Abstract
Primary cilia have essential roles in transducing signals in eukaryotes. At their core is the ciliary axoneme, a microtubule-based structure that defines cilium morphology and provides a substrate for intraflagellar transport. However, the extent to which axonemal microtubules are specialized for sensory cilium function is unknown. In the nematode Caenorhabditis elegans, primary cilia are present at the dendritic ends of most sensory neurons, where they provide a specialized environment for the transduction of particular stimuli. Here, we find that three tubulin isotypes—the α-tubulins TBA-6 and TBA-9 and the β-tubulin TBB-4—are specifically expressed in overlapping sets of C. elegans sensory neurons and localize to the sensory cilia of these cells. Although cilia still form in mutants lacking tba-6, tba-9, and tbb-4, ciliary function is often compromised: these mutants exhibit a variety of sensory deficits as well as the mislocalization of signaling components. In at least one case, that of the CEM cephalic sensory neurons, cilium architecture is disrupted in mutants lacking specific ciliary tubulins. While there is likely to be some functional redundancy among C. elegans tubulin genes, our results indicate that specific tubulins optimize the functional properties of C. elegans sensory cilia.
THE fitness of all organisms depends on an ability to appropriately sense and respond to the environment. At the cellular level, many specific architectures have evolved to optimize these sensory functions. Prominent among these is the sensory cilium, a tubulin-based cytoplasmic extension that interrogates the extracellular environment in many biological contexts (Davenport and Yoder 2005; Berbari et al. 2009). Cilia are important for the transduction of a broad range of visual, auditory, mechanical, thermal, and chemical stimuli. They also function during development to receive a variety of signals, both chemical and mechanical, that regulate proliferation and differentiation (Goetz and Anderson 2010). Indeed, the disruption of cilium assembly and function can give rise to a spectrum of human diseases collectively known as ciliopathies (Berbari et al. 2009; Lancaster and Gleeson 2009). These disorders, which include autosomal dominant polycystic kidney disease (ADPKD) and autosomal recessive polycystic kidney disease (ARPKD), Bardet–Biedl syndrome, Meckel–Gruber syndrome, and Joubert syndrome, are associated with a variety of pathogenic conditions including polycystic kidneys and neurological impairments.
At the core of all cilia and flagella is the microtubule axoneme. This characteristic structural element comprises nine doublet outer microtubules that may surround a central pair, the presence of which often indicates a motile cilium/flagellum. Like all microtubule-based structures, ciliary axonemes are built of heterodimers of α- and β-tubulins, highly conserved small GTP-binding proteins. The recruitment of other cilium components, including signal transduction machinery, requires a conserved assembly and maintenance process called intraflagellar transport (IFT) (Blacque et al. 2008; Pedersen and Rosenbaum 2008). IFT employs two major complexes that transport ciliary cargo bidirectionally by traveling along the axonemal microtubules. Loss of individual IFT components can cause a broad spectrum of defects in the assembly, maintenance, and function of cilia.
Important insights into cilium structure and function have come from studies of genetically tractable organisms, particularly the green alga Chlamydomonas and the nematode Caenorhabditis elegans (Bae and Barr 2008; Pedersen and Rosenbaum 2008). In C. elegans, sensory cilia are found exclusively at the dendritic ends of sensory neurons. These cilia constitute a highly specialized sensory environment characterized by localized sensory receptors and specific signaling components. Cilium morphology is quite distinctive in many of these cells and likely contributes to their functional specialization (Ward et al. 1975). Recent progress has shed light on the mechanisms that confer this specialization onto more general pan-ciliary pathways (Evans et al. 2006; Mukhopadhyay et al. 2007; Jauregui et al. 2008; Mukhopadhyay et al. 2008; Silverman and Leroux 2009).
The genomes of many eukaryotes harbor multiple α- and β-tubulin genes. Two hypotheses, which are not mutually exclusive, have been proposed to account for these paralogs (Cleveland 1987; Wade 2007). At one extreme, different tubulin isotypes might be functionally redundant, such that their minor coding differences are largely irrelevant. According to this model, multiple genes allow the maintenance of a stable pool of available monomers and dimers. The small amount of sequence variation within the α- and β-tubulin families supports this idea, as do studies of functionally redundant mitotic tubulins in C. elegans (Ellis et al. 2004; Lu et al. 2004; Phillips et al. 2004; Lu and Mains 2005). The alternative hypothesis proposes that specific structures, e.g., ciliary axonemes or axonal microtubules, rely on tubulins optimized for specific roles. Support for this idea has come from studies of cultured mammalian neurons (Joshi and Cleveland 1989), Drosophila (Hutchens et al. 1997; Raff et al. 1997), and human tubulins (Vent et al. 2005; Jaglin et al. 2009). In Drosophila, studies of motile sperm flagella have revealed that the sperm-specific β2 tubulin isoform builds not only the specialized motile axoneme but also all other tubulin-based structures (Kemphues et al. 1982). However, sequences both within and outside the axoneme motif in the C-terminal tail of this tubulin isoform are required for the flagellar axoneme, and other closely related β-tubulins cannot support this role (Fuller et al. 1987; Raff et al. 1997; Popodi et al. 2008). Genetic interactions have provided evidence that β2 tubulin heterodimerizes with the α-tubulin 84B (Hays et al. 1989), which also possesses specific functional properties not provided by structurally similar α-tubulins (Hutchens et al. 1997). In C. elegans, a specific role for tubulin isoforms has been described in the six touch receptor neurons. These nonciliated cells harbor unusual 15-filament microtubules composed of dimers of the α-tubulin MEC-12 and the β-tubulin MEC-7. The loss of mec-7 or mec-12, the expression of which is largely restricted to these cells, results in the conversion of 15-filament microtubules to the standard 11-microfilament variety and a commensurate loss of light-touch response (Savage et al. 1989; Fukushige et al. 1999; Bounoutas et al. 2009). Thus experimental support exists for both of these opposing views, and it seems likely that the role of specific tubulin isoforms in regulating microtubule structure and function differs according to cell and organelle type.
The C. elegans genome encodes nine α- and six β-tubulin genes (Gogonea et al. 1999). Some of these genes, particularly tba-1, tba-2, tbb-1, and tbb-2, are expressed broadly during embryogenesis and function redundantly in spindle assembly and positioning (Ellis et al. 2004; Lu et al. 2004; Phillips et al. 2004; Lu and Mains 2005). tba-1 and tbb-2 have also been recently shown to be important for axon outgrowth and synaptogenesis (Baran et al. 2010). Several others, including mec-7, mec-12, and the β-tubulin ben-1, have been identified through genetic screens for particular phenotypes, such as touch insensitivity or benzimidazole resistance (Driscoll et al. 1989; Savage et al. 1989; Fukushige et al. 1999). However, the extent to which specific tubulin isoforms are required for structural and functional diversity in the C. elegans nervous system remains unknown. Here, taking advantage of several existing genome-wide data sets, we identify the α-tubulins TBA-6 and TBA-9 and the β-tubulin TBB-4 as strong candidates for tubulins that have roles in sensory cilia. We find that each of these genes are expressed in characteristic, partially overlapping, sets of sensory neurons, where their products localize to ciliary axonemes. While the loss of any one (or all three) of these genes does not abolish ciliogenesis, tubulin mutants exhibit significant defects in the localization of cilium proteins and in some cilium-dependent behavioral responses. Together, our results indicate that specific α- and β-tubulin isoforms are important, although not essential, for the efficient assembly and function of specific classes of C. elegans sensory cilia. Sensory cilia throughout the animal kingdom may therefore employ specific tubulin isoforms to optimize their function.
MATERIALS AND METHODS
Nematode culture:
C. elegans strains were cultured using standard techniques (Brenner 1974). To increase the frequency of males in self-progeny broods, him-5(e1490) was present in the background of all strains used in this study except those with mnIs17. tba-6(cxP4018), a Tc3 transposon insertion allele, which was generously provided by Laurent Segalat (CNRS-CGMC, Université de Lyon). tbb-4(ok1461) and tba-9(ok1858) are deletion alleles generated by the C. elegans Gene Knockout Consortium (Oklahoma Medical Research Foundation). Other mutants used here were provided by the Caenorhabditis Genetics Center (University of Minnesota). myIs4[PKD-2∷GFP] and myEx648[KLP-6∷GFP] were generously provided by Maureen Barr (Rutgers University), and bxIs14[Ppkd-2∷GFP] was provided by Scott Emmons (Albert Einstein College of Medicine). Strains harboring OSM-3∷GFP (Mukhopadhyay et al. 2007) and KAP-1∷GFP (Orozco et al. 1999) extrachromosomal arrays were kindly provided by Saikat Mukhodpadhyay and Piali Sengupta (Brandeis University). TRP-4∷GFP was a generous gift of Wei Li and Shawn Xu (University of Michigan).
Tubulin reporter transgenes:
Transcriptional and translational fluorescent protein fusions were created through PCR sequence overlap extension (Boulin et al. 2006). For tba-6, 1226 bases upstream of the start codon was amplified from genomic DNA and fused to the coding region for yellow fluorescent protein (YFP) and the unc-54 3′ UTR, which was amplified from pPD136.64 (Fire Lab Vector Kit) to generate a transcriptional fusion. The same upstream promoter region and the entire genomic coding region excluding the stop codon were fused in-frame to YFP∷unc-54 3′ UTR to generate a translational fusion. For tba-9, 2033 bases upstream of the start codon was used for both constructs. tbb-4 constructs have been previously described (Portman and Emmons 2004). To make mCherry transgenes, mCherry and the unc-54 3′ UTR were amplified from pCH1 (K. Nehrke, University of Rochester). Extrachromosomal array transgenes were generated by standard methods to yield fsEx165[Ptba-6∷mCh + Ptba-9∷YFP + Psulp-3∷GFP], fsEx260[Ptba-6∷mCherry], fsEx261[TBA-9∷YFP + pBX1], fsEx262[TBA-6∷YFP + Punc-122∷GFP + pBX1], fsEx263[TBB4∷YFP + pBX1], fsEx287[Ptba-9∷mCherry], fsEx288[Ptbb-4∷mCherry], fsEx289[Ptbb-4∷YFP + pBX1], and fsEx290[Ptba-9∷YFP + pBX1]. The chromosomal integrant fsIs14 was produced by UV irradiation of a strain carrying the extrachromosomal array fsEx165.
Dye filling:
Young adults were exposed to 50 μm DiD (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine) in M9 salts for 1 hr to stain amphid neurons (Starich et al. 1995) or 100 μm DiO (3,3′-dioctadecyloxacarbocyanine) in 50 mm calcium acetate for 2 hr to stain amphid and inner labial neurons (Burket et al. 2006).
Microscopy:
Images were collected with an Apotome-equipped Zeiss Axioplan 2 or a Leica SP1 confocal microscope. Brightness and γ adjustments were carried out in Adobe Photoshop (Adobe).
Behavioral assays:
For response assays, male worms were isolated daily at the fourth larval stage and transferred to fresh agar plates seeded with Escherichia coli OP50. Mating assays were performed the following day. Males were placed on a 1-cm lawn of OP50 dotted with eight paralyzed unc-31 hermaphrodites and observed for a 10-min period. Response consisted of cessation of forward swimming, placement of the ventral side of the tail against the hermaphrodite body, and beginning to swim backward. Response efficiency was calculated as the percentage of males that responded to contact with a hermaphrodite in the allowed time (Hart 2005). To test for differences between groups, we used Fisher's exact test, with a P-value threshold for significance of 0.05. Since response is a binary choice, data are reported without any associated error. Olfaction assays were carried out essentially as previously described (Hart 2005). For locomotion assays, animals were isolated to sex-segregated plates at the mid-L4 stage and assayed 20 hr later using an automated worm-tracking system (Cronin et al. 2005). Briefly, individual animals were placed on a lightly seeded agar plate, allowed to habituate for 5 min, and recorded for 2 min. Multiple parameters of locomotion were measured, including wavelength, frequency, flex, and velocity. For each sex and each genotype, 10 worms were assayed. Statistical comparisons were made between mutants and wild type (him-5 controls of the same sex) using a Student's t-test with a cutoff of P < 0.05. For nose-touch assays, hermaphrodites were isolated at the mid-L4 stage and assayed 20–24 hr later. To prepare the plates, 100 μl of an overnight OP50 bacterial culture was added to an agar plate and allowed to dry in a thin layer. Ten to 15 worms were picked to the assay plate and allowed to habituate for ∼15 min. The positive response to nose touch was scored if the animal reversed upon contacting a human arm hair with the nose at a perpendicular angle (Hart 2005). For each animal, 10 consecutive trials were carried out and the percentage of successful trials (reversals) was averaged among animals of the same genotype for statistical comparisons using an ANOVA with Bonferroni post-hoc tests. As a negative control, glr-1 mutants were assayed in parallel. All assays were scored with the observer blinded to the genotype of the animals.
RESULTS
Identifying candidate ciliary tubulins:
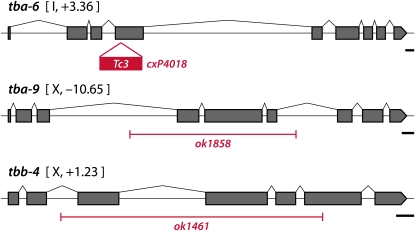
To identify candidate ciliary tubulins in C. elegans, we intersected data from four recent genomic analyses. Three of these were designed to identify genes expressed in all ciliated neurons (Blacque et al. 2005; Kunitomo et al. 2005; Chen et al. 2006), while the fourth analysis (Portman and Emmons 2004) sought genes expressed in the male tail sensory rays, which bear two classes of ciliated sensory neurons. Of the nine C. elegans α-tubulin genes, tba-6 appeared in the top 10th percentile in two of these four studies, while tba-9 ranked in the top 10th in three of the four studies (Table 1). Of the six C. elegans β-tubulin genes, only tbb-4 showed consistent enrichment, falling in the top first percentile in two of the four studies. Most other α- and β-tubulin genes, including the neuronally expressed tubulins mec-7, mec-12, and ben-1 (D. D. Hurd, unpublished data), did not show consistent enrichment in these studies. The only other good candidate, tba-8, appeared in the top 10th percentile in one of four studies. Reporters for this gene showed no detectable neural expression, instead being specifically expressed in the hypodermal seam cells that run laterally along the body of the animal (Portman and Emmons 2004). We therefore focused our efforts on tba-6, tba-9, and tbb-4 (Figure 1).
TABLE 1.
Genomic identification of candidate ciliary tubulins
| Percentile rank |
||||
|---|---|---|---|---|
| Gene | Study Aa | Study Bb | Study Cc | Study Dd |
| tba-6 | 61.0 | 1.1 | 28.0 | 1.0 |
| tba-8 | 1.7 | 14.2 | 5.8 | 45.4 |
| tba-9 | 0.1 | ND | 7.8 | 6.0 |
| tbb-4 | ND | ND | ND | 4.0 |
| ben-1 | 24.0 | 41.9 | 42.6 | 74.6 |
| n | 4,685 | 8,349 | 12,500 | 9,495 |
Genes were percentiled according to their rank in each data set. ND indicates that a gene was not present in the data set for a particular experiment. n, total number of genes in the percentile rankings.
Ciliated neuron genes by serial analysis of gene expression (SAGE) (Blacque et al. 2008).
Ciliated neuron genes by mRNA tagging and microarray (Kunitomo et al. 2005).
Genes downregulated in daf-19 mutants by microarray (Chen et al. 2006).
Ray-enriched genes (lin-22 vs. lin-32; hlh-2) by microarray (Portman and Emmons 2004).
Figure 1.—
Deletion alleles of tubulin genes. For each gene, black boxes indicate coding exons. The red lines depict the insertion of the Tc3 transposon (cxP4018) or the extent of the genomic deletion (ok1858 and ok1461). Scale bar in each panel, 100 bp.
tbb-4, tba-6, and tba-9 are combinatorially expressed in a variety of ciliated sensory neurons:
Using fluorescent transcriptional reporter genes, we examined the cellular expression pattern of each of these three genes. A preliminary expression pattern for tbb-4 has been previously reported (Portman and Emmons 2004), indicating expression in ciliated neurons. We found that tba-6 and tba-9 are also expressed in a variety of ciliated sensory neurons.
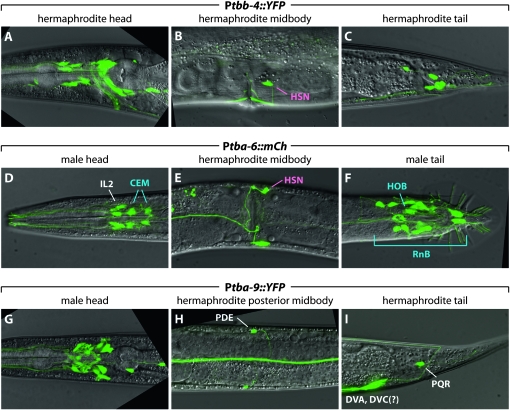
Of these three genes, tbb-4 was expressed most broadly (Figure 2, A–C). In the head of both males and hermaphrodites, we observed expression of a Ptbb-4∷YFP transcriptional reporter in many amphid neurons, likely ADL, AFD, ASE, ASG, ASH, ASJ, ASK, ASI, AWA, AWB, and AWC as judged by co-labeling with lipophilic dyes and/or cellular morphology (Figure 1). Other head neurons expressing Ptbb-4∷YFP include the cephalic CEP neurons, the outer labial quadrant OLQ neurons, and the mechanosensory FLP neurons. In the midbody and tail, Ptbb-4∷YFP expression was found in the PDE postdeirid neurons, PQR (a sensory neuron whose cilium is exposed to the pseudocoelomic fluid), and the phasmid neurons PHA and PHB. Ptbb-4∷YFP was also expressed in all 18 ray neurons in the male, consistent with a previous report (Portman and Emmons 2004). Because of abundant ray neuron expression, Ptbb-4∷YFP expression in male phasmid and hook neurons could not be reliably confirmed, although expression in these cells is not unlikely. We also observed Ptbb-4∷YFP expression in the hermaphrodite HSN motor neurons, the vulval muscles, and several unidentified nonsensory neurons. Expression was also occasionally apparent in the amphid sheath (AMsh) and socket (AMso) glial cells (data not shown).
Figure 2.—
tbb-4, tba-9, and tba-6 are expressed in partially overlapping sets of ciliated C. elegans sensory neurons. A–I show the expression of a transcriptional YFP reporter gene in the indicated body region. Selected cell assignments are shown, using pink for hermaphrodite-specific neurons, blue for male-specific neurons, and white for non-sex-specific neurons. See the text and Table 4 for a more thorough description of tbb-4, tba-9, and tba-6 expression.
Of the two α-tubulin genes that we examined, expression of tba-9 was more extensive (Figure 2, D–F). In the head, a Ptba-9∷YFP transcriptional reporter showed expression in amphid neurons, likely to be ADF, AFD, ASE, ASI, AWA, and AWC, in addition to the OLQ and CEP neurons, the anterior and posterior deirid neurons ADE and PDE, the RIG interneuron, and several additional unidentified nonsensory neurons. In the posterior of both sexes, this reporter was expressed in two tail interneurons, likely DVA, DVB, and/or DVC, as well as the sensory neuron PQR. In the male tail, Ptba-9∷mCherry was expressed in the hook HOA and the ray RnA neurons as judged by morphology, position, and lack of overlap with the RnB marker Ppkd-2∷GFP (data not shown).
The expression of tba-6 was much more circumscribed (Figure 2, G–I). In hermaphrodites, we observed expression of Ptba-6∷mCherry only in the IL2 inner labial sensory neurons and the HSN motor neurons. In males, this reporter was expressed in the IL2s, the cephalic companion CEM sensory neurons, the HOB hook neuron, and all ray RnB neurons, usually excluding those of ray 6. Interestingly, with the exception of the HSN neurons, this expression pattern is identical to that of the kinesin motor KLP-6 (Peden and Barr 2005). Moreover, all tba-6-positive male-specific sensory neurons also express the polycystin/TRPP-class proteins LOV-1 and PKD-2 (Barr and Sternberg 1999; Barr et al. 2001) as well as the five previously described cwp genes (Portman and Emmons 2004; Miller and Portman 2010), suggesting that tba-6 could be involved in building structures important for polycystin signaling.
Together, these results indicate that most ciliated neurons express tbb-4, often along with either tba-6 or tba-9. We found no co-expression of tba-6 and tba-9 in the same cell type, raising the possibility that TBB-4 is the β-tubulin partner for specific α-tubulins in certain cell types. Additionally, some ciliated sensory neurons expressed neither α-tubulin reporter, indicating either that other α-tubulin paralogs are expressed in these neurons or that our reporters do not fully recapitulate the expression patterns of tba-6 and tba-9.
Because many cilium genes in C. elegans are controlled by the RFX transcription factor DAF-19 (Swoboda et al. 2000), we looked for matches to the DAF-19-binding site, the X-box, in the promoters of ciliary tubulins. Of these three genes, only tbb-4 harbors a strong candidate X-box (data not shown). However, this motif was not required for expression of a transcriptional Ptbb-4∷YFP reporter construct, nor was expression of the Ptbb-4∷YFP altered in daf-19 mutants (D. D. Hurd, W.-S. Park and D. S. Portman, unpublished data). This is consistent with previous observations that some cilium-specific genes are not directly regulated by DAF-19 (Yu et al. 2003; Mukhopadhyay et al. 2007). One possibility is that daf-19 activates a more general cilium program, while genes that endow cilia with their functional specializations are under cell type-specific transcriptional control.
TBB-4, TBA-6, and TBA-9 are enriched in ciliary axonemes:
To examine the subcellular localization of TBA-6, TBA-9, and TBB-4, we studied animals harboring Tubulin∷YFP fusion proteins. Each of these proteins accumulated in the ciliated ends and distal dendrites of the sensory neurons in which they were expressed (Figure 2). This was particularly apparent in the amphid, CEM, IL2, and male tail ray neurons. The localization of these fusion proteins was not restricted to cilia, being frequently present in cell bodies, along the length of dendrites, and in transition zones. However, fluorescence was typically the most intense at the ciliated tips of these cells, suggesting that TBB-4, TBA-6, and TBA-9 could be important for the development, function, and diversity of primary cilia in C. elegans sensory neurons. We also observed the expression of both TBB-4∷YFP and TBA-9∷YFP in vulval muscles (data not shown).
None of these translational reporter transgenes rescued the mating behavior defects seen in tba-6, tba-9, or tbb-4 mutant males (see Ray neuron function is compromised in tbb-4, tba-6, and tba-9 mutants). Indeed, these transgenes dominantly disrupted male contact-response behavior from 89% in wild-type (him-5) males to 61%, 3.1%, and 52% in males carrying TBA-6∷YFP, TBA-9∷YFP, and TBB-4∷YFP, respectively (n = 21–36 animals/genotype). These results suggest that the C-terminal fusion of YFP to ciliary tubulins can impede microtubule formation, dynamics, and/or function.
Ciliary tubulins are required for each other's efficient ciliary localization:
To explore the potential roles of tbb-4, tba-6, and tba-9 in C. elegans cilia, we used existing mutant alleles disrupting each of these three genes (Figure 1). All three of these alleles, tbb-4(ok1461), tba-6(cxP4018), and tba-9(ok1858), are likely to be strong loss-of-function or null alleles. Animals homozygous for each of these mutations, as well as triple mutants lacking all three tubulin isotypes, were viable and superficially wild type.
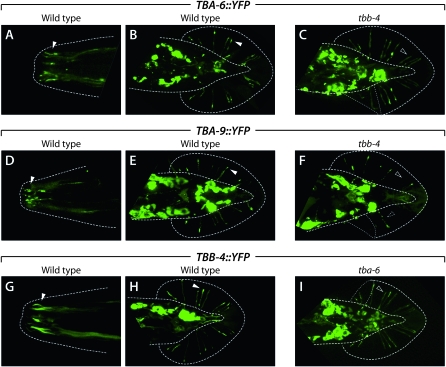
Because tubulins undergo obligate heterodimerization prior to assembly into microtubules, we asked whether the localization of TBA-6, TBA-9, and TBB-4 to the distal ends of sensory dendrites depended on each other's function. We focused on the ray neurons because the anatomy of the male tail allows relatively unobstructed visualization of ray sensory endings. Each of the 18 rays is innervated by one RnA and one RnB neuron. These two neuron types, whose dendritic processes run adjacent to each other in each ray, differ in ultrastructure (Sulston et al. 1980; Chow et al. 1995), gene expression (Barr and Sternberg 1999; Lints et al. 2004), and function (P. Koo and R. Lints, personal communication). As described above, the RnA neurons express tba-9 and tbb-4, while the RnB neurons express tba-6 and tbb-4. In the RnB neurons, we found that efficient distal accumulation of both TBA-6∷YFP and TBB-4∷YFP required each other's activity (Figure 3, C and I). While distal TBA-6∷YFP localization was apparent in 81% (n = 69) of rays in wild-type animals, this frequency was reduced to 60% (n = 87) in tbb-4 mutants. Conversely, ciliary enrichment of TBB-4∷YFP was observed in 67% (n = 108) of rays in wild-type males but in only 36% (n = 69) of tba-6 mutant male rays. This defect in TBB-4∷YFP localization is likely occurring specifically in the RnB neurons, although the close proximity of RnA and RnB dendrites makes this difficult to assess directly.
Figure 3.—
TBA-6, TBA-9, and TBB-4 fusion proteins localize to sensory cilia. A–I show the localization of the indicated tubulin∷YFP fusion protein expressed under the control of its own promoter. Genotypes, either wild-type or tubulin mutant, are shown above each image. Dotted lines indicate the outline of the body and the fan, the cuticular layer in which the rays are embedded. Thin dotted lines in C, F, and I indicate regions in which the fan is folded over onto itself. A, C, and E show nose cilia, while other panels show ventral or dorsal views of the male tail. All images are flattened stacks derived from multiple confocal sections spanning planes containing nose or ray cilia. Solid arrowheads indicate examples of the localization of tubulin fusion proteins to cilia. Open arrowheads highlight examples of dendritic endings that do not show significant tubulin localization. The dashed arrowhead in F indicates the beads-on-a-string appearance of TBA-9∷YFP accumulation in a tbb-4 mutant.
The relationship between TBA-9 and TBB-4 in the RnA neurons was somewhat different. Again, TBA-9∷YFP was clearly present at the distal ends of most RnA neurons (67%, n = 105) in wild-type animals. In tbb-4 mutants, the frequency of TBA-9∷YFP distal localization was reduced (41%, n = 46) and accumulation in large “beads-on-a-string”-like dendritic varicosities was observed (Figure 3F). We detected no apparent mislocalization of TBB-4∷YFP in tba-9 mutants (data not shown); however, any decrease in TBB-4 localization to the RnA cilium might have been obscured by the presence of this protein in RnB. Together, these results support the possibility that the formation of TBA-9:TBB-4 and TBA-6:TBB-4 heterodimers are important for their incorporation into the RnA and RnB cilia, respectively. However, since a significant number of ray neurons do appropriately localize ciliary tubulins in tba-6 and tbb-4 mutants, it is likely that there exists some functional redundancy among tubulins, such that specific isotypes are not absolutely required for building ciliary axonemes in the ray neurons.
Grossly normal cilia can form in the absence of tba-6, tba-9, and tbb-4:
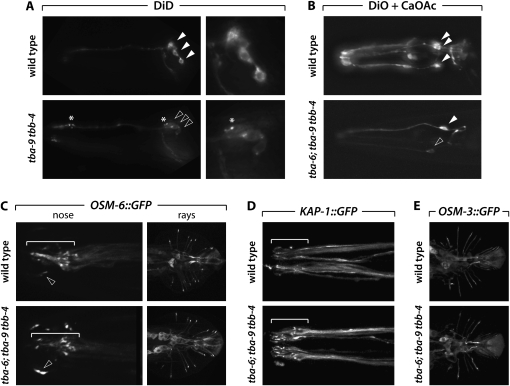
Dye filling, the ability of some of some C. elegans sensory neurons to take up lipophilic dyes from the environment, is commonly used to assess the integrity of sensory cilia and their exposure to the environment (Perkins et al. 1986). We found that uptake of the dye DiD by amphid and phasmid neurons was not grossly compromised in tbb-4, tba-9, or tba-9tbb-4 mutants. (We did not carefully examine dye filling in tba-6 mutants because we found no evidence for expression of this gene in dye-filling neurons.) However, we did find that the soma of amphid neurons sometimes stained more weakly in tbb-4 (data not shown) and tba-9tbb-4 (Figure 4A) animals than in controls. In these neurons, dendrites often exhibited large varicosities, and foci of dye accumulation were seen in cell bodies. Interestingly, these defects are reminiscent of those seen in mutants that perturb IFT but retain cilia (Williams et al. 2008). We also found that the ASI amphid neuron sometimes failed to fill with dye in tbb-4 and tba-9 mutants, although this defect was variable (data not shown). We obtained similar results when the IL2 neurons were labeled with DiO in the presence of calcium acetate (Burket et al. 2006). Under conditions in which ∼50% of the six IL2 neurons dye-filled in wild-type animals (3.2 DiO-positive IL2s per individual; n = 27), only 1.8 IL2s per animal dye-filled in tba-6; tba-9tbb-4 triple mutants (n = 27). Moreover, the intensity of DiO fluorescence in these mutant IL2s was often markedly reduced (Figure 4B). Together, these results indicate that amphid, phasmid, and IL2 cilia can form in the absence of tba-6, tba-9, and/or tbb-4, but their structure or function may be compromised.
Figure 4.—
Grossly normal cilia can form in the absence of tba-6, tba-9, and tbb-4. (A) Dye filling with DiD in the noses of wild-type and tba-9 tbb-4 mutant hermaphrodites. Note that the image for the mutant is typical only of the relatively small number of animals in which clear defects were observed. Solid and open arrowheads point to the cell bodies of three amphid neurons. Asterisks indicate puncta of dye accumulation. (Right) Higher-magnification views of amphid soma. (B) Dye filling with DiO under conditions in which IL2 neurons label (calcium acetate treatment). Top and bottom panels show a representative individual. Solid arrowheads indicate strongly staining IL2 neurons; the open arrowhead indicates a neuron with low dye uptake. (C) OSM-6∷GFP localization in the noses (left) and male tails (right) of wild-type and triple-tubulin mutants. The white bracket indicates the location of the amphid cilia bundle, which is slightly disorganized in the tba-6; tba-9 tbb-4 mutant. Arrowheads indicate the cilium of a CEP neuron. (D) KAP-1∷GFP in wild-type and triple-tubulin hermaphrodite noses. The position of the amphid cilia bundles are denoted with a white bracket. Note the slight disorganization and presence of varicosities in the triple mutant. (E) OSM-3∷GFP in the male tail ray neurons of wild-type and triple-tubulin mutant males.
As another means to assess the integrity of cilia, we examined the localization of several well-characterized ciliary markers in tba-6; tba-9tbb-4 triple mutants. We found that the localization of the IFT component OSM-6, visualized with an OSM-6∷GFP fusion protein, was grossly normal in head and ray sensory neurons, although cilia often appeared disorganized, especially in the amphid (Figure 4C). In these animals, we also consistently observed defects in the CEP neurons, whose swollen cilia exhibited significant increases of the amount of OSM-6∷GFP fluorescence (arrowheads in Figure 4C). We also examined the localization of KAP-1∷GFP and OSM-3∷GFP, components of the kinesin motors that build cilia. Again, these showed only subtle defects in amphid and ray neuron dendrites and cilia (Figure 4, D and E, and data not shown). Taken together, these data indicate that sensory cilia can be built and maintained in the absence of TBA-6, TBA-9, and TBB-4.
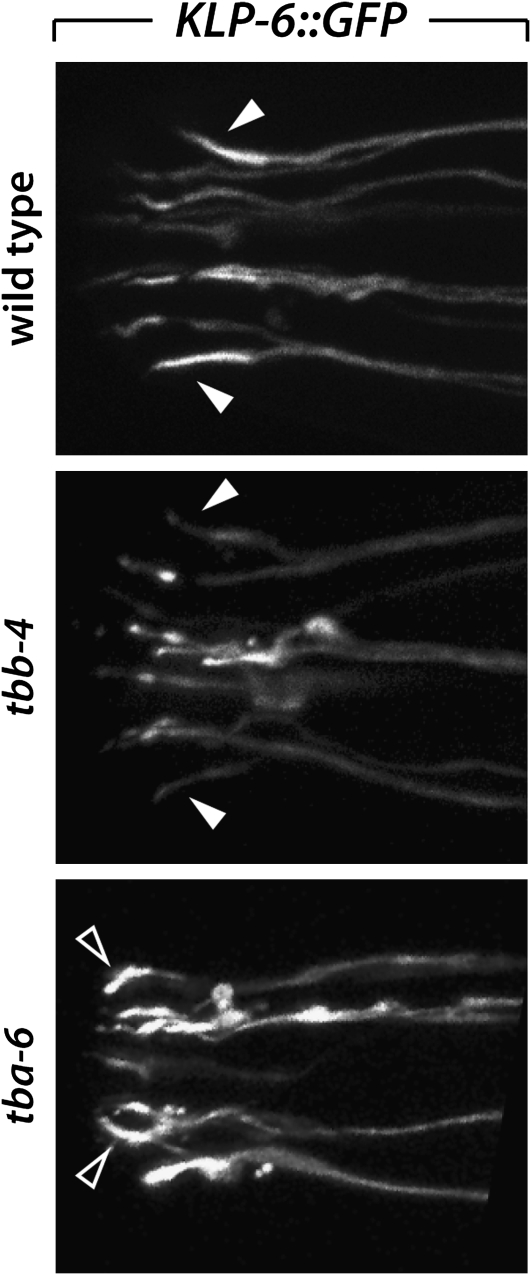
TBA-6 and TBB-4 are necessary for the specialized morphology of the CEM cilia:
The male RnB, HOB, and CEM neurons, as well as the non-sex-specific IL2 sensory neurons, all of which express tba-6, also express the kinesin-like gene klp-6, indicating that these neurons may utilize a specific mechanism to build and/or maintain functional cilia (Peden and Barr 2005). We found that the morphology of the CEM cilia, which are normally straight or bent slightly outward (Jauregui et al. 2008), requires both tbb-4 and tbb-6 (Figure 5). In tbb-4 mutants, 28% (n = 36) of CEM cilia, visualized with KLP-6∷GFP, appeared abnormal, often with a distorted base. Moreover, 78% (n = 36) of tba-6 mutant cilia were defective, exhibiting a gross mislocalization of KLP-6∷GFP and an inward-bending, hook-like appearance. In tba-6; tba-9tbb-4 triple mutants (n = 44), 100% of CEM cilia exhibited a defective club-like shape, with prominent varicosities of KLP-6∷GFP in the distal dendrite (data not shown). Thus the specific architecture of the CEM cilia, but not their presence, is strongly dependent on TBA-6 and TBB-4.
Figure 5.—
The morphology of the CEM neurons is defective in tubulin mutants. CEM cilia, as well as those of the IL2 neurons, are marked with KLP-6∷GFP. In wild-type animals, the CEM cilia exhibit brighter fluorescence and extend straight toward the nose or bend slightly outward (open arrowheads). In some tbb-4 and most tba-6 mutants, CEM cilium morphology is clearly abnormal, bending inwards and possessing fluorescent varicosities (outlined arrowheads).
Ray neuron function is compromised in tbb-4, tba-6, and tba-9 mutants:
Because ciliary axonemes are generally present in tubulin mutants, we asked whether these mutant cilia were able to support functional sensory transduction. We focused first on male mating behavior because tbb-4, tba-6, and tba-9 are all expressed in the male tail sensory rays. In the first step of the male-mating program, males respond to contact with a hermaphrodite by ceasing forward movement, placing the ventral side of the tail against the hermaphrodite body, and maintaining this contact while moving backward until the vulva is located (Liu and Sternberg 1995). Contact response is thought to be mediated largely by the ray neurons, with a minor contribution from the hook HOB neuron (Liu and Sternberg 1995). In particular, TRPP/polycystin-mediated sensory signaling in the RnB neurons is critical for efficient response behavior (Barr and Sternberg 1999; Barr et al. 2001; Miller and Portman 2010).
We found that the function of all three ray-expressed ciliary tubulins was necessary for optimal mating behavior. In contact-response assays, wild-type him-5 males responded to hermaphrodite contact with a frequency of 83% (n = 80). In contrast, single tbb-4, tba-6, or tba-9 mutant males exhibited significant, although relatively modest, defects in response behavior (Table 2). Both tbb-4 and tba-9 single mutant males responded with a frequency of 60% (n = 86 and 85, respectively). In tba-6 mutant males, response frequency was 73% (n = 96). Double and triple tubulin mutants had similar or more severe defects, although the further increases in severity did not reach statistical significance (Table 2). Because contact-response behavior was clearly not eliminated in tubulin mutants, our data again indicate that functional cilia can form in the absence of these genes. However, they also indicate that both RnA and RnB neurons likely require specific tubulins for optimal sensory function. These data, along with the ability of the TBA-9∷YFP translational reporter to dominantly disrupt contact response (see above), also suggest that the RnA neurons have an uncharacterized but important function in response behavior. Unexpectedly, in all of these mutants, males that responded to contact were able to locate the hermaphrodite vulva with wild-type efficiency and mate successfully (data not shown). Since vulva-location behavior is mediated predominantly by the HOB neuron (Liu and Sternberg 1995), our data indicate either that HOB cilium function is robust to the loss of these tubulins or that the HOB cilium is not essential for its sensory function.
TABLE 2.
tbb-4, tba-6, and tba-9 are required for optimal response behavior
| Genotype | Response (%) | na |
|---|---|---|
| Wild typeb | 82.5 | 80 |
| pkd-2c | 54.2* | 96 |
| tba-6 | 72.9* | 96 |
| tba-9 | 60.0* | 85 |
| tbb-4 | 59.3* | 86 |
| tba-6; tbb-4 | 50.0* | 42 |
| tba-6; tba-9 | 54.8* | 42 |
| tba-9 tbb-4 | 35.0* | 40 |
| tba-6; pkd-2 | 65.4* | 107 |
| pkd-2; tba-9 | 26.4*,** | 91 |
| pkd-2; tbb-4 | 57.7* | 97 |
| tba-6; tba-9 tbb-4 | 44.7* | 76 |
| tba-6; pkd-2; tba-9 tbb-4 | 32.6* | 46 |
*P < 0.01 vs. him-5 (Fisher's exact test); **P < 0.0001 vs. tba-9 or pkd-2 (Fisher's exact test).
n is the number of observations (see materials and methods).
him-5(e1490) is in the background of all strains and is used as the wild-type control.
The pkd-2 null allele sy606 was used for these experiments.
The RnB neurons contribute to response behavior through a pathway involving a TRPP/polycystin channel composed of LOV-1 and PKD-2, the C. elegans orthologs of the mammalian polycystins PC-1 and PC-2 (Barr and Sternberg 1999; Barr et al. 2001). As some response behavior is still observed in lov-1, pkd-2, and lov-1; pkd-2 null mutants, a secondary signaling pathway is also likely to exist (Barr and Sternberg 1999; Barr et al. 2001; Peden and Barr 2005). To ask whether these pathways require ciliary tubulin function, we examined genetic interactions between polycystin and tubulin mutants (Table 2). We found that the loss of either of the two RnB-expressed tubulins, tba-6 and tbb-4, had no further effect on the response defects of pkd-2 null mutants. However, pkd-2; tba-9 double mutant males exhibited a response efficiency significantly lower than each single mutant. This again suggests that the RnA neurons are important for efficient response behavior and that a tba-9-dependent ciliary function of the RnA neuron is important for the secondary pathway. However, the loss of ciliary tubulins does not abolish the secondary pathway because tba-6; pkd-2; tba-9tbb-4 quadruple mutant males still retain some ability to respond to contact with hermaphrodites. Again, this supports the idea that the loss of ciliary tubulins compromises, but does not eliminate, sensory neuron function.
Other sensory behaviors are also disrupted in tubulin mutants:
Sensory cilia are also important for the nose-touch response, an aversive withdrawal reflex mediated in parallel by three neuron types, ASH, FLP, and OLQ (Kaplan and Horvitz 1993). Each of these three neuron types expresses TBA-9 and/or TBB-4. We found a significant reduction in the response of tba-9, tbb-4, and tba-9tbb-4 mutants to nose touch when compared to wild-type controls (Table 3). Thus the optimal function of these head mechanosensory cells depends on specific tubulins, possibly reflecting a need for specific cilium morphology and/or the localization of signaling components in these cells.
TABLE 3.
tba-9 and tbb-4 are required for optimal nose-touch behavior
| Genotype | Nose-touch response (%) |
|---|---|
| Wild typea | 64.4 |
| glr-1b | 18.8* |
| tba-9 | 43.3* |
| tbb-4 | 44.4* |
| tba-9 tbb-4 | 35.6* |
*P < 0.01 vs. him-5.
him-5(e1490) is in the background of all strains except glr-1 and is used as the wild-type control.
glr-1 mutants were assayed in parallel as a negative control.
Another well-known role of sensory function in C. elegans is in modulating locomotion via proprioceptive feedback and in response to mechanical and chemical food-derived cues. Loss of proprioceptive function in the neuron DVA changes the waveform of animals during sinusoidal locomotion (Li et al. 2006b). Additionally, the disruption of ciliary function in multiple head sensory neurons leads to a “dwelling” phenotype, in which mutants fail to explore a food source to the extent exhibited by wild-type animals (Fujiwara et al. 2002). Using an automated locomotion-tracking system (Cronin et al. 2005), we examined locomotion in tubulin mutants. We found no consistent abnormalities in wavelength, amplitude, frequency, or bend angle (flex) in tba-6 or tbb-4 mutants. However, tba-9 hermaphrodites and males displayed increases in body bend frequency and flex, a measurement of bending angle (data not shown). These properties of tba-9 mutants are readily observable on a standard plate as a “loopy-Unc” phenotype. These animals also exhibited a hyper-exploratory phenotype: in exploratory behavior assays, these animals consistently moved through more areas of the plate than wild-type controls (data not shown). Together, these results indicate that tba-9 has a structural role in the transduction of sensory stimuli that regulates C. elegans motor behavior. Although it is beyond the scope of the current work, identifying the cellular focus of tba-9 in modulating locomotion may provide significant insight into the neural control of behavior in C. elegans.
In contrast, we found that several other sensory functions were not detectably affected by the loss of ciliary tubulins. AWA, an amphid neuron pair that senses multiple attractive odorants (Bargmann et al. 1993), co-expresses tba-9 and tbb-4. However, we found that tba-9, tbb-4, and tba-9tbb-4 double mutants all responded robustly to the AWA-sensed odorant diacetyl (data not shown). We also examined the function of ASE, a bilaterally asymmetric amphid neuron pair that mediates salt chemosensation (Bargmann and Horvitz 1991; Thiele et al. 2009). Like AWA, ASE also expresses both tba-9 and tbb-4. Again, we observed no differences in the response of tbb-4, tba-9, or tba-9tbb-4 double mutants using a four-quadrant salt chemotaxis assay (Hart 2005) compared to wild-type controls (data not shown). Thus at least some AWA and ASE functions are not critically dependent on the expression of specific tubulin isotypes.
Ciliary tubulins regulate the trafficking of signaling molecules to cilia:
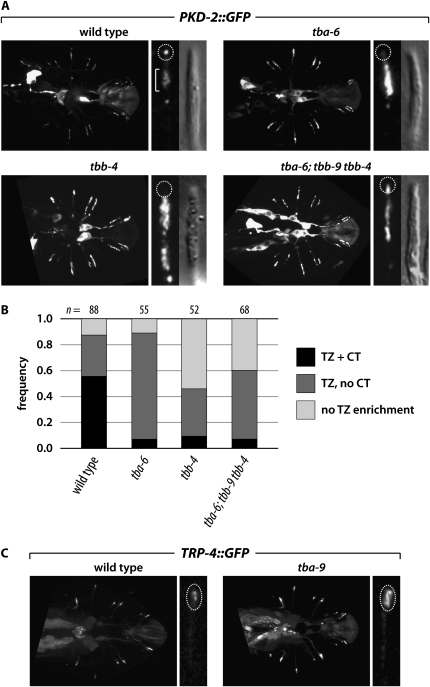
The sensory defects in tba-6, tba-9, and tbb-4 mutants indicate that these genes could play a role in generating and maintaining functional sensory cilia. To investigate this possibility, we asked whether ciliary tubulins are important for the localization of signaling molecules to cilia. As discussed above, localization of the TRPP channel PKD-2 to the distal tips of RnB neuron sensory cilia is necessary for efficient response behavior (Barr et al. 2001). We found that both TBA-6 and TBB-4, the two ciliary tubulins expressed in these cells, were important for this localization (Figure 6, A and B). In wild-type controls, 56% of rays (n = 88) had detectable PKD-2∷GFP in the RnB cilium tips. This frequently was significantly reduced in tubulin mutants to 9.6% in tbb-4 rays (n = 52) and 7.3% in tba-6 rays (n = 55). Interestingly, enrichment to the distal region of the dendrite (likely corresponding to the transition zone, or TZ) was still present in most tba-6 mutant rays (82%). However, tbb-4 mutants had a more severe phenotype: only 37% exhibited TZ enrichment, while 54% showed PKD-2∷GFP localization throughout the dendrite without specific TZ enrichment. Thus there seems to be a specific requirement for TBA-6 in trafficking PKD-2 to its presumptive site of action at the distal tip of the cilium, while TBB-4 likely has additional roles in trafficking PKD-2 along the dendrite. These results demonstrate that TBA-6 and TBB-4 are necessary to generate a functionally competent RnB cilium by enabling the appropriate localization of PKD-2.
Figure 6.—
Ciliary tubulins are required for normal localization of signaling components in cilia. (A) PKD-2∷GFP localization in the RnB neuron dendrites and cilia. (Left) For each genotype, the the entire set of rays is shown. (Right) GFP and DIC images of ray 4. The dashed circle indicates the location of the cilium tip (CT), while the bracket shows the location of the presumptive transition zone (TZ). In wild-type males, PKD-2∷GFP is enriched at the dendritic ends of the rays and exhibits prominent localization to the very tip of the ray neuron cilium. In tubulin mutants, PKD-2∷GFP localization to the CT is often absent, and PKD-2∷GFP often accumulates in the dendrite proximally to the TZ. (B) PKD-2∷GFP localization in individual rays was binned into three categories: “TZ + CT” (transition zone plus cilium tip), in which GFP was observed in a reasonably compact TZ region as well as a single discrete punctum at the end of the cilium; “TZ, no CT,” in which TZ localization was intact but CT localization was absent; and “no TZ enrichment,” in which the CT was generally absent and accumulation of PKD-2∷GFP was observed throughout the ray neuron dendrite rather than being specifically localized to the TZ region. The number of rays scored for each genotype is indicated above each bar. (C) TRP-4∷GFP localization in the RnA neuron cilia. In wild-type males, TRP-4∷GFP localizes to the cilia of the RnA neurons (dashed oval). tba-9 mutants often exhibit an excess accumulation of TRP-4∷GFP in ray sensory endings.
We also found that the amount of the TRP channel TRP-4 in the sensory endings of the RnA neurons depended on the RnA-expressed tubulin TBA-9. In wild-type males, TRP-4∷GFP accumulated in cilia; in mutants, ciliary localization was still present but TRP-4 existed in notably larger punctae (Figure 6C). Although a role for TRP-4 in RnA function has not been described, these data indicate that TBA-9 is also required for the generation of a sensory cilium that can support the appropriate localization and/or function of signaling components.
DISCUSSION
Sensory cilia possess remarkable structural and functional diversity, presumably enabling specialized responses to characteristic sensory stimuli. Although cilia play central roles in normal cellular function and in a variety of human diseases, the relationship between cytoskeletal architecture and cilium structure and function is not well understood. Here, we have identified three tubulin isoforms, TBA-6, TBA-9, and TBB-4, that localize to particular subsets of primary cilia in sensory neurons of the nematode C. elegans (Table 4). At the structural level, we have found that the loss of these tubulins, either alone or in combination, does not result in severe impairments in the formation or maintenance of cilia. However, both the localization of sensory components to cilia and their optimal function often require cilium-specific tubulins (Table 4). Taken together, our results indicate significant functional redundancy between tubulin paralogs in C. elegans. However, they also show that TBA-6, TBA-9, and TBB-4 possess some degree of functional specialization, optimizing processes important for the efficient transduction of environmental stimuli by sensory cilia. Given the conservation of ciliogenesis mechanisms from algae to mammals, these functions may be a general property of tubulin isoforms among a wide variety of species.
TABLE 4.
Characteristics of C. elegans ciliary tubulin mutants
| Tubulin gene | Anatomic expression | Tubulin localization defects in mutant | Cilium morphology defects in mutant | Cilium component localization defects in mutant | Signaling protein localization defects in mutant | Behavioral defects in mutant |
|---|---|---|---|---|---|---|
| tbb-4 | ADL, AFD, ASE, ASG, ASH, ASJ, ASK, ASI, AWA, AWB, AWC, CEP, OLQ, FLP, PDE, PQR, PHA, PHB, HSN, CEM, RnA, RnB, HOA(?), HOB(?), amphid glia, vulval muscle cells | Reduced accumulation of TBA-6 and TBA-9 in ray neuron cilia | CEM cilia disrupted, CEP cilia possibly abnormal | Subtle defects in OSM-6, KAP-1, and OSM-3 localization | PKD-2 mislocalized | Reduced male contact response, reduced nose-touch response |
| tba-6 | IL2, CEM, HSN, RnB, HOB | Reduced accumulation of TBB-4 in RnB cilia | CEM cilia disrupted | Subtle defects in OSM-6, KAP-1, and OSM-3 localization | PKD-2 mislocalized | Reduced male contact response |
| tba-9 | ADF, AFD, ASE, ASI, AWA, AWC, CEP, OLQ, ADE, PDE, RIG, PQR, RnA, amphid glia, HOA(?), DVA/B/C(?), ventral cord motor neurons, vulval muscle cells | No detectable mislocalization of TBB-4 in RnA cilia (possibly obscured by RnB cilia) | CEP cilia possibly abnormal | Subtle defects in OSM-6, KAP-1, and OSM-3 localization | TRP-4 mislocalized | Reduced male contact response, reduced nose-touch response, increased exploratory behavior, and locomotion defects |
Our studies do not allow us to determine whether TBA-6, TBA-9, and TBB-4 are the exclusive components of ciliary microtubules in wild-type animals. It is possible that TBA-6:TBB-4 or TBA-9:TBB-4 dimers contribute only to certain domains of the cilium, with the rest of the axoneme composed of more general “housekeeping” tubulins such as TBA-1/2 and TBB-1/2. It is also possible that the entire axoneme is built from a complex mixture of tubulin dimers using both cilium-specific and more general subunits. Either of these possibilities is consistent with our observations that the efficient localization of ciliary tubulins to sensory endings, particularly in the ray neurons, usually depends on the co-expressed ciliary tubulin partner. However, the fact that cilia can be built in the absence of these partners indicates that there are not absolute isoform requirements either for heterodimerization or for incorporation into a growing microtubule.
At the functional level, however, our studies clearly indicate that the sensory cilia generated in tba-6, tba-9, and tbb-4 mutants are abnormal. Several behaviors dependent on sensory input—male mating, nose touch, and basal locomotion—were compromised in tubulin mutants. We also found defects at the molecular level; the localization of the TRP channels TRP-4 and PKD-2 in the cilia of the RnA and RnB neurons, respectively, were disrupted in tba-6, tba-9, and tbb-4 mutants. Interestingly, these experiments revealed that the consequences of losing an α-tubulin (either tba-6 or tba-9) were often different from those of losing the β-tubulin tbb-4, indicating some cell-type specificity in the functions of ciliary microtubules. Together, these data show that the functional redundancy exhibited by tubulin isoforms in building the basic components of cilia does not extend to their more subtle functional properties and that α- and β-tubulins may play distinct roles in optimizing cilium specialization.
Cilia use kinesin- and dynein-based motors to move cargo to and from the distal end of the ciliary axoneme. Basal components of this intraflagellar transport process, including the kinesin OSM-3 (Snow et al. 2004), the kinesin accessory protein KAP-1 (Signor et al. 1999), and the IFT component OSM-6 (Collet et al. 1998), operate in all C. elegans sensory cilia. We found that the localization of these proteins was essentially undisturbed in ciliary tubulin mutants, consistent with the idea that TBA-6, TBA-9, and TBB-4 are more important for specialized cilium functions than for general aspects of ciliogenesis. In contrast, previous work in C. elegans has shown that cell type-specific factors, including the kinesin-like protein KLP-6, are important for the proper transport of signaling molecules in particular types of ciliated neurons (Peden and Barr 2005; Bae et al. 2006; Jauregui et al. 2008; Bae et al. 2009). Here, we find that some specialized cilia, especially those of the CEM neurons, are particularly dependent on tba-6 and tbb-4 function. Interestingly, klp-6 and tba-6 share an identical expression pattern that includes the IL2, CEM, HOB, and RnB neurons. Moreover, klp-6 is necessary for efficient male mating behavior and acts in both polycystin-dependent and -independent pathways (Peden and Barr 2005). One possibility is that KLP-6 is specialized to transport cargoes important for the function of these neurons, including the polycystin PKD-2. Indeed, in klp-6, tba-6, and tbb-4 mutants, PKD-2∷GFP fails to accumulate at the distal puncta of RnB cilia, with a resulting loss of sensory function (Peden and Barr 2005 and this study). Interestingly, we find that PKD-2∷GFP localization defects are more severe in tbb-4 mutants than in tba-6 mutants, consistent with previous work demonstrating that kinesins interact predominantly with β-tubulins (Marx et al. 2006). Together, these data support a model in which ciliary axonemes containing TBA-6 and TBB-4 provide an ideal substrate along which KLP-6 and other specialized motors transport cargoes necessary for unique cell functions.
Specialized ciliary microtubules could also play a more direct role in modulating sensory function. In particular, tubulins can play an important role in linking channel proteins with the cytoskeleton (Li et al. 2006a; Bounoutas et al. 2009). Many of the neurons in which we observed sensory defects are thought to sense mechanosensory stimuli, including the nose-touch neurons OLQ, ASH, and FLP (Kaplan and Horvitz 1993) and the RnB neurons (Barr and Sternberg 1999). One idea is that deformation of the cell (or ciliary) membrane might activate a stretch-sensitive ion channel. Specific ciliary tubulins could functionally or physically tether such ion channels to the axoneme, providing rigidity to the channel conformation and allowing for rapid and accurate responses to stimuli. A related possibility is that specific types of microtubules provide structural integrity to the cilium, rather than directly linking the cytoskeleton to membrane-embedded channels. Unfortunately, exploring these ideas more directly in C. elegans is limited by the small size of its neurons and the difficulty of mechanically stimulating and recording from these cells. Behavioral defects in tubulin mutants have also been reported in Drosophila, where a divergent β-tubulin is required for larval proprioception and photoreception (Dettman et al. 2001). However, the defects in this case arise not from abnormal sensory cilia but rather from defective development of non-neuronal support cells.
How might different tubulin isoforms confer different structural and functional properties on the cytoskeleton? One significant region of nonconservation among tubulin paralogues is the carboxyl-terminal tail. The C terminus is generally found either lying across or emanating from the surface of the tubulin heterodimer (Nogales et al. 1999; Gaertig and Wloga 2008). Further, C-terminal tails have been shown to be a site for post-translational modifications that regulate assembly of axonemes and/or have interactions with various microtubule-associated proteins, including motor proteins (Hammond et al. 2008; Bulinski 2009). Interestingly, C. elegans TBB-4 has a recognizable acidic axonemal motif at its carboxyl terminus (Raff et al. 1997), while the other five β-tubulins do not. This motif may help explain why TBB-4 is the only β-tubulin significantly enriched in cilia. Of the C. elegans α-tubulins, TBA-6 has the longest carboxyl terminus. While it is not homologous to an identified α-tubulin axonemal motif (Duan and Gorovsky 2002), TBA-6 possesses a long tail that contains several positively charged residues, a feature unique among tubulins (Baleanu-Gogonea and Siddiqui 2000). TBA-9 also has an extended C-terminal tail lacking a recognizable α-tubulin axoneme motif and instead has a serine and a histidine in positions that are acidic residues in other α-tubulins. Thus the requirements for TBA-6, TBA-9, and TBB-4 in optimizing the function of specific sensory neurons may stem from specific post-translational modifications and/or protein–protein interactions directed by unique sequence motifs at their C termini.
Together, our studies indicate that specific tubulin isotypes are important for optimizing the sensory functions of many primary cilia in C. elegans rather than for generating their basic architecture. Understanding how specialized microtubules facilitate and perhaps even direct the functional specialization of ciliary subtypes is a promising area for future investigation. Given the importance of primary cilia in the development and physiology of a wide variety of organisms, it seems likely that the use of specific tubulin isotypes to optimize cilium function may be a common biological principle.
Acknowledgments
We thank the members of our laboratory as well as Michel Leroux, Oliver Blacque, and Denise Ferkey for discussion and helpful suggestions. We are grateful to Stefani McGregor and Kwi Yeon Lee for excellent technical assistance. Some mutant strains were obtained from the Caenorhabditis Genetics Center, which is funded by the National Center for Research Resources. We thank Laurent Segalat for providing cxP4018, the C. elegans Gene Knockout Consortium for isolating ok1461 and ok1858, K. Nehrke for pCH1, and Piali Sengupta, Maureen Barr, X. Z. Shawn Xu, and Scott Emmons for strains. We thank Nikhil Bhatla for use of the Exon-Intron Graphic Maker (http://wormweb.org/exonintron) that was used to produce Figure 1. This work was supported by National Institutes of Health (NIH) grant R01 NS050268 to D.S.P. and NIH grant F32 DK075270 to R.M.M.
References
- Bae, Y., and M. Barr, 2008. Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans. Front. Biosci. 13 5959–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, Y., H. Qin, K. Knobel, J. Hu, J. Rosenbaum et al., 2006. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133 3859–3870. [DOI] [PubMed] [Google Scholar]
- Bae, Y. K., E. Kim, S. W. L'Hernault and M. M. Barr, 2009. The CIL-1 PI 5-phosphatase localizes TRP polycystins to cilia and activates sperm in C. elegans. Curr. Biol. 19 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baleanu-Gogonea, C., and S. S. Siddiqui, 2000. Molecular cloning and three-dimensional structure prediction of a novel alpha-tubulin in Caenorhabditis elegans. J. Cell. Mol. Med. 4 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran, R., L. Castelblanco, G. Tang, I. Shapiro, A. Goncharov et al., 2010. Motor neuron synapse and axon defects in a C. elegans alpha-tubulin mutant. PLoS One 5 e9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann, C. I., and H. R. Horvitz, 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7 729–742. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., E. Hartwieg and H. R. Horvitz, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74 515–527. [DOI] [PubMed] [Google Scholar]
- Barr, M. M., and P. W. Sternberg, 1999. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401 386–389. [DOI] [PubMed] [Google Scholar]
- Barr, M. M., J. DeModena, D. Braun, C. Q. Nguyen, D. H. Hall et al., 2001. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr. Biol. 11 1341–1346. [DOI] [PubMed] [Google Scholar]
- Berbari, N. F., A. K. O'Connor, C. J. Haycraft and B. K. Yoder, 2009. The primary cilium as a complex signaling center. Curr. Biol. 19 R526–R535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque, O. E., E. A. Perens, K. A. Boroevich, P. N. Inglis, C. Li et al., 2005. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 15 935–941. [DOI] [PubMed] [Google Scholar]
- Blacque, O. E., S. Cevik and O. I. Kaplan, 2008. Intraflagellar transport: from molecular characterisation to mechanism. Front. Biosci. 13 2633–2652. [DOI] [PubMed] [Google Scholar]
- Boulin, T., J. F. Etchberger and O. Hobert, 2006. Reporter gene fusions (April 5, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.106.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Bounoutas, A., R. O'Hagan and M. Chalfie, 2009. The multipurpose 15-protofilament microtubules in C. elegans have specific roles in mechanosensation. Curr. Biol. 19 1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski, J. C., 2009. Tubulin posttranslational modifications: a Pushmi-Pullyu at work? Dev. Cell 16 773–774. [DOI] [PubMed] [Google Scholar]
- Burket, C. T., C. E. Higgins, L. C. Hull, P. M. Berninsone and E. F. Ryder, 2006. The C. elegans gene dig-1 encodes a giant member of the immunoglobulin superfamily that promotes fasciculation of neuronal processes. Dev. Biol. 299 193–205. [DOI] [PubMed] [Google Scholar]
- Chen, N., A. Mah, O. E. Blacque, J. Chu, K. Phgora et al., 2006. Identification of ciliary and ciliopathy genes in Caenorhabditis elegans through comparative genomics. Genome Biol. 7 R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, K. L., D. H. Hall and S. W. Emmons, 1995. The mab-21 gene of Caenorhabditis elegans encodes a novel protein required for choice of alternate cell fates. Development 121 3615–3626. [DOI] [PubMed] [Google Scholar]
- Cleveland, D. W., 1987. The multitubulin hypothesis revisited: What have we learned? J. Cell Biol. 104 381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet, J., C. A. Spike, E. A. Lundquist, J. E. Shaw and R. K. Herman, 1998. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics 148 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin, C., J. Mendel, S. Mukhtar, Y. Kim, R. Stirbl et al., 2005. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 6 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport, J. R., and B. K. Yoder, 2005. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am. J. Physiol. Renal Physiol. 289 F1159–F1169. [DOI] [PubMed] [Google Scholar]
- Dettman, R. W., F. R. Turner, H. D. Hoyle and E. C. Raff, 2001. Embryonic expression of the divergent Drosophila β3-tubulin isoform is required for larval behavior. Genetics 158 253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll, M., E. Dean, E. Reilly, E. Bergholz and M. Chalfie, 1989. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J. Cell Biol. 109 2993–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, J., and M. A. Gorovsky, 2002. Both carboxy-terminal tails of alpha- and beta-tubulin are essential, but either one will suffice. Curr. Biol. 12 313–316. [DOI] [PubMed] [Google Scholar]
- Ellis, G. C., J. B. Phillips, S. O'Rourke, R. Lyczak and B. Bowerman, 2004. Maternally expressed and partially redundant beta-tubulins in Caenorhabditis elegans are autoregulated. J. Cell Sci. 117 457–464. [DOI] [PubMed] [Google Scholar]
- Evans, J. E., J. J. Snow, A. L. Gunnarson, G. Ou, H. Stahlberg et al., 2006. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 172 663–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara, M., P. Sengupta and S. L. McIntire, 2002. Regulation of body size and behavioral state of C. elegans by sensory perception and the EGL-4 cGMP-dependent protein kinase. Neuron 36 1091–1102. [DOI] [PubMed] [Google Scholar]
- Fukushige, T., Z. K. Siddiqui, M. Chou, J. G. Culotti, C. B. Gogonea et al., 1999. MEC-12, an alpha-tubulin required for touch sensitivity in C. elegans. J. Cell Sci. 112 395–403. [DOI] [PubMed] [Google Scholar]
- Fuller, M. T., J. H. Caulton, J. A. Hutchens, T. C. Kaufman and E. C. Raff, 1987. Genetic analysis of microtubule structure: a beta-tubulin mutation causes the formation of aberrant microtubules in vivo and in vitro. J. Cell Biol. 104 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig, J., and D. Wloga, 2008. Ciliary tubulin and its post-translational modifications. Curr. Top. Dev. Biol. 85 83–113. [DOI] [PubMed] [Google Scholar]
- Goetz, S. C., and K. V. Anderson, 2010. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 11 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogonea, C. B., V. Gogonea, Y. M. Ali, K. M. Merz, Jr., and S. S. Siddiqui, 1999. Computational prediction of the three-dimensional structures for the Caenorhabditis elegans tubulin family. J. Mol. Graph. Model. 17 90–100, 126–130. [DOI] [PubMed] [Google Scholar]
- Hammond, J. W., D. Cai and K. J. Verhey, 2008. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart, A. (Editor), 2005. Behavior (July 3, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.87.1, http://www.wormbook.org.
- Hays, T. S., R. Deuring, B. Robertson, M. Prout and M. T. Fuller, 1989. Interacting proteins identified by genetic interactions: a missense mutation in alpha-tubulin fails to complement alleles of the testis-specific beta-tubulin gene of Drosophila melanogaster. Mol. Cell. Biol. 9 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens, J. A., H. D. Hoyle, F. R. Turner and E. C. Raff, 1997. Structurally similar Drosophila alpha-tubulins are functionally distinct in vivo. Mol. Biol. Cell 8 481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglin, X. H., K. Poirier, Y. Saillour, E. Buhler, G. Tian et al., 2009. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat. Genet. 41 746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui, A. R., K. C. Nguyen, D. H. Hall and M. M. Barr, 2008. The Caenorhabditis elegans nephrocystins act as global modifiers of cilium structure. J. Cell Biol. 180 973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, H. C., and D. W. Cleveland, 1989. Differential utilization of beta-tubulin isotypes in differentiating neurites. J. Cell Biol. 109 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, J. M., and H. R. Horvitz, 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues, K. J., T. C. Kaufman, R. A. Raff and E. C. Raff, 1982. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell 31 655–670. [DOI] [PubMed] [Google Scholar]
- Kunitomo, H., H. Uesugi, Y. Kohara and Y. Iino, 2005. Identification of ciliated sensory neuron-expressed genes in Caenorhabditis elegans using targeted pull-down of poly(A) tails. Genome Biol. 6 R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, M. A., and J. G. Gleeson, 2009. The primary cilium as a cellular signaling center: lessons from disease. Curr. Opin. Genet. Dev. 19 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q., N. Montalbetti, Y. Wu, A. Ramos, M. K. Raychowdhury et al., 2006. a Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J. Biol. Chem. 281 37566–37575. [DOI] [PubMed] [Google Scholar]
- Li, W., Z. Feng, P. Sternberg and X. Xu, 2006. b A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature 440 684–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lints, R., L. Jia, K. Kim, C. Li and S. W. Emmons, 2004. Axial patterning of C. elegans male sensilla identities by selector genes. Dev. Biol. 269 137–151. [DOI] [PubMed] [Google Scholar]
- Liu, K. S., and P. W. Sternberg, 1995. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14 79–89. [DOI] [PubMed] [Google Scholar]
- Lu, C., and P. E. Mains, 2005. Mutations of a redundant alpha-tubulin gene affect Caenorhabditis elegans early embryonic cleavage via MEI-1/katanin-dependent and -independent pathways. Genetics 170 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C., M. Srayko and P. E. Mains, 2004. The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol. Biol. Cell 15 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, A., J. Muller, E. M. Mandelkow, A. Hoenger and E. Mandelkow, 2006. Interaction of kinesin motors, microtubules, and MAPs. J. Muscle Res. Cell Motil. 27 125–137. [DOI] [PubMed] [Google Scholar]
- Miller, R. M., and D. S. Portman, 2010. A latent capacity of the C. elegans polycystins to disrupt sensory transduction is repressed by the single-pass ciliary membrane protein CWP-5. Dis. Model. Mech. 3 (in press). [DOI] [PMC free article] [PubMed]
- Mukhopadhyay, S., Y. Lu, H. Qin, A. Lanjuin, S. Shaham et al., 2007. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 26 2966–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay, S., Y. Lu, S. Shaham and P. Sengupta, 2008. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev. Cell 14 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogales, E., M. Whittaker, R. A. Milligan and K. H. Downing, 1999. High-resolution model of the microtubule. Cell 96 79–88. [DOI] [PubMed] [Google Scholar]
- Orozco, J. T., K. P. Wedaman, D. Signor, H. Brown, L. Rose et al., 1999. Movement of motor and cargo along cilia. Nature 398 674. [DOI] [PubMed] [Google Scholar]
- Peden, E., and M. Barr, 2005. The KLP-6 kinesin is required for male mating behaviors and polycystin localization in Caenorhabditis elegans. Curr. Biol. 15 394–404. [DOI] [PubMed] [Google Scholar]
- Pedersen, L. B., and J. L. Rosenbaum, 2008. Intraflagellar transport (IFT): role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85 23–61. [DOI] [PubMed] [Google Scholar]
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson and J. G. Culotti, 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117 456–487. [DOI] [PubMed] [Google Scholar]
- Phillips, J. B., R. Lyczak, G. C. Ellis and B. Bowerman, 2004. Roles for two partially redundant alpha-tubulins during mitosis in early Caenorhabditis elegans embryos. Cell Motil. Cytoskeleton 58 112–126. [DOI] [PubMed] [Google Scholar]
- Popodi, E. M., H. D. Hoyle, F. R. Turner, K. Xu, S. Kruse et al., 2008. Axoneme specialization embedded in a “generalist” beta-tubulin. Cell Motil. Cytoskeleton 65 216–237. [DOI] [PubMed] [Google Scholar]
- Portman, D. S., and S. W. Emmons, 2004. Identification of C. elegans sensory ray genes using whole-genome expression profiling. Dev. Biol. 270 499–512. [DOI] [PubMed] [Google Scholar]
- Raff, E. C., J. D. Fackenthal, J. A. Hutchens, H. D. Hoyle and F. R. Turner, 1997. Microtubule architecture specified by a beta-tubulin isoform. Science 275 70–73. [DOI] [PubMed] [Google Scholar]
- Savage, C., M. Hamelin, J. G. Culotti, A. Coulson, D. G. Albertson et al., 1989. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3 870–881. [DOI] [PubMed] [Google Scholar]
- Signor, D., K. P. Wedaman, J. T. Orozco, N. D. Dwyer, C. I. Bargmann et al., 1999. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, M. A., and M. R. Leroux, 2009. Intraflagellar transport and the generation of dynamic, structurally and functionally diverse cilia. Trends Cell Biol. 19 306–316. [DOI] [PubMed] [Google Scholar]
- Snow, J., G. Ou, A. Gunnarson, M. Walker, H. Zhou et al., 2004. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nat. Cell Biol. 6 1109–1113. [DOI] [PubMed] [Google Scholar]
- Starich, T. A., R. K. Herman, C. K. Kari, W. H. Yeh, W. S. Schackwitz et al., 1995. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston, J. E., D. G. Albertson and J. N. Thomson, 1980. The Caenorhabditis elegans male: postembryonic development of nongonadal structures. Dev. Biol. 78 542–576. [DOI] [PubMed] [Google Scholar]
- Swoboda, P., H. T. Adler and J. H. Thomas, 2000. The RFX-type transcription factor DAF-19 regulates sensory neuron cilium formation in C. elegans. Mol. Cell 5 411–421. [DOI] [PubMed] [Google Scholar]
- Thiele, T. R., S. Faumont and S. R. Lockery, 2009. The neural network for chemotaxis to tastants in Caenorhabditis elegans is specialized for temporal differentiation. J. Neurosci. 29 11904–11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vent, J., T. A. Wyatt, D. D. Smith, A. Banerjee, R. F. Luduena et al., 2005. Direct involvement of the isotype-specific C-terminus of beta tubulin in ciliary beating. J. Cell Sci. 118 4333–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, R. H., 2007. Microtubules: an overview. Methods Mol. Med. 137 1–16. [DOI] [PubMed] [Google Scholar]
- Ward, S., N. Thomson, J. White and S. Brenner, 1975. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 160 313–337. [DOI] [PubMed] [Google Scholar]
- Williams, C. L., M. E. Winkelbauer, J. C. Schafer, E. J. Michaud and B. K. Yoder, 2008. Functional redundancy of the B9 proteins and nephrocystins in Caenorhabditis elegans ciliogenesis. Mol. Biol. Cell 19 2154–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H., R. F. Pretot, T. R. Burglin and P. W. Sternberg, 2003. Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development 130 5217–5227. [DOI] [PubMed] [Google Scholar]