Abstract
Introduction
Thymosin β4 (Tβ4) is a developmentally expressed 43-amino acid peptide that inhibits organization of the actin-cytoskeleton by sequestration of G-actin monomers. Tβ4 improves cardiac function after myocardial infarction in adult mice and promotes healing properties in both dermal and corneal wounds. We tested the hypothesis that Tβ4 improves functional neurological outcome in a rat model of embolic stroke.
Experimental Procedures
Male Wistar rats (n=18) were subjected to embolic middle cerebral artery occlusion (MCAo). Tβ4 (6 mg/kg, IP) was administered 24 hours after MCAo and then every 3 days for 4 additional doses (n=9). Rats treated with saline were used as a control (n=9). The adhesive-removal test (ART) and modified Neurological Severity Score (mNSS) were performed to measure functional outcome. Rats were sacrificed 56 days after MCAo. Immunostaining was performed with antibodies against NG-2 (chondroitin sulfate proteoglycan), CNPase (2″, 3″-cyclic nucleotide 3′-phosphodiesterase) to detect immature and mature oligodendrocytes. Neurofilament-H (NF-H) antibodies were used to detect axons while myelinated axons were identified with Bielschowsky/Luxol (B/L) blue staining. EBA (endothelial barrier antigen) was used for detection of mature vessels
Results
Ischemic rats treated with Tβ4 demonstrated a significant overall improvement (p<0.01) in the ART and the mNSS when compared to controls. Significant improvement was observed beginning at 14 days and 35 days, respectively. Lesion volumes showed no significant differences between the two groups. Treatment with Tβ4 increased myelinated axons and increased vessel density in the ischemic boundary (p<0.05) and augmented remyelination which was associated with an increase of oligodendrocyte progenitor cells (OPCs) and myelinating oligodendrocytes (p<0.05).
Conclusions
The present study suggests that Tβ4 improves neurological functional outcome after embolic stroke in rats. Axonal remodeling from mobilization of OPCs is proposed as contributing to Tβ4 induced functional improvement.
Keywords: axonal remodeling, oligodendrocyte, rat, stroke, Thymosin, Thymosin beta4
Thymosin β4 (Tβ4) is a developmentally expressed 43-amino acid peptide that was originally isolated from thymic extract and exists in numerous tissues and isoforms (Goldstein et al., 1966). Tβ4 inhibits organization of the actin-cytoskeleton by sequestration of G-actin monomers thereby enabling cells to migrate (Goldstein et al., 2005). Additionally, Tβ4 may regulate nuclear actin thereby influencing chromatin remodeling and ultimately gene expression (Huff et al., 2004). Tβ4 has multiple biological functions in addition to its actin binding properties. Tβ4 is an angiogenic promoting molecule which stimulates endothelial cell migration and tubule formation (Malinda et al., 1999, Smart et al., 2007). Tβ4 also promotes healing properties in both wounds and corneal injury by increasing cell migration and reducing inflammation. (Malinda et al., 1999, Sosne et al., 2002). Clinically, Tβ4 promotes wound healing and is being tested in a clinical trial of wound healing (Guarnera et al., 2007).
Tβ4 promotes cardiomyocyte survival and improves cardiac function after myocardial infarction (MI) in experimental adult mice (Bock-Marquette et al., 2004). Tβ4 administration reduces left ventricular scar volume and promotes cardiomyocyte cell survival within 24 hours of coronary ligation. Tβ4 activation of Akt has been proposed as a potential mechanism that increases cell survival after acute MI. Hinkel et al, demonstrated in a pig MI model that downregulation of Tβ4 in embryonic endothelial progenitor cells used to treat experimental MI confers a loss of cardiomyocyte survival, increases infarct volume and reduces left ventricle function (Hinkel et al., 2008). Furthermore, Smart et al, demonstrated that epicardial progenitor cells isolated from wild type adult hearts differentiate into smooth muscle and endothelial cells when cultured in the presence of Tβ4 (Smart et al., 2007). Collectively, Tβ4 may improve cardiac function by promoting cardiomyocyte survival and may stimulate epicardial progenitor cells to differentiate into smooth muscle and endothelial cell types to repair damaged myocardium.
Over the past two decades, research on treatment of stroke has focused on neuroprotection and revascularization strategies. The only successful clinical trial that has resulted from this research is the use of tissue plasminogen activator (rt-PA) within 4.5 hours of symptom onset (The NINDS rt-PA Stroke Study Group 1995, Hacke et al., 2008). Use of rt-PA has been limited because of its narrow time-dependent treatment window as most stroke patients present to the Emergency Department well beyond six hours of symptom onset (California Acute Stroke Pilot Registry (CASPR) Investigators, 2005, Kleindorfer et al., 2007) Moreover, use of rt-PA is complicated by a 6.4% symptomatic intracerbral hemmorhage (ICH) rate which has caused considerable controversy regarding its practical use, particularly amongst Emergency physicians. The restriction on time and potential adverse effects have limited the use of rt-PA to approximately 3% of stroke patients (California Acute Stroke Pilot Registry (CASPR) Investigators, 2005, Kleindorfer et al., 2009). Thus, it is imperative to develop therapies for ischemic stroke designed specifically to reduce neurological deficits, which can be employed to treat the vast majority of patients.
Tβ4 is expressed in both the developing and adult brain (Carpintero et al., 1999, Gomez-Marquez and Anadon, 2002). Specifically, Tβ4 is localized in growing neurites of neurons (van Kesteren et al., 2006, Yang et al., 2008). In many disease states such as focal ischemia and Huntington’s disease, gene expression of Tβ4 is upregulated (Vartiainen et al., 1996, Sapp et al., 2001). Moreover, Tβ4 is upregulated 12 hours to 7 days after focal ischemia (Vartiainen et al., 1996). Since actin dynamics contribute to cell migration and synaptogenesis, we speculate that administration of Tβ4 could enhance recovery. Using a rat model of embolic stroke, we tested the hypothesis that treatment with Tβ4 improves neurological functional outcome when administered 24 hours after onset of ischemia.
EXPERIMENTAL PROCEDURES
All experimental procedures were approved by the Institutional Animals Care and Use Committee of Henry Ford Hospital.
Embolic stroke rat model
The middle cerebral artery (MCA) of male Wistar rats (320 to 380 g, n=18) was occluded by placement of an embolus at the origin of the MCA, as previously described (Zhang et al., 1997).
Tβ4 treatment
Twenty four hours after stroke, Tβ4 provided by RegeneRx, Inc. was administered intraperitonal (IP) and then every 3 days (6 mg/kg, IP) for 4 additional doses (n=9). These doses and the route were chosen based on previous studies of Tβ4 biodistribution that showed increased concentrations of Tβ4 in the brains of Swiss-Webster mice between 40 minutes and 2 hours following IP injection (Mora et al., 1997). Moreover, the blood brain barrier (BBB) in this rat model is disrupted. Three dimensional immunofluorescent analyses revealed spatial BBB leakage in the ischemic core and corresponding white matter volume (Zhang et al., 2002b, Ding et al., 2006). Saline was administered IP to stroke rats as a control group (n=9). For investigating the long term effect of Tβ4 on brain remodeling and functional outcome, all rats were sacrificed 56 days after MCAo.
Functional tests
In the embolic stroke rat model, a battery of behavioral tests was performed before MCA occlusion and at 1, 7, 14, 21, 28, 35, 42, 49 and 56 days after MCAo by an investigator who was blinded to the experimental groups. The battery of tests consisted of the adhesive-removal test (ART) and the modified Neurological Severity Score (mNSS) (Cenci et al., 2002, Chen et al., 2006a). Briefly, the rats were removed from their home cages so that the adhesive paper dots could be firmly and accurately attached. Two small pieces of adhesive-backed paper dots (of equal size, 113.1 mm2) were used as bilateral tactile stimuli occupying the distal-radial region on the wrist of each forelimb. The rats were then returned to their cages and they typically contacted and removed each stimulus one at a time using their teeth. The time required to remove both stimuli from each limb was recorded in 5 trials per day. Before surgery, the rats were trained 5 times a day for 3 d and all rats were able to remove the dots within 10 sec at the end of training. The rats, therefore, were familiarized with the testing environment. Each animal received 5 trials on all testing days after MCA occlusion and the mean time required to remove both stimuli from limbs was recorded. In order to increase sensitivity of the test, the adhesive-backed paper dots were reduced in size by one-half at day 35. The mNSS test is a composite score in which motor, sensory, balance and reflex measures are used to calculate a value ranging from 1 to 18, with the higher score implying greater neurological injury. Points are awarded for the inability to perform the tasks or for the lack of a tested reflex. (normal score 1, maximal deficit score 18).
Neurosphere and RNA isolation
Subventricular zone (SVZ) cells were dissociated from normal (n=21) and 7 day MCA occlusion (n=21) rats, as previously described (Zhang et al., 2004). Three separate cultures each containing SVZ cells from seven rats were grown. The cells were plated at a density of 20,000/ml in medium containing 20 ng/ml epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF). The generated neurospheres (primary spheres) were passed by mechanical dissociation and reseeded as single cells at a density of 20/ul in EGF-containing media. Passage 2 and 3 neurospheres were used in the present study. For differentiation assay, the cells were grown in media containing no growth factors (differentiation media) for an additional 7 days. The neurospheres were then incubated at 37°C for 7 days with 0, 25 or 50 ng/ml of Tβ4. Total RNA from the cells was isolated using RNeasy Micro Kit (Qiagen, Inc). cDNA was prepared from total RNA using oligo (dT), dNTP mix, First-Strand Buffer, DTT, RNaseOut and Superscript III (Invitrogen). Gene expression assays were then performed.
Real-Time PCR
Real Time PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems). The RT-PCR reaction system contained SYBR Green® Universal Master Mix. Gene expression was normalized to the internal control gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative gene expression was determined using the 2 −ΔΔCT method. Primers designed to test for gene expression of oligodendrogenesis (NG2, Oligo2 (bHLH transcription factor)) and EGFR (epidemeral growth factor receptor) and neurogenesis (Mash1 (mammalian homolog of achaete-scute complex-like 1)) were constructed. Primer sequences (5-3″) for GAPDH, NG2, Oligo2, Mash1 and EGFR are listed in table 1.
Table 1.
| cDNA | Primer sequence (5″-3″) |
|---|---|
| GAPDH | GTG-GAC-CTC-ATG-GCC-TAC-AT TGT-GAG-GGA-GAT-GCT-CAG-TG |
| NG2 | AAA-CTC-TCC-CTC-CCT-GGT-GT TTA-ACC-AAC-CCCAGA-GCA-AC |
| OLIGO2 | GGG-TCC-TGT-GGT-CTC-AGA-AG GCT-TGC-TCA-TGT-GGT-CTG-AA |
| MASH1 | GGC-TCA-ACT-TCA-GTG-GCT-TC GCC-CAG-GTT-AAC-CAA-CTT-GA |
| EGFR | CTG-CCA-AGG-CAC-AAG-TAA-CA CCC-AAG-GAC-CAC-TTC-ACA-GT |
Histological and immunohistochemical assessment
After neurological testing was completed on day 56, rats were sacrificed. Rat brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde, and the brains were embedded in paraffin. The cerebral tissues were cut into seven equally spaced (2 mm) coronal blocks. A series of adjacent 6 μm-thick sections was cut from each block in the coronal plane and was stained with hematoxylin and eosin (H&E). Seven brain sections were traced using a microcomputer imaging device (MCID) image analysis system (Imaging Research, St. Catharines, Canada). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated. Lesion volume is represented as a volume percentage of the lesion compared with the contralateral hemisphere.
Standard paraffin blocks were obtained from the center of the lesion, corresponding to coronal coordinates for bregma -1-1 mm. A series of 6 μm thick sections at various levels (100 μm interval) were cut. Immunostaining was performed on these sections. Antibodies were used for identification of CNPase (2″,3″-cyclic nucleotide 3′-phosphodiesterase), (1:200, Monoclonal, incubated for 2 days at 4°C, Chemicon, CA), a marker for maturing oligodendrocytes (OL), NG-2 (chondroitin sulfate proteoglycan) (1:800, Polyclonal rabbit, incubated overnight at 4°C, Chemicon, CA), a marker of oligodendrocyte progenitor cells (OPC), and neurofilament-H (NF-H, 1:10,000, Polyclonal chicken, incubated for one hour at room temperature, ABR, Co.), a marker of axons and EBA (endothelial barrier antigen) (1:1000, IgM mouse, incubated overnight at 4°C) for detection of mature vessels with intact BBB. Following sequential incubation with biotin-conjugated anti mouse or goat IgG (dilution 1:200, Vector laboratories, INC), the sections were treated with an ABC kit (Vector laboratories, INC). Diaminobenzidine (DAB) was then used as a sensitive chromogen for light microscopy. Bielschowsky and Luxol (B/L) fast blue staining were used to identify myelinated axons. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitting the primary antibodies.
Image acquisition and quantification
Coronal sections were digitized using a 40x objective using a three-CCD color video camera (Sony DXC-970MD) interfaced with a MCID system. For quantification of axon and myelinating OLs, the density of NF-H, B/L blue staining and CNP-ase immunoreactive area throughout the SVZ, peri-infarct corpus callosum and striatum, and the contralateral homologous area were digitized. Two coronal sections (100 μm interval) from the center of the ischemic core (bregma 0.2 mm) were used per staining from each animal. For quantification of axon and myelinating OLs, NF-H, B/L blue staining and CNP-ase immunoreactive areas were measured in eight fields of view within peri-infarct corpus callosum and striatum. The contralateral homologous areas were used as reference because axons and myelinating OLs vary with anatomic regions. To minimize variation of anatomic structures among slices, we measured the contralateral homologous regions as a reference and determined that Tβ4 treatment did not change these parameters used in the present study in the contralateral hemisphere. Data are presented as the percentage of changes in the areas of staining at the peri-infarct corpus callosum and striatum compared with the contralateral homologous region on the same section. For quantitative analysis of OPCs, the numbers of NG2 immunoreactive cells were counted throughout the ipsilateral SVZ of the lateral ventricular wall and peri-infarct area at the corpus callosum and striatum. The number of positive cells for the two coronal sections per rat was averaged to obtain a mean number of cells. Data are presented as the density of immunoreactive cells relative to the area of the SVZ and peri-infarct corpus callosum and striatum. For quantification of vessels, the number of EBA positive vessels was measured in eight fields of view within the peri-infarct corpus callosum and striatum. The number of positive vessels for the two coronal sections per rat was averaged to obtain a mean number of vessels. Data are presented as the density of EBA immunoreactive vessels relative to the area of the peri-infarct corpus callosum and striatum.
Statistical Analysis
Animals were treated with either TB4 or placebo at day 1 after MCAo. Functional tests (ART and mNSS) were performed and measured day 1 (baseline) before the study treatment and at days 7, 14, 21, 28, 35, 42, 49 and day 56 after the stroke onset. Data were evaluated for normality. Data transformation was considered if data were not normal. As the result, ranked ART and mNSS were used for the analysis, given the data were not normal, respectively. We first tested the balance of the data at baseline among the groups using two-sample t-test. The imbalanced variable would be included as covariate to test the treatment and/or dose effect. For each functional outcome, repeated measures analysis of variance (ANCOVA) was used to study the TB4 effect and its effect over time. The analysis began testing for 2-way interaction of TB4, and time, followed by testing the main effect of time at the 0.05 level. Least-square mean treatment comparison was conducted if there was 2-way interaction or the main effect of time/TB4. Two-sample t-test was used to test the effect of TB4 on cell measurements, compared to controls. Results are presented as mean ± S.E for data illustration.
RESULTS
A total of 18 rats were subjected to embolic middle cerebral artery occlusion (MCAo). Tβ4 (6 mg/kg, IP) was administered 24 hours after MCAo and then every 3 days for 4 additional doses (n=9). All the baseline data (for stroke severity) were balanced (p>=0.50).
Neurological functional outcome and lesion volume
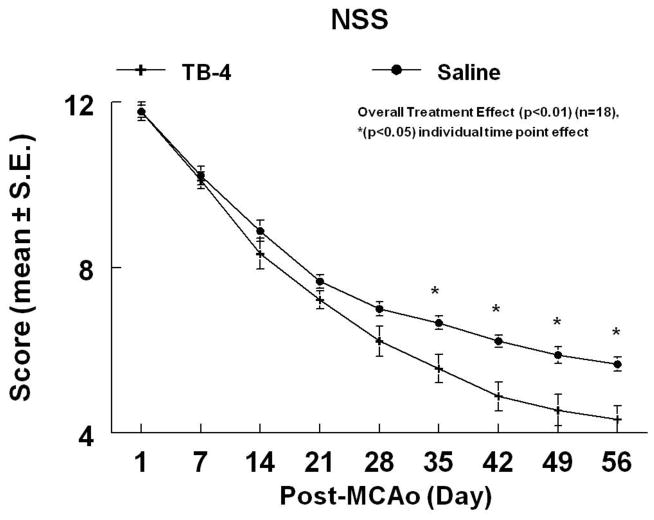
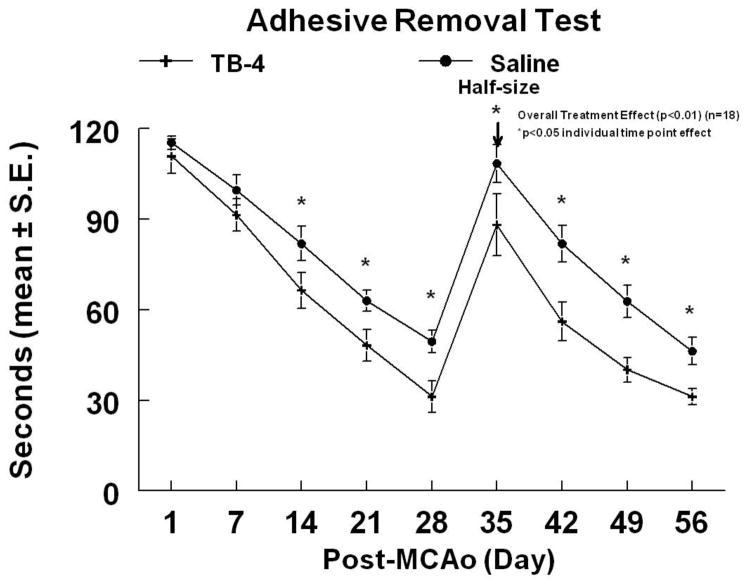
To test whether Tβ4 promotes functional recovery after stroke, ischemic rats were treated with Tβ4 starting 24h after MCAo and then every 3 days (6 mg/kg, IP) for 4 additional doses. Figures 1 and 2 demonstrate the time course of improvement on the mNSS and the ART. Tβ4 treated rats demonstrated a 24.2% and a 29.9% overall improvement in the ART and mNSS scores (at time of sacrifice), respectively, when compared to controls (overall treatment effect, p<0.01). Functional improvements persisted for at least 56 days after MCAo. There were no significant differences of ischemic lesion volumes between the rats treated with Tβ4 (35.2% ± 6.7%) and with saline (33.1 %± 7.8%, p>0.05).
Figure 1.
The mNSS of embolic stroke rats treated with or without Tβ4. Overall treatment effect (p<0.01) was observed with significant effect (p<0.05) at individual time points indicated.
Figure 2.
The adhesive removal test of embolic stroke rats treated with or without Tβ4. Overall treatment effect (p<0.01) was observed with significant effect (p<0.05) at individual time points indicated. Adhesive-backed paper dots were reduced in size by one-half at day 35 (arrow).
Neuronal and oligodendrocyte gene expression
Gene expression in normal non-ischemic neurospheres cultured in differentiation media in the presence of Tβ4 was measured. Tβ4 (50 ng/ml) increased Mash1 expression, a gene that regulates neuronal and OL differentiation, by a factor of 1.39 ± 0.61. NG2 and Oligo2 gene expression, both of them expressed in OPCs, was increased by a factor of 1.97 ± 0.46 and 1.32 ± 0.18, respectively. EGFR gene expression was increased by a factor of 1.47 ± 0.51. Based on the 2-fold increase in gene expression of NG2, we further investigated myelination and OPC proliferation in Tβ4 treated rats.
Tβ4 regulates axonal remodeling after stroke
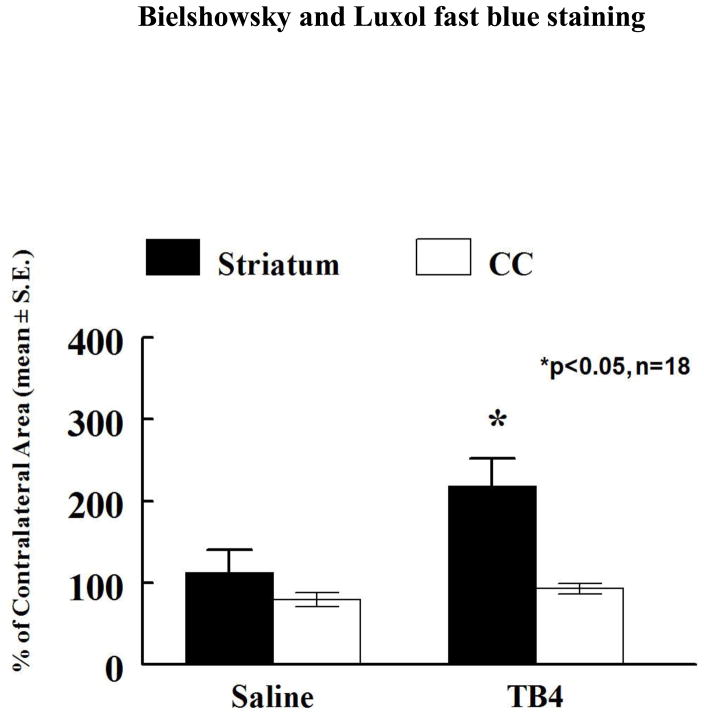
To test whether Tβ4 promotes axonal remodeling after stroke, brain sections were stained using the B/L fast blue staining to detect myelinated axons. Figure 3 demonstrates significant increases in myelinated axons in the striatal ischemic boundary in the Tβ4 treatment group (215.3 ± 29.9%) when compared to the control group (115.2 ± 9.0%) (p<0.05). No differences were detected in the corpus collosum. In addition, immunostaining revealed that NF-H immunoreactivity, a marker of axons, was increased in the treatment group (143.5 ± 10.2%) when compared to control (104.1% ± 5.1%, p<0.05, Fig. 4).
Figure 3.
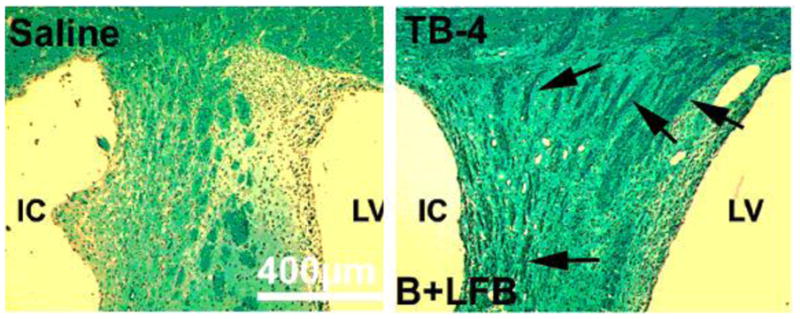
The staining by Bielshowsky and Luxol fast blue shows the myelin and axons in the white matter bundles of the striatum of saline and Tβ4 treated rats (see arrows). There is an increased area of staining in the Tβ4 treated rats compared to the demyelination of the saline control. Quantitative data show significantly increased staining in the Tβ4 treated rats compared to the saline control. CC= corpus collosum, LV=lateral ventricle and IC=ischemic core.
Figure 4.
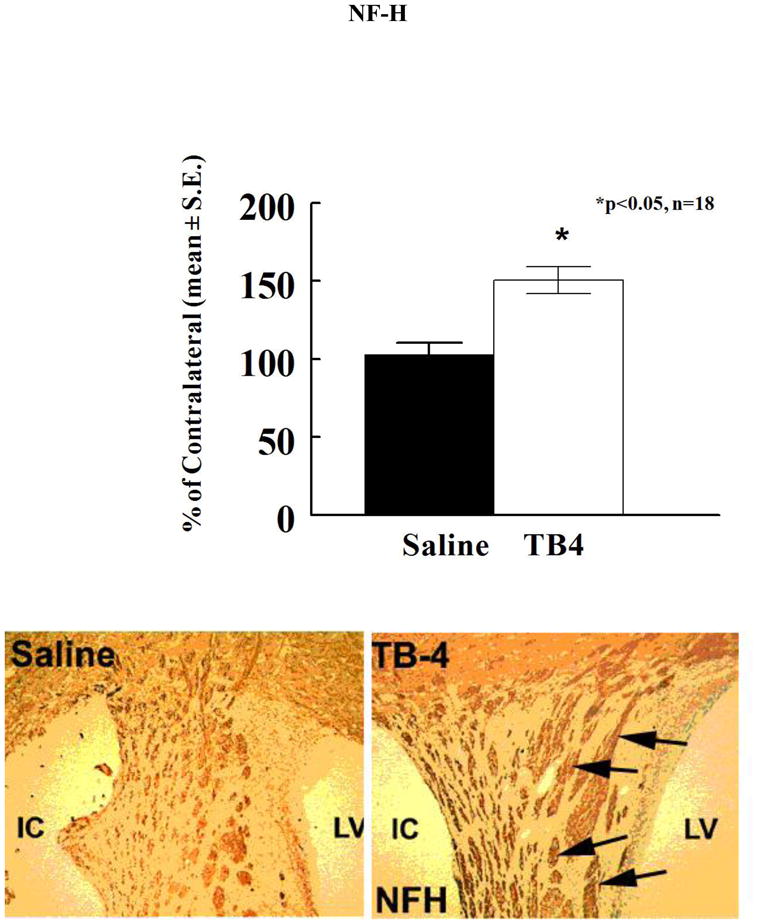
The neurofilament-H (NF-H) shows the axons in the white matter bundles of the striatum of saline and Tβ4 treated rats (see arrows). There is an increased area of staining in the Tβ4 treated rats compared to the saline control. Quantitative data show significantly increased staining in the Tβ4 treated rats compared to the saline control. LV=lateral ventricle and IC=ischemic core
Tβ4 increases SVZ oligodendrocyte progenitor cell proliferation
To test whether Tβ4 treatment increases the number OPCs after stroke, NG-2, a marker of OPCs and CNPase, a marker of myelinating OLs, were measured. Figures 5 and 6 demonstrate the expression of these two markers. When compared to controls, Tβ4 treatment significantly increased the density (cells/mm2) of NG-2 positive cells in the SVZ (396.6 ± 19.6 vs 209.1 ± 42.7) and striatum (130 ± 15.3 vs 61.0 ± 7.6) (p<0.05). NG-2 immunoreactivity was also increased in the corpus collosum (166.8 ± 26.0 vs 78.3 ± 12.2, p<0.05). CNPase immunoreactivity was increased in the striatum (149.1%± 9.4% vs 115.2%± 7.1%, p<0.05).
Figure 5.
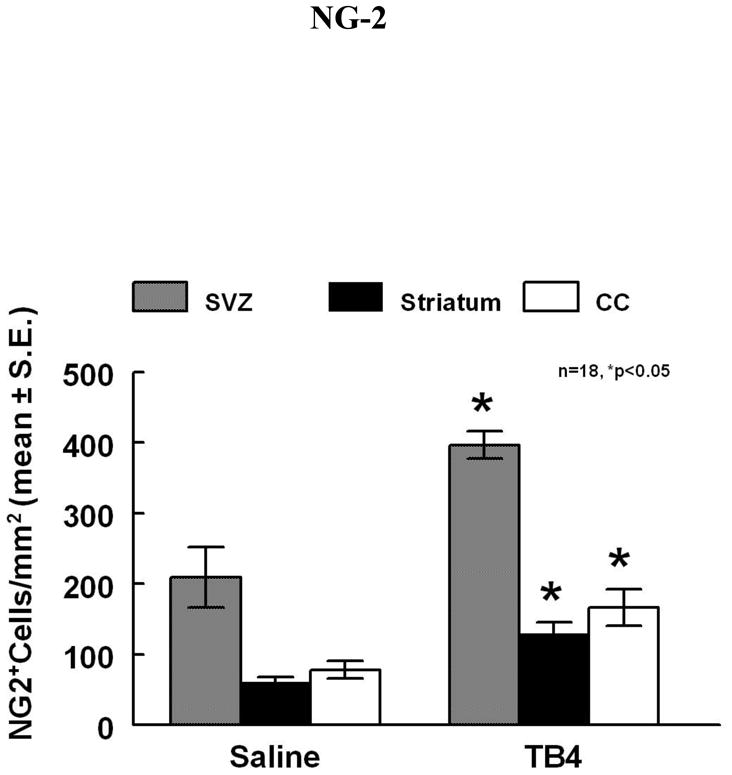
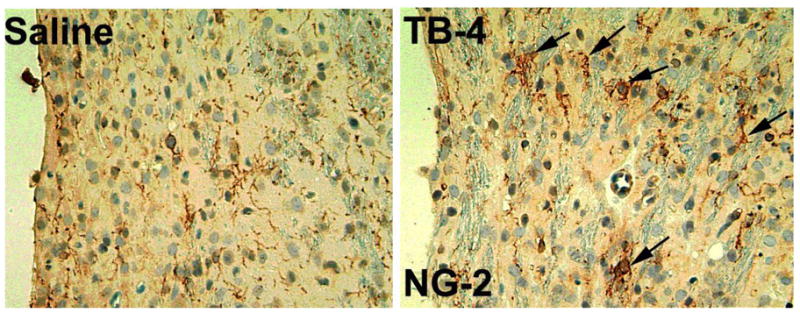
NG-2 staining is increased in the ipsilateral SVZ and striatum adjacent to the ischemic core of Tβ4 treated rats when compared to saline control (see arrows). Quantitative data show significantly increased density in these areas in the Tβ4 treated rats compared to the saline control.
Figure 6.
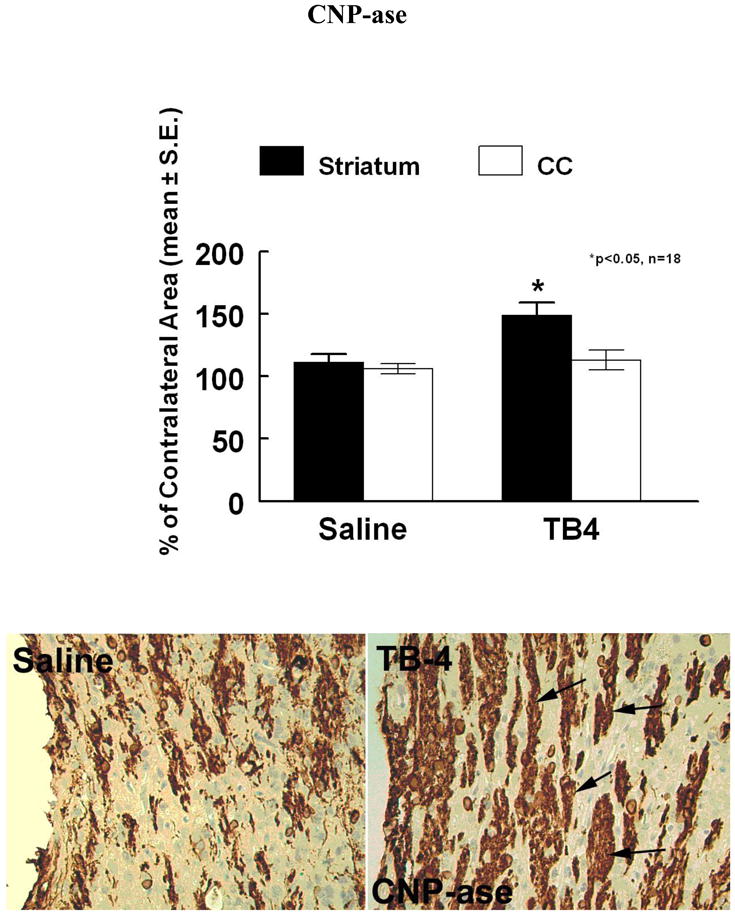
CNPase is increased in the striatum of Tβ4 treated rats when compared to saline control (see arrows). Quantitative data show significantly increased staining in the striatum in the Tβ4 treated rats compared to the saline control. CC= corpus collosum,
Tβ4 increases vessel density in the ischemic boundary zone
To test whether Tβ4 increases vessel density after stroke, EBA, a marker of vessels, were measured. When compared to controls, Tβ4 treatment significantly increased the density (vessels/mm2) of EBA positive vessels in the peri-infarct corpus callosum and striatum area (419.2 ± 50.9 vs 289.9 ± 22.8) (p<0.05), Figure 7.
Figure 7.
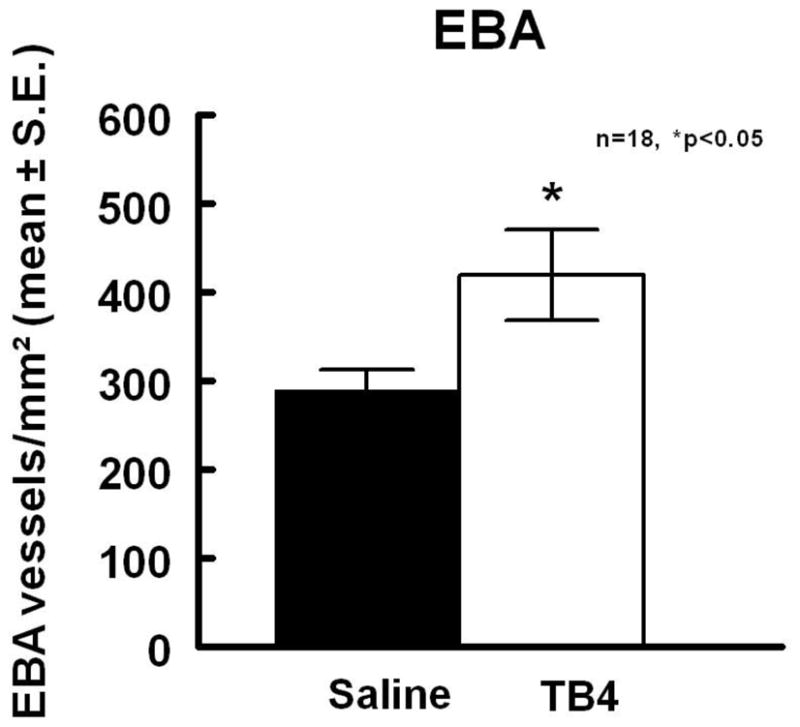
Tβ4 treatment significantly increased the density (vessels/mm2) of EBA positive vessels in the peri-infarct corpus callosum and striatum area when compared to saline
DISCUSSION
We demonstrate that administration of TB4 to a rat model of embolic stroke improved neurological outcome when administered 24 hours after MCAo. Significant improvement in functional outcome was observed as early as 14 days after stroke in the ART and 35 days in the comprehensive mNSS test. Infarction volume was unchanged, signifying a mechanism of action unrelated to a neuroprotective mechanism. Our data suggest a different mechanism, a mechanism that appears to stimulate axonal remodeling at the ischemic boundary as evidenced by the increased presence of B/L fast blue and NF-H staining in treated animals. Additional evidence to support this hypothesis is the increase of OPCs in the SVZ, striatum and corpus collosum. A significant increase of mature OLs as demonstrated by increased CNPase further supports the evidence that axonal remodeling contributes to the delayed improvement in functional outcome. Furthermore, an increase in vessel density as measured by EBA also provides evidence that Tβ4 promotes angiogenesis in the ischemic boundary zone.
Stroke increases neurogenesis in the SVZ, specifically type A and C cells (Arvidsson et al., 2002, Zhang et al., 2004). Our fundamental hypothesis, based on our described data, is that treatment with Tβ4 promotes axonal repair by stimulation of OPCs in the SVZ (subpopulation of type C cells) and OPCs in the intact white matter. OLs are highly susceptible to focal cerebral ischemia and our data suggest that Tβ4 increases differentiation of OLs as evidenced by increased CNPase staining. It was once thought that white matter was resistant to hypoxic injury, however, evidence now suggests that injury to OL leads to axonal damage, affecting motor, sensory and cognitive function (Pantoni et al., 1996, Dewar et al., 2003). In our model, Tβ4 improved functional outcome in the mNSS, a comprehensive motor and sensory test, and in the ART somatosensory test (sensory and motor).
OLs are highly vulnerable to focal cerebral ischemia (Pantoni et al., 1996). Pantoni, et al demonstrated that as early as 30 minutes after MCAo, diffuse injury to OLs occurred in the area of infarction. Swelling of OLs followed by vacuolation of the white matter with segmental swelling of myelinated axons was observed. Although the ischemic damage to OLs was assumed to be lethal, Gregersen et al found myelin basic protein (MBP) and growth-associated protein-43 (GAP-43) gene transcription in OLs located in the peri-infarct areas three days after the MCAo, suggesting a remyelination process was occurring (Gregersen et al., 2001). In general, mature OLs are unable to migrate or divide (Franklin and Ffrench-Constant, 2008). However, new OLs can be generated by OPCs that are present in the SVZ and white matter of adult rodent brain (Franklin and Ffrench-Constant, 2008). Our data show that OPCs assayed by NG2 positive cells and OLs detected by CNPase immunoreactivity were present in the SVZ and the peri-infarct white matter areas. Collectively, these observations suggest that ischemic injury severely damages OLs, however, a regeneration process is initiated. The success of this regeneration process is likely dependant on the duration and intensity of ischemia. We propose that the regeneration process can be improved and augmented by Tβ4. Similar to the cardiac and skin research whereby respective tissue progenitor cells migrate to areas of injury and differentiate, we suggest that Tβ4 stimulates OPCs to migrate to the area of infarction and subsequently differentiate into mature myelin secreting OLs.
Remyelination has been well studied in various adult animal models and involves the generation of new mature OLs (Horner et al., 2000, Sim et al., 2002). Our data show that treatment with Tβ4 increased the number of mature OLs leading to an increase in myelinated axons after injury as evidenced by the increased in B/L fast blue staining, suggesting that Tβ4 enhances remyelination. Further investigations using higher and more frequent doses of Tβ4 are warranted given the fact that the doses used in this study were small. The improved neurological outcome supports the hypothesis that axonal remodeling may be occurring in these Tβ4 treated embolic stroke rats.
TB4 was also observed to increase vessel density which is consistent with the observations in the cardiac models. Smart et al, demonstrated that TB4 is an essential factor for all aspects of coronary vessel development in mice. Vascular progenitor cells isolated from the adult epicardium differentiated into smooth muscle and endothelial cells when cultured in the presence of Tβ4. Tβ4 may improve cardiac function by acting in conjunction with other angiogenic factors (i.e. VEGF) to revascularize damaged myocardium through stimulation of adult vascular progenitor cells. Furthermore, Tβ4’s wound healing properties can be attributed to its angiogenic actions. Tβ4 specifically stimulates endothelial cell migration and was found to be upregulated 5-fold after endothelial cells began to make capillary-like structures on Matrigel (Malinda et al., 1997). In a rat wound assay, Tβ4 increased the number of blood vessels in healing wounds 4 to 7 days after injury when compared to controls (Malinda et al., 1999).
The specific receptor for Tβ4 has not yet been discovered. Molecular mechanisms underlying Tβ4-increased OLs remain to be investigated. However, Our RT-PCR analysis revealed that Tβ4 at concentration of 50ng/ml upregulated EGFR and Mash1 gene expression by a factor of 1.5 and 1.4, respectively, in SVZ neural progenitor cells, which was coupled with upregulation of OPC marker genes, NG2 and Oligo2. Aguirre et al demonstrated that NG2 cells in the adult SVZ migrate to the corpus callosum and differentiate into OLs in response to EGFR signaling under white matter demyelination (Aguirre and Gallo, 2007). Ivkovic et al observed that overexpression of EGFR produces diffuse hyperplasia of white matter composed of OPCs in a 3-day post-natal rat forebrain model (Ivkovic et al., 2008). Upregulation of Mash1 promotes neural progenitor cell differentiation into the OLs (Aguirre et al., 2007). Collectively, these data suggest that EGFR and Mash1 upregulated by Tβ4 in neural progenitor cells could promote generation of OPCs and maturation of OLs observed in our stroke model. Although our findings are preliminary, the present data provide a basis for initiating investigations for the cause effect of EGFR and Mash1 on Tβ4-induced OLs.
Other mechanisms may be involved in the observed functional recovery. This study provides evidence that remyelination and angiogenesis are potential mechanisms of recovery, however, we cannot exclude the possibility that Tβ4 may enhance other restorative processes in the ischemic brain such as activation of cellular survival mechanisms that was observed in the cardiac literature. Further study of these mechanisms are warranted; however, Tβ4’s potential for restoration of myelination with an increase of intact vessel density after stroke, especially in a time window of administration of 24 hours demonstrates its enormous potential for the treatment of the vast majority of stroke patients.
The observation that treatment with Tβ4 improved functional neurological outcome when initiated 24 hours after the onset of injury adds to a body of literature on restorative therapy for stroke and neural injury. Previous studies in the treatment of stroke and brain trauma demonstrated that the injured brain can be stimulated to improve neurological function. Pharmacological based therapies such as phosphodiesterase 5-inhibitors (Zhang et al., 2002a), nitric oxide donors (Chen et al., 2006b), statins (Chen et al., 2003) and erythropoietin (Wang et al., 2004, Lu et al., 2005) all improved neurological outcome in stroke and trauma. These treatments induce changes in neurogenesis (Zhang et al., 2006), angiogenesis (Chen et al., 2003), synaptogenesis (Shen et al., 2006) in the injured brain. Tβ4 selectively actsi to promote maturation of the OPCs and may in fact be useful in other demyelinating diseases.
In conclusion, we demonstrate that administration of Tβ4 24 hours after stroke improved functional neurological outcome. Our data suggests that a remyelination repair process by the increase in OPCs and mature OLs contributes to functional improvement. Our study is a proof-of-concept study and further preclinical dose response studies are warranted to investigate Tβ4 and its potential role in the treatment of neurological injuries.
Acknowledgments
Support
This work was support by NINDS grants: PO1 NS23393, RO1 NS062832 and R01 HL64766
We would like to thank Cynthia Roberts for technical assistance
List of Abbreviations
- ART
adhesive-removal test
- ANOVA
analysis of variance
- bFGF
basic fibroblast growth factor
- B//L
Bielschowsky/Luxol
- CCD
charge couple device
- CNPase
2″, 3″-cyclic nucleotide 3′-phosphodiesterase
- DAB
diaminobenzidine
- DTT
dithiothreitol
- EBA
endothelial barrier antigen
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GAP-43
growth-associated protein-43
- H/E
hematoxylin and eosin
- IP
intraperitonal
- MASH1
mammalian homolog of achaete-scute complex-like 1
- MBP
myelin basic protein
- MCAo
middle cerebral artery occlusion
- MCID
microcomputer imaging device
- MI
myocardial infarction
- mNSS
modified Neurological Severity Score
- NF-H
Neurofilament-H
- NG-2
chondroitin sulfate proteoglycan
- OL
oligodendrocyte
- OPC
oligodendrocyte progenitor cells
- SVZ
subventricular zone
- Tβ4
Thymosin β4
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
A US Provisional Patent 61/163,556 has been filed for this discovery.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- Aguirre A, Gallo V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007;3:209–220. doi: 10.1017/S1740925X08000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- California Acute Stroke Pilot Registry (CASPR) Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- Carpintero P, Anadon R, Diaz-Regueira S, Gomez-Marquez J. Expression of thymosin beta4 messenger RNA in normal and kainate-treated rat forebrain. Neuroscience. 1999;90:1433–1444. doi: 10.1016/s0306-4522(98)00494-1. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006a;141:737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li Y, Li A, Wang L, Katakowski M, Roberts C, Lu M, Chopp M. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006b;140:377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Gang Zhang Z, Ledbetter KA, Ewing JR, Li Q, Chopp M. Detection of BBB disruption and hemorrhage by Gd-DTPA enhanced MRI after embolic stroke in rat. Brain Res. 2006;1114:195–203. doi: 10.1016/j.brainres.2006.07.116. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Hannappel E, Kleinman HK. Thymosin beta4: actin-sequestering protein moonlights to repair injured tissues. Trends Mol Med. 2005;11:421–429. doi: 10.1016/j.molmed.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Goldstein AL, Slater FD, White A. Preparation, assay, and partial purification of a thymic lymphocytopoietic factor (thymosin) Proc Natl Acad Sci U S A. 1966;56:1010–1017. doi: 10.1073/pnas.56.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marquez J, Anadon R. The beta-thymosins, small actin-binding peptides widely expressed in the developing and adult cerebellum. Cerebellum. 2002;1:95–102. doi: 10.1007/BF02941895. [DOI] [PubMed] [Google Scholar]
- Gregersen R, Christensen T, Lehrmann E, Diemer NH, Finsen B. Focal cerebral ischemia induces increased myelin basic protein and growth-associated protein-43 gene transcription in peri-infarct areas in the rat brain. Exp Brain Res. 2001;138:384–392. doi: 10.1007/s002210100715. [DOI] [PubMed] [Google Scholar]
- Guarnera G, ADER, Camerini R. Thymosin beta-4 and venous ulcers: clinical remarks on a European prospective, randomized study on safety, tolerability, and enhancement on healing. Ann N Y Acad Sci. 2007;1112:407–412. doi: 10.1196/annals.1415.003. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Muller S, Willhauck M, Spitzweg C, Gildehaus FJ, Munzing W, Hannappel E, Bock-Marquette I, DiMaio JM, Hatzopoulos AK, Boekstegers P, Kupatt C. Thymosin beta4 is an essential paracrine factor of embryonic endothelial progenitor cell-mediated cardioprotection. Circulation. 2008;117:2232–2240. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff T, Rosorius O, Otto AM, Muller CS, Ballweber E, Hannappel E, Mannherz HG. Nuclear localisation of the G-actin sequestering peptide thymosin beta4. J Cell Sci. 2004;117:5333–5341. doi: 10.1242/jcs.01404. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. J Neurosci. 2008;28:914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleindorfer D, Xu Y, Moomaw CJ, Khatri P, Adeoye O, Hornung R. US geographic distribution of rt-PA utilization by hospital for acute ischemic stroke. Stroke. 2009;40:3580–3584. doi: 10.1161/STROKEAHA.109.554626. [DOI] [PubMed] [Google Scholar]
- Kleindorfer DO, Broderick JP, Khoury J, Flaherty ML, Woo D, Alwell K, Moomaw CJ, Pancioli A, Jauch E, Miller R, Kissela BM. Emergency department arrival times after acute ischemic stroke during the 1990s. Neurocrit Care. 2007;7:31–35. doi: 10.1007/s12028-007-0029-5. [DOI] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Goldstein AL, Kleinman HK. Thymosin beta 4 stimulates directional migration of human umbilical vein endothelial cells. FASEB J. 1997;11:474–481. doi: 10.1096/fasebj.11.6.9194528. [DOI] [PubMed] [Google Scholar]
- Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, Kleinman HK. Thymosin beta4 accelerates wound healing. J Invest Dermatol. 1999;113:364–368. doi: 10.1046/j.1523-1747.1999.00708.x. [DOI] [PubMed] [Google Scholar]
- Mora CA, Baumann CA, Paino JE, Goldstein AL, Badamchian M. Biodistribution of synthetic thymosin beta 4 in the serum, urine, and major organs of mice. Int J Immunopharmacol. 1997;19:1–8. doi: 10.1016/s0192-0561(97)00005-2. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1646. doi: 10.1161/01.str.27.9.1641. discussion 1647. [DOI] [PubMed] [Google Scholar]
- Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K, Bhide PG, Vonsattel JP, DiFiglia M. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol. 2001;60:161–172. doi: 10.1093/jnen/60.2.161. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature. 2007;445:177–182. doi: 10.1038/nature05383. [DOI] [PubMed] [Google Scholar]
- Sosne G, Hafeez S, Greenberry AL, 2nd, Kurpakus-Wheater M. Thymosin beta4 promotes human conjunctival epithelial cell migration. Curr Eye Res. 2002;24:268–273. doi: 10.1076/ceyr.24.4.268.8414. [DOI] [PubMed] [Google Scholar]
- The NINDS rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- van Kesteren RE, Carter C, Dissel HM, van Minnen J, Gouwenberg Y, Syed NI, Spencer GE, Smit AB. Local synthesis of actin-binding protein beta-thymosin regulates neurite outgrowth. J Neurosci. 2006;26:152–157. doi: 10.1523/JNEUROSCI.4164-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen N, Pyykonen I, Hokfelt T, Koistinaho J. Induction of thymosin beta(4) mRNA following focal brain ischemia. Neuroreport. 1996;7:1613–1616. doi: 10.1097/00001756-199607080-00017. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Yang H, Cheng X, Yao Q, Li J, Ju G. The promotive effects of thymosin beta4 on neuronal survival and neurite outgrowth by upregulating L1 expression. Neurochem Res. 2008;33:2269–2280. doi: 10.1007/s11064-008-9712-y. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002a;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006;83:1213–1219. doi: 10.1002/jnr.20813. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang RL, Jiang Q, Raman SB, Cantwell L, Chopp M. A new rat model of thrombotic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:123–135. doi: 10.1097/00004647-199702000-00001. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, Goussev A, Powers C, Yeich T, Chopp M. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002b;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]