Abstract
Cyclin D1 is a cell cycle control protein that plays an important role in regenerating liver and many types of cancer. Previous reports have shown that cyclin D1 can directly enhance estrogen receptor activity and inhibit androgen receptor activity in a ligand-independent manner and thus may play an important role in hormone-responsive malignancies. In this study, we examine a distinct mechanism by which cyclin D1 regulates sex steroid signaling, via altered metabolism of these hormones at the tissue and cellular level. In male mouse liver, ectopic expression of cyclin D1 regulated genes involved in the synthesis and degradation of sex steroid hormones in a pattern that would predict increased estrogen and decreased androgen levels. Indeed, hepatic expression of cyclin D1 led to increased serum estradiol levels, increased estrogen-responsive gene expression, and decreased androgen-responsive gene expression. Cyclin D1 also regulated the activity of several key enzymatic reactions in the liver, including increased oxidation of testosterone to androstenedione and decreased conversion of estradiol to estrone. Similar findings were seen in the setting of physiological cyclin D1 expression in regenerating liver. Knockdown of cyclin D1 in HuH7 cells produced reciprocal changes in steroid metabolism genes compared with cyclin D1 overexpression in mouse liver. In conclusion, these studies establish a novel link between the cell cycle machinery and sex steroid metabolism and provide a distinct mechanism by which cyclin D1 may regulate hormone signaling. Furthermore, these results suggest that increased cyclin D1 expression, which occurs in liver regeneration and liver diseases, may contribute to the feminization seen in these settings.
Keywords: cyclin D1b, liver regeneration, steroid 5-α reductase, 17β-hydroxysteroid dehydrogenase, 3β-hydroxysteroid dehydrogenase
in many circumstances, the actions of sex steroids are determined by their local synthesis and inactivation rather than the circulating levels of these hormones (29). Biologically active hormones are produced from circulating precursors such as dehydroepiandrosterone (DHEA) and act in a paracrine or intracrine manner. In addition, the level of these compounds is controlled by inactivating enzymes that often act within the tissue microenvironment (29). Although the enzymatic pathways that control sex steroid hormone production and inactivation have been extensively studied, relatively little is known about their regulation at the tissue level. This is an issue of some importance given the significant role that estrogens and androgens play in diverse processes including cell proliferation, differentiation, metabolism, and cancer. A better understanding of how key enzymes are regulated at the cellular and tissue level may provide insight into new therapeutic targets for diseases such as cancer.
Sex steroids potently promote the proliferation of some types of cells. For example, activation of the androgen receptor (AR) and estrogen receptor α (ERα) in prostate and breast epithelial cells, respectively, triggers the cell cycle machinery, consisting of cyclin/cyclin-dependent kinase (cdk) complexes (3, 13). A key target of both of these receptors is cyclin D1, which is induced by mitogens during G1 phase and plays a pivotal role in cell cycle progression. In many types of cancers, aberrant expression of cyclin D1 contributes to uncontrolled proliferation and other effects that underlie the malignant phenotype (20, 25). In androgen-responsive prostate cancer cells and estrogen-responsive breast cancer cells, blockade of their respective receptors leads to decreased cyclin D1 expression and diminished proliferation (3, 13).
Interestingly, cyclin D1 appears to enhance ERα activity and inhibit AR activity, thereby producing potential positive and negative feedback signals to these respective receptors. This may occur through several potential mechanisms, including direct binding to the receptors, inactivation of the retinoblastoma protein (Rb), and regulation of transcriptional coregulators (3, 10, 20). However, the existing literature contains some conflicting information regarding the underlying mechanisms, and it is possible that cyclin D1 regulates the activity of these receptors via additional pathways.
The liver plays an important role in the biotransformation of steroid hormones. Men with advanced liver diseases can develop a feminization syndrome with increased estradiol (E2) and decreased androgen levels, and similar effects are seen acutely after major liver resection (17, 18, 41). However, the mechanisms that modulate hepatic sex steroid metabolism under these circumstances have not been established. In addition to regulating sex steroid hormones, hepatocytes express ERα and AR and respond to estrogens and androgens (21). For example, E2 has both proliferative and metabolic effects on hepatocytes in culture and in vivo (4, 19, 22). E2 has been shown to promote proliferation of male primary hepatocytes and hepatocellular carcinoma (HCC) cells in culture (4, 19). Studies have also suggested that increased estrogen levels may correlate with an increased risk of HCC in men and postmenopausal women (8, 40). Although increased serum androgen levels have also been linked to HCC development, recent studies indicate that altered metabolism in liver diseases and HCC can lead to conversion of androgens to estrogens with a net “estrogenic” effect at the cellular and tissue level (7, 23). Notably, HCC almost always occurs in cirrhotic livers, which frequently display ongoing hepatocyte proliferation and increased cyclin D1 expression (2, 32).
Although they rarely divide in normal adult liver, differentiated hepatocytes readily enter the cell cycle in response to liver injuries that diminish functional hepatic mass (15). Previous studies have shown that cyclin D1 plays an important role in hepatocyte proliferation and is sufficient to promote cell cycle progression in the absence of other stimuli (1, 34). As part of an effort to define novel effects of this protein in the liver (33), we have examined the regulation of enzymes that control the synthesis and inactivation of sex steroid hormones by cyclin D1. Our results indicate that cyclin D1 regulates key enzymes involved in the metabolism of these compounds in a manner that favors increased estrogen and decreased androgen accumulation in the liver and in HCC cells. These studies suggest that cyclin D1 significantly modulates sex steroid metabolism at the cellular and tissue level and thus provides a potential mechanism for the feminization syndrome seen in males with advanced liver disease or major liver resection. In addition, local regulation of hormone ligand levels by cyclin D1 may help to explain its effects on ERα and AR activity. These findings offer further insight into the complex relationship between sex steroids, cyclin D1, and cell cycle control in liver diseases and cancer.
EXPERIMENTAL PROCEDURES
Adenoviruses.
Adenoviruses encoding cyclin D1, cyclin D2, cyclin D3, and β-galactosidase were prepared as previously described (33). Adenoviruses encoding human cyclin D1b and cyclin D1-KE were constructed by Vector Biolabs (Philadelphia, PA) from plasmids kindly provided by Erik S. Knudsen (Thomas Jefferson University, Philadelphia, PA) and Philip W. Hinds (Tufts-New England Medical Center, Boston, MA).
Animal procedures.
All animal studies were approved by the Institutional Animal Care and Use Committee and were completed following National Institutes of Health guidelines. Eight-week-old male BALB/c (Harlan Sprague Dawley) mice were injected with 7–9 × 109 plaque-forming units via tail vein injection of recombinant adenovirus as previously described (34), and livers were harvested after 24 h. Letrozole (Novartis) was suspended in ethanol and administered at a concentration of 20 μg/animal at 24 and 2 h prior to harvest as indicated. Ethanol was injected in other animals as a vehicle control. A 70% partial hepatectomy (PH) followed by liver harvest at 42 h was performed as previously described (34).
Western blots.
Protein isolated from liver tissue was used for Western blot analysis as described previously (1, 34). Antibodies used for Western blot include cyclin D1 (UBI, Temecula, CA), cdk1 (Santa Cruz Biotechnology), and actin (Sigma). Cyclin D1b antibody was kindly provided by Erik S. Knudsen.
BrdU labeling.
Liver samples were fixed in 10% formalin, paraffinized, and sectioned. Samples were stained using the Mouse on Mouse IgG (Vector Laboratories) and the anti- bromodeoxyuridine (BrdU) primary antibody (GE Healthcare). Peroxidase was activated by using the DAB kit (Vector Laboratories) and counterstain was performed with hematoxylin (Sigma). Positive nuclei were counted per 100 hepatocytes.
Quantification of mRNA by real-time RT-PCR.
Total RNA from each liver was isolated and quantified as previously described (35). RNA (5 μg) was treated with DNase I (DNA-free, Ambion) according to the manufacturer's instructions. cDNA was synthesized with a TaqMan reverse transcriptase reagent kit (Applied Biosystems) primed with oligo(dT). Primers specific for genes of interest were previously published or designed using Primer Depot (primerdepot.nci.nih.gov) and purchased from Integrated DNA Technologies. Sequences and references are provided in Supplementary Table S1. Real-time PCR was performed using the Light Cycler DNA Master SYBR Green I kit (Roche Applied Sciences). Primers were used at a concentration of 0.5 μM and MgCl2 at 2.4 mM. Samples were denatured for 10 min at 95°C and then 40 cycles of 95°C for 20 s, 60°C for 20 s, and 72°C for 20 s. Results were normalized to GAPDH. The RT-PCR data for each figure represent three to four samples, and a separate experiment using different specimens provided similar results (data not shown).
Analysis of serum steroid levels.
Blood samples were taken and serum was prepared and frozen at −80°C until assayed. Serum E2 was measured according to the manufacturer's instructions using an enzyme immunoassay kit from Cayman Chemical (Ann Arbor, MI). Prior to measurement, serum was extracted with ether and reconstituted in EIA buffer according to the manufacturer's directions.
Cell culture.
HuH7 cells were purchased from Riken BioResource Center (Japan) and routinely cultured in DMEM with 10% FBS. For experiments, cells were seeded at a density of 400,000 cells per well in six-well plates with 3 ml of phenol red-free MEM with 10% charcoal-stripped FBS per well. Cells were cultured in the presence or absence of DHEA or E2 as indicated in the figures.
Cyclin D1 siRNA.
Small interfering RNA (siRNA) transfer into HuH7 cells was administered using DharmaFECT4 (Thermo Scientific) according to manufacturer's instructions. Briefly, cells were plated as described above and incubated overnight. The following day, cells were treated with a 400-μl reaction mixture containing 1 nM control siRNA [NonTargeting pool cat. no. D-001810-10-20 (Thermo Scientific Dharmacon)] or 1 nM cyclin D1 siRNA [On-Target plus Smart Pool cat. no. L-003210-00]. After an overnight incubation, fresh medium was added and the cells were cultured overnight with DHEA, E2, or vehicle control. Two days after siRNA treatment, protein lysates were prepared or total RNA was isolated as described above.
Enzyme assays used to measure conversion of testosterone and DHEA to androstenedione.
Flash-frozen livers were homogenized in a Dounce homogenizer at a concentration of 100 mg/ml in an ice-cold buffer containing 20% (vol/vol) glycerol, 10 mM 2-mercaptoethanol and 40 mM potassium phosphate, pH 7.0, and centrifuged at 1,000 g for 10 min. The supernatants were stored at 4°C and used within 5 h. Assays were optimized to be in the linear range of product formation and performed as described previously (5). For 3β-HSD assays using DHEA as substrate (Fig. 3A), a 10-μl aliquot of lysate was incubated with 10-μl reaction mixture containing 1.0 mM NAD+ and 2.0 μM 3H-DHEA ([1,2,6,7-3H]dehydroepiandrosterone, 100 Ci/mmol, from PerkinElmer Life Sciences, Boston, MA) in 0.08 M bicine, pH 9.0, and incubated at 37°C for 30 min. Oxidation of testosterone to androstenedione (17β-HSDox enzyme activity) (Fig. 3B) was assayed by using liver lysates diluted 1:10. Lysates (10 μl) were combined with 10-μl reaction mixture containing 1.0 mM NAD+ and 2.0 μM 3H-testosterone ([1,2,6,7-3H]testosterone, 95.0 Ci/mmol from Amersham Pharmacia Biotech, Arlington Heights, IL) in 0.08 M bicine, pH 9.0, and incubated at 37°C for 6 min. The reaction products were analyzed as previously described (5). The percent conversion of substrate was calculated from [cpm product/cpm product + substrate] × 100 and converted to picomoles per milligram tissue per 6 or 30 min as shown in Figs. 3, A and B. The data are presented as the mean of two or three livers assayed in duplicate for each condition.
Fig. 3.
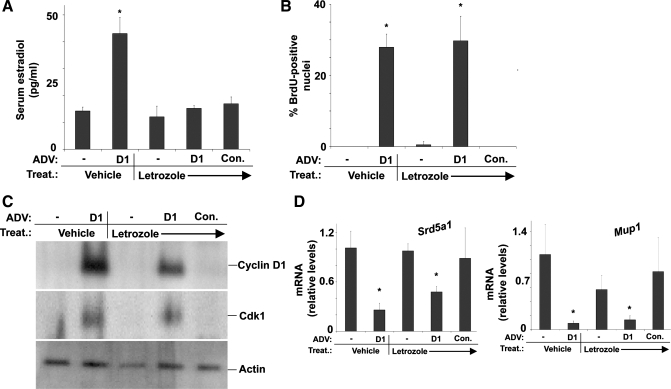
The aromatase inhibitor letrozole prevents induction of E2 levels but does not affect cell cycle progression or expression of SRD5A1 and MUP1 in response to cyclin D1. Mice were treated (Treat.) with letrozole as outlined in experimental procedures and transfected with the cyclin D1 or control adenoviruses as in Fig. 1. A: serum E2 levels. B: hepatocyte DNA synthesis as assessed by BrdU uptake. C: Western blot of cyclin D1 and cdk1. D: RT-PCR for SRD5A1 and MUP1.
Enzyme assays used to measure aromatase, steroid sulfatase, and 17β-HSD activities.
Flash-frozen livers were homogenized in a Dounce homogenizer in RIPA buffer (50 mM Tris·HCl, pH 7.4 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA). Nondissolved cell remnants were removed through centrifugation at 15,000 g and the supernatant was stored at −80°C. Protein concentration was determined by using the BC Assay Protein Quantitation Kit (Uptima, Interchim). A recently developed HPLC method was used to measure the amounts of estrone (E1) or E2 in the reaction mixtures (11, 12). Aromatase activity (Fig. 3C) was assayed by using 50–75 μg protein lysate incubated with 1 mM NADPH and 9 nmol androstenedione in 100 mM potassium phosphate buffer (pH 7.4) for 24 h at 37°C in a 500-μl reaction. The conversion of E2 to E1 (17β-HSD oxidative activity) (Fig. 3D) was carried out as described (11, 12). Fifty to 75 μg lysate was incubated with a 500-μl reaction mixture containing 5 mM NADP+ and 10 nmol E2 in 100 mM potassium phosphate buffer (pH 7.4) for 24 h. The conversion of E1 to E2 (17β-HSD reductive activity) (Fig. 3E) was assayed using 50–75 μg liver lysate combined with 500-μl reaction mixture containing 2.6 mM NADP+, 3.3 mM glucose-6-phosphate, 1 U glucose-6-phosphate dehydrogenase, 3.3 mM magnesium chloride, and 10 nmol E1 in 100 mM potassium phosphate buffer (pH 7.4) for 24 h. Steroid sulfatase (STS) activity was measured as E1 produced from E1–1-sulfate (Fig. 3F), as previously described (12). In brief, a 750-μl reaction mixture containing 4 mM NADP+, 25 mM sucrose, and 10 nmol E1–3-sulfate in PBS (pH 7.4) was incubated with 50–75 μg lysate for 24 h. For Fig. 3, C–F, three to four livers were assayed for each condition.
Statistics.
Statistical significance was defined as P < 0.05 by the Student's t-test. In each study, three to five independent mice or cell culture replicates were used.
RESULTS
Cyclin D1 regulates the expression of key sex steroid metabolism genes in the liver.
Cyclin D1 is not expressed in normal male adult liver but is induced in proliferating hepatocytes following 70% PH or other liver injury (15). Previous studies have shown that transient transduction of the liver with an adenoviral vector expressing cyclin D1 leads to robust hepatocyte proliferation within 24 h and marked liver growth (1, 34). Intravenously injected recombinant adenoviruses primarily target hepatocytes, and this technique has been used extensively to study the effect of single-gene expression in the liver. We have recently shown that at 1 day after transfection, cyclin D1 regulates genes involved in diverse pathways in the livers of male mice (33). Further evaluation of these results unexpectedly revealed that cyclin D1 regulated a number of transcripts involved in androgen and estrogen metabolism. Table 1 shows selected genes involved in the metabolism of sex steroid hormones that were modulated by cyclin D1 in the liver on the microarray (33). In this analysis, cyclin D1 regulated the expression of several key genes involved in steroid hormone biosynthesis, including GSTA3, HSD3B2, HSD17B6, HSD17B10, SRD5A1, StARD4, StARD5, and STS, and enzymes that catalyze the inactivation of steroid hormones, such as HSD3B5, AKR1C19, CYP1A2, UGT2B1, and UGT2B38. These findings suggest that cyclin D1 may modulate the level of sex steroid hormone ligands at the tissue level.
Table 1.
Cyclin D1 regulates genes involved in estrogen and androgen metabolism
| Gene Symbol | Gene Name | Fold Change |
|---|---|---|
| Akr1c19 | aldo-keto reductase family 1, member C19 | −14.49 |
| Arsg | arylsulfatase G | 4.83 |
| Cyp1a2 | cytochrome P450, family 1, subfamily a, polypeptide 2 | −3.07 |
| Gsta3 | glutathione S-transferase 3 | −10.05 |
| Hsd3b2 | hydroxy-δ-5-steroid dehydrogenase, 3β- and steroid δ-isomerase 2 | −16.96 |
| Hsd3b5 | hydroxy-δ-5-steroid dehydrogenase, 3β- and steroid δ-isomerase | −71.91 |
| Hsd17b6 | hydroxysteroid (17β) dehydrogenase 6 | −18.04 |
| Hsd17b10 | hydroxysteroid (17β) dehydrogenase 10 | −3.32 |
| Rdh16 | Retinol dehydrogenase 16 | −17.94 |
| Srd5a1 | steroid 5 α-reductase 1 | −19.79 |
| Srd5a2l | steroid 5 α-reductase 2-like | 2.04 |
| Stard4 | StAR-related lipid transfer (START) domain containing 4 | −5.46 |
| Stard5 | StAR-related lipid transfer (START) domain containing 5 | −3.19 |
| Sts | Steroid sulfatase | 2 |
| Ugt2b1 | UDP glucuronosyltransferase 2 family | −4.12 |
| Ugt2b38 | UDP glucuronosyltransferase 2 family, polypeptide B38 | −2.33 |
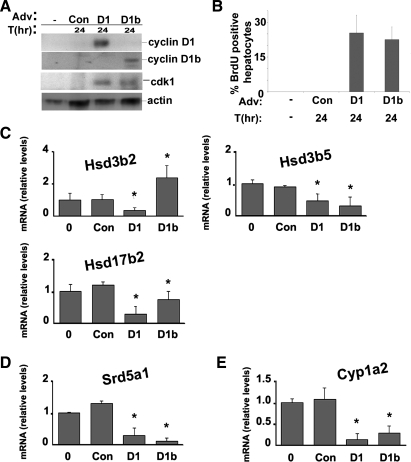
To confirm these effects, RT-PCR was performed for key sex steroid metabolism genes by using RNA isolated from male mouse livers harvested 1 day after cyclin D1 or control transfection (Fig. 1). We also examined the effect of cyclin D1b, a common polymorphism of cyclin D1, on the expression of these transcripts. Cyclin D1b is a splice variant that possesses a distinct COOH terminus and is associated with an increased risk of some cancers (28). Recent studies have suggested that cyclin D1b may regulate ERα and AR activity differently than cyclin D1 (6, 42). Expression of the cyclin D1 variants was confirmed by Western blot using COOH-terminal antibodies that specifically recognize each (Fig. 1A). Transfection of cyclin D1b induced hepatocyte proliferation (as measured by DNA synthesis) and expression of cell cycle proteins (such as cdk1) in a manner similar to cyclin D1 (34). As is shown in Fig. 1B, cyclins D1 and D1b regulated the expression of several hydroxysteroid dehydrogenase (HSD) genes, which catalyze the conversion of androgens and estrogens. Specifically, both downregulated the expression of 3β-hydroxysteroid dehydrogenase type 5 (HSD3B5) and 17β-hydroxysteroid dehydrogenase type 2 (HSD17B2). In contrast, cyclin D1 inhibited expression of the 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) mRNA, whereas cyclin D1b induced this transcript.
Fig. 1.
Cyclin D1 and D1b regulate the expression of transcripts encoding key enzymes involved in androgen and estrogen metabolism in the liver. A: Western blot analysis for cyclin D1, cyclin D1b, cdk1, and actin using liver lysates from normal male mice or from livers 1 day after transfection with the indicated adenoviruses. T(hr), hours; Adv, Adenovirus. B: DNA synthesis as estimated by bromodeoxyuridine (BrdU) immunohistochemistry of hepatocyte nuclei (expressed as BrdU-positive nuclei per 100 hepatocytes). Con, control. C: RT-PCR for selected hydroxysteroid dehydrogenase (HSD) genes using RNA isolated from untreated normal or transfected livers. D: RT-PCR for SRD5A1. E: RT-PCR for CYP1A2. RT-PCR data were normalized to Gapdh and expressed as relative change compared with untreated livers. Results are means ± SE of at least 3 independent mouse livers performed in triplicate. (*P < 0.05 in each figure).
Cyclins D1 and D1b each inhibited expression of the mRNA encoding steroid 5 α-reductase 1 (SRD5A1), which catalyzes the conversion of testosterone to the more potent androgen dihydrotestosterone (DHT) (Fig. 1C) (38). CYP1A2, which metabolically inactivates estrogens, was inhibited by both cyclin D1 variants (Fig. 1D). The results in Fig. 1 suggest that cyclin D1 may regulate sex steroid metabolism enzymes in a manner that would favor increased estrogen and decreased androgen levels in the liver.
Cyclin D1 leads to elevated serum E2 levels and regulates hepatic estrogen- and androgen-responsive genes in males.
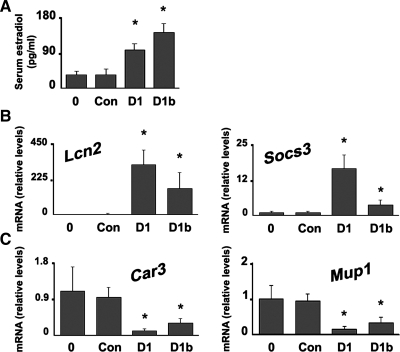
We next examined whether the regulation of sex steroid metabolism genes by cyclin D1 had a biological effect. At 1 day after transfection with cyclin D1 or D1b, serum E2 levels were significantly increased in the male mice (Fig. 2A). This suggests that the increase in serum E2 seen after major liver resection or in liver disease in males may be due in part to the induction of cyclin D1 in these settings (17, 18, 41). We also examined whether cyclin D1 regulated the expression of estrogen- and androgen-responsive genes in the liver. As is shown in Fig. 2B, cyclins D1 and D1b led to markedly increased expression of LCN2 and SOCS3 mRNA, which are known to be induced by estrogen in the breast (39) and liver (31), respectively. Conversely, cyclins D1 and D1b led to decreased expression of CAR3 and MUP1 (Fig. 2C), which are androgen-responsive genes in the liver (16). These results suggest that cyclin D1 increases estrogenic and decreases androgenic signaling in the liver.
Fig. 2.
Liver-specific expression of cyclin D1 increases serum estradiol (E2) levels and regulates estrogen- and androgen-responsive gene expression. A: serum E2 was measured in serum from untreated male mice or mice transfected with the cyclin D1, cyclin D1b, or control vectors. B: RT-PCR for estrogen-responsive genes LCN2 and SOCS3. C: RT-PCR for hepatic androgen-responsive genes CAR3 and MUP1.
Since E2 has been reported to induce hepatocyte proliferation in culture, we asked whether the increase in estrogen levels played a role in the proliferative response induced by hepatic cyclin D1 expression. To test this, we treated mice with the aromatase inhibitor letrozole, which is used to suppress estrogen synthesis in hormone-responsive breast cancer. As shown in Fig. 3A, letrozole completely prevented the induction of serum E2 levels by cyclin D1. However, letrozole did not affect the induction of hepatocyte DNA synthesis or cdk1 expression (Fig. 3, B and C). Furthermore, suppression of the increased E2 production by letrozole did not prevent the downregulation of SRD5A1 or the androgen target gene MUP1 transcripts.(Fig. 4D). These data suggest that the increased E2 levels are not required for the induction of hepatocyte proliferation and that cyclin D1 promotes proliferation via the canonical Rb-E2F pathway. The data also suggest that cyclin D1 regulates hepatic androgen metabolism via an estrogen-independent mechanism.
Fig. 4.
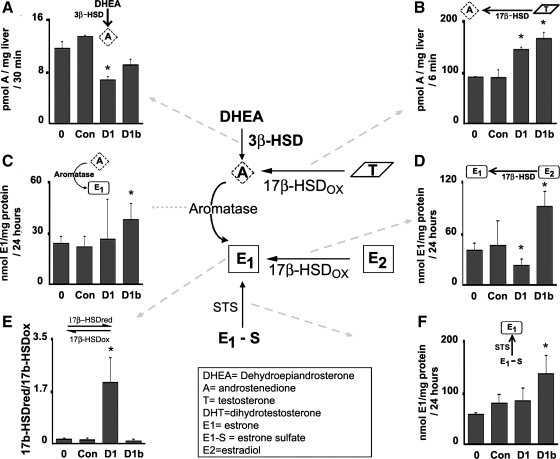
Androgen and estrogen-converting enzyme-specific activities are regulated by cyclins D1 and D1b. A: specific activities of 3β-HSDs with dehydroepiandrosterone (DHEA) as a substrate were measured in livers from untreated male mouse livers or livers expressing cyclin D1, cyclin D1b, or control vector. B: specific activity measurements of 17β-HSDs with testosterone as a substrate. C: aromatase activity was measured as the conversion of androstenedione (A) to estrone (E1). D: activity measurements of 17β-HSDs responsible for the oxidation of E2 into the less active E1. E: activities of 17β-HSDs responsible for the reduction of E1 to E2 and the oxidation of E2 to E1 were measured and expressed as activity ratios of activating (reducing) vs. inactivating (oxidizing) 17β-HSDs. F: steroid sulfatase (STS) activities were measured as conversion of E1–1-sulfate (E1-S) to E1. *P < 0.05 between livers transfected with the cyclin D1 or D1b adenoviruses compared with untreated or control-transfected livers. Results are means ± SE of at least 3 independent mouse livers performed in duplicate.
Cyclin D1 regulates the activity of steroid metabolizing enzymes.
The data in Fig. 1 indicate that cyclin D1 significantly modulates the expression of a number of key sex steroid metabolism genes. However, enzyme activities may be regulated distinctly from transcript levels. To examine this further, we performed assays for key enzymatic reactions on extracts from male mouse livers as outlined in Fig. 4. To investigate the production of androstenedione by 3β-HSD or 17β-HSD activities, enzyme assays were performed using either testosterone or DHEA as substrate. Cyclins D1 and D1b led to decreased conversion of DHEA to androstenedione (Fig. 4A), indicating a decrease in 3β-HSD activity. In comparison, there was a much higher basal rate of conversion of testosterone to androstenedione by 17β-HSD in normal liver, and this was significantly increased by both cyclins D1 and D1b (Fig. 4B). This suggests that cyclin D1 may shift testosterone utilization away from androgen synthesis and toward androstenedione production in the liver.
Aromatase activity, measured as the conversion of androstenedione to E1, was present in normal male liver (Fig. 4C). This activity was highly variable in livers transfected with cyclin D1, and no statistically significant change was detected. In livers transfected with cyclin D1b, aromatase activity was significantly increased. In Fig. 4D, the conversion of E2 to the less potent estrogen ligand E1 by 17β-HSD was examined. As was the case for the oxidation of testosterone to androstenedione (Fig. 4B), there was a relatively high rate of conversion of E2 into E1 in normal liver (Fig. 4D). Cyclin D1 decreased this activity, suggesting that it may cause a relative increase in E2 content at the tissue level. On the other hand, cyclin D1b triggered a higher rate of E2 to E1 conversion.
In contrast to the high level of activities of inactivating 17β-HSDs in normal liver, there was a 10-fold lower basal level of activity of 17β-HSDs responsible for the conversion of E1 to E2 in the liver samples (data not shown). To examine this further, the net conversion between E2 and E1 was calculated for the liver samples by examining the ratio of activating (reducing) to inactivating (oxidizing) 17β-HSD enzyme activities, as has been previously described (24, 27). Cyclin D1 caused a substantial increase in this ratio, whereas cyclin D1b had no significant effect (Fig. 4E). Finally, the activity of STS, which converts sulfated estrogens into nonconjugated estrogens, was measured in livers expressing cyclins D1 or D1b. As measured by E1 production from the inactive sulfated form (E1–1-sulfate), there was a high level of STS activity in the liver samples (Fig. 4F). STS activity was significantly increased by cyclin D1b but not cyclin D1. Taken together, these data confirm that cyclin D1 regulates several key enzymes that favor local E2 production. Interestingly, cyclin D1b differentially regulates some of these enzymes and may favor greater production of E1 and lower production of E2 at the tissue level than does cyclin D1. This may be an additional explanation for the recently published finding that cyclin D1b does not regulate ERα activity similarly to cyclin D1 in breast cancer cells (6).
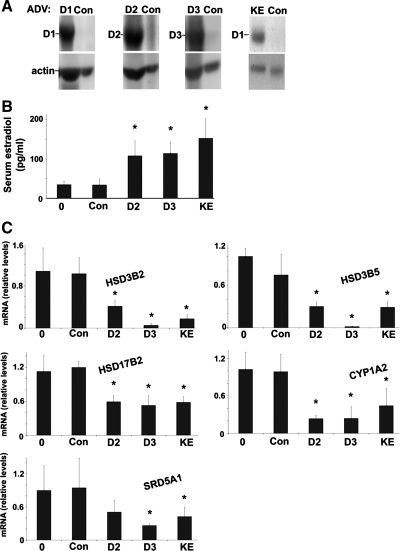
Effect of cyclins D2, D3, and D1-KE.
We next examined whether the closely related D-type cyclins (D2 and D3) had a similar effect in our model and whether cdk4 activation was required for the effect. Cyclin D1 regulates cell cycle progression via activation of cdk4, but it can regulate other cellular effects, such as transcriptional regulation, through cdk-independent mechanisms (10, 20). In Fig. 5, mice were transfected with adenoviruses encoding cyclin D2, cyclin D3, or a mutant cyclin D1 with a lysine to glutamine point mutation in the cyclin box, called cyclin D1-KE. This mutant does not activate cdk4 (26, 30) and did not promote DNA synthesis or cell cycle gene expression at 1 day after transfection (E. A. Hanse and J. H. Albrecht, unpublished data). The cyclins D2, D3, and D1-KE increased serum E2 levels to a similar degree as wild-type cyclin D1 (Fig. 5B). Furthermore, each of these D-type cyclins regulated the expression of key sex steroid metabolism mRNAs in a pattern similar to that seen after cyclin D1 transfection (Fig. 5C). These data suggest that each of the D-type cyclins can regulate aspects of steroid metabolism in the liver and that the mechanism is independent of cdk4 activation or cell cycle progression.
Fig. 5.
Regulation of hepatic steroid metabolism by cyclins D2, D3, and D1-KE. Mice were transfected as in Figs. 1–3 with adenoviruses expressing cyclin D2, cyclin D3, or the KE mutant of cyclin D1 and harvested at 1 day after transfection. A: expression of the indicated transfected proteins by Western blot. B: serum E2 levels. C: RT-PCR of the indicated sex steroid metabolism mRNA.
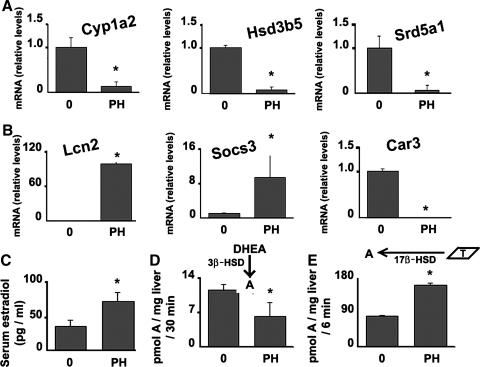
Regulation of sex steroid metabolism in regenerating liver.
We next examined whether the physiological induction of cyclin D1 following 70% PH was associated with changes in sex steroid metabolism similar to that observed with cyclin D1 transfection. The level of cyclin D1 induction after PH is less than that seen after adenoviral transfection (34). We examined liver harvested 42 h after PH, at which time cyclin D1 is significantly induced (34). As was seen after cyclin D1 transfection, PH led to decreased expression of CYP1A2, HSD3B5, and SRD5A1 mRNA (Fig. 6A). PH similarly induced the expression of estrogen-responsive genes LCN2 and SOCS3 and decreased expression of androgen-responsive CAR3 (Fig. 6B). As has been previously reported (9), PH stimulated an increase in serum E2 (Fig. 6C). PH led to a decrease in the conversion of DHEA to androstenedione by 3β-HSD activity (Fig. 6D) and an increase in testosterone to androstenedione conversion by 17β-HSD activity (Fig. 6E), similar to the effects seen after cyclin D1 transduction. These data provide insight into the mechanisms underlying the shift in sex steroid metabolism after PH in males that favor increased estrogen and decreased androgen levels. Taken together with the data in Figs. 1–5, these results suggest that cyclin D1 expression in the regenerating liver may play a role in the feminization syndrome seen after major liver resection in men.
Fig. 6.
Steroid-converting enzymes expression and activities in the liver and serum E2 levels are regulated in during liver regeneration. RNA was isolated from normal livers or 42 h after partial hepatectomy (PH) and used for RT-PCR of the indicated genes. A: steroid-converting enzymes. B: androgen and estrogen-responsive genes. C: serum E2 levels. D: specific activities of 3β-HSD with DHEA as a substrate. E: specific activity measurements of 17β-HSDs, with testosterone as a substrate.
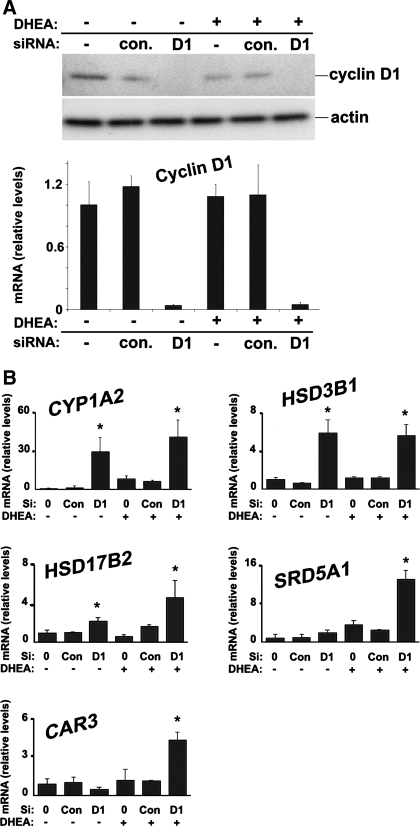
Cyclin D1 regulates key sex steroid metabolism genes in HuH7 cells.
The regulation of sex steroid metabolism by cyclin D1 in mouse liver led us to investigate whether it has similar effects in the well-differentiated human HCC cell line HuH7. Because these cells express a high level of cyclin D1, we used a knockdown approach to study the role of this protein. As is shown in Fig. 7A, cyclin D1 mRNA and protein expression was almost completely ablated by its siRNA. In Fig. 7B, the expression of sex steroid metabolism transcripts was analyzed in the presence or absence of the steroid precursor DHEA. Cyclin D1 knockdown led to increased CYP1A2 expression, which correlates well with the inhibition of this mRNA by cyclin D1 expression and PH in the liver (Figs. 1 and 6). The genes encoding 3β-HSD enzyme dehydrogenases differ between mice and humans; human 3β-HSD types 1 and 2 are steroidogenic dehydrogenases that catalyze the conversion of DHEA to androstenedione and appear to be similar to the mouse 3β-HSD type 2 dehydrogenase (37), which was downregulated by cyclin D1 (Fig. 1). Inhibition of cyclin D1 led to increased HSD3B1 expression in HuH7 cells (Fig. 7B) but did not affect HSD3B2 (data not shown). This suggests that cyclin D1 may regulate 3β-HSD activity in human cells via modulation of HSD3B1. HSD17B2 catalyzes the conversion of E2 to the less potent estrogen E1 (37), and expression of this transcript was increased by knockdown of cyclin D1. Consistent with the data in mouse liver (Figs. 1–6), these findings suggest that cyclin D1 affects E2 and E1 levels via regulation of HSD17B2.
Fig. 7.
Cyclin D1 regulates sex steroid converting enzymes in HuH7 cells. Cells were cultured in the presence of 10% charcoal-stripped FBS. Cells were treated with cyclin D1 or control small interfering RNA (siRNA) 2 days prior to harvest and cultured with 10 nM DHEA for the final 24 h as indicated. A: Western blot analysis (top) and RT-PCR (bottom) of cyclin D1 expression. B: RT-PCR for the indicated genes from cells cultured in the presence and absence of DHEA as described above.
Steroid 5α-reductase 1 (SRD5A1) plays a key role in androgen production, and its expression was induced by DHEA (Fig. 7B). In the presence of DHEA, cyclin D1 knockdown significantly promoted SRD5A1 expression, whereas this transcript is downregulated in cyclin D1-transfected mouse liver (Fig. 1). Expression of the androgen-responsive CAR3 gene was induced by cyclin D1 siRNA in the presence of DHEA but not in its absence. This suggests that direct modulation of the AR by cyclin D1 does not account for the regulation of CAR3 in the absence of ligand. However, these data support the concept that increased production of androgen (via induction of SRD5A1 and potentially other mechanisms) promotes CAR3 expression following cyclin D1 knockdown in the presence of steroid precursor (DHEA).
DISCUSSION
In this study, we provide evidence for a novel interaction between the cell cycle machinery and sex steroid signaling, whereby cyclin D1 regulates key enzymes involved in the synthesis and degradation of these hormones. Although previous studies have documented that cyclin D1 controls the activity of ERα and AR through direct binding or by modulating transcriptional coregulators, the present experiments suggest that this protein can also regulate the levels of their respective ligands. We found that cyclin D1 affected several different enzymes that play an important role in sex steroid metabolism, suggesting that it may have a coordinated effect on these pathways.
Abnormal regulation of sex steroid metabolism in the setting of liver diseases or resection is a well-described phenomenon, but the underlying mechanisms have not been established. In men with cirrhosis, hypogonadism and alterations in the hypothalamic-pituitary axis may explain some of the features of feminization (40). However, these changes would not likely account for the increased estrogen levels seen in men and postmenopausal women that occur acutely after major liver resection (17, 18, 41), nor the increased levels seen in male mice after PH (Fig. 5C) (9). After major hepatectomy, a large number of hepatocytes enter the cell cycle as part of the regenerative response, and this is accompanied by marked induction of cyclin D1 (15, 34). Cyclin D1 expression is also elevated in patients with cirrhosis due to ongoing hepatocyte proliferation that helps to sustain functional liver mass (2, 15, 32). In this study, we provide evidence that cyclin D1 may account for changes in sex steroid metabolism as follows: 1) Cyclin D1 regulated the expression of a number of genes involved in steroid synthesis and degradation in a pattern that would predict increased estrogen and decreased androgen production in the liver. 2) Short-term cyclin D1 expression in the liver led to increased estrogen levels similar to those seen after PH. 3) Cyclin D1 promoted expression of estrogen-responsive genes and downregulated androgen-responsive genes in the liver. 4) Cyclin D1 regulated the hepatic activity of key enzymes involved in sex steroid synthesis in a manner that favors increased estrogen and decreased androgen synthesis. 5) PH in mice led to similar changes in gene expression and enzyme activity to those induced by cyclin D1. 6) Knockdown of cyclin D1 expression in the well-differentiated HCC line HuH7 had reciprocal effects on several key genes compared with cyclin D1 transfection in the liver. Although it is highly likely that the hormonal changes seen in the setting of liver disease or PH are regulated by several factors, the data presented here suggest that expression of cyclin D1 in hepatocytes plays an important role.
A handful of prior studies have suggested a link between the cell cycle machinery and sex steroid metabolism, although a causative role has not been previously established. For example, in the fetal baboon adrenal gland, cyclin D1 expression decreases during gestation whereas 3β-HSD expression increases (14). Treatment of human ovarian cells with the antiproliferative agents TGF-β1 and all-trans retinoic acid induces HSD3B1 expression, suggesting that cell cycle inhibition promotes its expression (36). The data presented here demonstrate that cyclin D1 inhibits 3β-HSD activity (Fig. 3) and downregulates the expression of key 3β-HSD genes (HSD3B1 and murine HSD3B2, Figs. 1 and 7). In addition, we found that cyclin D1 modulated 17β-HSD mRNA expression and activity in a manner that favors increased E2 and decreased testosterone synthesis (Figs. 1 and 4). To our knowledge, no previous studies have shown that SRD5A1 varies during the cell cycle, and thus our finding that SRD5A1 was regulated by cyclin D1 in mouse liver and human cancer cells lines is of particular interest.
Previous studies have shown that a central “repressor domain” of cyclin D1 can inhibit AR function by direct binding to the receptor and by recruiting histone deacetylases to repress transcription (3). Of note, this mechanism has been established by using transfection systems to overexpress cyclin D1, and we are unaware of prior studies looking at knockdown of endogenous cyclin D1 expression. The data presented here suggest that cyclin D1 may inhibit AR activity through an additional mechanism, by reducing the availability of androgen ligands. Although we have not successfully performed SRD5A1 Western blot or 5α-reductase enzyme assays (data not shown), data from mouse liver and human HuH7 cells show that this gene is inhibited by cyclin D1 expression (Figs. 1 and 6). The downregulation of SRD5A1 by cyclin D1 does not require the induction of E2 (Fig. 3) or the activation of cdk4 (Fig. 5). 5α-Reductase plays a pivotal role in androgen synthesis, and inhibitors of this enzyme are used for therapy of androgen-responsive prostate cancer. Interestingly, we found that SRD5A1 expression was negligible in cultured HuH7 cells but was induced by DHEA, suggesting that its expression is dependent on steroid ligands. In the presence of DHEA (but not in its absence), cyclin D1 siRNA significantly increased the expression of AR target gene CAR3 (Fig. 7). Thus modulation of endogenous cyclin D1 levels per se was not sufficient to regulate CAR3, but the combination of a steroid precursor and knockdown of cyclin D1 induced this gene. These data suggest that cyclin D1 may regulate AR activity, in part, by modulating synthesis of androgens at the tissue and cellular level.
In cell culture systems, cyclin D1 has also been shown to regulate the activity of ERα through at least two different ligand-independent mechanisms (10, 20, 42). The data presented here provide evidence that cyclin D1 also regulates the availability of E2, thereby providing an additional potential mechanism by which it can activate ERα. Further study will be required to determine the relative contribution of each mechanism, which may vary between cell types, tissues, and experimental systems. A better understanding of the functional relationship between cyclin D1 and ERα may aid the development of additional therapies for estrogen-responsive malignancies.
The basis for performing the present studies was the unexpected finding that transient cyclin D1 expression in the liver regulated the expression of a significant number of genes involved in sex steroid metabolism (Table 1) (33). We have not yet examined other potentially relevant enzymes and sex steroids that may be affected by cyclin D1. For example, cyclin D1 downregulated the StARD4 and StARD5 mRNA on the gene array; these encode StAR lipid transfer proteins that control a rate-limiting step in the biogenesis of steroid hormones. Similarly, cyclin D1 inhibited expression of GSTA3, which also plays an important role in steroid hormone synthesis. Cyclin D1 also downregulated expression of transcripts encoding glucuronyltransferases (UGT2B1 and UGT2B38) involved in steroid deactivation. Furthermore, many of these same enzymes are involved in the synthesis of progesterone, which may also be affected by cyclin D1 expression. Although these findings require further study, they suggest that cyclin D1 significantly regulates these enzyme pathways.
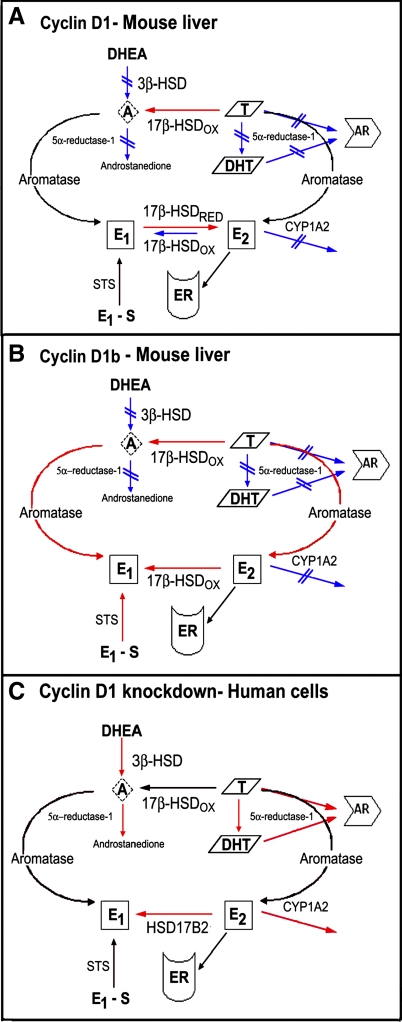
Taken together, our data are consistent with the theme that cyclin D1 regulates hepatic sex steroid synthesis and degradation at several levels (Fig. 8). Further study is required to determine the mechanism(s) by which these changes occur, but the findings suggest cyclin D1 may trigger a coordinated response to alter the bioavailability of these ligands in tissues and cells. Relatively little is known about the transcriptional and posttranscriptional mechanisms that regulate key enzymes involved in steroid hormone synthesis (37). Cyclin D1 is known to modulate gene transcription through phosphorylation or direct binding to transcription factors and coregulators (10) and thus could be inducing a coordinated transcriptional response. Notably, we found that the cyclin D1-KE mutant, which does not activate cdk4 or promote cell cycle progression at the time point studied, produced similar changes in serum E2 and sex steroid metabolism transcript expression. Cdk4-independent actions of cyclin D1 include transcriptional regulation and modulation of certain metabolic functions (3, 10, 20). Cyclins D2 and D3 also induced serum estrogen levels and regulated key metabolic genes, suggesting that a shared domain may affect the response. The central “repressor domain” of cyclin D1 (which is intact in cyclin D1-KE) is highly homologous to the corresponding regions in cyclins D2 and D3 (3, 13), and thus each of the D-type cyclins may be affecting hormone metabolism through a transcriptional mechanism. However, further investigation will be required to determine the mechanisms by which cyclin D regulates hormone metabolism in the liver.
Fig. 8.
Proposed model for the regulation of sex steroid metabolism by cyclin D1. Enzymatic reactions that are increased or decreased are labeled in red and blue, respectively. A: expression of cyclin D1 in the liver leads to diminished 3β-HSD activity converting DHEA to androstenedione but increases the oxidation of testosterone to androstenedione by 17β-HSD. Inhibition of 5α-reductase further diminishes production of dihydrotestosterone (DHT) from testosterone, with the net result being decreased androgen receptor (AR) activation. Cyclin D1 promotes accumulation of E2 from E1 by increasing the relative reduction/oxidation activity of 17β-HSD enzymes converting these compounds and by decreasing CYP1A2 expression that normally inactivates E2. The net result is increased estrogen stimulation of estrogen receptor α (ERα). B: cyclin D1b produces several similar effects, except that it also increases aromatase activity, favors conversion of E2 to E1 by 17β-HSD, and increases STS activity. This results in less E2 and more E1 production than that induced by cyclin D1, and relatively less ERα activation. C: knockdown of cyclin D1 in human cancer cells induces 5α-reductase (SRD5A1) expression, increases DHT levels, and activates AR. Depletion of cyclin D1 also promotes 3β-HSD type 1 (HSD3B1) and 17β-HSD type 2 (HSD17B2) expression. The induction of HSD17B2 leads to increased conversion of E2 to E1, resulting in diminished ERα activation. Cyclin D1 may regulate other key enzymes in these cell lines that remain to be studied.
In summary, the present studies indicate that cyclin D1 regulates sex steroid metabolism in the liver and in human cancer cells. Our findings present a potential mechanism for the increased estrogen and decreased androgen levels seen in males in the setting of liver resection or diseases. Furthermore, these data suggest that cyclin D1 may enhance ERα activity and inhibit AR activity, at least in part, by modulating androgen and estrogen ligands at the tissue or cellular level. These actions of cyclin D1 may be highly relevant to its role in hormone-responsive tissues and cancer.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK54921 (J. H. Albrecht) and F32DK074320 (L. K. Mullany), internal grants from University Hospital Maastricht (B. Delvoux and A. Romano), MRC grant 0500047 (J. I. Mason), Gynecologic Cancer Foundation and Susan G. Komen Foundation (C. H. Blomquist).
DISCLOSURES
No conflicts of interest are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Charles H. Blomquist, Ph.D. (1933–2009) for inspiration. We thank Erik Knudsen and Phil Hinds for providing reagents used in this study and Ashley Solomonson for technical assistance.
REFERENCES
- 1.Albrecht JH, Hansen LK. Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ 10: 397–404, 1999 [PubMed] [Google Scholar]
- 2.Albrecht JH, Hu MY, Cerra FB. Distinct patterns of cyclin D1 regulation in models of liver regeneration and human liver. Biochem Biophys Res Commun 209: 648–655, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Balk SP, Knudsen KE. AR, the cell cycle, and prostate cancer. Nucl Recept Signal 6: e001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone M, Ladisa R, Di Leo A, Spano D, Francioso D, Aglio V, Amoruso A, Francavilla A, Iolascon A. Estrogen-induced proliferation in cultured hepatocytes involves cyclin D1, p21(Cip1) and p27(Kip1). Dig Dis Sci 51: 580–586, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Blomquist CH, Bonenfant M, McGinley DM, Posalaky Z, Lakatua DJ, Tuli-Puri S, Bealka DG, Tremblay Y. Androgenic and estrogenic 17beta-hydroxysteroid dehydrogenase/17-ketosteroid reductase in human ovarian epithelial tumors: evidence for the type 1, 2 and 5 isoforms. J Steroid Biochem Mol Biol 81: 343–351, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Burd CJ, Petre CE, Morey LM, Wang Y, Revelo MP, Haiman CA, Lu S, Fenoglio-Preiser CM, Li J, Knudsen ES, Wong J, Knudsen KE. Cyclin D1b variant influences prostate cancer growth through aberrant androgen receptor regulation. Proc Natl Acad Sci USA 103: 2190–2195, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carruba G. Aromatase in nontumoral and malignant human liver tissues and cells. Ann NY Acad Sci 1155: 187–193, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Castagnetta LA, Agostara B, Montalto G, Polito L, Campisi I, Saetta A, Itoh T, Yu B, Chen S, Carruba G. Local estrogen formation by nontumoral, cirrhotic, and malignant human liver tissues and cells. Cancer Res 63: 5041–5045, 2003 [PubMed] [Google Scholar]
- 9.Castellaneta A, Di Leo A, Francavilla R, Margiotta M, Barone M, Amoruso A, Troiani L, Thomson AW, Francavilla A. Functional modification of CD11c+ liver dendritic cells during liver regeneration after partial hepatectomy in mice. Hepatology 43: 807–816, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Coqueret O. Linking cyclins to transcriptional control. Gene 299: 35–55, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Delvoux B, Groothuis P, D'Hooghe T, Kyama C, Dunselman G, Romano A. Increased production of 17β-estradiol in endometriosis lesions is the result of impaired metabolism. J Clin Endocrinol Metab 94: 876–883, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Delvoux B, Husen B, Aldenhoff Y, Koole L, Dunselman G, Thole H, Groothuis P. A sensitive HPLC method for the assessment of metabolic conversion of estrogens. J Steroid Biochem Mol Biol 104: 246–251, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Doisneau-Sixou SF, Sergio CM, Carroll JS, Hui R, Musgrove EA, Sutherland RL. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr Relat Cancer 10: 179–186, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Dumitrescu A, Aberdeen GW, Pepe GJ, Albrecht ED. Developmental expression of cell cycle regulators in the baboon fetal adrenal gland. J Endocrinol 192: 237–247, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43: S45–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Zhu Y, Chen X, Sha J, Fan L, Chen Q. Effects of diet-induced hypercholesterolemia on testosterone-regulated protein expression in mice liver. J Nanosci Nanotechnol 5: 1273–1276, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Francavilla A, Gavaler JS, Makowka L, Barone M, Mazzaferro V, Ambrosino G, Iwatsuki S, Guglielmi FW, Dileo A, Balestrazzi A. Estradiol and testosterone levels in patients undergoing partial hepatectomy. A possible signal for hepatic regeneration? Dig Dis Sci 34: 818–822, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francavilla A, Panella C, Polimeno L, Giangaspero A, Mazzaferro V, Pan CE, Van Thiel DH, Starzl TE. Hormonal and enzymatic parameters of hepatic regeneration in patients undergoing major liver resections. Hepatology 12: 1134–1138, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francavilla A, Polimeno L, DiLeo A, Barone M, Ove P, Coetzee M, Eagon P, Makowka L, Ambrosino G, Mazzaferro V. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology 9: 614–620, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: Cyclin D1: normal and abnormal functions. Endocrinology 145: 5439–5447, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Giannitrapani L, Soresi M, La Spada E, Cervello M, D'Alessandro N, Montalto G. Sex hormones and risk of liver tumor. Ann NY Acad Sci 1089: 228–236, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Gowri PM, Sengupta S, Bertera S, Katzenellenbogen BS. Lipin1 regulation by estrogen in uterus and liver: implications for diabetes and fertility. Endocrinology 148: 3685–3693, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Granata OM, Cocciadifero L, Campisi I, Miceli V, Montalto G, Polito LM, Agostara B, Carruba G. Androgen metabolism and biotransformation in nontumoral and malignant human liver tissues and cells. J Steroid Biochem Mol Biol 113: 290–295, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Gunnarsson C, Hellqvist E, Stal O. 17β-Hydroxysteroid dehydrogenases involved in local oestrogen synthesis have prognostic significance in breast cancer. Br J Cancer 92: 547–552, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer 2: 331–341, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Hinds PW, Dowdy SF, Eaton EN, Arnold A, Weinberg RA. Function of a human cyclin gene as an oncogene. Proc Natl Acad Sci USA 91: 709–713, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansson A, Gunnarsson C, Stal O. Proliferative responses to altered 17beta-hydroxysteroid dehydrogenase (17HSD) type 2 expression in human breast cancer cells are dependent on endogenous expression of 17HSD type 1 and the oestradiol receptors. Endocr Relat Cancer 13: 875–884, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Knudsen KE. The cyclin D1b splice variant: an old oncogene learns new tricks. Cell Div 1: 15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol 22: 185–212, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Landis MW, Pawlyk BS, Li T, Sicinski P, Hinds PW. Cyclin D1-dependent kinase activity in murine development and mammary tumorigenesis. Cancer Cell 9: 13–22, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Leong GM, Moverare S, Brce J, Doyle N, Sjogren K, Dahlman-Wright K, Gustafsson JA, Ho KK, Ohlsson C, Leung KC. Estrogen up-regulates hepatic expression of suppressors of cytokine signaling-2 and -3 in vivo and in vitro. Endocrinology 145: 5525–5531, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Marshall A, Rushbrook S, Davies SE, Morris LS, Scott IS, Vowler SL, Coleman N, Alexander G. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 128: 33–42, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Mullany LK, White P, Hanse EA, Nelsen CJ, Goggin MM, Mullany JE, Anttila CK, Greenbaum LE, Kaestner KH, Albrecht JH. Distinct proliferative and transcriptional effects of the D-type cyclins in vivo. Cell Cycle 7: 2215–2224, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res 61: 8564–8568, 2001 [PubMed] [Google Scholar]
- 35.Nelsen CJ, Rickheim DG, Tucker MM, Hansen LK, Albrecht JH. Evidence that cyclin D1 mediates both growth and proliferation downstream of TOR in hepatocytes. J Biol Chem 278: 3656–3663, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Papacleovoulou G, Edmondson RJ, Critchley HO, Hillier SG, Mason JI. 3β-Hydroxysteroid dehydrogenases and pre-receptor steroid metabolism in the human ovarian surface epithelium. Mol Cell Endocrinol 301: 65–73, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev 25: 947–970, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem 63: 25–61, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Seth P, Porter D, Lahti-Domenici J, Geng Y, Richardson A, Polyak K. Cellular and molecular targets of estrogen in normal human breast tissue. Cancer Res 62: 4540–4544, 2002 [PubMed] [Google Scholar]
- 40.Villa E. Role of estrogen in liver cancer. Womens Health (Lond Engl) 4: 41–50, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Villa E, Baldini GM, Pasquinelli C, Melegari M, Cariani E, Di Chirico G, Manenti F. Risk factors for hepatocellular carcinoma in Italy. Male sex, hepatitis B virus, non-A non-B infection, and alcohol. Cancer 62: 611–615, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Dean JL, Millar EK, Tran TH, McNeil CM, Burd CJ, Henshall SM, Utama FE, Witkiewicz A, Rui H, Sutherland RL, Knudsen KE, Knudsen ES. Cyclin D1b is aberrantly regulated in response to therapeutic challenge and promotes resistance to estrogen antagonists. Cancer Res 68: 5628–5638, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.