF.
In the present study, we examined whether renal pelvic administration of an ETA receptor antagonist restored the ARNA responses to graded increases in renal pelvic pressure in CHF rats towards that in Sham-CHF rats in vivo. Because our previous studies showed that the impaired activation of renal mechanosensory nerves in CHF is related to a mechanism(s) at the peripheral renal sensory nerve endings, we also examined whether an ETA receptor antagonist enhanced the renal pelvic release of substance P produced by PGE2 using an isolated renal pelvic wall preparation.
Material and methods
The experimental protocols were approved by the Institutional Animal Care and Use Committee and performed according to the AGuide for the Care and Use of Laboratory Animals@ from the National Institutes of Health.
Male Sprague-Dawley rats allowed free access to normal sodium pellets (Teklad, sodium content=163 meq/kg) and tap water to drink were used in the study. CHF was induced by left coronary artery ligation (DiBona et al., 1998, Kopp et al., 2002c, Pfeffer et al., 1979). Briefly, the rats, 5–6 weeks of age, were anesthetized with pentobarbital sodium (0.2 mmol/kg i.p. Abbott Laboratories) and an oral endotrachial tube was inserted. The rats were artificially ventilated with room air. The heart was exposed via a left thoracotomy and the left coronary artery was ligated between the pulmonary outflow tract and left atrium.Thorax was closed in layers and negative pressure created in the pleural cavity to inflate the lungs. Buprenorphine (0.05 mg/kg) was administered to relieve postoperative pain. Following recovery from anesthesia and removal from the ventilator, the rats were returned to their cages. Sham operated rats were exposed to similar procedures except left coronary artery was not ligated.
The studies were performed six to seven weeks following coronary artery ligation or sham ligation.
In vivo Studies
Anesthesia was induced with pentobarbital sodium, 0.2 mmol/kg i.p., and maintained with an i.v. infusion of pentobarbital sodium, 0.04 mmol·kg−1·hr−1, in isotonic saline at 50 µl/min into the femoral vein. Arterial pressure was recorded from a catheter in the femoral artery. The procedures for stimulating and recording ARNA have been previously described in detail (e.g., Kopp et al., 2002a, 2002b, 2003, 2006). In short, left kidney was approached by a flank incision, a PE-10 catheter was placed in the right ureter for collection of urine and a PE-60 catheter was placed in the left ureter with its tip in the pelvis. The left renal pelvis was perfused, via a PE-10 catheter placed inside the PE-60 catheter, at 20 µl/min with vehicle or the ETA receptor antagonist BQ123 (Ihara et al. 1992), see below. ARNA was stimulated by increasing renal pelvic pressure. ARNA was recorded from the peripheral portion of the cut end of one renal nerve branch placed on a bipolar silver wire electrode. ARNA was integrated over 1-second intervals, the unit of measure being microvolts per second per 1 second. All data were collected at 500 Hz and averaged over 2 seconds. Postmortem renal nerve activity, assessed by crushing the renal nerve bundles central or peripheral to the recording electrode, was subtracted from all values of ARNA. Renal nerve activity was expressed in percentage of its baseline value during the control period (e.g., Kopp et al., 2002a, 2002b, 2003, 2006).
Experimental Protocols
Approximately 1.5 h elapsed after the end of surgery and the start of the experiment to allow the rat to stabilize as evidenced by 30 min of steady-state urine collections and ARNA recordings.
Effects of an ETA receptor antagonist on the ARNA response to increased renal pelvic pressure in CHF and Sham-CHF rats
The experiment was divided into two parts separated by a 10-min interval. Each part consisted of two 10-min control, 3-min experimental and 10-min recovery periods. The left ureteral catheter was raised to increase renal pelvic pressure 2.5 and 7.5 mm Hg during the two experimental periods. In two groups of rats, 14 CHF rats and 13 Sham-CHF rats, the renal pelvis was perfused with vehicle during the first part of the experiment and BQ123, 5 µM, during the second part.
Repeated increases in renal pelvic pressure in CHF and Sham-CHF rats - time controls
In 6 CHF and 8 Sham-CHF rats, renal pelvic pressure was increased 2.5 and 7.5 mmHg twice in the presence of renal pelvic perfusion with vehicle (NaCl). Thus, these experiments were performed identically to those above, except BQ123 was not administered.
At the end of each experiment, a catheter was inserted into the right carotid artery and advanced into the left ventricle for measurement of left ventricular end-diastolic pressure (LVEDP).
In vitro studies
Effects of an ETA receptor antagonist on the increases in PGE2-mediated release of substance P from pelvises derived from CHF and Sham-CHF rats
To examine whether activation of ETA receptors contributed to the suppressed responsiveness of the renal mechanosensory nerves in CHF by a mechanism at the peripheral sensory nerve endings, an isolated renal pelvic wall preparation was used. The sensory nerve endings were stimulated by PGE2 added to the incubation bath containing isolated renal pelvises from CHF rats and Sham-CHF rats and the release of substance P was measured.
After induction of anesthesia and before the kidneys were removed, arterial blood pressure and LVEDP were measured in each rats for 15 min. The isolated renal pelvic wall preparation has previously been described in detail (e.g., Kopp et al., 2002a, 2002b, 2003, 2006). In brief, renal pelvises dissected from the kidneys were placed in wells containing 400 µl HEPES buffer maintained at 37°C. Each well contained the pelvic wall from one kidney. Throughout the experiment, the incubation medium was replaced with fresh incubation media every 5 min. After a 2-hrs= equilibration period, the experiment started with four 5-min control periods followed by one 5-min experimental period and four 5-min recovery periods. PGE2, 0.14 µM, was added to the incubation bath to both pelvises during the experimental periods. One pelvis was incubated in HEPES buffer and the other pelvis in HEPES buffer containing 1 µM BQ123 throughout the control, experimental and recovery periods. Renal pelvic release of substance P into the incubation bath was measured throughout the experiment. The incubation medium was collected in siliconized vials and stored at −80 °C for later analysis of substance P.
Drugs
Substance P antibody (IHC 7451) was acquired from Penninsula Laboratories (San Carlos, CA) and PGE2 from Cayman Chemicals (Ann Arbor, MI). All other reagents/chemicals were from Sigma Aldrich (St. Louis, MO) unless otherwise stated. All agents were dissolved in incubation buffer (in vitro studies) or 0.15 M NaCl (in vivo studies).
Analytical Procedures
Substance P in the incubation medium was measured by ELISA, as previously described in detail (Kopp et al., 2002a, 2002b, 2003, 2006).
Statistical Analysis
Ipsilateral ARNA, systemic hemodynamics, renal excretion and substance P release were measured and averaged over each period. The effects of activation of renal mechanosensory nerves were calculated by comparing the experimental value with the average value of the bracketing control and recovery periods. Normally distributed data were analyzed using Student=s paired t-test, one- or two-ways analysis of variance for repeated measurements followed by Bonferroni correction for multiple comparisons. Data which were not normally distributed were analyzed using Wilcoxon signed rank test and Friedman one-way analysis of variance with repeated measures followed by Dunn=s multiple comparison test (GraphPad Prism version 5.02 for Windows, GraphPad Software, San Diego, CA). A significance level of 5% was chosen. Data in text and figures are expressed as means ∀ SE.
Results
LVEDP and heart weight were greater in CHF rats than in Sham-CHF rats (P<0.01, Table 1). Bodyweight, mean arterial pressure, systolic and diastolic blood pressure and heart rate were similar in the two groups of rats. At the time of surgery, the CHF rats were characterized by ascites. It has previously been demonstrated in this model of heart failure that rats with LVEDP ranging from 10 – 20 mmHg have left ventricular infarct size of 35–46% of the left ventricular endocardial circumference and about 25% reduction in maximal dp/dt (Pfeffer et al., 1979) .
Table 1.
Basal values in CHF and Sham CHF rats
| n | Body Wt, g |
Heart Wt, g |
LVEDP, mmHg |
MAP, mmHg |
SBP mmHg |
DBP mmHg |
HR, beats/min |
|
|---|---|---|---|---|---|---|---|---|
| CHF | 29 | 372±5 | 1.95±0.08** | 10.3±0.6** | 111±3 | 131±4 | 87±4 | 267±10 |
| Sham CHF | 30 | 370±5 | 1.27±0.03 | 3.1±0.3 | 111±3 | 137±5 | 89±3 | 286±11 |
Values are means±SE; CHF, congestive heart failure, LVEDP, left ventricular end diastolic pressure; MAP, mean arterial pressure;SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate
P<0.01 vs. sham CHF rats.
In vivo studies
Effects of an ETA receptor antagonist on the ARNA response to increased renal pelvic pressure in CHF and Sham-CHF rats
Activation of ETA receptors contributes to the impaired responsiveness of renal mechanosensory nerves in rats fed low sodium diet (Kopp et al., 2006). Endothelin contributes to the pathophysiology of CHF (Agapitov and Haynes, 2002). Renal ET-1 content and expression of renal cortical ETA receptors are increased in CHF (Motte et al., 2003). Interestingly, systemic treatment with an ETA- receptor antagonist has been shown to decrease the elevated ERSNA in rabbits with heart failure (Liu et al., 2001). In view of these studies, we hypothesized that activation of renal pelvic ETA receptors may contribute to the impaired responsiveness of renal mechanosensory nerves in CHF rats (Kopp et al., 2002c). To localize possible beneficial effects of ETA-R blockade to the renal pelvic afferent renal nerves, the ETA-R antagonist BQ123 was perfused into the renal pelvis in vivo or added to an incubation bath containing renal pelvic wall tissue in vitro.
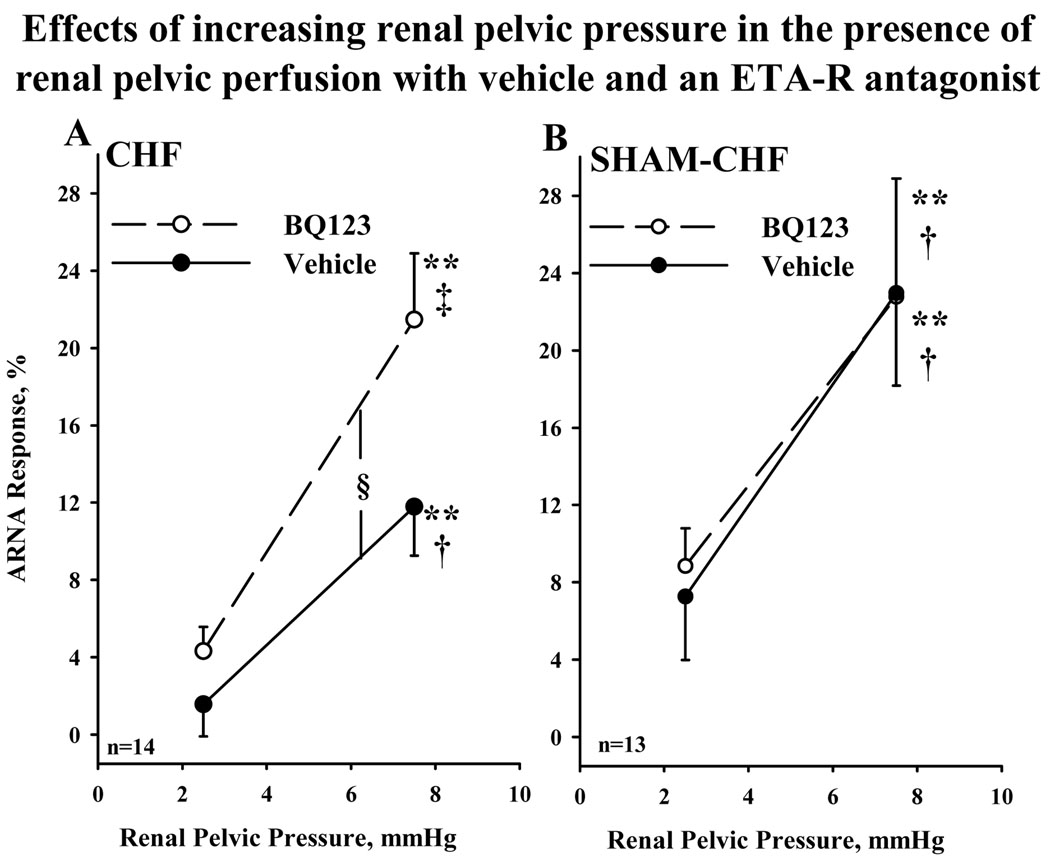
In CHF rats, the ARNA responses to increasing renal pelvic pressure 2.5 and 7.5 mmHg were suppressed compared to those in Sham-CHF rats (P<0.05) in the presence of vehicle (Fig.1). Perfusing the renal pelvis with BQ123 enhanced the ARNA responses to increasing renal pelvic pressure 2.5 and 7.5 mmHg in CHF rats but not in Sham-CHF rats. In the presence of BQ123, the ARNA responses were similar in the two groups of rats. In CHF rats in the presence of vehicle, increasing renal pelvic pressure 2.5 or 7.5 mmHg failed to increase contralateral urinary sodium excretion (Table 2) which was in contrast to Sham-CHF rats in which contralateral urinary sodium excretion increased in response to 2.5 and 7.5 mmHg increases in renal pelvic pressure. However in the presence of BQ123, increasing renal pelvic pressure 7.5 mmHg resulted in a significant increase in contralateral urinary sodium excretion (P<0.01) in CHF rats, the natriuretic response being greater in the presence than in the absence of BQ123 (P<0.05). In Sham- CHF rats, BQ123 had no effect on the natriuretic responses to increases in renal pelvic pressure. Baseline urinary sodium excretion was not altered by BQ123 in either group. Mean arterial pressure and heart rate were unaffected by increases in renal pelvic pressure and renal pelvic perfusion with BQ123 in either group.
Figure 1.
Effects of increasing renal pelvic pressure 2.5 and 7.5 mmHg on ipsilateral ARNA during renal pelvic perfusion with vehicle (solid lines) and 5 µM BQ123 (dashed lines) (paired experimental design) in CHF rats (A) and Sham-CHF rats (B). CHF, congestive heart failure; ARNA, afferent renal nerve activity; **P<0.01 vs. 0; H P<0.05, I P<0.01 vs. ARNA response to increasing renal pelvic pressure 2.5 mmHg; 'P< 0.05 the ARNA response curves to increases in renal pelvic pressure in the presence of BQ123 vs. vehicle.
Table 2.
Effects of increasing renal pelvic pressure on contralateral urinary sodium excretion in CHF and Sham CHF rats during renal pelvic perfusion with vehicle and BQ123, 5 µM.
| Renal Pelvic Perfusion |
Control | 8RPP 2.5 mmHg |
Control |
8RPP 7.5 mmHg |
|
|---|---|---|---|---|---|
| CHF, n=13 | Vehicle | 1.63±0.33 | 1.61±0.30 | 1.77±0.35 | 1.88±0.37 |
| BQ123 | 1.84±0.29 | 1.93±0.29 | 1.99±0.31 | 2.23±0.39** | |
| Sham CHF, n=13 | Vehicle | 1.09±0.29 | 1.28±0.33* | 1.09±0.28 | 1.37±0.31* |
| BQ123 | 1.18±0.19 | 1.34±0.22* | 1.37±0.20 | 1.77±0.27* |
Values are means±SE in µmol·min−1·g−1
Control, average of control and recovery periods; 8RPP , increased renal pelvic pressure;
P<0.05
P<0.01 vs. control.
Repeated increases in renal pelvic pressure in CHF and Sham-CHF rats - time controls
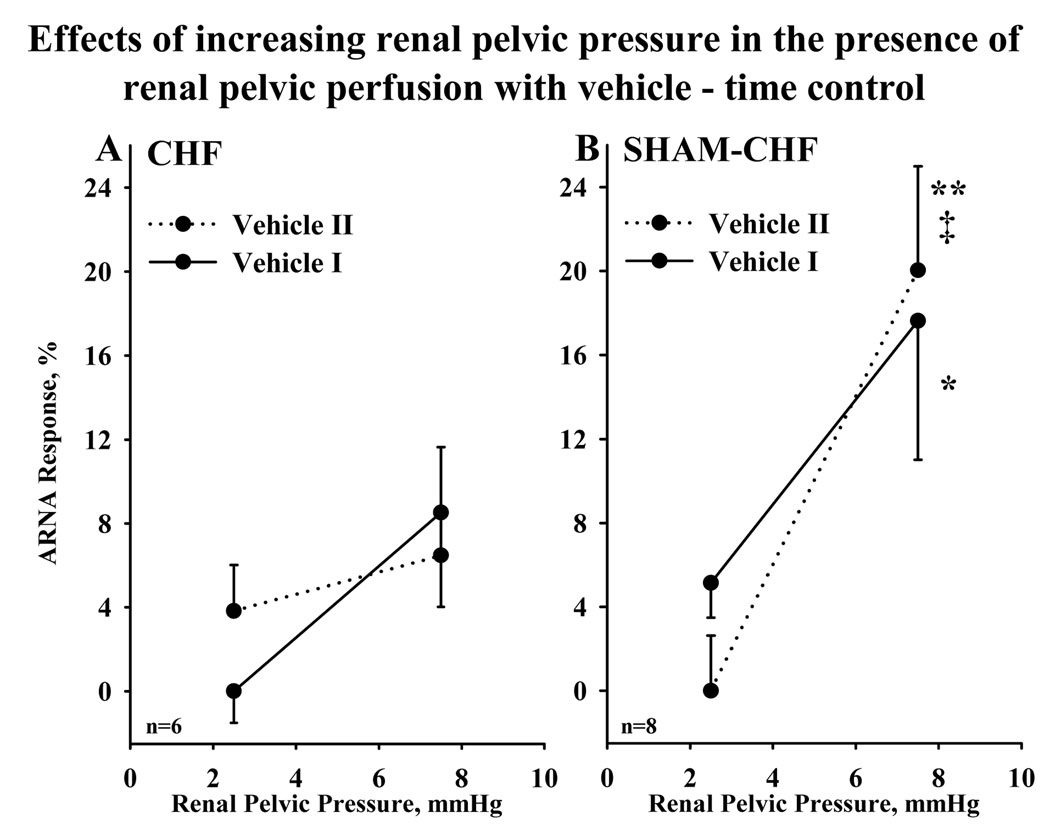
In CHF and Sham-CHF rats, increasing renal pelvic pressure 2.5 and 7.5 mmHg resulted in reproducible increases in ARNA in the presence of renal pelvic perfusion with vehicle (Fig.2).
Figure 2.
Effects of increasing renal pelvic pressure 2.5 mmHg and 7.5 mmHg twice in the presence of vehicle (time control experiments) in CHF rats (A) and Sham-CHF rats (B). Vehicle I (solid lines) and vehicle II (dotted lines) refer to first part and second parts, respectively, of the experiment. * P<0.05; **P<0.01 vs. 0; I P<0.01 vs. ARNA response to increasing renal pelvic pressure 2.5 mmHg. For abbreviations, see legends for Figure 1.
In vitro studies
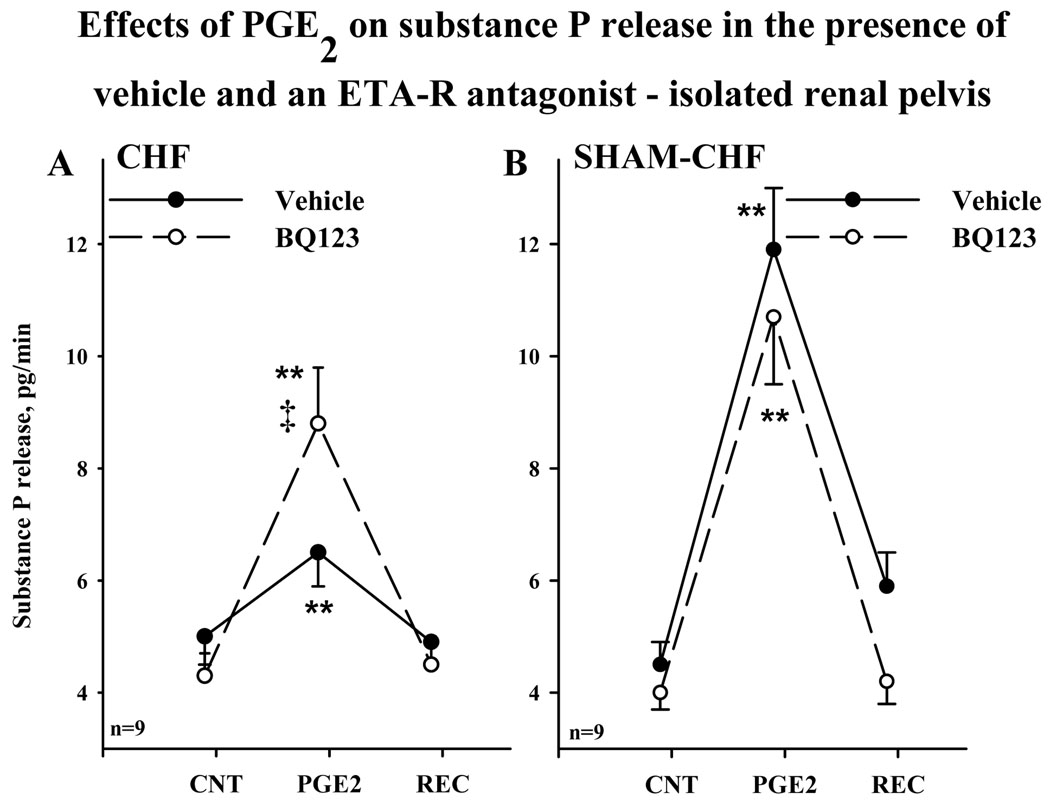
Renal pelvic perfusion with BQ123 enhanced the ARNA responses to increased renal pelvic pressure in CHF but not in Sham-CHF rats in the absence of systemic hemodynamic changes (Fig. 1). These data suggested that ET-1 mediated activation of ETA receptors suppressed the responsiveness of the renal mechanosensory nerves in CHF by a mechanism involving the peripheral renal sensory nerve fibers. ETA receptors have been localized to the renal pelvic smooth muscle cells where the majority of the substance P containing renal sensory nerve fibers is found (Kopp et al., 2009). Therefore, we tested the idea that ET-1 mediated activation of ETA receptors modulates the PGE2-mediated release of substance P (Kopp et al., 2002a) in an isolated renal pelvic wall preparation by comparing the substance P release from renal pelvic wall tissue incubated in the presence and absence of BQ123. In the absence of BQ123 in the bath, 0.14 µM PGE2 resulted in a suppressed release of substance P from CHF pelvises compared to Sham-CHF pelvises (P<0.01) (Fig. 3). In the presence of BQ123 in the bath, the increases in PGE2-mediated release of substance P release from CHF and Sham- CHF pelvises were similar; the PGE2-mediated release of substance P being enhanced by BQ123 in CHF but not in Sham- CHF pelvises.
Figure 3.
Effects of 0.14 µM PGE2 on renal pelvic release of substance P from an isolated renal pelvic wall preparation derived from CHF rats (A) and Sham-CHF rats (B) in the absence (solid line) and presence (dashed lines) of 1 µM BQ123 in the incubation bath. ** P<0.01 vs average substance P release during control and recovery periods; I P<0.01 vs. PGE2-mediated substance P release in the presence vs. absence of BQ123.
Discussion
The results of the present studies show that the responsiveness of the renal mechanosensory nerves is impaired in CHF rats compared to their sham operated litter mates. Renal pelvic administration of an ETA receptor antagonist enhances the increases in renal pelvic release of substance P produced by PGE2 and the increases in ARNA and urinary sodium excretion produced by increased renal pelvic pressure. Taken together these studies suggest that ET-1 mediated activation of ETA receptors contributes to the impaired responsiveness of renal mechanosensory nerves in CHF.
Heart failure is characterized by increased ERSNA and sodium retention (DiBona and Kopp, 1997). It has long been known that the activation of the carotid and arterial baroreflexes are impaired in heart failure. In addition, our previous and current studies suggest an impairment of the renorenal reflexes in CHF rats (Kopp et al. 2002c). Increases in renal pelvic pressure result in increases in ARNA and urinary sodium excretion of a lesser magnitude in CHF rats than in their sham operated litter mates. It unlikely that the impaired responsiveness of the renal mechanosensory nerves in CHF rats is related to the basal level of ERSNA because activation of renal sensory nerves is positively correlated with ERSNA (Kopp et al., 2007b), basal ERSNA being increased, not decreased, in heart failure. This conclusion is further supported by our studies in the isolated renal pelvic wall preparation which showed markedly impaired increases in the release of substance P from the renal pelvic sensory nerves in response to PGE2 from pelvises derived from CHF rats compared to those from Sham-CHF rats. The isolated renal pelvic wall preparation is by design a sympathetically denervated preparation. The in vitro studies further suggested that the impaired responsiveness of the renal mechanosensory nerves in CHF was due to a defect in the mechanism(s) at the peripheral renal sensory nerve fiber endings. Taken together these findings suggest that the impaired renorenal reflex control of ERSNA contributes to the increased ERSNA and sodium retention in heart failure.
Although much focus has been placed an ET-1 as a modulator of pain (Khodorova et al., 2009), there is also evidence for ET-1 modulating sensory nerves involved in the cardiovascular homeostasis, e.g., the arterial baroflexes. Whereas central administration of ET-1 and ET-3 increases baroreflex sensitivity (Itoh. and van den Buuse, 1991), local administration of ET-1 into an isolated baroreceptor preparation suppresses the baroreceptor activity (Chapleau et al., 1992). Interestingly, a role for ET-1 induced activation of ETA receptors in the increased ERSNA in heart failure was suggested by the fall in ERSNA by chronic administration of the ETA receptor antagonist BQ123 in rabbits with heart failure, most likely by a mechanism(s) involving improvement of the carotid baroreceptor function (Liu et al., 2001). Further evidence for a role for ET-1 in the pathogenesis of heart failure are derived from studies showing elevated cardiac and renal ET-1 levels in heart failure (Agapitov and Haynes, 2002, Motte et al., 2003, Luchner et al., 2000, Kobayashi et al., 1999). Plasma ET-1 levels have been shown to be correlated to LVEDP and the level of the percent infarct size (Sakai et al., 1996). Urinary ET-1 excretion has been shown to be increased in heart failure and negatively correlated with urinary sodium excretion (Motto et al., 2003, Modesti et al., 2000). There is an increased expression of cardiac (Luchner et al.,, 2000, Kobayashi et al., 1999) and renal ETA receptors in heart failure, the latter being correlated with worsening of cardiac function (Motte et al., 2003).
ET-1 is present in renal pelvic wall tissue (Kopp et al., 2006) and ETA receptors have been localized to smooth muscle cell fibers in the renal pelvic wall in close contact with sensory nerve fibers (Kopp et al., 2009). There is an increased expression of ETA receptor in renal pelvic tissue in normal rats fed low sodium diet (Kopp et al., 2009). Renal pelvic administration of an ETA receptor antagonist restored the suppressed responsiveness of renal mechanosensory nerves in these rats towards that in rats fed normal sodium diet (Kopp et al., 2006). Because these findings suggest a tonic inhibitory effect of ET-1 mediated activation of ETA receptors on ARNA in physiological condition of sodium retention, we hypothesized that increased activation of renal pelvic ETA receptors contributes to the impaired responsiveness of the renal mechanosensory nerves in pathological conditions of increased ERSNA and sodium retention, e.g. congestive heart failure. Because systemic administration of an ETA-R antagonist may influence the activity of renal pelvic mechanosensitive nerve fibers by its systemic and renal hemodynamic effects (e.g., Mulder et al., 2000; Xia et al., 2006, Spieker et al., 2001), the ETA-R antagonist BQ123 was administered directly into the renal pelvis in our studies designed to examine the mechanisms involved in the impaired responsiveness of the renal pelvic mechanosensory nerves in CHF. The results show that renal pelvic administration of the ETA receptor antagonist enhanced the ARNA and natriuretic responses to increases in renal pelvic pressure in CHF rats towards those produced in Sham-CHF in the absence of BQ123. BQ123 has no effect on the activation of renal mechanosensory nerves in Sham-CHF rats which is in agreement with our previous studies in normal rats fed normal sodium diet (Kopp et al., 2006). Because BQ123 was administrated directly into the renal pelvis, these studies suggested that the enhanced responsiveness of the renal sensory nerves produced by BQ123 was related to peripheral effects on or close to the renal pelvic sensory nerves. To support this conclusion, we used the isolated renal pelvic wall preparation to further minimize any possible renal hemodynamic and excretory effects by BQ123. Renal sensory nerves were activated by PGE2 at a concentration that increases substance P release from renal pelvises derived from rats fed normal sodium diet (Kopp et al., 2002b).This concentration of PGE2 resulted in a release of substance P from pelvises derived from the CHF rats that was markedly suppressed compared to that from Sham- CHF rats. However in the presence of BQ123 in the bath, the PGE2-mediated release of substance P from pelvises derived from CHF and Sham-CHF rats was similar. Importantly, BQ123 has no effect in Sham-CHF rats. Taken together, these studies support our in vivo studies and suggest that activation of ETA receptors suppresses the responsiveness of the renal mechanosensory nerves by a mechanism(s) at the renal sensory nerve fiber endings.
The mechanisms by which activation of ETA receptors may suppress the responsiveness of the renal mechanosensory nerves are currently unknown. An intriguing hypothesis is derived from the presence of ETA receptors on vascular smooth muscle cells on the vasculature in the renal pelvic wall close to the sensory nerves (Kopp et al., 2009). We hypothesize that the suppressed responsiveness of the renal mechanosensory nerves is, at least in part, related to ET-1 induced vasoconstriction producing ischemia-induced blockade of sensory nerve conduction. Support for this hypothesis is derived from studies showing that activation of ETA receptors suppresses the nociceptive response to activation of cutaneous afferent nerves in association with local vasoconstriction (Shrestha et al., 2009). Also, ET-1 induced conduction block of the sciatic nerve is associated with vasoconstriction of epineural microvessels (Zochodne et al., 1992).
Another, or additional, mechanism(s) involved in the ETA receptor induced suppression of renal mechanosensory nerves may involve endogenous angiotensin (ANG) II, known to be increased in heart failure (DiBona and Kopp, 1997). Our previous studies showed a similar suppressive effect of ANG II on the responsiveness of the renal mechanosensory nerves in CHF as produced by ET-1 in the current study (Kopp et al., 2002c). ANG II type 1 (AT1) receptors have also been localized in the renal pelvic wall (Healy et al., 1995). Studies examining the interaction between ANG II and ET-1 in normal rats showed that renal pelvic administration of BQ123 in combination with the AT1-receptor antagonist losartan produced no further inhibition of the PGE2-mediated release of substance P than either antagonist alone. These results together with those showing that BQ123 reduced the ANG II-mediated reduction in the PGE2-mediated release of substance P suggest that ET-mediated activation of ETA-receptors contributes to the ANG II induced reduction of the responsiveness of the renal mechanosensory nerves to PGE2 and increased renal pelvic pressure (Kopp et al., 2007a). ANG II has been shown to increase prepro-ET-1 mRNA and ET-1 content in various tissues, including the kidney and urinary bladder (Imai et al., 1992, Kohno et al., 1992) and ETA-R expression in cardiomyocytes (Xia and Karmazyn, 2004). These data together with the increased renal ET-1 content and renal expression of ETA receptors in heart failure (Motto et al., 2003) suggest that the suppression of the responsiveness of the renal mechanosensory nerves in CHF may also be due, at least in part, to ANG II increasing ET-1, ETA receptors and/or the activation of ETA receptors. ANG II suppresses the responsiveness of the renal sensory nerves by reducing PGE2-mediated activation of adenylyl cyclase via a pertussis toxin sensitive mechanism (Kopp et al., 2003). Therefore, we hypothesize that similar mechanisms may contribute to the ETA-R mediated reduction of the PGE2-mediated activation of renal sensory nerves in conditions of increased ET-1 mediated activation of renal pelvic ETA-R in physiological and pathophysiological conditions of sodium retention.
In summary, our in vivo and in vitro data suggest that ET-1 mediated activation of ETA receptors in the renal pelvic wall contributes to the suppressed responsiveness of renal mechanosensory nerves in heart failure. The impairment of the renorenal reflex control of ERSNA may contribute to the increased ERSNA and sodium retention in heart failure.
ACKNOWLEDGMENT
This work was supported by grants from the Department of Veterans Affairs and The National Institutes of Health, Heart, Lung and Blood Institute, RO1 HL66068.
References
- Agapitov AA, Haynes WG. Role of endothelin in cardiovascular disease. J. Renin-Angiotensin-Aldosterone Syst. 2002;3:1–15. doi: 10.3317/jraas.2002.001. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Hajduczok G, Abboud FM. Suppression of baroreceptor discharge by endothelin at high carotid sinus pressure. Am. J. Physiol. 1992;263:R103–R108. doi: 10.1152/ajpregu.1992.263.1.R103. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp UC. Neural control of renal function. Physiol. Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Jones SY, Sawin LL. Angiotensin receptor antagonist improves cardiac reflex control of renal sodium handling in heart failure. Am. J. Physiol. 1998;274:H636–H641. doi: 10.1152/ajpheart.1998.274.2.H636. [DOI] [PubMed] [Google Scholar]
- Healy DP, Ye M-Q, Troyanovskaya M. Localization of angiotensin II type 1 receptor subtype mRNA in rat kidney. Am. J. Physiol. 1995;268:F220–F226. doi: 10.1152/ajprenal.1995.268.2.F220. [DOI] [PubMed] [Google Scholar]
- Ihara M, Noguchi K, Saeki T, Fukuroda T, Tsuchida S, Kimura S, Fukami T, Ishikawa K, Nishikibe M, Yano M. Biological profiles of highly potent endothelin antagonists selective for the ETA receptor. Life Sci. 1991;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992;19:753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- Itoh S, van den Buuse M. Sensitization of baroreflex by central endothelin in conscious rats. Am. J. Physiol. 1991;260:H1106–H1112. doi: 10.1152/ajpheart.1991.260.4.H1106. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Montmayeur J-P, Strichartz G. Endothelin receptors and pain. J. Pain. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Miyauchi T, Sakai S, Kobayashi M, Yamaguchi I, Goto K, Sugishita Y. Expression of endothelin-1, ETA and ETB receptors and ECE and distribution of endothelin-1 in failing rat heart. Am. J. Phsyiol. 1999;276:H1197–H1206. doi: 10.1152/ajpheart.1999.276.4.H1197. [DOI] [PubMed] [Google Scholar]
- Kohno M, Horio T, Ikeda M, Yokokawa K, Fukui T, Yasanari K, Kurihara N, Takeda T. Angioternsin II stimulates endothelin-1 secretion in cultured rat mesangial cells. Kid. Int. 1992;42:860–866. doi: 10.1038/ki.1992.361. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA, Haeggström JZ, Samuelsson B, Hökfelt T. Cyclooxygenase-2 involved in stimulation of renal mechanosensitive neurons. Hypertension. 2000a;35:373–378. doi: 10.1161/01.hyp.35.1.373. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. PGE2 increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am. J. Physiol. 2002a;282:R1618–R1627. doi: 10.1152/ajpregu.00701.2001. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE2-mediated release of substance P from renal mechanosensory nerve fibers. Am. J. Physiol. 2002b;282:R19–R30. doi: 10.1152/ajpregu.2002.282.1.R19. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am. J. Physiol. 2002c;284:R116–R124. doi: 10.1152/ajpregu.00336.2002. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Angiotensin blocks substance P release from renal sensory nerves by inhibiting PGE2-mediated activation of cAMP. Am. J. Physiol. 2003;285:F472–F483. doi: 10.1152/ajprenal.00399.2002. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Nakamura K, Nusing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2 mediated stimulation of renal sensory nerves. Am. J. Physiol. 2004;287:F1269–F1282. doi: 10.1152/ajprenal.00230.2004. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Differential effects of endothelin on the activation of renal mechanosensory nerves: stimulatory in high and inhibitory in low sodium diet. Am. J. Physiol. 2006;291:R1545–R1556. doi: 10.1152/ajpregu.00878.2005. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA. Activation of endothelin-A receptors contributes to angiotensin-induced suppression of renal sensory nerve activation. Hypertension. 2007a;49:141–147. doi: 10.1161/01.HYP.0000249634.46212.7b. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA, Mulder J, Hokfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of _1- and _2- adrenoceptors on renal sensory nerve fibers. Am. J. Physiol. 2007b;293:R1561–R1572. doi: 10.1152/ajpregu.00485.2007. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Farley DM, Cicha MZ, Smith LA. Activation of renal mechanosensitive neurons involves bradykinin, protein kinase C, PGE2 and substance P. Am. J. Physiol. 2000b;278:R937–R946. doi: 10.1152/ajpregu.2000.278.4.R937. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Grisk O, Cicha M, Smith L, Steinbach A, Schlüter T, Mähler N, Hökfelt T. Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: role of endothelin. Am J Physiol. 2009;297:R337–R351. doi: 10.1152/ajpregu.91029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Jones SY, DiBona GF. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am. J. Physiol. 2008;295:R1882–R1890. doi: 10.1152/ajpregu.90529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano-and chemoreceptor stimulation in the dog and rat. Am. J. Physiol. 1984;246:F67–F77. doi: 10.1152/ajprenal.1984.246.1.F67. [DOI] [PubMed] [Google Scholar]
- Liu L, Barajas L. The rat renal nerves during development. Anat Embryol. 1993;188:345–361. doi: 10.1007/BF00185944. 1993. [DOI] [PubMed] [Google Scholar]
- Liu J-L, Pliquett RU, Brewer E, Cornish KG, Shen Y-T, Zucker IH. Chronic endothelin-1 blockade reduces sympathetic nerve activity in rabbits with heart failure. Am. J. Physiol. 2001;280:R1906–R1913. doi: 10.1152/ajpregu.2001.280.6.R1906. [DOI] [PubMed] [Google Scholar]
- Luchner A, Jougasaki M, Friedrich E, Borgeson DD, Stevens TL, Redfield MM, Riegger GAJ, Burnett JC. Activation of cardiorenal and pulmonary tissue endothelin-1 in experimental heart failure. Am. J. Physiol. 2000;279:R974–R979. doi: 10.1152/ajpregu.2000.279.3.R974. [DOI] [PubMed] [Google Scholar]
- Modesti PA, Cecioni I, Costoli A, Pogessi L, Galanti G, Serneri GGN. Renal endothelin in heart failure and its relation to sodium excretion. Am. Heart J. 200;0140:617–623. doi: 10.1067/mhj.2000.109917. [DOI] [PubMed] [Google Scholar]
- Motte S, van Beneden R, Mottet J, Rondolet B, Mathieu M, Havaux X, Lause P, Clercx C, Ketelslegers JM, Naeije R, McEntee K. Early activation of cardiac and renal endothelin systems in experimental heart failure. Am. J. Physiol. 2003;285:H2482–H2491. doi: 10.1152/ajpheart.00419.2003. [DOI] [PubMed] [Google Scholar]
- Mulder P, Boujedaini H, Richard V, Derumeaux G, Henry JP, Renet S, Wessale J, Opgenorth T, Thuillez C. Selective endothelin-A versus combined endothelin-A/endothelin-B receptor blockade in rat chronic heart failure. Circ. 2000;102:491–493. doi: 10.1161/01.cir.102.5.491. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Pfeffer JM, Fishbein MC, Fletcher PJ, Spadoro J, Kloner RA, Braunwald E. Myocardial infarct size and ventricular function in rats. Circ. Res. 1979;44:503–512. doi: 10.1161/01.res.44.4.503. [DOI] [PubMed] [Google Scholar]
- Sakai S, Miyauchi T, Sakurai T, Kasuya Y, Ihara M, Yamaguchi I, Goto K, Sugishita Y. Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circ. 1996;93:1069–1072. doi: 10.1161/01.cir.93.6.1214. [DOI] [PubMed] [Google Scholar]
- Shresta A, Gracias NG, Mujemda F, Khodorova A, Vasko MR, Strichartz GR. Local antinociception induced by endothelin-1 in the hairy skin of the rat=s back. J. Pain. 2009;10:702–714. doi: 10.1016/j.jpain.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker LE, Noll G, Ruschitzka FT, Lüscher TF. Endothelin receptor antagonists in congestive heart failure: a new therapeutic principle for the future? J. Am. Col. Cardiol. 2001;37:1493–1505. doi: 10.1016/s0735-1097(01)01210-4. [DOI] [PubMed] [Google Scholar]
- Xia Y, Karmazyn M. Obligatory role for endogenous endothelin in mediating the hypertrophic effects of phenylephrine and angiotensin II in neonatal rat ventricular myocytes: evidence for two distinct mechanisms for endothelin regulation. J. Pharmacol. Exp. Ther. 2004;310:43–51. doi: 10.1124/jpet.104.065185. [DOI] [PubMed] [Google Scholar]
- Xia Q-G, Reinecke A, Morenkamp M, Daemen MJ, Simon R, Unger T. Effects of endothelin ETA receptor blocker LU 135252 on cardiac remodeling and survival in a hypertensive rat model of chronic heart failure. Acta Pharmacol. Sin. 2006;27:1417–1422. doi: 10.1111/j.1745-7254.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ho LT, Gross PM. Acute endoneural ischemia induced by epineural endothelin in the rat sciatic nerve. Am. J. Physiol. 1992;263:H1806–H1810. doi: 10.1152/ajpheart.1992.263.6.H1806. [DOI] [PubMed] [Google Scholar]