Abstract
Sphingolipids in the membranes of neurons play important roles in signal transduction, either by modulating the localization and activation of membrane-associated receptors or by acting as precursors of bioactive lipid mediators. Activation of cytokine and neurotrophic factor receptors coupled to sphingomyelinases results in the generation of ceramides and gangliosides, which in turn, modify the structural and functional plasticity of neurons. In aging and neurodegenerative conditions such as Alzheimer’s disease (AD), there is increased membrane-associated oxidative stress and excessive production and accumulation of ceramides. Studies of brain tissue samples from human subjects, and of experimental models of the diseases, suggest that perturbed sphingomyelin metabolism is a pivotal event in the dysfunction and degeneration of neurons that occurs in AD and HIV dementia. Dietary and pharmacological interventions that target sphingolipid metabolism should be pursued for the prevention and treatment of neurodegenerative disorders.
Keywords: Alzheimer’s Disease, sphingolipid, sphingomylein, ceramide, sphingosine, ganglioside, synapse, amyloid
Introduction
Nearly two decades of research on roles for lipids in Alzheimer’s disease (AD) suggest that a progressive disturbance in the composition of brain lipids may play important roles in the neuropathological process. These findings identify a disease-associated disruption in brain lipid biochemistry that deregulates levels of particular phospholipid, sphingomylein, ceramide and ganglioside species in the brains of subjects with AD. Since a wide variety of signaling events are regulated by these lipids, a shift in the composition of lipids in brain cells would have profound effects on neural function. In this review we summarize our current state of knowledge for how disruptions in sphingolipid metabolism may promote aberrant amyloid processing, β-amyloid oligomerization and disruption of synaptic function in AD.
Overview of Sphingolipid Metabolism
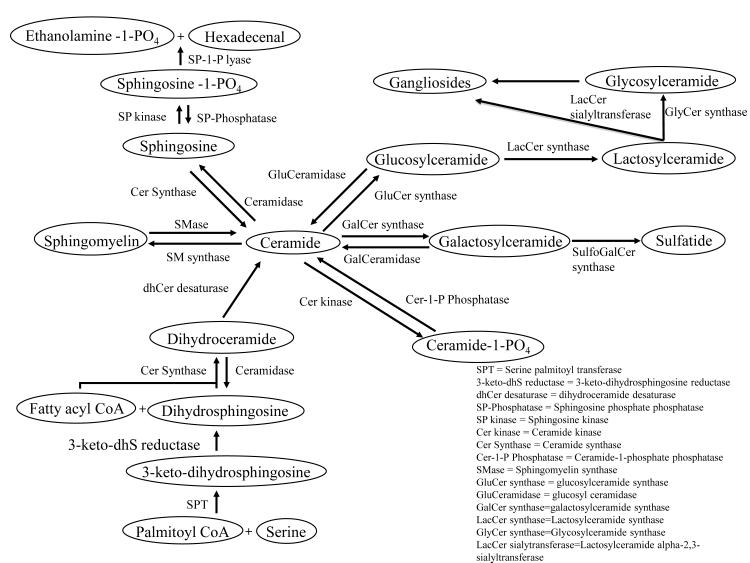
Sphingolipid metabolism is complex and involves hundreds of molecular species and metabolic pathways. We do not attempt to fully address the complexities of sphingolipid metabolism in this section, rather it is intended to familiarize the reader with some relevant reactions involved in the metabolism of the major classes of sphingolipids (Figure 1). Knowledge of sphingolipid metabolism is essential when considering how perturbations in these pathways may contribute to aberrant amyloid processing and synaptic failure in AD.
Figure 1.
Pathways of Sphingolipid Metabolism
Sphingolipids are derived from the alipathic amino alcohol sphingosine. The sphingosine backbone is O-linked to a charged head group such as ethanolamine, serine or choline, and amide-linked to an acyl group, such as a fatty acid. Ceramides are the simplest sphingolipids, consisting of a fatty acid chain attached by an amide linkage to sphingosine. Ceramide is a precursor to sphingomyelin, which has a phosphorylcholine or phosphoethanolamine esterfied to the 1-hydroxy group of ceramide. Ceramide can be deacylated to produce sphingosine that can then be phosphorylated to create sphingosine 1-phosphate. Glycosphingolipids are also derived from ceramides with the addition of one or more sugar residues, joined with a α-glycosidic linkage at the 1-hydroxyl position. Sphingolipids are enriched in the central nervous system (CNS), where in addition to important structural roles, sphingolipid metabolites function as second messengers to modulate a wide variety of signaling events. The major enzymes that regulate the metabolism of sphingolipids highlighted above are briefly discussed below.
Ceramide and Sphingomyelin
Ceramide is synthesized de novo in the endoplasmic reticulum with the condensation of serine and palmitoyl-CoA by serine palmitoyltransferase to produce 3-keto dihydrospingosine. 3-keto dihydrospingosine is then reduced to dihydrospingosine by 3-keto dihydrospingosine reductase. Dihydrosphingosine is N-acylated by ceramide synthase to create dihydroceramide. A final conversion of dihydroceramide to ceramide by dihydroceramide desaturase completes ceramide synthesis. In mammals, ceramide acyl chain length can vary from 16 to 26 carbons, depending on the particular ceramide synthase involved in their synthesis. There are 6 known ceramide synthases (CerS1-CerS6). These enzymes are also known as longevity assurance genes (LASS1-LASS6; ee [1] for a review), with each mammalian enzyme utilizing a relatively restricted subset of fatty acyl-CoAs. The preferred fatty acid substrates are as follows: CerS1:C18, CerS2:C20-C26, CerS3:C18 & C24, CerS4:C18 & C20, CerS5:C16 and CerS6:C14 & C16. It is currently assumed that the six known mammalian CerS account for the synthesis of all known ceramides, but it is possible that other proteins, such as sphingomyelinases and ceramidases contribute to the synthesis of ceramides with restricted fatty acid composition.
Ceramide can also be created by the catabolism of sphingomyelin via a family of sphingomyelinases that hydrolyze the phosphodiester bond of sphingomyelin to create phosphocholine and ceramide. Sphingomyelinases are categorized based on optimal pH for activity into acidic- (aSMase), alkaline- (alkSMase) and neutral (nSMase). aSMase are Zn2+ dependent and primarily located to the lysosomal compartment [2], although there is also a secreted form of aSMase [3]. The alkSMase enzyme shares no structural similarity with other SMases (it belongs to the nucleotide pyrophosphatase/phosphodiesterase family), but does possess enzymatic properties similar to other sphingomyelinases [4]. Alkaline sphingomyelinase is located in the intestinal mucosa and bile where it functions in the conversion of dietary sphingomyelin (see [5] for a review). There are currently three known nSMases that differ in subcellular location and ion dependence. nSMase1 is Mg2+ dependent and located to the endoplasmic reticulum [6]. nSMase2 is located in the Golgi apparatus [6, 7], and this nSMase can also translocate to perinuclear regions in response to the antioxidant glutathione and to the plasmamembrane in response to oxidative stress [8-10]. nSMase 3 is located to in the golgi apparatus, endoplasmic reticulum and plasma membrane [11].
Ceramide can be converted to sphingomylein by the transfer of a phosphocholine head group from phosphatidylcholine (Glycerophosphocholine) onto ceramide by the enzyme phosphatidylcholine transferase (sphingomyelin synthase). Currently, two sphingomyelin synthases designated as 1 and 2 (SMS1, SMS2) have been identified. These enzymes play critical roles in sphingolipid metabolism by catalyzing the conversion of ceramide and phosphatidylcholine to sphingomyelin and diacylglycerol (DAG). Human SMS1 is localized to the Golgi, while SMS2 resides primarily at the plasma membrane [12-15].
Sphingosine and Sphingosine 1-Phosphate
Ceramide can be converted into sphingosine and a fatty acid by ceramidases. Sphingosine can be further converted to an anti-apoptotic lipid, sphingosine 1-phosphate by the enzyme sphingosine kinase. Similar to the sphingomyelins, ceramidases are categorized based on pH optima and cellular localization. There are 5 known human ceramidases that include acid ceramidase localized to lysosomal compartments, neutral ceramidase that localizes largely to the plasma membrane, an alkaline ceramidases 1, 2 and 3 which localize to the golgi apparatus and the plasma membrane (see [16] for a recent review of ceramidases).
Gangliosides
Gangliosides are a heterogeneous family of glycosphingolipids abundant in the brain. These glycolipids are composed of a glycosphingolipid (ceramide + oligosaccharide) with one or more sialic acids (n-acetlyneuraminic acid) attached to the sugar chain. Over 40 gangliosides have been identified that differ mainly in the position and number of sialic acid residues. Gangliosides are important components of cellular membranes, and comprise ~ 6% of the total lipid content in brain. Gangliosides are differentially distributed in cells. For example, GD1a is more enriched in granule cells than in Purkinje cells, whereas the opposite is true for GT1a [17-19]. The ganglioside GD3 is heavily enriched in reactive and radial glia [17, 20]. Glucosylceramide synthase (GCS; also known as glucosylceramide transferase) catalyzes the first glycosylation step in the biosynthesis of glycosphingolipids [21]. Human GCS was cloned in 1996 [22] and is primarily located to the golgi apparatus [21].
Evidence for Disturbance of Brain Lipid Content in Alzheimer’s Disease
Phospholipids
It was fist reported early in the 1990’s that brain phospholipid content was decreased in AD. In particular, there were decreases in the phospholipid precursors choline, and ethanolamine with consequent decreases in levels of the associated phospholipids phosphatidylcholine (PC) and phosphotidlyethanolamine (PE) in frontal and parietal cortex (brain regions severely damage in AD), but not in the primary auditory cortex (a relatively unaffected brain region) [23]. Decreases in PC and PE were accompanied by increases in the phospholipid catabolite glycerophosphocholine, suggesting that decreases in these phospholipid species were due to increases in phospholipid turnover. Subsequent studies also found decreases in phosphatidylinositol that were specific to the fatty acids oleic and arachidonic in the hippocampus and parahippocampal gyrus of AD brains [24]. A number of these findings from autopsy brain have been supported by studies of cerebrospinal fluid (CSF) that used this compartment as a window to study brain lipid metabolism in living patients. In CSF of AD patients, the choline metabolites glycerophosphocholine, phosphocholine and choline were decreased, as was the lysophospatidylcholine/choline ratio compared with non-neurological disease controls [25, 26], supporting data from brain tissues that there is an increased turnover of particular phospholipid species in AD. In addition to these region-specific losses of particular phospholipids, there may also be differences in brain phospholipid content that depend on the form of AD. Measures that determined total phospholipid content found decreases in frontal, temporal, caudate nucleus and hippocampus of patients with early onset AD, but no change in patients with late onset AD [27]. Although intriguing, it is difficult to determine if these differences are due to AD subtype or reflect the duration of AD, since there is evidence that oxidative stress and perturbed lipid metabolism peak early in the course of AD [28]. Moreover, measuring total phospholipid content could easily mask decreases in particular species of phospholipid. Thus, it is not clear at this time if there are differences in brain phospholipid metabolism that can be attributed to AD type. Together these results suggest that there is an increased metabolism of phospholipids in brain regions involved by AD, and that phospholipids containing choline and ethanolamine are most prominently involved. To date, few studies have addressed what particular phospholipid metabolic pathways are perturbed in AD, or the relationship of these pathways to the neuropathological correlates of AD.
Sphingomyelin, Ceramide, Sphingosine
While relatively few reports have directly examined sphingomyelin and ceramide levels in AD brain tissue and CSF samples, results thus far have consistently demonstrated that sphingomyelins are decreased and ceramides are increased in AD [29-32]. The most prominent changes appear to be in the very long-chain C24:0 and C24:1 species [31] that are enriched in endosomal lysosomal compartments, consistent with roles for ceramide in regulating β- and γ-secretases and APP processing in these compartments (sphingolipid regulation of APP processing is discussed in detail below). A recent report also demonstrated increased sphingosine with decreased sphingosine 1-phosphate (S1P) in AD brains compared with age-matched neurologically normal control subjects [30]. In general, higher S1P levels are protective to neurons [33, 34] and elevations in sphingosine and ceramide can be toxic to neurons [31, 35-38], suggesting that both increased levels of ceramide and and a sphingosine/S1P rheostat imbalance contribute to neuronal dysfunction in AD. Indeed, expression of several genes involved in sphingomylein and ceramide metabolism, including sphingosine 1-phosphate lyase, CerS1, 2 and 5, are increased in AD brain, as are genes involved in sphingomylein catabolism including aSMase and factors associated with nSMase activation [39]. In addition, brain cells in AD patients exhibit decreased expression of genes involved in ceramide transformation that include acid ceramidase, ceramide kinase and genes involved in glycosphingolipid synthesis such as glucosylceramidetransferase [39]. However, only increased aSMase and nSMase have been confirmed at the level of enzyme activity and aSMase at the protein level [30]. In contrast to the findings from gene array, activity and protein levels of acid ceramidase were increased in AD brain [30]. While a great deal of work remains to positively identify sphingolipid metabolizing enzymes that are dysfunctional in AD, these initial reports suggest a pattern of enzyme dysfunction that favors ceramide generation at the expense of S1P and ganglioside synthesis. This altered sphingolipid profile is consistent with signaling that favors neuronal dysfunction and apoptosis.
Gangliosides
Reports of disturbances in glycolipids from AD brain first appeared in the late 1960s, when it was reported that particular fatty acid compositions of gangliosides, cerebrosides and cerebroside sulfatide levels were abnormal in AD brain [40, 41]. Subsequent studies found that the gangliosides GM1, GD1a, GD1b and GT1b were decreased in multiple brain regions including temporal white matter, temporal cortex and frontal cortex, whereas the gangliosides GM2, GM3, GQ1b and GT1L were increased in AD compared with age-matched control tissues [18, 27, 42, 43]. Similar to the phospholipid findings discussed in the preceding section, disturbances in gangliosides are more profound in brain tissues from subjects with early onset disease, in which reductions of 58-70% were found in grey matter from nearly all brain regions, and an 81% reduction of total gangliosides in frontal white matter[44]. In late onset AD, gangliosides were selectively reduced in the temporal cortex, hippocampus, and frontal white matter [45]. Since GM1, GD1 and GT1 are enriched in neurons, reductions in these ganglioside species are thought to reflect synaptic and dendritic damage and frank neuronal loss in AD brain. Studies using CSF from living AD patients supports this hypothesis, with the observation that GM1 was increased in CSF of subjects with early-onset AD compared to late-onset AD and age-matched controls, likely due to a removal of GM1 from brain parenchyma [46]. Recent findings suggest that alterations in ganglioside metabolism may contribute to the development of neuropathologies associated with AD. For instance, GM1 is increased in detergent resistant membrane fractions isolated from frontal cortex of AD brain, and the gangliosides GM1 and GD1a have been found to co-label with dystrophic neurites, neurofibrillary tangles and Aβ-plaques [47-50]. Indeed, numerous studies in tissue culture and rodent models have shown that lipid composition is important for regulating pathways that promote APP cleavage and in regulating numerous aspects of synaptic function.
Membrane Lipids Regulate APP Processing
Specialized membrane domains known as lipid rafts are thought to play important roles in regulating the trafficking and proteolytic processing of the b-amyloid precursor protein (APP). These membrane microdomains contain mostly unsaturated lipids and are rich in sphingomylein, gangliosides and cholesterol. This more “ordered” molecular arrangement results in a rigid structure that exhibits decreased lateral mobility compared with the surrounding phospholipid bilayer. Nevertheless, lipid rafts are dynamic structures that can coalesce to form larger platforms and separate into their component rafts within seconds to minutes [51, 52]. There is evidence of considerable heterogeneity in the composition of lipid rafts, including variation in the lipid and sterol composition that is dependent on cell type, cellular location and activation state of the cell [51]. These dynamic membrane domains are involved in the regulation of protein trafficking and protein compartmentalization, and serve as organizational centers for the assembly of signaling complexes.
In AD, lipid rafts appear to be critical sites where the proteolytic processing of APP is regulated (Figure 2). Current evidence suggests that the non-amyloidogenic α-cleavage of APP occurs outside of lipid raft domains, where the disintegrin and metalloproteinase 10 (ADAM10; a major α-secretase in brain) is exclusively located [53]. Amyloidogenic processing of APP is thought to occur primarily in lipid rafts [54, 55], where all relevant proteins appear to be concentrated including: BACE1, presenilins (PS1 and PS2), nicastrin, APH-1, PEN-2 (components of γ-secretase), APP, APP N- and N-terminal fragments and Aβ peptides [56-60]. These observations suggest that lipid rafts are major sites for amyloidogenic processing of APP. In this context, it is the trafficking of APP and secretases that regulate the form of APP processing. For instance, cellular glycosphingolipid levels are critical for regulating the maturation and cell surface transport of APP. Inhibition of glycosyl ceramide synthase (catalyzes the first step in glycosphingolipid biosynthesis) markedly reduced the secretion of APP and Aβ-peptide production, whereas the addition of exogenous gangliosides reversed these effects [61]. There is in addition, evidence that BACE1 and γ-secretase traffic in and out of lipid raft domains. While numerous studies found that BACE1 is located to membrane fractions consistent with lipid raft domains [62-65], a closer examination has revealed that activation state plays a major role in regulating the membrane location of BACE1. Inactive BACE1 is located outside of lipid rafts, and active BACE1 is located to lipid rafts [66, 67]. Forced targeting of BACE1 to lipid rafts by replacing the transmembrane and C-terminal domains with a glycosylphosphatidylinositol (GPI) anchor substantially upregulates the production of both sAPPb and Aβ, compared with cells over-expressing wild-type BACE1, consistent with a requirement for BACE1 to traffic into lipid rafts for β-cleavage of APP. Ceramide, a major bioactive component of lipid rafts, also appears to be important for regulating stabilizing BACE by facilitating the carrier-mediated translocation of acetyl-CoA into the ER lumen, where BACE1 is acetylated on seven lysine residues of the N-terminal portion of the protein. Site-directed mutagenesis experiments have shown that lysine acetylation is a necessary step for BACE1 to leave the ER and move ahead in the secretory pathway[68, 69].
Figure 2. Amyloid Processing in Lipid Rafts.
Amyloid precursor protein (APP) cleavage by β- and γ-secretases occurs most efficiently in lipid rafts. Aβ binds to membranes with a preference for anonic lipid head groups and can be translocated into GM1-rich lipid rafts where Aβ undergos a conformational shift that disrupts membrane stability, promotes peptide-peptide interactions and Aβ oligomer formation.
Similar to BACE1, γ-secretase is known to exist in both non-raft and lipid raft membrane domains and its activity is upregulated in lipid rafts. Increasing the sphingolipid and cholesterol content of proteoliposomes containing γ-secretase increased enzyme acitivy promoting the formation of lipid rafts [70]. In healthy cells and tissues γ-secretase is primarily located outside of lipid raft domains [62, 66], where it is known to cleave the C-terminal fragments (CTFs) of Notch1, Jagged2, N-cadherin and deleted in colorectal cancer (DCC) [65]. Loss of γ-secretase activity by gene deletion or chemical inhibition results in the accumulation of APP CTFs in lipid rafts, indicating that γ-secretase cleavage occurs in these membrane microdomains [65]. With advancing age, and in AD, an increasing fraction of γ-secretase becomes located to lipid raft microdomains in post-Golgi and endosomal compartments [55, 62, 65, 71]. Thus, the mislocalization of BACE1 and γ-secretase to lipid rafts may promote Aβ formation. Lastly, the membrane location of APP is important for determining the form of proteolytic processing, although little is known about the mechanisms that regulate the raft versus non-raft targeting of APP. One potential mechanism involves the interaction of APP with the cytoplasmic domain of low-density lipoprotein receptor-related protein (LRP), which is thought to promote BACE1-APP interaction by trafficking to lipid raft domains [72]. Thus, one potential explanation for the altered processing of APP to a pro-amyloidogenic pathway is that APP, β- and γ-secretases are increasingly localized to lipid rafts with increasing age and in neurodegenerative diseases [66]. However, there is also evidence that excluding BACE1 or γ-secretase from lipid rafts does not appreciably alter Aβ formation. In these experiments, it was found that BACE1 is S-palmitoylated at Cys474, Cys478, Cys482, and Cys485, nicastrin is S-palmitoylated at Cys689, and APH-1 is S-palmitoylated at Cys182 and Cys245. Site-directed mutagenesis of these sites to Ala prevented S-palmitoylation and localized BACE1 and γ-secretase to non-raft membrane regions, but did not entirely disrupt the β- or γ-cleavage of APP or release of Aβ [73, 74]. However, APP processing appears to occur less efficiently outside of rafts. Together, these data suggest that amyloidogenic processing of APP occurs most efficiently [62, 66, 75], but not exclusively [62] in lipid rafts.
Roles for Lipids in Seeding Aβ Aggregation
The self-aggregation of Aβ into oligomers and fibrils is a pivotal step in the pathogenesis of AD. There is considerable evidence that suggests particular lipids play key roles in regulating the conformational shift in Aβ from helix- to beta-sheet-rich structures (Figure 2). Aβ directly interacts with lipid membranes and disrupts the biophysical properties of the bilayer by disordering nearby lipids. Multinuclear NMR studies in model membranes have shown that the interaction of Aβ with lipid bilayers disrupts the lamellar phase of membranes and promotes a hexagonal phase with a significant number of non-oriented lipids [76]. The means by which Aβ interacts with lipid membranes involves a destabilization of Aβ structure, which leads to an extended peptide conformation that increases the probability of electrostatic and hydrogen bonding interactions between the peptide and the head groups of lipids [77]. Aβ interacts with membranes in an electrostatic-independent manner [78], showing a preference for the head group of anionic lipids [79, 80]. Using X-ray and neutron scattering techniques, it was found that Aβ exhibited enhanced interactions with charged lipids compared with zwitterionic lipids, and that these interactions promoted the formation of amyoid fibrils, suggesting that the interaction of Aβ with membranes seeded fibril formation [79]. Indeed, there is considerable evidence that lipid-interactions with Aβ are critical for fibril formation [80-85]. A recent study shed some light on how these complex interactions between lipids and Aβ are regulated. It was found that Aβ selectively bound to glycosphingolpids that contained a 2-OH group on the acyl chain of the ceramide backbone and did not effectively interact with glycosphingolipids that contained a nonhydroxylated fatty acid. Cholesterol inhibited the interaction of Aβ with glycosphingolipids that contained a 2-OH group, but increased the ability of Aβ to bind glycosphingolipids that contained nonhydroxylated fatty acids. Thus, cholesterol could either inhibit or facilitate interactions of Aβ with the membrane by regulating the form of glycosphingolipid that Aβ interacted with [86]. These studies are in agreement that the interaction of Aβ with membranes dramatically increases peptide aggregation rate, and induces a structural conversion in Aβ that favors peptide-peptide interactions to increases the probability of Aβ oligomerization from a random coil to the beta-structure observed in mature fibrils [80, 81].
While Aβ interactions with anionic phospholipid head groups appear to be important for the interaction of Aβ with membranes, the interactions of Aβ with the ganglioside GM1 present in lipid rafts appears to have the greatest effect on Aβ aggregation and fibril formation. Aβ oligomers appeared rapidly after incubation with lipid rafts isolated from rodent brain, and this process was not disrupted by treatment with heat or porteinase K, suggesting that proteins contained in this lipid fraction were not critical for Aβ oligomerization [82]. However, increasing concentrations of the ganglioside GM1, either by direct addition or by incubating Aβ peptides with lipid raft fractions isolated from C2C12 cells rich in GM1 increases the rate of Aβ oligomerization, while incubation with ganglioside poor SK-N-MC cells slows the rate of Aβ oligomerization [82, 83]. Interestingly, the removal of cholesterol from membranes does not eliminate the formation of Aβ oligomers [82]. Rather, the role for cholesterol in Aβ-oligomerization seems to be in the sterols’ ability to promote the formation of GM1 clusters that preferentially interact with Aβ, compared with monomeric GM1, or other ganglioside species [84, 85]. Recently, an intriguing study provided evidence that integrates Aβ interaction with phospholipid head groups (most abundant in less-ordered membrane regions) and GM1 (most abundant in ordered lipid raft regions). In this study it was found that exogenously applied Aβ can traffic on neuronal membranes and accumulate in lipid rafts by a mechanism dependent on the protein-tyrosine-kinase fyn [87]. Together these data suggest that Aβ binds to membranes with a preference for anonic lipid head groups, and Aβ is then incorporated into GM1-rich membrane regions where the peptides undergo a conformational shift that disrupts membrane stability and promotes peptide-peptide interaction and oligomer formation.
Roles for Deregulated Sphingolipid Metabolism in Disrupting Synaptic Activity
Synaptic dysfunction is an early and seminal event in the pathogenesis of AD. Disturbances of sphingolipid metabolism that occur in AD are likely to disrupt a number of protein-lipid interactions and perturb protein and lipid trafficking, and in so doing would dysregulate multiple cellular signaling events including those involved in synaptic plasticity/cognition (Figure 3). Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites including ceramide, ceramide-1-phosphate, sphingosine and sphingosine 1-phosphate. It is becoming increasingly recognized that these sphingolipids play critical and complex roles in regulating neuronal excitability. The biophysical properties of sphingolipids play important roles in the regulation of membrane shape, endo- and exocytotic events, and vesicle and protein trafficking; several lipid species also function as second messengers that regulate neuronal functions including pre- and post-synaptic processes involved in synaptic plasticity [88-91].
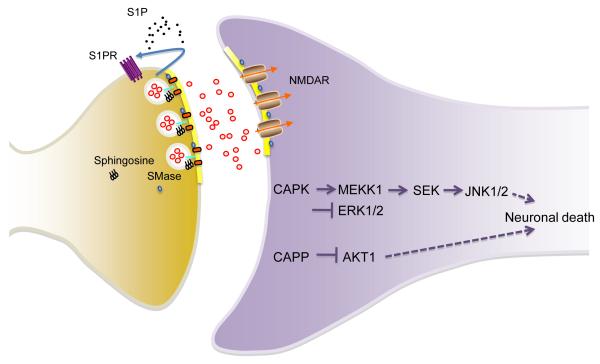
Figure 3. Roles for Sphingolipids in the Regulation and Dysregulation of Synaptic Functions.
Sphingosine can regulate neurotransmitter release by neutralizing interactions of snaptobrevin with phospholipids in synaptic vesicles. This action allows synaptobrevin to engage syntaxin/SNAP-25 on the inner leaflet of pre-synaptic membranes. A rapid and focal generation of ceramide by SMase promotes vesicle fusion with the plasma membrane and release of neurotransmitters. Sphingosine 1-phosphate (S1P) can signal in an autocrine manner through S1P receptors to further enhance the neurotransmitter release. At the post-synaptic terminal, rapid and transient increases of ceramide and DAG regulates plasma membrane trafficking of NMDA receptors by promoting the fusion of receptor-laden vesicles with the plasma membrane. In AD, disruptions of sphingolipid metabolism perturb these sphingolipid-regulated pre- and post-synaptic functions, and sustained increases of ceramide may activate ceramide associated protein kinases (CAPK) and protein phosphateases (CAPP) to promote death signaling in neurons.
At the presynaptic terminal, docking and fusion of synaptic vesicles with the plasma membrane is regulated by a highly conserved family of SNARE proteins that include synaptobrevin in vesicles and syntaxin and SNAP-25 at the plasma membrane. Recent findings have demonstrated that formation of the SNARE complex, synaptic vesicle fusion and exocytosis is regulated by sphingosine. Synaptobrevin adheres tightly to synaptic vesicles through electrostatic and hydrophobic interactions of the cytoplasmic part of synaptobrevin with vesicular membranes. Sphingosine activates synaptobrevin by neutralizing interactions of snaptobrevin with phospholipids, thus allowing synaptobrevin to engage syntaxin/SNAP-25, resulting in SNARE assembly, vesicle fusion and transmitter release [92]. These results are consistent with an earlier report that identified ceramidase (catalyzes the deacylation of ceramide to produce a free fatty acid and sphingosine) as an important regulator of synaptic vesicle exocytosis [93]. Sphingosine can be rapidly converted S1P by sphingosine kinase 1 (SK1), and this more soluble product has also been implicated in regulating transmitter release. SK1 is formed in the presynaptic terminal in an activity-dependent manner, and S1P applied to hippocampal neurons promotes transmitter release that is dependent on the S1P 1 receptor [94, 95]. Roles for ceramide in neurotrasmitter release that involve SMase activity have also been suggested in which the fusogenic properties of ceramide are thought to promote vesicle fusion with the plasma membrane [96-98].
Ceramide is emerging as an important regulator of synaptic function and has been implicated in synapse formation, transmitter release and plasticity [36, 99-105] (Figure 3). Early evidence for the regulation of synaptic activity by ceramide was provided by experiments that used synthetic cell-permeable short (C2 – C6) ceramide analogs to demonstrate that ceramide could directly increase excitatory postsynaptic currents without affecting paired-pulse facilitation [104, 106, 107]. Interestingly, these ceramide-associated enhancements of excitatory currents were often transient and followed by sustained depression of excitatory postsynaptic currents [100, 104, 106, 108, 109]. One mechanism by which ceramide may be involved regulating synaptic activity is by controlling the spatial and temporal location of receptors at postsynaptic sites. In neurons, the sphingomyelin-, ceramide- and ganglioside-rich membrane regions known as lipid rafts have been identified as important sites for the docking and insertion of both NMDA and AMPA subtypes of glutamate receptors [52, 99, 110]. For instance, disrupting lipid rafts by removal of cholesterol from membranes inhibits NMDA receptor currents and calcium flux, and increases the basal internalization rate of AMPA receptors [111-113].
Although the precise mechanisms that regulate synaptic events by controlling lipid metabolism are still largely unknown, accumulating evidence suggests that the sphingomyelin catabolizing enzyme nSMase2 and its reaction product ceramide may play important roles. For instance, nerve growth factor (NGF) is known to regulate neurite outgrowth, synaptogenesis and to enhance the rate of depolarization-induced action potentials by signaling through nSMase2 and ceramide [36, 105, 114] [115-117]. In a similar fashion, TNFα can also enhance excitatory post-synaptic currents and NMDA-evoked calcium bursts by mechanisms that require nSMase2-regulated generation of ceramide and concurrent generation of diacylglycerol [99]. Ceramide’s role in regulating synaptic activity may involve its fusogenic properties that would allow receptor insertion and removal. For instance, ceramide may mediate focal changes in the biophysical properties of membranes that facilitate the traffic of transmembrane receptors [99]. Thus, a dysfunction of sphingolipids metabolism in AD could disrupt these sphingolipid-regulated pre- and post-synaptic events to perturb synaptic function.
Evidence for Ceramide-Assisted Neuronal Death in AD
There is considerable experimental evidence that cytokine dysregulation and increased cellular oxidation play key roles in neuronal death associated with AD [118, 119]. There is also considerable evidence that pro-inflammatory cytokines, and cellular oxidants are important modulators of enzymes involved in sphingomyelin and ceramide metabolism. For instance, there is a bidirectional relationship between cytokine balance and ceramide levels. TNFα, IL-1, and Fas/FasL are potent inducers of ceramide production, and increased concentrations of ceramide can stimulate the production of IL-2 and IL-6 [120]. Oxidative stress is known to generate ceramide [38, 121-124], and antioxidants such as N-acetlycysteine (NAC), pyrrolidine dithiocarbamate (PDTC), glutathione and α-tocopherol (vitamin E) have been shown to prevent the generation of ceramide induced by TNFα and Fas-ligand [122, 125-128]. Sustained or excessive increases of ceramide can activate pro-apoptotic pathways. For example, Fas/FasL interaction can activate a caspase-8-dependent increase in SMase activity that increases ceramide that then promotes the formation of large lipid platforms and the assembly of cell death signaling protein complexes [129-132].
There are also several ceramide-regulated protein kinases (CAPK) and phosphatases (CAPP) that when activated can evoke signaling that triggers apoptosis. Pro-apoptotic CAPK signaling involves recruitment of MAPK/ERK kinase kinase (MEKK1), activation of SAPK-kinase (SEK1), Jun N-terminal kinases (JNK 1 and JNK2) and inhibition of the survival factor extracellular signal-regulated kinase-1 and 2 (ERK1 and ERK2) [131, 133-137]. Signaling through the JNKs is thought to trigger apoptosis [136]. CAPK-induced apoptosis may also involve a Raf-1 kinase, mitogen-activated kinase (MEK-1) / ERK pathway that was first demonstrated in glia [138]. However, the contribution of Raf-1 signaling to neuronal death is unclear, and ERK can have protective or apoptotic effects depending on the mechanism of activation and duration of action. Potential roles for CAPPs in neuronal survival are not yet well defined, but may involve inactivation of the survival factor Akt1, p38 MAPK [139]. When taken together with the considerable evidence for the involvement of neuronal apoptosis in AD [119], the data described in this section support roles for perturbed membrane ceramide metabolism in this form of cell death.
Implications for Therapeutic Interventions that Target Shingolipid Metabolism
A working strategy for shingolipid metabolism-based interventions for the prevention and treatment of AD would be to develop drugs that suppress the excessive SMase-mediated cleavage of sphingomyelins that apparently occurs relatively early in the AD process. Preclinical proof-of-concept for the latter approach comes from studies showing that agents that inhibit sphingomyelin production (the serine palmitoyl-CoA-transferase inhibitor myriocin/ISP-1) [31] or an acid sphingomyelin hydrolysis (using D609) [140] can protect neurons against damage caused by Ab and oxidative stress. Another approach, more tailored towards disease prevention would be to prescribe diets that would be expected to lower levels sphingomyelin in brain cells. For example, diets rich in omega-3 fatty acids (fish, for example) would be expected to inhibit sphingomyelin metabolism and ceramide production [141], and may thereby protect neurons against synaptic dysfunction in AD as suggested from the results of preclinical trials in a mouse model of AD [142].
Summary
Our understanding of roles for sphingolipids in regulating neuronal function is increasing at a rapid rate, in part due to recent advances in our ability to measure and quantitate individual lipid species by mass spectrometry techniques and the availability of reagents to visualize and accurately modulate subcellular targets involved in sphingolipid metabolism. These advances have in addition led to a greater understanding of how perturbations in sphingolipid metabolism could contribute to the pathogenesis of neurodegenerative conditions such as Alzheimer’s disease. Increased knowledge of the pathways that regulate sphingolipid metabolism and how these systems become dysregulated in Alzheimer’s disease will ultimately lead to new therapeutics and disease prevention strategies that target these pathways.
Acknowledgements
These studies were supported by National Institutes of Health grants, MH077542, AA0174078 and AG034849 to NJH, and by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- [2].Gatt S. Enzymic Hydrolysis and Synthesis of Ceramides. J Biol Chem. 1963;238:3131–3133. [PubMed] [Google Scholar]
- [3].Spence MW, Byers DM, Palmer FB, Cook HW. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264:5358–5363. [PubMed] [Google Scholar]
- [4].Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969;176:339–347. doi: 10.1016/0005-2760(69)90192-1. [DOI] [PubMed] [Google Scholar]
- [5].Duan RD. Alkaline sphingomyelinase: an old enzyme with novel implications. Biochim Biophys Acta. 2006;1761:281–291. doi: 10.1016/j.bbalip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- [6].Hofmann K, Tomiuk S, Wolff G, Stoffel W. Cloning and characterization of the mammalian brain-specific, Mg2+-dependent neutral sphingomyelinase. Proc Natl Acad Sci U S A. 2000;97:5895–5900. doi: 10.1073/pnas.97.11.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W. Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci U S A. 1998;95:3638–3643. doi: 10.1073/pnas.95.7.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344:900–905. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castillo SS, Levy M, Thaikoottathil JV, Goldkorn T. Reactive nitrogen and oxygen species activate different sphingomyelinases to induce apoptosis in airway epithelial cells. Exp Cell Res. 2007;313:2680–2686. doi: 10.1016/j.yexcr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- [10].Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- [11].Krut O, Wiegmann K, Kashkar H, Yazdanpanah B, Kronke M. Novel tumor necrosis factor-responsive mammalian neutral sphingomyelinase-3 is a C-tail-anchored protein. J Biol Chem. 2006;281:13784–13793. doi: 10.1074/jbc.M511306200. [DOI] [PubMed] [Google Scholar]
- [12].Takeuchi J, Okada M, Toh-e A, Kikuchi Y. The SMS1 gene encoding a serine-rich transmembrane protein suppresses the temperature sensitivity of the htr1 disruptant in Saccharomyces cerevisiae. Biochim Biophys Acta. 1995;1260:94–96. doi: 10.1016/0167-4781(94)00188-9. [DOI] [PubMed] [Google Scholar]
- [13].Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. Embo J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khoury CM, Yang Z, Ismail S, Greenwood MT. Characterization of a novel alternatively spliced human transcript encoding an N-terminally truncated Vps24 protein that suppresses the effects of Bax in an ESCRT independent manner in yeast. Gene. 2007;391:233–241. doi: 10.1016/j.gene.2006.12.039. [DOI] [PubMed] [Google Scholar]
- [15].Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–17547. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- [16].Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seyfried TN, Yu RK. Cellular localization of gangliosides in the mouse cerebellum: analysis using neurological mutants. Adv Exp Med Biol. 1984;174:169–181. doi: 10.1007/978-1-4684-1200-0_15. [DOI] [PubMed] [Google Scholar]
- [18].Brooksbank BW, McGovern J. Gangliosides in the brain in adult Down’s syndrome and Alzheimer’s disease. Mol Chem Neuropathol. 1989;11:143–156. doi: 10.1007/BF03160048. [DOI] [PubMed] [Google Scholar]
- [19].Palestini P, Masserini M, Fiorilli A, Calappi E, Tettamanti G. Age-related changes in the ceramide composition of the major gangliosides present in rat brain subcellular fractions enriched in plasma membranes of neuronal and myelin origin. J Neurochem. 1993;61:955–960. doi: 10.1111/j.1471-4159.1993.tb03608.x. [DOI] [PubMed] [Google Scholar]
- [20].Cammer W, Zhang H. Ganglioside GD3 in radial glia and astrocytes in situ in brains of young and adult mice. J Neurosci Res. 1996;46:18–23. doi: 10.1002/(SICI)1097-4547(19961001)46:1<18::AID-JNR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [21].Paul P, Kamisaka Y, Marks DL, Pagano RE. Purification and characterization of UDP-glucose:ceramide glucosyltransferase from rat liver Golgi membranes. J Biol Chem. 1996;271:2287–2293. doi: 10.1074/jbc.271.4.2287. [DOI] [PubMed] [Google Scholar]
- [22].Ichikawa S, Sakiyama H, Suzuki G, Hidari KI, Hirabayashi Y. Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc Natl Acad Sci U S A. 1996;93:12654. doi: 10.1073/pnas.93.22.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nitsch RM, Blusztajn JK, Pittas AG, Slack BE, Growdon JH, Wurtman RJ. Evidence for a membrane defect in Alzheimer disease brain. Proc Natl Acad Sci U S A. 1992;89:1671–1675. doi: 10.1073/pnas.89.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 1998;23:81–88. doi: 10.1023/a:1022457605436. [DOI] [PubMed] [Google Scholar]
- [25].Walter A, Korth U, Hilgert M, Hartmann J, Weichel O, Fassbender K, Schmitt A, Klein J. Glycerophosphocholine is elevated in cerebrospinal fluid of Alzheimer patients. Neurobiol Aging. 2004;25:1299–1303. doi: 10.1016/j.neurobiolaging.2004.02.016. [DOI] [PubMed] [Google Scholar]
- [26].Mulder C, Wahlund LO, Teerlink T, Blomberg M, Veerhuis R, van Kamp GJ, Scheltens P, Scheffer PG. Decreased lysophosphatidylcholine/phosphatidylcholine ratio in cerebrospinal fluid in Alzheimer’s disease. J Neural Transm. 2003;110:949–955. doi: 10.1007/s00702-003-0007-9. [DOI] [PubMed] [Google Scholar]
- [27].Svennerholm L, Gottfries CG. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II) J Neurochem. 1994;62:1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- [28].Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Han X, D MH, McKeel DW, Jr., Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- [30].He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, Oka N, Akiguchi I, Furuya S, Hirabayashi Y, Okazaki T. Astroglial expression of ceramide in Alzheimer’s disease brains: a role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- [33].Rius RA, Edsall LC, Spiegel S. Activation of sphingosine kinase in pheochromocytoma PC12 neuronal cells in response to trophic factors. FEBS Lett. 1997;417:173–176. doi: 10.1016/s0014-5793(97)01277-5. [DOI] [PubMed] [Google Scholar]
- [34].Edsall LC, Pirianov GG, Spiegel S. Involvement of sphingosine 1-phosphate in nerve growth factor-mediated neuronal survival and differentiation. J Neurosci. 1997;17:6952–6960. doi: 10.1523/JNEUROSCI.17-18-06952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dawson G, Goswami R, Kilkus J, Wiesner D, Dawson S. The formation of ceramide from sphingomyelin is associated with cellular apoptosis. Acta Biochim Pol. 1998;45:287–297. [PubMed] [Google Scholar]
- [36].Brann AB, Scott R, Neuberger Y, Abulafia D, Boldin S, Fainzilber M, Futerman AH. Ceramide signaling downstream of the p75 neurotrophin receptor mediates the effects of nerve growth factor on outgrowth of cultured hippocampal neurons. J Neurosci. 1999;19:8199–8206. doi: 10.1523/JNEUROSCI.19-19-08199.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Toman RE, Movsesyan V, Murthy SK, Milstien S, Spiegel S, Faden AI. Ceramide-induced cell death in primary neuronal cultures: upregulation of ceramide levels during neuronal apoptosis. J Neurosci Res. 2002;68:323–330. doi: 10.1002/jnr.10190. [DOI] [PubMed] [Google Scholar]
- [38].Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, Turchan J, Nath A, Mattson MP. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Ann Neurol. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- [39].Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- [40].Cherayil GD. Fatty acid composition of brain glycolipids in Alzheimer’s disease, senile dementia, and cerebrocortical atrophy. J Lipid Res. 1968;9:207–214. [PubMed] [Google Scholar]
- [41].Suzuki K, Katzman R, Korey SR. Chemical Studies on Alzheimer’s Disease. J Neuropathol Exp Neurol. 1965;24:211–224. doi: 10.1097/00005072-196504000-00004. [DOI] [PubMed] [Google Scholar]
- [42].Kracun I, Kalanj S, Talan-Hranilovic J, Cosovic C. Cortical distribution of gangliosides in Alzheimer’s disease. Neurochem Int. 1992;20:433–438. doi: 10.1016/0197-0186(92)90058-y. [DOI] [PubMed] [Google Scholar]
- [43].Op Den Velde W, Hooghwinkel GJ. The brain ganglioside pattern in presenile and senile dementia. J Am Geriatr Soc. 1975;23:301–303. doi: 10.1111/j.1532-5415.1975.tb00217.x. [DOI] [PubMed] [Google Scholar]
- [44].Kalanj S, Kracun I, Rosner H, Cosovic C. Regional distribution of brain gangliosides in Alzheimer’s disease. Neurol Croat. 1991;40:269–281. [PubMed] [Google Scholar]
- [45].Svennerholm L. Gangliosides--a new therapeutic agent against stroke and Alzheimer’s disease. Life Sci. 1994;55:2125–2134. doi: 10.1016/0024-3205(94)00393-9. [DOI] [PubMed] [Google Scholar]
- [46].Blennow K, Davidsson P, Wallin A, Fredman P, Gottfries CG, Karlsson I, Mansson JE, Svennerholm L. Gangliosides in cerebrospinal fluid in ‘probable Alzheimer’s disease’. Arch Neurol. 1991;48:1032–1035. doi: 10.1001/archneur.1991.00530220048018. [DOI] [PubMed] [Google Scholar]
- [47].Tooyama I, Yamada T, Kim SU, McGeer PL. Immunohistochemical study of A2B5-positive ganglioside in postmortem human brain tissue of Alzheimer disease, amyotrophic lateral sclerosis, progressive supranuclear palsy and control cases. Neurosci Lett. 1992;136:91–94. doi: 10.1016/0304-3940(92)90655-q. [DOI] [PubMed] [Google Scholar]
- [48].Nishinaka T, Iwata D, Shimada S, Kosaka K, Suzuki Y. Anti-ganglioside GD1a monoclonal antibody recognizes senile plaques in the brains of patients with Alzheimer-type dementia. Neurosci Res. 1993;17:171–176. doi: 10.1016/0168-0102(93)90093-6. [DOI] [PubMed] [Google Scholar]
- [49].Takahashi H, Hirokawa K, Ando S, Obata K. Immunohistological study on brains of Alzheimer’s disease using antibodies to fetal antigens, C-series gangliosides and microtubule-associated protein 5. Acta Neuropathol. 1991;81:626–631. doi: 10.1007/BF00296372. [DOI] [PubMed] [Google Scholar]
- [50].Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Mansson JE, Fredman P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J Neurochem. 2005;92:171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- [51].Jin S, Zhang Y, Yi F, Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Fullekrug J, Simons K. Lipid rafts and apical membrane traffic. Ann N Y Acad Sci. 2004;1014:164–169. doi: 10.1196/annals.1294.017. [DOI] [PubMed] [Google Scholar]
- [53].Harris B, Pereira I, Parkin E. Targeting ADAM10 to lipid rafts in neuroblastoma SH-SY5Y cells impairs amyloidogenic processing of the amyloid precursor protein. Brain Res. 2009;1296:203–215. doi: 10.1016/j.brainres.2009.07.105. [DOI] [PubMed] [Google Scholar]
- [54].Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee SJ, Liyanage U, Bickel PE, Xia W, Lansbury PT, Jr., Kosik KS. A detergent-insoluble membrane compartment contains A beta in vivo. Nat Med. 1998;4:730–734. doi: 10.1038/nm0698-730. [DOI] [PubMed] [Google Scholar]
- [56].Watanabe N, Araki W, Chui DH, Makifuchi T, Ihara Y, Tabira T. Glypican-1 as an Abeta binding HSPG in the human brain: its localization in DIG domains and possible roles in the pathogenesis of Alzheimer’s disease. FASEB J. 2004;18:1013–1015. doi: 10.1096/fj.03-1040fje. [DOI] [PubMed] [Google Scholar]
- [57].Parkin ET, Hussain I, Karran EH, Turner AJ, Hooper NM. Characterization of detergent-insoluble complexes containing the familial Alzheimer’s disease-associated presenilins. J Neurochem. 1999;72:1534–1543. doi: 10.1046/j.1471-4159.1999.721534.x. [DOI] [PubMed] [Google Scholar]
- [58].Wahrle S, Das P, Nyborg AC, McLendon C, Shoji M, Kawarabayashi T, Younkin LH, Younkin SG, Golde TE. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol Dis. 2002;9:11–23. doi: 10.1006/nbdi.2001.0470. [DOI] [PubMed] [Google Scholar]
- [59].Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, Matsubara E, Abe K, Ashe KH, Younkin SG. Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2004;24:3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Marlow L, Cain M, Pappolla MA, Sambamurti K. Beta-secretase processing of the Alzheimer’s amyloid protein precursor (APP) J Mol Neurosci. 2003;20:233–239. doi: 10.1385/JMN:20:3:233. [DOI] [PubMed] [Google Scholar]
- [61].Tamboli IY, Prager K, Barth E, Heneka M, Sandhoff K, Walter J. Inhibition of glycosphingolipid biosynthesis reduces secretion of the beta-amyloid precursor protein and amyloid beta-peptide. J Biol Chem. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- [62].Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem. 2004;279:44945–44954. doi: 10.1074/jbc.M407986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kametaka S, Shibata M, Moroe K, Kanamori S, Ohsawa Y, Waguri S, Sims PJ, Emoto K, Umeda M, Uchiyama Y. Identification of phospholipid scramblase 1 as a novel interacting molecule with beta -secretase (beta -site amyloid precursor protein (APP) cleaving enzyme (BACE)) J Biol Chem. 2003;278:15239–15245. doi: 10.1074/jbc.M208611200. [DOI] [PubMed] [Google Scholar]
- [64].Riddell DR, Christie G, Hussain I, Dingwall C. Compartmentalization of beta-secretase (Asp2) into low-buoyant density, noncaveolar lipid rafts. Curr Biol. 2001;11:1288–1293. doi: 10.1016/s0960-9822(01)00394-3. [DOI] [PubMed] [Google Scholar]
- [65].Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cordy JM, Hussain I, Dingwall C, Hooper NM, Turner AJ. Exclusively targeting beta-secretase to lipid rafts by GPI-anchor addition up-regulates beta-site processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2003;100:11735–11740. doi: 10.1073/pnas.1635130100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ebina M, Futai E, Tanabe C, Sasagawa N, Kiso Y, Ishiura S. Inhibition by KMI-574 leads to dislocalization of BACE1 from lipid rafts. J Neurosci Res. 2009;87:360–368. doi: 10.1002/jnr.21858. [DOI] [PubMed] [Google Scholar]
- [68].Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. J Biol Chem. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- [69].Costantini C, Ko MH, Jonas MC, Puglielli L. A reversible form of lysine acetylation in the ER and Golgi lumen controls the molecular stabilization of BACE1. Biochem J. 2007;407:383–395. doi: 10.1042/BJ20070040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Osenkowski P, Ye W, Wang R, Wolfe MS, Selkoe DJ. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J Biol Chem. 2008;283:22529–22540. doi: 10.1074/jbc.M801925200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hur JY, Welander H, Behbahani H, Aoki M, Franberg J, Winblad B, Frykman S, Tjernberg LO. Active gamma-secretase is localized to detergent-resistant membranes in human brain. FEBS J. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]
- [72].Yoon IS, Chen E, Busse T, Repetto E, Lakshmana MK, Koo EH, Kang DE. Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 2007;21:2742–2752. doi: 10.1096/fj.07-8114com. [DOI] [PubMed] [Google Scholar]
- [73].Vetrivel KS, Meckler X, Chen Y, Nguyen PD, Seidah NG, Vassar R, Wong PC, Fukata M, Kounnas MZ, Thinakaran G. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J Biol Chem. 2009:3793–3803. doi: 10.1074/jbc.M808920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Cheng H, Vetrivel KS, Drisdel RC, Meckler X, Gong P, Leem JY, Li T, Carter M, Chen Y, Nguyen P, Iwatsubo T, Tomita T, Wong PC, Green WN, Kounnas MZ, Thinakaran G. S-palmitoylation of gamma-secretase subunits nicastrin and APH-1. J Biol Chem. 2009;284:1373–1384. doi: 10.1074/jbc.M806380200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, Simons K. Lipids as modulators of proteolytic activity of BACE: involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. J Biol Chem. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- [76].Nakazawa Y, Suzuki Y, Williamson MP, Saito H, Asakura T. The interaction of amyloid Abeta(1-40) with lipid bilayers and ganglioside as studied by 31P solid-state NMR. Chem Phys Lipids. 2009;158:54–60. doi: 10.1016/j.chemphyslip.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [77].Lemkul JA, Bevan DR. Perturbation of membranes by the amyloid beta- peptide--a molecular dynamics study. FEBS J. 2009;276:3060–3075. doi: 10.1111/j.1742-4658.2009.07024.x. [DOI] [PubMed] [Google Scholar]
- [78].Yoda M, Miura T, Takeuchi H. Non-electrostatic binding and self-association of amyloid beta-peptide on the surface of tightly packed phosphatidylcholine membranes. Biochem Biophys Res Commun. 2008;376:56–59. doi: 10.1016/j.bbrc.2008.08.093. [DOI] [PubMed] [Google Scholar]
- [79].Chi EY, Ege C, Winans A, Majewski J, Wu G, Kjaer K, Lee KY. Lipid membrane templates the ordering and induces the fibrillogenesis of Alzheimer’s disease amyloid-beta peptide. Proteins. 2008;72:1–24. doi: 10.1002/prot.21887. [DOI] [PubMed] [Google Scholar]
- [80].Davis CH, Berkowitz ML. Interaction between amyloid-beta (1-42) peptide and phospholipid bilayers: a molecular dynamics study. Biophys J. 2009;96:785–797. doi: 10.1016/j.bpj.2008.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lau TL, Ambroggio EE, Tew DJ, Cappai R, Masters CL, Fidelio GD, Barnham KJ, Separovic F. Amyloid-beta peptide disruption of lipid membranes and the effect of metal ions. J Mol Biol. 2006;356:759–770. doi: 10.1016/j.jmb.2005.11.091. [DOI] [PubMed] [Google Scholar]
- [82].Kim SI, Yi JS, Ko YG. Amyloid beta oligomerization is induced by brain lipid rafts. J Cell Biochem. 2006;99:878–889. doi: 10.1002/jcb.20978. [DOI] [PubMed] [Google Scholar]
- [83].Chi EY, Frey SL, Lee KY. Ganglioside G(M1)-mediated amyloid-beta fibrillogenesis and membrane disruption. Biochemistry. 2007;46:1913–1924. doi: 10.1021/bi062177x. [DOI] [PubMed] [Google Scholar]
- [84].Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 2002;41:7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- [85].Kakio A, Nishimoto SI, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. J Biol Chem. 2001;276:24985–24990. doi: 10.1074/jbc.M100252200. [DOI] [PubMed] [Google Scholar]
- [86].Yahi N, Aulas A, Fantini J. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer’s beta amyloid peptide (Abeta1-40) PLoS One. 5:e9079. doi: 10.1371/journal.pone.0009079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Williamson R, Usardi A, Hanger DP, Anderton BH. Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB J. 2008;22:1552–1559. doi: 10.1096/fj.07-9766com. [DOI] [PubMed] [Google Scholar]
- [88].Stahelin RV. Lipid binding domains: more than simple lipid effectors. J Lipid Res. 2009;50(Suppl):S299–304. doi: 10.1194/jlr.R800078-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Day CA, Kenworthy AK. Tracking microdomain dynamics in cell membranes. Biochim Biophys Acta. 2009;1788:245–253. doi: 10.1016/j.bbamem.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Swartz KJ. Sensing voltage across lipid membranes. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Owen DM, Williamson D, Rentero C, Gaus K. Quantitative microscopy: protein dynamics and membrane organisation. Traffic. 2009;10:962–971. doi: 10.1111/j.1600-0854.2009.00908.x. [DOI] [PubMed] [Google Scholar]
- [92].Darios F, Wasser C, Shakirzyanova A, Giniatullin A, Goodman K, Munoz-Bravo JL, Raingo J, Jorgacevski J, Kreft M, Zorec R, Rosa JM, Gandia L, Gutierrez LM, Binz T, Giniatullin R, Kavalali ET, Davletov B. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron. 2009;62:683–694. doi: 10.1016/j.neuron.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Rohrbough J, Rushton E, Palanker L, Woodruff E, Matthies HJ, Acharya U, Acharya JK, Broadie K. Ceramidase regulates synaptic vesicle exocytosis and trafficking. J Neurosci. 2004;24:7789–7803. doi: 10.1523/JNEUROSCI.1146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kajimoto T, Okada T, Yu H, Goparaju SK, Jahangeer S, Nakamura S. Involvement of sphingosine-1-phosphate in glutamate secretion in hippocampal neurons. Mol Cell Biol. 2007;27:3429–3440. doi: 10.1128/MCB.01465-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bajjalieh SM, Martin TF, Floor E. Synaptic vesicle ceramide kinase. A calcium-stimulated lipid kinase that co-purifies with brain synaptic vesicles. J Biol Chem. 1989;264:14354–14360. [PubMed] [Google Scholar]
- [96].Numakawa T, Nakayama H, Suzuki S, Kubo T, Nara F, Numakawa Y, Yokomaku D, Araki T, Ishimoto T, Ogura A, Taguchi T. Nerve growth factor-induced glutamate release is via p75 receptor, ceramide, and Ca(2+) from ryanodine receptor in developing cerebellar neurons. J Biol Chem. 2003;278:41259–41269. doi: 10.1074/jbc.M304409200. [DOI] [PubMed] [Google Scholar]
- [97].Jeon HJ, Lee DH, Kang MS, Lee MO, Jung KM, Jung SY, Kim DK. Dopamine release in PC12 cells is mediated by Ca(2+)-dependent production of ceramide via sphingomyelin pathway. J Neurochem. 2005;95:811–820. doi: 10.1111/j.1471-4159.2005.03403.x. [DOI] [PubMed] [Google Scholar]
- [98].Blochl A, Thoenen H. Localization of cellular storage compartments and sites of constitutive and activity-dependent release of nerve growth factor (NGF) in primary cultures of hippocampal neurons. Mol Cell Neurosci. 1996;7:173–190. doi: 10.1006/mcne.1996.0014. [DOI] [PubMed] [Google Scholar]
- [99].Wheeler D, Knapp E, Bandaru VV, Wang Y, Knorr D, Poirier C, Mattson MP, Geiger JD, Haughey NJ. Tumor necrosis factor-alpha-induced neutral sphingomyelinase-2 modulates synaptic plasticity by controlling the membrane insertion of NMDA receptors. J Neurochem. 2009;109:1237–1249. doi: 10.1111/j.1471-4159.2009.06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yang SN. Ceramide-induced sustained depression of synaptic currents mediated by ionotropic glutamate receptors in the hippocampus: an essential role of postsynaptic protein phosphatases. Neuroscience. 2000;96:253–258. doi: 10.1016/s0306-4522(99)00582-5. [DOI] [PubMed] [Google Scholar]
- [101].Ping SE, Barrett GL. Ceramide can induce cell death in sensory neurons, whereas ceramide analogues and sphingosine promote survival. J Neurosci Res. 1998;54:206–213. doi: 10.1002/(SICI)1097-4547(19981015)54:2<206::AID-JNR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [102].Inokuchi J, Mizutani A, Jimbo M, Usuki S, Yamagishi K, Mochizuki H, Muramoto K, Kobayashi K, Kuroda Y, Iwasaki K, Ohgami Y, Fujiwara M. A synthetic ceramide analog (L-PDMP) up-regulates neuronal function. Ann N Y Acad Sci. 1998;845:219–224. doi: 10.1111/j.1749-6632.1998.tb09674.x. [DOI] [PubMed] [Google Scholar]
- [103].Furuya S, Mitoma J, Makino A, Hirabayashi Y. Ceramide and its interconvertible metabolite sphingosine function as indispensable lipid factors involved in survival and dendritic differentiation of cerebellar Purkinje cells. J Neurochem. 1998;71:366–377. doi: 10.1046/j.1471-4159.1998.71010366.x. [DOI] [PubMed] [Google Scholar]
- [104].Furukawa K, Mattson MP. The transcription factor NF-kappaB mediates increases in calcium currents and decreases in NMDA- and AMPA/kainate-induced currents induced by tumor necrosis factor-alpha in hippocampal neurons. J Neurochem. 1998;70:1876–1886. doi: 10.1046/j.1471-4159.1998.70051876.x. [DOI] [PubMed] [Google Scholar]
- [105].Ito A, Horigome K. Ceramide prevents neuronal programmed cell death induced by nerve growth factor deprivation. J Neurochem. 1995;65:463–466. doi: 10.1046/j.1471-4159.1995.65010463.x. [DOI] [PubMed] [Google Scholar]
- [106].Coogan AN, O’Neill LA, O’Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- [107].Fasano C, Miolan JP, Niel JP. Modulation by C2 ceramide of the nicotinic transmission within the coeliac ganglion in the rabbit. Neuroscience. 2003;116:753–759. doi: 10.1016/s0306-4522(02)00760-1. [DOI] [PubMed] [Google Scholar]
- [108].Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [109].Davis CN, Tabarean I, Gaidarova S, Behrens MM, Bartfai T. IL-1beta induces a MyD88-dependent and ceramide-mediated activation of Src in anterior hypothalamic neurons. J Neurochem. 2006;98:1379–1389. doi: 10.1111/j.1471-4159.2006.03951.x. [DOI] [PubMed] [Google Scholar]
- [110].Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- [111].Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Frank C, Giammarioli AM, Pepponi R, Fiorentini C, Rufini S. Cholesterol perturbing agents inhibit NMDA-dependent calcium influx in rat hippocampal primary culture. FEBS Lett. 2004;566:25–29. doi: 10.1016/j.febslet.2004.03.113. [DOI] [PubMed] [Google Scholar]
- [113].Abulrob A, Tauskela JS, Mealing G, Brunette E, Faid K, Stanimirovic D. Protection by cholesterol-extracting cyclodextrins: a role for N-methyl-D-aspartate receptor redistribution. J Neurochem. 2005;92:1477–1486. doi: 10.1111/j.1471-4159.2005.03001.x. [DOI] [PubMed] [Google Scholar]
- [114].Hirata H, Hibasami H, Yoshida T, Ogawa M, Matsumoto M, Morita A, Uchida A. Nerve growth factor signaling of p75 induces differentiation and ceramide-mediated apoptosis in Schwann cells cultured from degenerating nerves. Glia. 2001;36:245–258. doi: 10.1002/glia.1113. [DOI] [PubMed] [Google Scholar]
- [115].Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang YH, Nicol GD. NGF-mediated sensitization of the excitability of rat sensory neurons is prevented by a blocking antibody to the p75 neurotrophin receptor. Neurosci Lett. 2004;366:187–192. doi: 10.1016/j.neulet.2004.05.042. [DOI] [PubMed] [Google Scholar]
- [117].Nicol GD. Nerve growth factor, sphingomyelins, and sensitization in sensory neurons. Sheng Li Xue Bao. 2008;60:603–604. [PubMed] [Google Scholar]
- [118].Mark RJ, Blanc EM, Mattson MP. Amyloid beta-peptide and oxidative cellular injury in Alzheimer’s disease. Mol Neurobiol. 1996;12:211–224. doi: 10.1007/BF02755589. [DOI] [PubMed] [Google Scholar]
- [119].Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ballou LR, Laulederkind SJ, Rosloniec EF, Raghow R. Ceramide signalling and the immune response. Biochim Biophys Acta. 1996;1301:273–287. doi: 10.1016/0005-2760(96)00004-5. [DOI] [PubMed] [Google Scholar]
- [121].Goldkorn T, Ravid T, Khan EM. Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Antioxid Redox Signal. 2005;7:119–128. doi: 10.1089/ars.2005.7.119. [DOI] [PubMed] [Google Scholar]
- [122].Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- [123].Ditaranto-Desimone K, Saito M, Tekirian TL, Berg M, Dubowchik G, Soreghan B, Thomas S, Marks N, Yang AJ. Neuronal endosomal/lysosomal membrane destabilization activates caspases and induces abnormal accumulation of the lipid secondary messenger ceramide. Brain Res Bull. 2003;59:523–531. doi: 10.1016/s0361-9230(02)00948-6. [DOI] [PubMed] [Google Scholar]
- [124].Denisova NA, Fisher D, Provost M, Joseph JA. The role of glutathione, membrane sphingomyelin, and its metabolites in oxidative stress-induced calcium “dysregulation” in PC12 cells. Free Radic Biol Med. 1999;27:1292–1301. doi: 10.1016/s0891-5849(99)00163-x. [DOI] [PubMed] [Google Scholar]
- [125].Yoshimura S, Banno Y, Nakashima S, Hayashi K, Yamakawa H, Sawada M, Sakai N, Nozawa Y. Inhibition of neutral sphingomyelinase activation and ceramide formation by glutathione in hypoxic PC12 cell death. J Neurochem. 1999;73:675–683. doi: 10.1046/j.1471-4159.1999.0730675.x. [DOI] [PubMed] [Google Scholar]
- [126].Ayasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25-35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic Biol Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- [127].Pannu R, Won JS, Khan M, Singh AK, Singh I. A novel role of lactosylceramide in the regulation of lipopolysaccharide/interferon-gamma-mediated inducible nitric oxide synthase gene expression: implications for neuroinflammatory diseases. J Neurosci. 2004;24:5942–5954. doi: 10.1523/JNEUROSCI.1271-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Furuk K, Bloom ET. Redox-sensitive events in Fas-induced apoptosis in human NK cells include ceramide generation and protein tyrosine dephosphorylation. Int Immunol. 1998;10:1261–1272. doi: 10.1093/intimm/10.9.1261. [DOI] [PubMed] [Google Scholar]
- [129].Schwandner R, Wiegmann K, Bernardo K, Kreder D, Kronke M. TNF receptor death domain-associated proteins TRADD and FADD signal activation of acid sphingomyelinase. J Biol Chem. 1998;273:5916–5922. doi: 10.1074/jbc.273.10.5916. [DOI] [PubMed] [Google Scholar]
- [130].Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- [131].Bourbon NA, Yun J, Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J Biol Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- [132].Bourbon NA, Yun J, Berkey D, Wang Y, Kester M. Inhibitory actions of ceramide upon PKC-epsilon/ERK interactions. Am J Physiol Cell Physiol. 2001;280:C1403–1411. doi: 10.1152/ajpcell.2001.280.6.C1403. [DOI] [PubMed] [Google Scholar]
- [133].Huwiler A, Xin C, Brust AK, Briner VA, Pfeilschifter J. Differential binding of ceramide to MEKK1 in glomerular endothelial and mesangial cells. Biochim Biophys Acta. 2004;1636:159–168. doi: 10.1016/j.bbalip.2003.08.010. [DOI] [PubMed] [Google Scholar]
- [134].Oh HL, Seok JY, Kwon CH, Kang SK, Kim YK. Role of MAPK in ceramide-induced cell death in primary cultured astrocytes from mouse embryonic brain. Neurotoxicology. 2006;27:31–38. doi: 10.1016/j.neuro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [135].Detre C, Kiss E, Varga Z, Ludanyi K, Paszty K, Enyedi A, Kovesdi D, Panyi G, Rajnavolgyi E, Matko J. Death or survival: membrane ceramide controls the fate and activation of antigen-specific T-cells depending on signal strength and duration. Cell Signal. 2006;18:294–306. doi: 10.1016/j.cellsig.2005.05.012. [DOI] [PubMed] [Google Scholar]
- [136].Stoica BA, Movsesyan VA, Lea P.M.t., Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- [137].Shen YH, Godlewski J, Zhu J, Sathyanarayana P, Leaner V, Birrer MJ, Rana A, Tzivion G. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J Biol Chem. 2003;278:26715–26721. doi: 10.1074/jbc.M303264200. [DOI] [PubMed] [Google Scholar]
- [138].Blazquez C, Galve-Roperh I, Guzman M. De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. Faseb J. 2000;14:2315–2322. doi: 10.1096/fj.00-0122com. [DOI] [PubMed] [Google Scholar]
- [139].Salinas M, Lopez-Valdaliso R, Martin D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- [140].Yu ZF, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15:85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- [141].Opreanu M, Lydic TA, Reid GE, McSorley KM, Esselman WJ, Busik J. Docosahexaenoic Acid Inhibits Cytokine Signaling in Human Retinal Endothelial Cells by Downregulating Sphingomyelinases. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.09-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–9089. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]