Abstract
Unilateral incompatibility (UI) is a prezygotic reproductive barrier in plants that prevents fertilization by foreign (interspecific) pollen through the inhibition of pollen tube growth. Incompatibility occurs in one direction only, most often when the female is a self-incompatible species and the male is self-compatible (the “SI × SC rule”). Pistils of the wild tomato relative Solanum lycopersicoides (SI) reject pollen of cultivated tomato (S. lycopersicum, SC), but accept pollen of S. pennellii (SC accession). Expression of pistil-side UI is weakened in S. lycopersicum × S. lycopersicoides hybrids, as pollen tube rejection occurs lower in the style. Two gametophytic factors are sufficient for pollen compatibility on allotriploid hybrids: ui1.1 on chromosome 1 (near the S locus), and ui6.1 on chromosome 6. We report herein a fine-scale map of the ui6.1 region. Recombination around ui6.1 was suppressed in lines containing a short S. pennellii introgression, but less so in lines containing a longer introgression. More recombinants were obtained from female than male meioses. A high-resolution genetic map of this region delineated the location of ui6.1 to ∼0.128 MU, or 160 kb. Identification of the underlying gene should elucidate the mechanism of interspecific pollen rejection and its relationship to self-incompatibility.
FLOWERING plants have evolved several reproductive barriers for preventing illegitimate hybridization with related species. These barriers may be expressed prefertilization and/or postfertilization. Unilateral incompatibility or incongruity (UI) is a prefertilization barrier that occurs when pollen of one species is rejected on pistils of a related species, while no rejection occurs in the reciprocal cross (De Nettancourt 1977). In theory, unilateral incompatibility should reinforce species identity in natural, sympatric populations of related taxa. This barrier also impedes the efforts of plant breeders to transfer traits from wild species into related crop plants. For example, the transfer of cytoplasmic traits from species with maternally inherited chloroplasts and mitochondria may be prevented by unilateral crossing barriers. Nuclear-encoded traits may also be inaccessible if F1 interspecific hybrids are both male sterile and incompatible as female parents.
In the Solanaceae, unilateral incompatibility is observed in crosses between cultivated tomato (Solanum lycopersicum, formerly Lycopersicon esculentum) and some related wild species. In general, pistils of the cultivated tomato act as a “universal acceptor,” in that they fail to recognize and reject pollen of other tomato species. In the reciprocal crosses, pollen of S. lycopersicum is rejected on styles of virtually all of the green-fruited species, but not on styles of other red or orange-fruited species (reviewed by Mutschler and Liedl 1994). This pattern is mostly consistent with the “SI × SC” rule, wherein pollen of self-compatible (SC) species (including cultivated tomato) are rejected on pistils of self-incompatible (SI) species, but not in the reverse direction (Lewis and Crowe 1958). Exceptions to the SI × SC rule in the tomato clade include species or populations that have lost self-incompatibility but retain the ability to reject pollen of tomato. This is the case for the facultative outcrossing species S. chmielewskii, the autogamous S. neorickii (formerly L. parviflorum), as well as marginal SC populations of normally SI species such as S. pennellii and S. habrochaites (formerly L. hirsutum). An SC accession of S. pennellii, LA0716, is exceptional in having lost the ability to reject self pollen, while retaining the ability to serve as pollen parent on styles of SI accessions of this species (and other SI species, including S. peruvianum and S. lycopersicoides) (Hardon 1967; Rick 1979; Quiros et al. 1986). In this regard, S. pennellii LA0716 conforms to the Lewis and Crowe (1958) model in that it behaves like a transitional form lacking SI function in the pistil but not in the pollen.
Unilateral incompatibility may also occur in crosses between populations or races of a single species. In S. habrochaites for example, pollen from SC biotypes located at the northern or southern margins of its geographic range is rejected on pistils of the central, SI populations (Martin 1961, 1963). Furthermore, pollen from the northern SC group is rejected by styles of the southern SC populations. Yet pistils of both SC biotypes are able to reject pollen of cultivated tomato. Thus there appear to be at least three distinct unilateral crossing barriers, just within S. habrochaites, possibly indicating different pollen tube recognition and rejection systems. The F1 N × S hybrid is SC, as expected, but SI plants are recovered in the F2 generation, suggesting that the loss of SI occurred via independent mutations in the north and the south (Rick and Chetelat 1991).
Interspecific F1 hybrids between SI wild species and SC cultivated tomato are self-incompatible and reject pollen of cultivated tomato, indicating both traits are at least partially dominant (McGuire and Rick 1954; Martin 1963; Hardon 1967). Interestingly, pollen of the F1 hybrids is incompatible on pistils of the wild species parent (i.e., including other individuals of the same accessions, but with nonmatching S alleles). This observation suggests that there are dominant factors from cultivated tomato that lead to pollen rejection on styles of the wild species, regardless of the pollen genotype. This apparent sporophytic effect contrasts with the purely gametophytic nature of pollen SI specificity in the Solanaceae (De Nettancourt 1977).
Early studies of the inheritance of unilateral incompatibility in tomato suggested the involvement of several genes controlling the pistil response; however, the genetics of pollen responses have received little attention. In F2 S. habrochaites (northern SC accession) × S. habrochaites (central SI accession), the rejection of pollen from the SC parent segregated as if controlled by one to two dominant genes from the SI accession (Martin 1964). In crosses of S. lycopersicum to both SI and SC accessions of S. pennellii, the intra- and interspecific crossing relations were largely consistent with the Lewis and Crowe (1958) model of stepwise mutation at the S locus (Hardon 1967); there was also evidence of a second barrier in the SC S. pennellii accession. In F1 and BC1 hybrids of S. lycopersicum × S. habrochaites, the segregation of unilateral and self-incompatibilities was consistent with the action of two major genes, with minor polygenes indicated as well (Martin 1967). More recently, several QTL underlying pistil-side unilateral and self-incompatibilities were mapped in BC1 S. lycopersicum × S. habrochaites (Bernacchi and Tanksley 1997); the major QTL for both forms of pollen rejection was located at or near the S locus on chromosome 1, which controls SI specificity (Tanksley and Loaiza-Figueroa 1985).
There are little data on pollen-side unilateral incompatibility factors in the tomato clade, or any other system. Our previous work identified two to three genetic loci from S. pennellii that are required for pollen to overcome incompatibility on pistils of S. lycopersicum × S. lycopersicoides or S. lycopersicum × S. sitiens hybrids (Chetelat and Deverna 1991; Pertuze et al. 2003). One of these factors mapped to the S locus, the other two were on chromosomes 6 and 10. In this system the female tester stocks were either diploid or allotriploid hybrids, the latter containing one genome of the wild, SI parent, plus two genomes of cultivated tomato; both types of hybrids reject pollen of cultivated tomato. The pollen parents were either F1 S. lycopersicum × S. pennellii or bridging lines developed by backcrossing the F1 to cultivated tomato and selecting for the ability to overcome stylar incompatibility. In the progeny, distorted segregation ratios were observed in which the S. pennellii alleles were preferentially transmitted, indicating linkage to gametophytic factors required forcompatibility.
This experimental system has several advantages for detecting pollen (gametophytic) unilateral incompatibility genes. First, pollen-expressed factors are readily distinguished from pistil factors because only the former show linkage to S. pennellii specific markers. Second, pollen rejection is by unilateral, not self-incompatibility, since both species contributing to the pollen genotype, S. lycopersicum and S. pennellii, are SC. Finally, as we describe herein, the rejection of tomato pollen by pistils of the interspecific hybrids is weakened by the decreasing dosage of the S. lycopersicoides genome, which reduces the number of pollen factors required for compatibility. Thus, the gametophytic factors on chromosomes 1 and 6 (denoted hereinafter ui1.1 and ui6.1), when present in the same pollen, are sufficient for full compatibility on pistils of allotriploid interspecific hybrids, whereas they confer only partial compatibility on diploid hybrids.
Our overall objectives are to identify the genes underlying both the chromosome 1 and chromosome 6 pollen-specific unilateral incompatibility factors from S. pennellii and to determine the nature of their interaction. Toward this goal, we report herein the high-resolution genetic and physical mapping of the ui6.1 region.
MATERIALS AND METHODS
Plant materials:
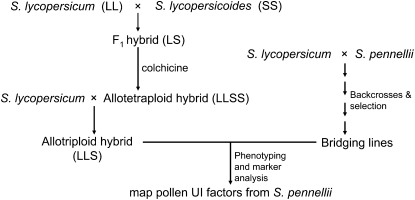
The parental species and accessions used were S. lycopersicum cultivars ‘Vendor Tm-2a’ (accession LA2968), ‘UC-82B’ (LA2801), and ‘VF-36’(LA0490), S. pennellii (LA0716), and S. lycopersicoides (LA1964, LA1991, and LA2951). Seed of these lines was obtained from the C. M. Rick Tomato Genetics Resource Center (http://tgrc.ucdavis.edu). An allotriploid interspecific hybrid (GH266, Rick et al. 1986), containing two genomes of S. lycopersicum (cv. UC82B) and one genome of S. lycopersicoides (LA1964), was used as a female tester line to detect pollen unilateral incompatibility factors (Figure 1, ‘LLS’). For comparison purposes, pollination tests were also carried out with pistils of the 2x F1 interspecific hybrid (90L4178 = S. lycopersicum VF36 × S. lycopersicoides LA2951) and pure S. lycopersicoides (LA1991). Our previous studies (Chetelat and Deverna 1991) indicated that two pollen factors from S. pennellii, located on chromosomes 1 and 6, were required and sufficient for overcoming incompatibility on styles of the allotriploid hybrid. The chromosome 1 factor, ui1.1, mapped close to the S locus controlling self-incompatibility. The chromosome 6 factor, ui6.1, was located on the short arm of chromosome 6. The phenotypes of recombinants around ui6.1 were determined by test pollinations onto the allotriploid hybrid (Figure 1).
Figure 1.—
Crossing scheme used to develop S. lycopersicum × S. lycopersicoides interspecific hybrids and bridging lines containing gametophytic unilateral incompatibility factors from S. pennellii. L, genome of S. lycopersicum; S, genome of S. lycopersicoides. Pollen of S. lycopersicum is rejected on pistils of S. lycopersicoides and its interspecific hybrids with cultivated tomato, whereas “bridging lines” containing specific gametophytic factors from S. pennellii are compatible.
To fine map ui6.1, two F2 populations were developed from S. pennellii-derived bridging lines, obtained after several backcrosses from the wild species to S. lycopersicum (cv. Vendor or UC-82B) (Chetelat and Deverna 1991, Figure 1). Initial mapping of ui6.1 was based on a mapping population (denoted “F2-a”) of 1167 plants derived from BC7F2 bridging lines heterozygous at both the ui1.1 and ui6.1 loci. To simplify phenotyping at ui6.1, a second population (“F2-b”) of 1920 plants was obtained from F2-a individuals that were homozygous for the S. pennellii allele at ui1.1 and heterozygous at ui6.1.
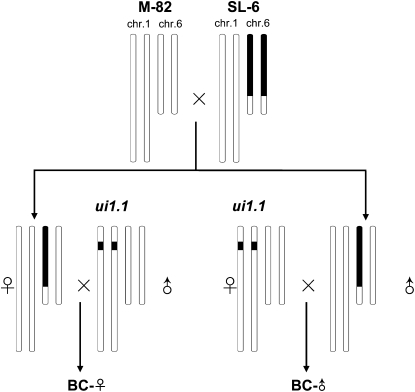
Severe recombination suppression was observed in these two mapping populations. To increase recombination rates, we created a heterozygous substitution line (SL-6), carrying a nearly intact (∼80 cM, Van Wordragen et al. 1996) S. pennellii chromosome 6 in the background of S. lycopersicum (Figure 6). The heterozygous SL-6 was then crossed as female (BC-♀) or male (BC-♂) to a bridging line, which was homozygous for the S. pennellii allele at ui1.1 and homozygous for the S. lycopersicum allele at ui6.1. This provided independent estimates of recombination frequency in female and male gametes.
Figure 6.—
Crossing scheme used to optimize the rate of recombination around ui6.1. A substitution line containing a nearly intact S. pennellii chromosome 6 (Van Wordragen et al. 1996) was crossed to tomato cv. M-82. The resulting hybrid, heterozygous for the pennellii chromosome, was backcrossed as male (BC-♂) or female (BC-♀) parent to a line homozygous for the S-locus region from S. pennellii. Recombinants were identified by marker analysis in the two BC populations and then tested for compatibility of pollen on pistils of the allotriploid hybrid.
Pollinations and pollen tube growth studies:
All crosses were performed in the greenhouse using standard pollination techniques. The compatibility or incompatibility of crosses was determined by visualizing pollen tubes within pistils of the allotriploid hybrid 24 hr after pollination. The staining method was according to Martin (1959), except that pistils were fixed in ethanol: glacial acetic acid (3:1) solution (instead of FAA). After rinsing three times in water, pistils were transferred to 8 N NaOH for 8 hr to clear and soften the tissue. After triple rinsing in water, the softened pistils were soaked in aniline blue solution (0.1% aniline blue in 0.1 N K3PO4) for 8–24 hr. Observation and measurements of pollen tubes were carried out under UV light fluorescence microscopy using a Zeiss Axioskop. Pollinations were judged incompatible when the growth of all pollen tubes was arrested in the upper half of the style. In contrast, compatible pollinations resulted in at least some pollen tubes reaching the ovaries. For each cross, the length of the longest pollen tubes was measured and expressed as a percentage of the style length (from stigma surface to base of style). At least three pistils were examined per cross, from which the average maximum pollen tube length was calculated.
For imaging, pistils were fixed in 3:1 ethanol: glacial acetic acid for 24 hr or more. Following a rinse with deionized water, the pistils were soaked overnight in 10 N NaOH, rinsed three times with deionized water, and immersed in a 0.005 g/ml solution of Aniline Blue Fluorochrome (Biosupplies Australia) in 0.1 M K2HPO4 buffer, pH 8.5. Pistils were stained overnight in the dark at room temperature. Stained pistils were mounted on glass slides in 50% glycerol, covered with a glass cover slip, and flattened with gentle pressure on the cover slip. Observation and photography of pollen tubes were carried out using a Leica DM5500 B fluorescence microscope with a DAPI filter. Images were captured with a Hamamatsu digital camera and IPLab software (BD Biosciences). Individual micrographs were assembled into whole pistil montages using Adobe Photoshop CS2.
DNA isolations:
A small scale DNA extraction method was adopted to isolate DNA from the mapping populations using 96-well microtiter plates. Seeds were treated with 50% household bleach for 15 min, rinsed thoroughly under running tap water, and germinated on blotter paper (Anchor Paper) in acrylic sandwich boxes (10.8 × 10.8 × 3.4 cm, Hoffman Manufacturing) for 7–10 days. Seedlings with fully expanded cotyledons were transplanted to soil in plastic seedling trays and grown in the greenhouse. After 2 or 3 weeks, single expanding leaflets of ∼1 cm2 in area were collected from each plant into 96-well microtiter plates. After adding 400 μl of extraction buffer (200 mm Tris–HCl pH 7.5, 250 mm NaCl, 25 mm EDTA, 0.5% SDS and ∼5% sodium metabisulfite) and two levitation stir balls (3.97 mm, cat. no. VP725E, V&P Scientific), leaf tissues were ground on a paint shaker (Skandex SO-10m, Fluid Management Europe) for 8 min and then incubated at 65° for 1 hr. After cooling, 150 μl of chilled 5 m KAc buffer was added and centrifuged at 4000 RPM (3000 × g) for 15 min. The supernatant (200 μl) was transferred to a new 96-well microtiter plate. DNA was precipitated by adding 100 μl 100% isopropanol, followed by centrifugation at 4000 RPM for 10 min. The supernatant was poured out and pellets were washed once using 200 μl of 70% ethanol and allowed to dry at room temperature. The pellets were resuspended in 200 μl distilled H2O. Each PCR reaction used 2 μl (100–200 ng) of resuspended DNA in a total volume of 20 μl.
CAPS markers:
Initial mapping of ui6.1 relied on previously mapped CAPS markers obtained from the Solanaceae Genomics Network (SGN, http://solgenomics.net). Fine-scale mapping was based on markers developed from the sequences of BACs located in the candidate region, also downloaded from SGN. PCR primers of predicted amplification size ranging from 700 to 1000 bp were picked out using the software Primer3. PCR conditions were 94° for 2 min, followed by 35 cycles of 94° for 30 sec, 55° for 30 sec, and 72° for 2 min, with a final extension of 5 min at 72°. Amplification products were separately digested with eight 4-bp recognizing restriction enzymes (AluI, HaeIII, HinfI, MboI, MseI, MspI, RsaI, and TaqI) to identify polymorphisms. A total of 142 markers were designed for the candidate region, of which 18 polymorphic markers were used in the final linkage analysis (supporting information, Table S1). All CAPS markers were resolved on 2% agarose gels.
Isolation and analysis of recombinants:
Marker analysis of the bridging lines indicated the size of the ui6.1 introgression from S. pennellii was ∼32 cM on the EXPEN 2000 reference map of tomato (data not shown). To identify the approximate location of ui6.1, two markers, SSR47 and T834, located at positions 6.5 and 32 cM on chromosome 6, were selected to genotype the first mapping population, F2-a. Recombinants identified with these two markers were then subjected to compatibility evaluation by measuring pollen tube growth on pistils of the allotriploid hybrid. The first round of screening indicated that ui6.1 is closely linked but distal to marker SSR47. Therefore, a CAPS marker nearer the end of chromosome 6, T1928, was used in combination with T834 to screen all subsequent mapping populations. Relatively high recombination frequencies were observed near ui6.1 in two populations (BC-♀ and BC-♂) derived from a S. pennellii substitution line. We focused on the recombinants identified among 1824 individuals of BC-♀. Hundreds of publically available markers, as well as markers developed from sequenced BACs and BAC ends, were used to conduct the linkage analysis. On the basis of the mapping results from this phase, flanking markers 73H07-3 and Mi were chosen to analyze the recombinants between T1928 and T834 from the other populations, i.e., the F2-a, F2-b, BC-♂, and the remainder of the BC-♀. The additional recombinants were then tested for compatibility on the allotriploid.
Linkage analysis was based on the recombinants isolated from all mapping populations. Map distances were expressed in units of recombination frequency, i.e., (RF = no. of recombinants/total no. of gametes) × 100%, where the no. of gametes = (no. of BC progeny), or (2 × no. of F2 progeny).
RESULTS
Pollen tube growth in pistils of S. lycopersicoides and F1 hybrids with tomato:
Like other SI wild tomato relatives, pistils of S. lycopersicoides reject pollen of cultivated tomato (S. lycopersicum, SC) (Table 1). No pollen rejection occurs in the other direction, consistent with the SI × SC rule. Rejection of tomato pollen occurs early during growth on the S. lycopersicoides pistil, with pollen tubes reaching no further than the stigma or uppermost portion of the style (<5% of style length). In contrast, self pollen tubes are arrested midway down the S. lycopersicoides style (∼50% of style length). Diploid and allotriploid S. lycopersicum × S. lycopersicoides hybrids also reject pollen of cultivated tomato, but the rejection occurs lower in the style: ∼15% of style length for the 2x hybrid, and ∼50% for the 3x hybrid. Thus, the interspecific hybrids have weakened or delayed incompatibility responses compared to S. lycopersicoides; rejection of tomato pollen tubes on the allotriploid occurs in about the same position as self pollen tubes on S. lycopersicoides. Pollen of S. pennellii (SC accession LA0716) is compatible on styles of S. lycopersicoides and the diploid or allotriploid hybrids.
TABLE 1.
Pollen tube growth following compatible and incompatible pollinations of Solanum lycopersicoides and interspecific Solanum hybrids
| Pistil genotype | Pollen genotype | Length of longest tube (% of style) | Location of rejection | No. tubes in ovaries |
|---|---|---|---|---|
| S. lycopersicoides LA2951 | S. lycopersicoides LA2951 (self) | 50.2 ± 2.76 | Style | 0 |
| S. lycopersicoides LA2951 (sib) | 100 | N/A | >50 | |
| S. pennellii LA0716 | 100 | N/A | 4.9 ± 2.91 | |
| S. lycopersicum cv. VF-36 | 5.6 ± 1.88 | Stigma | 0 | |
| S. lycopersicum (ui1.1) | 5.3 ± 0.67 | Stigma | 0 | |
| S. lycopersicum (ui6.1) | 4.7 ± 1.52 | Stigma | 0 | |
| S. lycopersicum (ui1.1 + ui6.1) | 10.9 ± 0.96 | Stigma/style | 0 | |
| S. lycopersicum × S. lycopersicoides (diploid hybrid 90L4178) | S. lycopersicoides LA2951 | 100 | N/A | >50 |
| S. pennellii LA0716 | 100 | N/A | >50 | |
| S. lycopersicum cv. VF-36 | 16.6 ± 1.86 | Style | 0 | |
| S. lycopersicum (ui1.1) | 13.7 ± 0.71 | Style | 0 | |
| S. lycopersicum (ui6.1) | 15.3 ± 3.14 | Style | 0 | |
| S. lycopersicum (ui1.1 + ui6.1) | 42.3 ± 14.5 | Style | 0 | |
| S. lycopersicum × S. lycopersicoides (allotriploid hybrid GH266) | S. lycopersicoides LA2951 | 100 | N/A | >50 |
| S. pennellii LA0716 | 100 | N/A | >50 | |
| S. lycopersicum cv. VF-36 | 47.8 ± 4.93 | Style | 0 | |
| S. lycopersicum (ui1.1) | 45.9 ± 6.40 | Style | 0 | |
| S. lycopersicum (ui6.1) | 53.0 ± 11.4 | Style | 0 | |
| S. lycopersicum (ui1.1 + ui6.1) | 100 | N/A | >50 |
Pollen compatibility is indicated by the length of the longest pollen tube expressed as a percentage of the total length of the style and stigma (a value of 100% indicates a compatible cross). Values are the averages (±SE) of at least 3 replicate pistils for each cross. On the female side, the genotypes were S. lycopersicoides, and diploid and allotriploid F1 S. lycopersicum × S. lycopersicoides hybrids. On the male side, crosses were made with pollen from S. lycopersicoides, S. lycopersicum, S. pennellii, and bridging lines containing the ui1.1 and/or ui6.1 factors from S. pennellii introgressed into cultivated tomato. These genotypes are described in materials and methods.
Two pollen factors, ui1.1 and ui6.1, are required to overcome stylar UI:
We previously showed that two S. pennellii gametophytic factors, ui1.1 and ui6.1, are required for pollen to overcome the stylar incompatibility barrier of diploid and allotriploid S. lycopersicum × S. lycopersicoides hybrids (Chetelat and Deverna 1991). These two factors, when bred into S. lycopersicum, confer full compatibility on pistils of the allotriploid (Table 1). On pistils of the diploid hybrid or S. lycopersicoides, pollen containing both ui1.1 and ui6.1 penetrate further into the style than pollen lacking either factor, yet do not reach the ovaries.
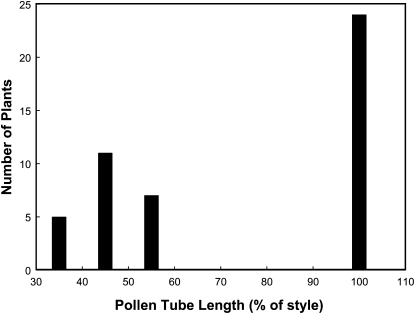
To elucidate the inheritance of pollen compatibility in this system, we genotyped BC7F2 progeny of bridging lines heterozygous for the S. pennellii alleles at both loci (the F2-a population), using markers SSR47 and T834 on the short arm of chromosome 6 and two S-locus flanking markers, TG184 and SSR98, on chromosome 1. Plants heterozygous or homozygous for the S. pennellii alleles at only ui1.1, or only ui6.1, or both loci, in the genetic background of S. lycopersicum, were selected from this population. Compatibility of pollen from the various genotypes was tested on pistils of the allotriploid hybrid. The distribution of maximum pollen tube lengths was bimodal, with no intermediate plants; thus pollen compatibility can be treated as a qualitative trait in this system (Figure 2).
Figure 2.—
Histogram of pollen tube lengths following pollinations of allotriploid S. lycopersicum × S. lycopersicoides hybrids (♀) with bridging lines segregating for two pollen compatibility factors from S. pennellii. Pollen tube lengths are measured from the position where the majority of tubes are arrested, excluding outlier tubes that may grow a little further (see Figure 3). Measurements were taken 24 hr after pollination.
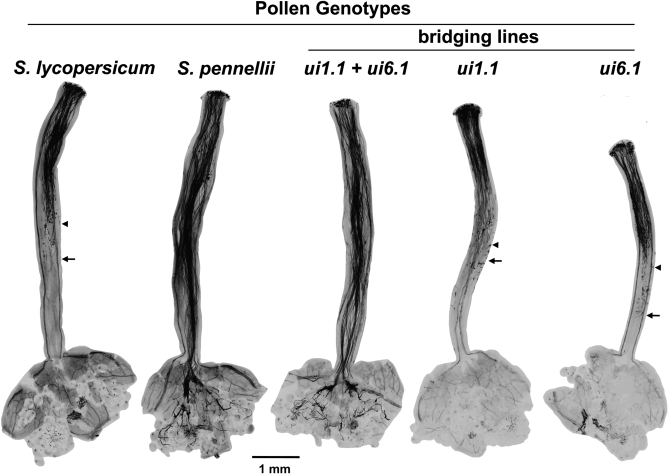
Pollen of S. lycopersicum cv. VF-36 and bridging containing a single S. pennellii factor (ui1.1 or ui6.1) were incompatible on pistils of the allotriploid, as the longest pollen tubes grew to only half the length of the style after 24 hr (Figure 3). In contrast, both S. pennellii and the ui1.1 + ui6.1 bridging line showed a compatible phenotype, with pollen tubes reaching the ovaries. These results confirmed that either S. pennellii factor alone is insufficient to overcome incompatibility, and that both are required to elicit a compatible reaction.
Figure 3.—
Pollen tube growth on pistils of allotriploid S. lycopersicum × S. lycopersicoides hybrids pollinated by different species or genotypes. Pistils were fixed 24 hr after pollination and stained with aniline blue to visualize pollen tubes. (Note: the color of images was inverted, thus pollen tubes appear darkly stained). For the incompatible crosses, arrowheads indicate the zone of the style where growth of most tubes stopped, and arrows indicate the longest pollen tube. The bridging line genotypes contained one or both pollen compatibility factors (ui1.1, ui6.1) from S. pennellii introgressed into the background of S. lycopersicum.
The pollen-side factors ui1.1 and ui6.1 do not confer UI or SI in the pistil:
Importantly, the two gametophytic factors from S. pennellii do not confer a stylar UI or SI response. Bridging lines homozygous or heterozygous for the S. pennellii introgressions containing ui1.1 and ui6.1 set fruit and seeds following self-pollination (data not shown), indicating they are SC. Furthermore, the bridging lines set fruit and seed following pollinations with cultivated tomato, providing strong evidence that pistil-side and pollen-side UI responses are controlled by different sets of genes.
Map location of ui6.1:
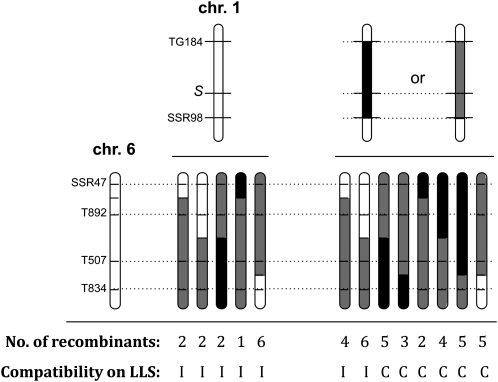
To determine the approximate genetic location of ui6.1, 1167 F2-a plants were genotyped with markers SSR47 and T834 on the short arm of chromosome 6. Recombinant plants were genotyped with two additional markers in this interval, T892 and T507, and two S-locus markers, TG184 and SSR98, on chromosome 1. Compatibility tests of the recombinants were carried out on pistils of the allotriploid. A total of 55 recombinants were identified between SSR47 and T834, of which 47 were tested for compatibility (Figure 4). Thirteen recombinants lacking the S. pennellii allele at ui1.1 showed an incompatible phenotype, regardless of their genotype at ui6.1. The average “maximum” pollen tube length in these plants was 48.2% of style length. The recombinants heterozygous or homozygous for the S. pennellii allele at ui1.1 segregated for compatibility, depending on their genotype for ui6.1. The 10 recombinants lacking the S. pennellii allele of SSR47 were incompatible, with an average pollen tube length of 47.4%, whereas the remaining 24 recombinants (i.e., those that were at least heterozygous) were all compatible on the pistils of the allotriploid.
Figure 4.—
Genotypes of recombinants recovered in the ui6.1 region in F2 progeny of bridging lines containing both the chromosome 1 and 6 factors. The compatibility or incompatibility of recombinants was determined by pollinations onto pistils of the allotriploid S. lycopersicum × S. lycopersicoides hybrid. Open bars, homozygous for the S. lycopersicum alleles; gray, heterozygous; and black, homozygous for the S. pennellii alleles. In the absence of the S. pennellii chromosome 1 factor, all plants elicit an incompatible reaction, regardless of their genotype at ui6.1. Recombinants that were heterozygous for the parental S. pennellii introgression were progeny tested to determine the phenotype of ui6.1.
Some F2-a recombinants did not provide information on the map location of ui6.1. All 13 plants lacking the S. pennellii allele at ui1.1 locus were incompatible, as expected, so they were uninformative regarding ui6.1. Recombinants that carried a parental (nonrecombinant) S. pennellii segment were also uninformative. These were self-pollinated and plants that carried only the recombinant segment were tested for compatibility. The results showed that ui6.1 is closely linked to marker SSR47 (Figure 5).
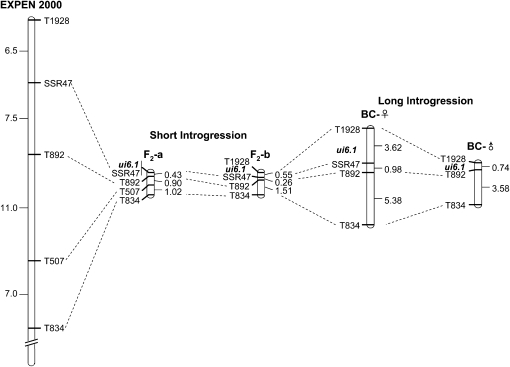
Figure 5.—
Comparison of recombination frequencies near ui6.1 in different mapping populations. The F2 maps are based on recombination within a relatively short (32 cM) S. pennellii segment, whereas the BC maps are derived from a longer segment (∼80 cM) in a substitution line. The reference map is the tomato EXPEN 2000 map (http://solgenomics.net). Genetic distances are in units of percentage of recombination, or, on the EXPEN 2000 map, in centimorgans. On the F2-b and BC maps, ui6.1 is shown at its approximate location because not all plants in these populations were phenotyped.
To avoid the problem of segregation for ui1.1, a second population (F2-b), homozygous for the S. pennellii allele at ui1.1 and segregating at ui6.1, was analyzed. This population of 1920 plants was screened with a distal marker, T1928 and T834; recombinants were then genotyped with SSR47 and T892 (Figure 5). A total of 86 recombinants were identified, of which 31 had crossovers between T1928 and T892. Only the latter were tested for compatibility phenotype on the allotriploid, since the F2-a population indicated a location for ui6.1 closer to SSR47. Of the 31 tested recombinants, only 13 were informative regarding the position of ui6.1; the other 18 recombinants also carried a parental S. pennellii segment, and thus required an additional generation to obtain an informative phenotype. The results indicated ui6.1 lies between T1928 and T892 (Figure 5).
Recombination near ui6.1 is suppressed:
Recombination in the ui6.1 region was strongly suppressed in the two F2 populations derived from the bridging lines (Figure 6). The total genetic distances observed in the two populations were 2.35 and 2.32 MU, between T834 and either SSR47 or T1928, respectively. These values are less than one-tenth of the recombination frequencies reported for the same markers on the reference map, EXPEN 2000, based on an interspecific F2 S. lycopersicum × S. pennellii cross (http://solgenomics.net; Fulton et al. 2002). This degree of recombination suppression could impede the fine-scale mapping and cloning of ui6.1, so we designed a mapping strategy to elevate crossover frequency in this region. Our previous studies showed that recombination frequency is higher in lines with long introgressed segments or whole chromosome substitutions, relative to short introgressions (Canady et al. 2006).
We therefore took advantage of a partial substitution line, SL-6, which contains a long S. pennellii introgression spanning most of chromosome 6 (Figure 6). A line heterozygous for SL-6 was crossed as female (BC-♀) or male (BC-♂) to a line homozygous for the ui1.1 region from S. pennellii. All plants in these two populations were screened with the flanking markers T1928 and T834 to identify recombinants, which were genotyped with T892 and SSR47. In 2946 BC-♀ and 672 BC-♂ plants, 294 and 29 recombinants, respectively, were found. The BC-♀ data were derived from two independent reciprocal crosses in which a homozygous SL-6 was crossed as male or female to cultivar M-82 to create the heterozygous stock; since no differences were seen between the reciprocal crosses (data not shown), the mapping data were pooled. The genetic distance between T1928 and T834 in the BC-♀ population was 9.98 MU, or over fourfold higher than observed in F2-a and F2-b (Figure 5). Recombination frequency was significantly lower in BC-♂, but the total distance of 4.32 MU was still nearly twice that of the F2 populations. These results confirmed that longer alien introgressions recombine more readily than short ones. Furthermore, recombination in female gametes of SL-6 heterozygotes (BC-♀) was over twice that observed in male gametes (BC-♂).
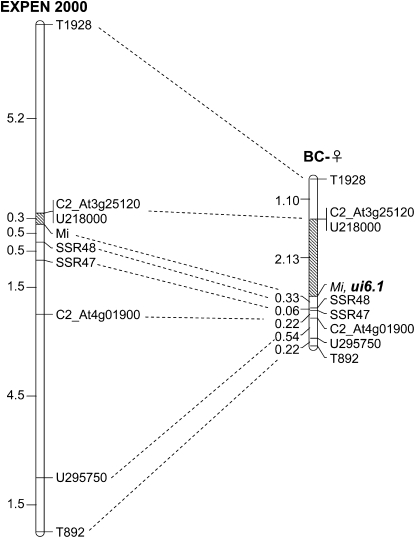
Heterogeneity for recombination rates along the chromosome:
We also observed that recombination rates were not uniform along the chromosome. For example, the genetic distance between T1928 and T892 in the BC-♀ population was 4.60 MU, more than six times the distance in BC-♂ (Figure 5). In contrast, the relative increase in recombination between T892 and T834 was only ∼1.5-fold. Fortuitously, ui6.1 was located in a region of relatively high crossover frequency (Figure 7). When additional markers were placed onto a genetic map derived from selective genotyping of the BC-♀ population, a region of elevated recombination was observed between markers U218000 and Mi. The distance between these two markers on our map was 2.13 MU, more than seven times the value on the reference map (Figure 7).
Figure 7.—
Genetic map of the ui6.1 region on the short arm of chromosome 6. Results are based on analysis of progeny from the substitution line, used as female parent (BC-♀), crossed to a line homozygous for the S. pennellii factor on chromosome 1. Markers are from the SGN database (http://solgenomics.net). The shaded region of the chromosomes indicates a zone of elevated recombination in the progeny of SL-6 relative to the EXPEN 2000 reference map.
Fine-scale mapping of ui6.1:
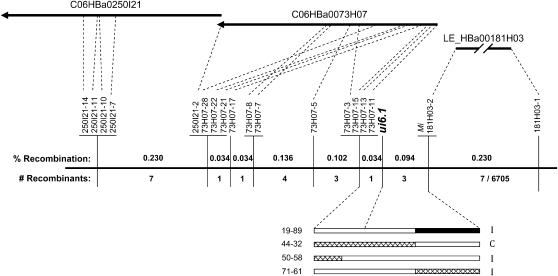
Several hundred plants with crossovers between T834 and T1928 or SSR47 were isolated from the four mapping populations. We initially focused on the recombinants isolated from the partial BC-♀, which produced the highest recombination frequency. The ui6.1 phenotypes of these were determined by test pollinations on the allotriploid. On the basis of the mapping results, we used two closely flanking markers to further characterize the recombinants isolated from the other populations. These recombinants were selectively phenotyped, focusing on those plants most likely to further resolve the position of ui6.1.
Our initial results showed ui6.1 is located between markers U218000 and Mi (Figure 7), based on 39 recombinants in this interval from the partial BC-♀ population. To further resolve the location of ui6.1, hundreds of markers based on the sequences of overlapping BACs were developed. Of these, 18 polymorphic CAPS markers, developed from the sequences of two overlapping BACs, C06HBa0250I21 and C06HBa0073H07, and the end sequences of LE_HBa00181H03 (Figure 8, Table S1), were used. A fine-scale map of the ui6.1 region was constructed from these recombinants and included six flanking markers (73H07-3, 73H07-11, 73H07-13, 73H07-15, Mi, and 181H03-2), with one crossover on each side (recombinant 44-32 and 50-58) of ui6.1 (Figure 8).
Figure 8.—
Fine-scale genetic and physical map of the ui6.1 region and phenotypes of recombinants with crossovers in the flanking intervals. The position of three BACs is shown relative to a fine-scale map of the region based on the analysis of recombinants. For each marker interval, the percentage of recombination and the number of recombinants obtained from all mapping populations are shown. Markers were derived from the S. lycopersicum BAC sequences. The genotypes of four recombinants (solid, homozygous S. pennellii; hatched, heterozygous; and open, homozygous S. lycopersicum) and their pollen phenotypes on pistils of the allotriploid (I, incompatible; C, compatible) are shown.
The closely linked markers 73H07-3 and Mi were then used to genotype the recombinants from the other populations. Two more recombinants, 19-89 from F2-b and 71-61 from BC-♀, were found in this region (Figure 8). The compatibility of these recombinants was evaluated on the allotriploid (recombinant 19-89 was tested using a homozygous F3 progeny). Linkage analysis using all the recombinants indicated that the interval spanning ui6.1 comprised ∼0.128 MU. An estimate for the corresponding physical distance was obtained from the prepublication draft genome sequence of tomato, made available by the International Tomato Genome Sequencing Consortium at http://solgenomics.net. This showed that the flanking markers 73H07-11 and Mi are separated by ∼160 kb.
DISCUSSION
Relationship between unilateral and self-incompatibility:
A basic question about the nature of prezygotic interspecific reproductive barriers in plants is their relationship to self-incompatibility. As described by Lewis and Crowe (1958), unilateral crossing barriers often follow the SI × SC rule, wherein pollen of a self-compatible species is rejected on styles of a related self-incompatible species, but no rejection occurs in the reciprocal cross. The general validity of this rule in many plant families, including the Solanaceae, suggests that mutation at the S locus is an important element of interspecific barriers. This hypothesis is supported by experiments in Nicotiana in which ectopic expression of S-RNase's from an SI species (Nicotiana alata) in the background of an SC species (N. tabacum) is sufficient to confer on the pistil the ability to reject pollen from several SC species (Murfett et al. 1996). However the same experiments also demonstrated pollen rejection pathways that did not depend on S-RNase expression. Thus, on the pistil side, the S locus is strongly implicated in interspecific pollen rejection, but other genetic factors are also involved. In crosses between S. lycopersicum (SC) and S. habrochaites (SI), several independent QTL were detected, of which the S-locus region had the largest phenotypic effect (Bernacchi and Tanksley 1997).
On the other hand, interspecific pollen tube rejection differs from SI in timing and location. Pollen tubes of cultivated tomato are rejected in the upper portion of the S. pennellii style (Hardon 1967; Liedl et al. 1996), similar to our results with S. lycopersicoides pistils (the “early” rejection). In contrast, “self” pollen tubes penetrate further into the style. This early rejection of interspecific pollen tubes superficially resembles sporophytic SI, wherein self pollen is recognized and rejected on the stigma. Further evidence for a sporophytic component of interspecific incompatibility comes from the crossing relationships of F1 hybrids between cultivated tomato and SI wild relatives: backcrosses to the SI species are only possible when the F1's are used as female parent (McGuire and Rick 1954; Hardon 1967). In other words, if the compatibility of pollen were purely determined by gametophytic factors, a significant fraction of pollen from the F1 hybrid should be compatible on pistils of the SI species. Thus, expression of interspecific incompatibility is genetically complex and may involve more than one mechanism for recognizing and rejecting foreign pollen.
In the present study, we report the fine mapping of ui6.1, a pollen compatibility factor on chromosome 6 that interacts with ui1.1, located at or near the S locus on chromosome 1 of tomato. We show that only pollen bearing the S. pennellii alleles of both ui1.1 and ui6.1 are compatible on styles of S. lycopersicum × S. lycopersicoides hybrids. These results strongly implicate the S locus in pollen-side unilateral incompatibility. They also provide the first genetic evidence (to our knowledge) that factors other than the product of the S locus are required for pollen function in self- or interspecific incompatibility.
Patterns of segregation distortion in various interspecific crosses of tomato support these conclusions. They provide further evidence that pollen bearing the cultivated tomato alleles at ui1.1 and ui6.1 are selectively eliminated on styles of interspecific hybrids, but only when the wild parent is SI. For example, a pseudo-F2 S. lycopersicum × S. chilense (SI) population, obtained by crossing two independent F1's (to avoid self-incompatibility) exhibited a deficiency of plants homozygous for the S. lycopersicum alleles of markers near ui1.1 and ui6.1 (Graham 2005). The likely explanation is that pollen lacking either factor from S. chilense are eliminated on styles of the F1 hybrid. In contrast, interspecific F2's between tomato and S. chmielewskii or S. pennellii LA0716, both of which are self-compatible yet reject tomato pollen, showed normal Mendelian segregation for markers near ui1.1 and ui6.1 (Paterson et al. 1991; http://solgenomics.net). Mendelian segregation is also observed in interspecific F2 populations when both parents are SI, consistent with the hypothesis that the SI species each contain functional alleles at ui1.1 and ui6.1 (Pertuze et al. 2002; Albrecht and Chetelat 2009). These results suggest the existence of an S-RNase dependent pathway for interspecific pollen rejection in pistils of the SI species, as well as an S-RNase independent mechanism in the SI and some SC species.
Thus, both the genetic evidence and the developmental timing of interspecific pollen rejection support the existence of at least two pathways. The first (early) mechanism rejects tomato pollen tubes in the stigma and upper styles on pistils of SI and some SC species; pollen containing the ui1.1 and ui6.1 factors does not overcome this barrier. The second mechanism causes pollen tube rejection in the mid position of styles from interspecific SC × SI hybrids, and is overcome by pollen containing ui1.1 and ui6.1. We found evidence of overlap between these two pathways: pollen of bridging lines containing both ui1.1 and ui6.1 grow farther into pistils of S. lycopersicoides than does pollen of tomato lines lacking either or both factors (10 vs. 5% of style length). Further research is clearly needed to understand the genetic and physiological basis of these interspecific pollen rejection pathways.
Relevance to other Solanaceae:
We believe our experimental system provides a useful model for studying unilateral incompatibility in other Solanaceae. On the pistil side, the diploid and allotriploid S. lycopersicum × S. lycopersicoides hybrids are SI and reject pollen of cultivated tomato, indicating these traits are effectively dominant, as they are in other Solanaceae hybrids. For example, diploid F1 hybrids between cultivated tomato and SI accessions of S. habrochaites, S. pennellii, S. peruvianum, or S. chilense are all SI and reject pollen of cultivated tomato (McGuire and Rick 1954; Martin 1961, 1963; Hardon 1967). Allotriploids composed of two genomes from tomato and one from potato (S. tuberosum, SI) also reject pollen of S. lycopersicum and accept pollen of S. pennellii LA0716 (Schoenmaker et al. 1993), i.e., the same crossing relationships we report herein. In other solanaceous genera, including Nicotiana (Murfett et al. 1996) and Capsicum (Pickersgill 1997), self- and unilateral incompatibility are also dominant in interspecific SC × SI hybrids.
Furthermore, if the underlying pathways for pollen tube rejection by unilateral incompatibility are conserved, then the results we present herein should be relevant to other systems. For example, allotriploid S. lycopersicum × S. sitiens hybrids also reject pollen of cultivated tomato, and bridging lines containing ui1.1 and ui6.1 from S. pennellii are compatible (Pertuze et al. 2003). In instances where unilateral incompatibility results from the breakdown of self-incompatibility, there are likely to be common mechanisms. Self-incompatibility systems are strongly conserved within plant families, and related families often have the same system. For example, all SI members of the Solanaceae employ the S-RNase based gametophytic system, under the control of a single S locus determining specificity (De Nettancourt 1977, 1997). While the S-RNases are highly divergent, each allele (S1, S2, etc.) is strongly conserved, even across plant families (Igic and Kohn 2001).
A weakened UI response in the allotriploid hybrid simplifies the genetics:
The ability to reject pollen of cultivated tomato is effectively dominant in pistils of S. lycopersicum × S. lycopersicoides hybrids. However, expression of the pistil response in the hybrids is weaker than in the wild parent, in two ways. First, pistils of the 3x hybrid arrest pollen tubes of cultivated tomato near the middle of the style, and lack the early (i.e., stigmatic) pollen tube rejection phenotype of S. lycopersicoides pistils, suggesting the latter barrier is additive or recessive. Second, bridging lines containing both the ui1.1 and ui6.1 genes from S. pennellii were fully compatible (i.e., many pollen tubes in the ovaries) with pistils of the allotriploid hybrid, but incompatible with the diploid hybrid or S. lycopersicoides. We hypothesize that additional genetic factors from S. pennellii contribute to compatibility with these two genotypes; a locus on chromosome 10 was identified in an earlier study (Chetelat and Deverna 1991). Thus, the use of allotriploid hybrids as tester stocks provides a simpler genetic system in which to isolate pollen-side unilateral incompatibility factors. This method may provide a useful route for genetic analysis of other interspecific incompatibility systems.
The causes of the weakened expression in the allotriploid are unknown, but presumably result from the reduced gene dosage of the SI parent (i.e., one genome of S. lycopersicoides, vs. two genomes of the SC parent, S. lycopersicum). We hypothesize that the different pathways for pollen tube rejection are differentially affected by gene dosage in the pistil. The action of a factor at or near the S locus in pollen implies that the S-RNase dependent pathway is fully dominant in pistils of the allotriploid hybrid. However, the early rejection pathway may be additive or recessive in hybrids. There are many examples of gene-specific dosage effects in aneuploids and triploids. Genes that are dominant over wild type in normal (2x) heterozygotes may show reduced expression in trisomics containing two doses of the wild-type allele (Khush and Rick 1968). Some protein coding loci are expressed at levels proportional to their gene dosage, while other loci maintain expression at the diploid level via dosage compensation (Smith and Conklin 1975; Birchler 1979; Tanksley 1979). In allotriploid hybrid fish, gene expression is reduced to the diploid level through allele-specific gene silencing (Pala et al. 2008). Finally, trisomic dosage may alter gene expression through genic interactions, for example if modifier loci are more sensitive to dosage (Gill 1974).
Toward map-based cloning of ui6.1:
To decipher the molecular pathways of interspecific pollen tube rejection, it is necessary to clone the underlying genes. Toward this goal, we mapped the ui6.1 gene to an interval of ∼0.128 MU or 160 kb on the short arm of chromosome 6 of tomato. The efficiency of linkage mapping was limited initially by low recombination compared to the reference map of tomato. However, the degree of recombination suppression varied along the chromosome, and crossover frequencies close to ui6.1 were higher than normal. The causes of recombination suppression (or enhancement) in this region of the genome are unknown, but may reflect the degree of divergence (structural or sequence) between the S. lycopersicum and S. pennellii chromosomes. The two species show colinearity of chromosome 6S, with no rearrangements (Peters et al. 2009). In S. peruvianum, a large paracentric inversion on chromosome 6S was responsible for recombination suppression around the Mi gene (for nematode resistance) in crosses to tomato (Seah et al. 2004). The Mi locus is close to ui6.1, but we found no evidence for an inversion in S. pennellii. While a few BAC-derived markers were inverted in our genetic map of the ui6.1 region compared to their order on the tomato BAC sequence, they were in a region of relatively high recombination. The discrepancy suggests a rearrangement may have occurred in this particular BAC. Furthermore, FISH mapping of many other BACs on chromosome 6 showed an area (position 0–5 cM) where the linear order of markers predicted from the genetic map (EXPEN 2000) is inverted relative to their actual physical arrangement along the chromosome (Peters et al. 2009), pointing to a likely error in the linkage map.
We obtained much higher recombination frequencies starting with a nearly intact S. pennellii chromosome 6, compared to the shorter introgressed segments present in the bridging lines. This agrees with our previous observations of a positive correlation between crossover frequency and the length of alien chromosome segments (Canady et al. 2006). Higher recombination rates were seen in female gametes than in male gametes, as has been reported before (Rick 1969; De Vicente and Tanksley 1991).
Thus, despite an overall suppression of recombination in this region, the efficiency of mapping could be maximized by choice of starting materials and the direction of the cross. In this way, the chromosomal position of ui6.1 was refined from a relatively imprecise interval, encompassing the entire short arm of chromosome 6, to only 0.128 MU (160 kb). We anticipate that isolation of the ui6.1 gene will shed light on the mechanism of pollen tube rejection in unilateral incompatibility, and its relationship to self-incompatibility. From a practical standpoint, a more complete understanding of the genetic factors underlying interspecific crossing barriers may eventually enable wider crosses. For example, overcoming the rejection of S. lycopersicum pollen on pistils of related SI species might facilitate the development of cytoplasmic male sterility in tomato.
Acknowledgments
The authors gratefully acknowledge Peter March, Katie Smith, and the C. M. Rick Tomato Genetics Resource Center staff for providing seed of key genotypes, and for growing and maintaining plants in the greenhouse. We thank Sheh May Tam for helpful discussions. BAC sequences were obtained from http://solgenomics.net. Funding for this research was provided by grant DBI 0605200 from the National Science Foundation, Plant Genome Program.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.116343/DC1.
References
- Albrecht, E., and R. T. Chetelat, 2009. Comparative genetic linkage map of Solanum sect. Juglandifolia: evidence of chromosomal rearrangements and overall synteny with the tomatoes and related nightshades. Theor. Appl. Genet. 118 831–847. [DOI] [PubMed] [Google Scholar]
- Bernacchi, D., and S. D. Tanksley, 1997. An interspecific backcross of Lycopersicon esculentum × L. hirsutum: linkage analysis and a QTL study of sexual compatibility factors and floral traits. Genetics 147 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1979. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics 92 1211–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canady, M. A., Y. Ji and R. T. Chetelat, 2006. Homeologous recombination in Solanum lycopersicoides introgression lines of cultivated tomato. Genetics 174 1775–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat, R. T., and J. W. Deverna, 1991. Expression of unilateral incompatibility in pollen of Lycopersicon pennellii is determined by major loci on chromosomes 1, 6 and 10. Theor. Appl. Genet. 82 704–712. [DOI] [PubMed] [Google Scholar]
- De Nettancourt, D., 1977. Incompatibility in Angiosperms. Springer-Verlag, Berlin.
- De Nettancourt, D., 1997. Incompatibility in angiosperms. Sex. Pl. Reprod. 10 185–199. [Google Scholar]
- De Vicente, M. C., and S. D. Tanksley, 1991. Genome-wide reduction in recombination of backcross progeny derived from male versus female gametes in an interspecific backcross of tomato. Theor. Appl. Genet. 83 173–178. [DOI] [PubMed] [Google Scholar]
- Fulton, T. M., R. Van Der Hoeven, N. T. Eannetta and S. D. Tanksley, 2002. Identification, analysis and utilization of conserved ortholog set (COS) markers for comparative genomics in higher plants. Plant Cell 14 1457–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill, B. S., 1974. Dosage-dependent epistasis in the tomato: its bearing on trisomy abnormalities and evolution. J. Hered. 65 130–132. [Google Scholar]
- Graham, E. B., 2005. Genetic diversity and crossing relationships of Lycopersicon chilense. Ph.D. Thesis, University of California, Davis.
- Hardon, J. J., 1967. Unilateral incompatibility between Solanum pennellii and Lycopersicon esculentum. Genetics 57 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igic, B., and J. R. Kohn, 2001. Evolutionary relationships among self-incompatibility RNAses. Proc. Natl. Acad. Sci. USA 98 13167–13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush, G. S., and C. M. Rick, 1968. Tomato telotrisomics: origin, identification, and use in linkage mapping. Cytologia 33 137–148. [Google Scholar]
- Lewis, D., and L. K. Crowe, 1958. Unilateral interspecific incompatibility in flowering plants. Heredity 12 233–256. [Google Scholar]
- Liedl, B. E., S. McCormick and M. A. Mutschler, 1996. Unilateral incongruity in crosses involving Lycopersicon pennellii and L. esculentum is distinct from self-incompatibility in expression, timing and location. Sex. Plant Reprod. 9 299–308. [Google Scholar]
- Martin, F. W., 1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technol. 34 125–128. [DOI] [PubMed] [Google Scholar]
- Martin, F. W., 1961. The inheritance of self-incompatibility in hybrids of Lycopersicon esculentum Mill. × L. chilense Dun. Genetics 46 1443–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F. W., 1963. Competition of pollen containing different S alleles in L. esculentum-L. hirsutum crosses. Tomato Genet. Coop. Report 13 14–15. [Google Scholar]
- Martin, F. W., 1964. The inheritance of unilateral incompatibility in Lycopersicon hirsutum. Genetics 50 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, F. W., 1967. The genetic control of unilateral incompatibility between two tomato species. Genetics 56 391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire, D. C., and C. M. Rick, 1954. Self-incompatibility in species of Lycopersicon Sect. Eriopersicon and hybrids with L. esculentum. Hilgardia 23 101–123. [Google Scholar]
- Murfett, J., T. J. Strabala, D. M. M. B. Zurek and B. M. B. A. Beecher, 1996. S RNase and interspecific pollen rejection in the genus Nicotiana: multiple pollen-rejection pathways contribute to unilateral incompatibility between self-incompatible and self-compatible species. Plant Cell 8 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutschler, M. A., and B. E. Liedl, 1994. Interspecific crossing barriers in Lycopersicon and their relationship to self-incompatibility, pp. 164–188 in Genetic Control of Self-incompatibility and Reproductive Development in Flowering Plants, edited by G. Williams, A. E. Clarke and B. R. Knox. Kluwer, The Netherlands.
- Pala, I., M. M. Coelho and M. Schartl, 2008. Dosage compensation by gene-copy silencing in a triploid hybrid fish. Curr. Biol. 18 1344–1348. [DOI] [PubMed] [Google Scholar]
- Paterson, A. H., S. Damon, J. D. Hewitt, D. Zamir, H. D. Rabinowitch et al., 1991. Mendelian factors underlying quantitative traits in tomato: comparison across species, generations, and environments. Genetics 127 181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertuze, R. A., Y. Ji and R. T. Chetelat, 2002. Comparative linkage map of the Solanum lycopersicoides and S. sitiens genomes and their differentiation from tomato. Genome 45 1003–1012. [DOI] [PubMed] [Google Scholar]
- Pertuze, R. A., Y. Ji and R. T. Chetelat, 2003. Transmission and recombination of homeologous Solanum sitiens chromosomes in tomato. Theor. Appl. Genet. 107 1391–1401. [DOI] [PubMed] [Google Scholar]
- Peters, S. A., E. Datema, D. Szinay, M. J. Van Staveren, E. G. W. M. Schijlen et al., 2009. Solanum lycopersicum cv. Heinz 1706 chromosome 6: distribution and abundance of genes and retrotransposable elements. Plant J. 58 857–869. [DOI] [PubMed] [Google Scholar]
- Pickersgill, B., 1997. Genetic resources and breeding of Capsicum spp. Euphytica 96 129–133. [Google Scholar]
- Quiros, C., O. Ochoa and D. Douches, 1986. L. peruvianum × L. pennellii sexual hybrids. Report Tomato Genetics Coop. 36 31–32. [Google Scholar]
- Rick, C. M., 1969. Controlled introgression of chromosomes of Solanum pennellii into Lycopersicon esculentum: segregation and recombination. Genetics 62 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick, C. M., 1979. Biosystematic studies in Lycopersicon and closely related species of Solanum, pp. 667–678 in: The Biology and Taxonomy of the Solanaceae. Edited by J. G. Hawkes, R. N. Lester and A. D. Skelding. Academic Press, New York.
- Rick, C. M., and R. T. Chetelat, 1991. The breakdown of self-incompatibility in Lycopersicon hirsutum, pp. 253–256 in Solanaceae III: Taxonomy, Chemistry, Evolution, edited by J. G. Hawkes, R. N. Lester, M. Nee and N. Estrada. Kew Publishing, Royal Botanic Gardens, UK.
- Rick, C. M., J. W. DeVerna, R. T. Chetelat and M. A. Stevens, 1986. Meiosis in sesquidiploid hybrids of Lycopersicon esculentum and Solanum lycopersicoides. Proc. Natl. Acad. Sci. USA 83 3580–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmaker, H. C. H., A. M. A. Wolters, E. M. Nobel, C. M. J. Deklein and M. Koornneef, 1993. Allotriploid somatic hybrids of diploid tomato (Lycopersicon esculentum) and monoploid potato (Solanum tuberosum). Theor. Appl. Genet. 87 328–336. [DOI] [PubMed] [Google Scholar]
- Seah, S., J. Yaghoobi, M. Rossi, C. A. Gleason and V. M. Williamson, 2004. The nematode-resistance gene, Mi-1, is associated with an inverted chromosomal segment in susceptible compared to resistant tomato. Theor. Appl. Genet. 108 1635–1642. [DOI] [PubMed] [Google Scholar]
- Smith, H. H., and M. E. Conklin, 1975. Effects of gene dosage on peroxidase isozymes in Datura stramonium trisomics, pp. 603–618 in: Isozymes: Developmental Biology. Edited by C. L. Markert. Academic Press, London.
- Tanksley, S. D., 1979. Linkage, chromosomal association, and expression of Adh-1 and Pgm-2 in tomato. Biochem. Genet. 17 1159–1167. [DOI] [PubMed] [Google Scholar]
- Tanksley, S. D., and F. Loaiza-Figueroa, 1985. Gametophytic self-incompatibility is controlled by a single major locus on chromosome 1 in Lycopersicon peruvianum. Proc. Natl. Acad. Sci. USA 82 5093–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wordragen, M. F., R. L. Weide, E. Coppoolse, M. Koornneef and P. Zabel, 1996. Tomato chromosome 6: a high resolution map of the long arm and construction of a composite integrated marker-order map. Theor. Appl. Genet. 92 1065–1072. [DOI] [PubMed] [Google Scholar]