Abstract
In the intestinal epithelium, the Cdx, GATA, and HNF transcription factor families are responsible for the expression of differentiation markers such as sucrase-isomaltase. Although previous studies have shown that Cdx2 can induce differentiation in rat intestinal IEC-6 cells, no data are available concerning the direct implication of transcription factors on differentiation in human normal intestinal epithelial cell types. We investigated the role of Cdx2, GATA-4, and HNF-1α using the undifferentiated human intestinal epithelial crypt cell line HIEC. These transcription factors were tested on proliferation and expression of polarization and differentiation markers. Ectopic expression of Cdx2 or HNF-1α, alone or in combination, altered cell proliferation abilities through the regulation of cyclin D1 and p27 expression. HNF-1α and GATA-4 together induced morphological modifications of the cells toward polarization, resulting in the appearance of functional features such as microvilli. HNF-1α was also sufficient to induce the expression of cadherins and dipeptidylpeptidase, whereas in combination with Cdx2 it allowed the expression of the late differentiation marker sucrase-isomaltase. Large-scale analysis of gene expression confirmed the cooperative effect of these factors. Finally, although DcamKL1 and Musashi-1 expression were downregulated in differentiated HIEC, other intestinal stem cell markers, such as Bmi1, were unaffected. These observations show that, in cooperation with Cdx2, HNF-1α acts as a key factor on human intestinal cells to trigger the onset of their functional differentiation program whereas GATA-4 appears to promote morphological changes.
Keywords: transcription factors, gene expression, cell proliferation, cell differentiation, cell junction molecules
the crypt/villus functional unit is responsible for the continuous renewal of the intestinal epithelium. A dynamic system directs this phenomenon involving cell generation and migration from the stem cell population, located in the lower part of the crypt (10, 57), to extrusion of the terminally differentiated cells at the tip of the villus (3). The epithelia of the different compartments of the crypt-villus axis are characterized by differential properties in regard to cell proliferation as well as differentiation stage. Until now, no normal intestinal epithelial cell model able to proliferate under culture conditions and recapitulate normal human epithelial differentiation has been available. Transformed or cancerous cell lines have been extensively used in various studies but are not ideal to investigate mechanisms related to normal gene expression. Although the usefulness of the IEC-6 cell model (52) has been largely demonstrated, its rodent origin as well as its immortalized features limit extrapolation of the results to normal human physiological processes. Moreover, these cells cannot be considered to be completely undifferentiated since they harbor microvilli and functional cell-cell junctions.
In our laboratory, we isolated the first normal human intestinal epithelial crypt (HIEC) cell model from the fetal human intestine (49). HIEC cells harbor specific features of intestinal crypt cells, such as the expression of keratins 8, 18, and 20/21, the crypt cell-specific marker MIM-1/39 (49), the integrin-α8 and integrin-α9 subunits (7, 15), and an intestine-specific truncated crypt-form of the integrin-β4 subunit (5) as well as low levels of the brush border hydrolases aminopeptidase N and dipeptidylpeptidase IV (DPPIV)(46, 49, 51). Under standard culture conditions, HIEC cells can be expanded up to 30 passages without reaching senescence. However, they appear to be unable to spontaneously acquire the typical morphological and functional features of differentiated intestinal epithelial cells (46, 48, 49).
It is now obvious that epithelial differentiation along the crypt/villus axis is highly dependent on relations between epithelial and stromal cells that involve complex mechanisms of signaling (1, 62) resulting in the reprogramming of the genome via the combined action of various transcription factors (25, 34, 39, 69). The Cdx2 transcription factor, belonging to the mammalian homeobox gene family related to the Drosophila melanogaster gene caudal (17, 33), has been shown to induce primitive cell differentiation and polarization of the rat immortalized IEC-6 cell model (61). DNA microarray analysis (65) and other studies have shown that Cdx2 upregulates the expression of molecules involved in cell-cell or cell-substratum interactions such as LI-cadherin (32), E-cadherin, the integrin β4 subunit, α-actinin (37), and claudin-2 (55). Thus the data strengthen the idea that Cdx2 could be a central transcription factor in triggering epithelial differentiation in the intestine (27). However, physical interactions of Cdx2 with other transcription factors, namely GATA-4 and HNF-1α, are needed for the expression of intestinal differentiation-specific genes such as sucrase-isomaltase (13, 31) and lactase-phlorizin hydrolase (12, 36, 41, 64, 67). In this context, cooperation between Cdx2, HNF-1α, and GATA-4 may also be required to trigger intestine specific patterns of gene expression leading to differentiation. In human intestinal crypt cells, we found that Cdx2 alone exerted a limited effect, essentially restricted to the alteration of proliferation (23) indicating fundamental distinctions from their rat counterpart IEC-6 in which Cdx2 alone can trigger differentiation (61). In fact, although both cell lines lack Cdx2 expression, IEC-6 cells harbor significant endogenous levels of other important transcription factors, such as GATA-4 (39), in contrast to HIEC cells (22), suggesting that these factors may cooperate with exogenous Cdx2 to trigger intestinal terminal differentiation.
In the present study we sought to evaluate the contribution of three distinct intestine related transcription factors, Cdx2, GATA-4, and HNF-1α, normally all absent in HIEC cells (22), with the goal of creating a human intestinal cell model useful for the study of elements regulating cell commitment. In addition we investigated the potential cooperation between these three factors on intestinal cell function, using recently cloned human versions of Cdx2, GATA-4, and HNF-1α (22), and analyzed the cellular impact of their ectopic expression, alone and in combination, on the proliferation, polarization, and gene expression of HIEC cells.
MATERIALS AND METHODS
Cell culture.
Human intestinal crypt HIEC-6 cells have been characterized elsewhere (46, 49). They were used at passages 9–20 and grown as recently described (23, 24). Postconfluent Caco-2/15 cells were also used as positive differentiated control in some experiments. Their characterization and the culture conditions used have been previously described (6, 66).
Tissues.
Tissues from the healthy adult proximal ileum were obtained from Quebec Transplant (Quebec, Canada) in accordance with protocols approved by the local Institutional Human Research Review Committee for the use of human material. The preparation and embedding of tissues for cryosectioning was performed as previously described (22).
Western blot analysis.
Proteins were extracted in Laemmli buffer and 50 μg of each protein sample was submitted to 12.5% SDS-PAGE separation, transferred onto nitrocellulose, and processed for Cdx2, GATA-4, HNF-1α, and keratin 18 immunodetection as previously described (22, 23). CyclinD1 and p27Kip1 (Santa Cruz Biotechnology, Santa Cruz, CA) and E-cadherin (BD Biosciences, Mississauga, ON, Canada) were also detected by using corresponding secondary antibodies conjugated to horseradish peroxidase and an ECL detection kit (Amersham, Baie d'Urfé, QC, Canada).
Indirect immunofluorescence.
Cryosections (2–3 μm) of adult proximal ileum were fixed and processed as described previously for Cdx2, GATA-4, HNF-1α, and E-cadherin detection in the fetal intestine (22). 4,6-Diamidino-2-phenylindole (DAPI) was used to stain nuclei.
Electron microscopy.
The preparation of cell samples for ultrastructural analysis was performed as previously described (48, 51, 66). Briefly, HIEC-derived cell lines were grown for 30 days after confluence in the presence of doxycycline (1 μg/ml), washed twice with 0.1 M sodium cacodylate (pH 7.4), fixed in 2.5% glutaraldehyde for 1 h at room temperature, postfixed in 2% osmium tetroxide, and dehydrated. For transmission electron microscopy, samples were embedded under vacuum in araldite. Ultrathin sections of these samples were examined with a Hitachi 7500 microscope. For scanning electron microscopy, samples were processed for critical point drying, gold coated, and analyzed at 15 kV with a JEOL JSM 840 microscope.
Cell proliferation assays.
Inducible stable cell populations, with or without subsequent infection, were seeded in 35-mm dishes at 2 × 105 cells/dish in OptiMEM medium. At different intervals between days 2 and 20 after seeding, three plates of each cell line were washed in PBS and counted with a Z1 Coulter Counter (Beckman, Mississauga, ON, Canada).
RT-PCR and real-time quantitative RT-PCR.
RNA extraction, quality verification, reverse transcription, and cDNA amplification by PCR for the detection of Cdx2, GATA-4, HNF-1α, sucrase-isomaltase, DPPIV, and β-actin were performed as described (22, 23). For LI-cadherin detection, we used the forward 5′ -GAGTGAGAATTCCTTCAGTG-3′ and reverse 5′ -GTCCATCCATGGGAAGTTTGG-3′ primers, whereas for E-cadherin detection we used the forward 5′ -CCTTCCTCCCAATACATCTCCC-3′ and reverse 5′ -TCTCCGCCTCCTTCTTCATC-3′ primers.
For quantitative RT-PCR, the threshold cycle values were converted into relative expression values compared with a pooled RNA standard (QPCR Human Reference Total RNA, Stratagene, La Jolla, CA) before normalization of gene expression against the normalizing gene RPLPO as described previously (19). All sequences of the primer pairs used are listed in Table 1. The annealing temperature of the reactions was 55°C and the amplification efficiencies of the reactions were between 96 and 112% as determined by standard curve analysis.
Table 1.
Primers used for quantitative RT-PCR
| Gene | Sense Primer | Antisense Primer |
|---|---|---|
| E-cadherin | 5′ -TTGCAAATTCCTGCCATTC-3′ | 5′ -GCTGGCTCAAGTCAAAGTCC-3′ |
| Zonula occludens-2 | 5′ -GGGATATTGCAGGCACAGTT-3′ | 5′ -CGCTGTCTCCCTTCTTGAAC-3′ |
| Cingulin | 5′ -GCTCCTGTTAGCTCGTGGTCC-3′ | 5′ -GAAAAGGCTCAGTTGGCTTG-3′ |
| Villin-2 | 5′ -GGCTGCAGGACTATGAGGAG-3′ | 5′ -TGGCAGTGTATTCTGCAAGC-3′ |
| Claudin-11 | 5′ -CTGGTGGACATCCTCATCCT-3′ | 5′ -CCAGCAGAATGAGCAAAACA-3′ |
| Calbindin-2 | 5′ -GCTCCAGGAATACACCCAAA-3′ | 5′ -CAGCTCATGCTCGTCAATGT-3′ |
| Bmi1 | 5′ -TGTTCGTTACCTGGAGACC-3′ | 5′ -CAGCATCAGCAGAAGGATG-3′ |
| DcamKL1 | 5′ -GGGCAGCAGGTGTAATCACT-3′ | 5′ -TTAACCCAGGGATGCTCAAG-3′ |
| Musashi-1 | 5′ -TACGCCAGCCGGAGTTATAC-3′ | 5′ -ATTGGTCCGTAGGCAGTGAG-3′ |
| Lgr5 | 5′ -TGCTCTTCACCAACTGCATC-3′ | 5′ -CTCAGGCTCACCAGATCCTC-3′ |
Establishment of HIEC-derived cell lines.
The establishment of stable HIEC cell populations expressing human Cdx2, GATA-4, or HNF-1α in an inducible manner was performed by use of retroviral infection. First, an inducible HIECTeton stable population was obtained by viral infection as previously described (23). pRevTRE vectors (BD Biosciences) containing the cDNA of each factor, cloned as previously described (22), were used to produce viruses in 293T cells upon cotransfection with helper VPack-VSV-G vectors (Stratagene). The pRevTRE empty vector was used to produce virus for the establishment of a control cell line. HIECTeton cells were infected with the different viruses, and selection was performed with 10 μg/ml hygromycin (Invitrogen, Grand Island, NY). HIECIndEmpty, HIECIndCdx2, HIECIndGATA-4, and HIECIndHNF-1α cell lines were then used in different experiments under basal (0 μg/ml doxycycline) or optimum inducing (5 μg/ml doxycycline) conditions.
Stable double-inducible HIECIndHNF-1α/Cdx2 cell populations were established by infecting HIECIndHNF-1α cells with a virus encoding puromycin resistance and inducible Cdx2 (obtained by standard procedures using a pRevTRE-derived vector; details available upon request). Selection was performed by use of 1 μg/ml puromycin, and optimum induction of Cdx2 and HNF-1α was obtained by adding 5 μg/ml doxycycline. In several experiments, we constitutively expressed Cdx2 and/or GATA-4 in HIECIndHNF-1α cells. Viruses encoding pLPCX-derived vectors (BD Biosciences) were produced and used to infect HIECIndHNF-1α cells. Virus encoding the pLPCX empty vector was also generated and used as a control.
Cell cycle progression analysis by laser scanning cytometry.
Each cell line was treated with 5 μg/ml doxycycline for 10 days prior to being seeded onto 12-well plates (Falcon) at 5 × 104 cells per well, 48 h before methanol fixation and DAPI staining. DAPI-stained cells were scanned with an iCys imaging cytometer (Compucyte, Cambridge, MA) to measure DNA content by using violet diode laser excitation (405 nm) and appropriate filters (463/39 nm) for fluorescence detection. DNA content was measured for at least 3,000 isolated nuclei per sample in three separate experiments to assess cell cycle distribution.
Gene profiling using DNA microarrays.
RNA extraction and quality verification was done as described (63). RNA amplification was performed with the TargetAMP 1-round aRNA amplification kit (Epicentre Biotechnologies, Madison, WI) according to the manufacturer's instructions. Probe preparation and procedures for hybridization and image processing have been previously described (63). cDNA microarrays (sets SS-H19k8, representing 19,200 human cDNA clones) were obtained from the University Health Network of Toronto, Ontario (Canada). A total of four independent biological samples for HIECIndHNF-1α/Cdx2 and HIECIndHNF-1α/Cdx2+GATA-4 cell models were analyzed relative to control HIECIndEmpty. Hybridization protocol, image processing, and data analysis were performed as previously described (63). Gene ontology analysis and functional classification were performed by using the Database for Annotation, Visualization and Integrated Discovery (DAVID) and Kyoto Encyclopedia of Genes and Genomes (KEGG) bioinformatic resources.
Statistical analysis.
Data were expressed as means ± SE. Each experiment was repeated at least three times and representative results were shown. DNA microarray related data were statistically analyzed as previously described (63). All other values were subjected to the two-tailed Student's t-test. P < 0.05 was considered significant.
RESULTS
The undifferentiated state of HIEC cells correlates with a lack of Cdx2 and HNF-1α expression.
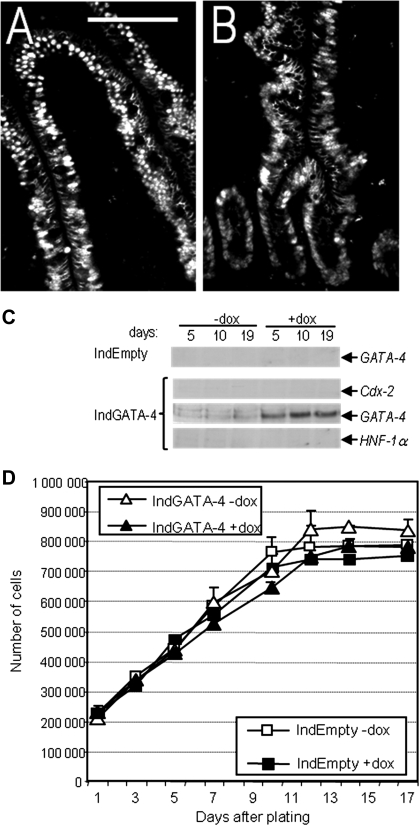
As shown previously, HIEC cells express intestinal cell features such as keratin 18 and 20 and the crypt cell marker MIM-1/39 but no true polarization nor differentiation features (24, 46, 49). Morphologically, HIEC cells display a flat surface and poorly organized junctions, whereas most differentiation markers are below the detection level, confirming their crypt-like status (46). Interestingly, Cdx2 and HNF-1α mRNAs, encoding transcription factors involved in the expression of intestine-specific genes in differentiated cells, are also lacking in HIEC (Fig. 1A). Thus the absence of these specific transcription factors suggests that they could be related to the inability of HIEC to undertake a differentiation program (24, 46). In this context, the absence of both Cdx2 and HNF-1α immunostaining observed in a limited number of cells located in the lower portion of the crypts in the adult normal small intestine (Fig. 1D, arrows), in contrast with the relatively strong and uniform distribution of the same factors in the differentiated cells of the epithelium (Fig. 1, B and C), could be consistent with the undifferentiated state of progenitor crypt cells in situ.
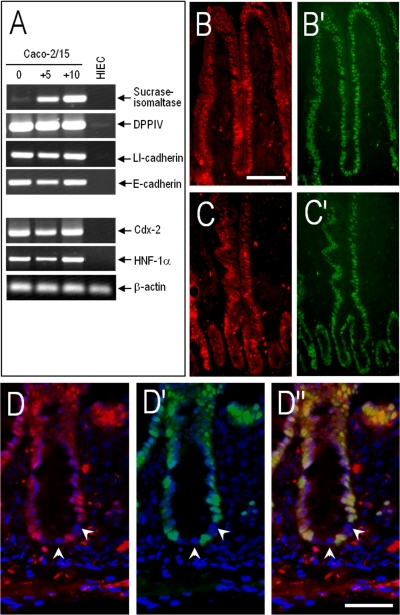
Fig. 1.
Undifferentiated human intestinal crypt cells lack Cdx2 and HNF-1α expression. A: representative RT-PCR analysis for detection at the transcript level of the differentiation markers sucrase-isomaltase, dipeptidylpeptidase IV (DPPIV), LI-cadherin and E-cadherin, and the transcription factors HNF-1α and Cdx2 in differentiated Caco-2/15 (5 and 10 days postconfluence) and undifferentiated human intestinal epithelial crypt (HIEC) cells. Although differentiated cells express all of these markers, undifferentiated HIEC cells express only low amounts of DPPIV. β-Actin was used for normalization. B and C: representative immunofluorescent staining on frozen sections of the adult small intestinal mucosa for the detection of HNF-1α in red (B and C) and Cdx2 in green (B′ and C′) showing nuclear expression in all differentiated cells of the villi (B and B′) and at the upper part of the crypts (C and C′); bar in B = 100 μm. D: higher magnification immunofluorescent pictures from the proximal ileum for the detection of HNF-1α in red (D), Cdx2 in green (D′), and merged HNF-1α/Cdx2 (D″). Nuclear staining by 4,6-diamidino-2-phenylindole (DAPI) was in blue; bar in D″ = 20 μm. Both transcription factors were found to be expressed relatively ubiquitously in the nuclei of upper crypt and villus epithelial cells (B and C), whereas a limited number of cells located in the lower portion of the crypts showed weak or negative staining (arrows in D, D′, and D″ denote negative nuclei).
Establishment and characterization of inducible Cdx2 or HNF-1α-expressing HIEC cell lines.
To analyze the role of these transcription factors in human intestinal crypt cells, we used viral strategies to first establish stable HIEC cell populations expressing each of the factors individually in an inducible manner. The stable inducible HIEC cell lines expressed relatively high amounts of the factors under a doxycycline-dependent manner (Fig. 2A), and indirect immunofluorescence revealed expression in 85–90% of the cells after doxycycline induction (not shown). In each case, expression of one factor did not induce expression of the others. Weak expression of transgenes can be detected in the absence of doxycycline for both constructs, leading to a basal level of transcription factor expression in uninduced cells (Fig. 2A), a phenomenon not observed under control conditions (IndEmpty). The effect of the expression of the individual transcription factors was first tested on HIEC cell proliferation (Fig. 2, B and C). Overexpression of Cdx2 or HNF-1α alone reduced cell proliferation by ∼50 and ∼35%, respectively, compared with control cells (Fig. 2, B and C). Basal levels of expression in uninduced HIECIndCdx2 cells were sufficient to inhibit cell proliferation by ∼20%, suggesting the activity of Cdx2 on cell proliferation is dosage dependent. Doxycycline had no effect on the control empty vector HIECIndEmpty cells (Fig. 2, B and C). Cdx2 overexpression led to a 46% decrease in cyclinD1 expression levels compared with control HIEC, reproducing what we have previously shown (23). Conversely, HNF-1α expression did not significantly affect the expression of cyclinD1 (Fig. 2D) but induced a twofold increase in p27KIP1 expression in HIEC, whereas Cdx2 had no significant effect (Fig. 2E). These results suggest that each transcription factor inhibits proliferation by different mechanisms.
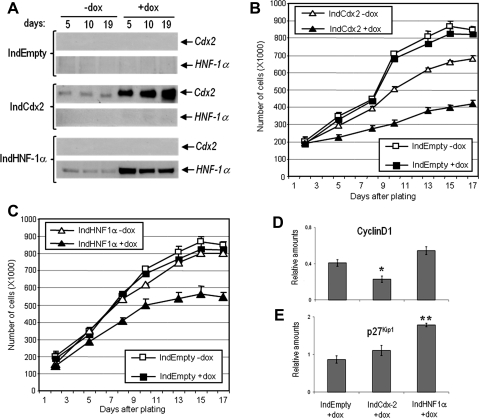
Fig. 2.
Characterization of HIEC cells expressing Cdx2 and HNF-1α individually under induced and uninduced conditions. A: Western-blot analysis of Cdx2 and HNF-1α expression in inducible HIEC cell lines. Cytokeratin-18 was used as a loading control (not shown). B and C: proliferation assay with inducible HIEC cell lines expressing Cdx2 or HNF-1α alone showing that both factors significantly slow down intestinal cell proliferation. Cells were grown up to day 17 after seeding and counted at the indicated times. HIECIndEmpty cells [presence or absence of doxycycline (+dox and −dox, respectively)] were grown in parallel with HIECIndCdx2 (B) and HIECIndHNF-1α (C). Relative amounts of cyclinD1 (D) and p27Kip1 (E) were evaluated by western blot analyses in HIEC induced for the expression of Cdx2 or HNF-1α by doxycycline. Cytokeratin-18 was used as a loading control (not shown) to determine the relative amounts. *P = 0.023, **P = 0.001.
To investigate whether the inhibitory effects of Cdx2 and HNF-1α are additive, constitutive expression of Cdx2 was forced in HIECIndHNF-1α cells (induced or not) and growth curves were established (Fig. 3A). Interestingly, although Cdx2 alone instigated a significant decrease in the growth rate, forced coexpression of Cdx2 in HNF-1α expressing cells greatly impaired the proliferation abilities (∼70%), compared with HIECIndHNF-1α uninduced cells. These observations suggest that the inhibitory effects of HNF-1α and Cdx2 on cell growth are additive and are consistent with the involvement of these transcription factors in distinct cell-cycle regulatory pathways. Laser scanning cytometry analysis of control, Cdx2, and HNF-1α/Cdx2 expressing HIEC populations showed that the decrease in cell growth observed in Fig. 3A was consistent with a significant and additive increase in the percentage of cells in G1 for Cdx2 and HNF-1α/Cdx2 compared with controls (Fig. 3B).
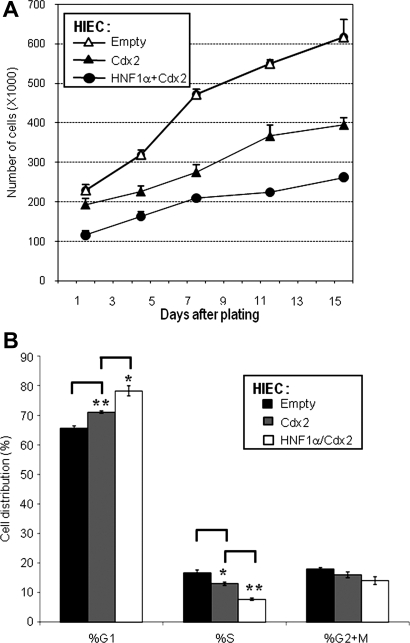
Fig. 3.
Effect of Cdx2 and HNF-1α combined expression on HIEC cell proliferation. A: proliferation assay with HNF-1α expressing HIEC cells (IndHNF-1α+dox) reinfected for the constitutive expression of Cdx2 (HNF1α+Cdx2) demonstrated an additive effect of the 2 factors on the inhibition of cell proliferation compared with cells that expressed Cdx2 alone (IndCdx2 +dox; Cdx2). A control infection was performed with the same virus containing the empty vector (IndEmpty +dox; Empty). B: histogram illustrating the percentage (%) of cells distributed in each of G1, S, and G2+M cell-cycle phases for HIEC control (IndEmpty +dox; Empty), Cdx2 alone (IndCdx2 +dox; Cdx2), and HNF-1α/Cdx2-expressing cells (IndHNF-1α/Cdx2 +dox; HNF1α/Cdx2) obtained by iCys laser scanning cytometry analysis (*P < 0.03, **P < 0.005).
Differentiation marker expression in Cdx2 and HNF-1α-expressing cells.
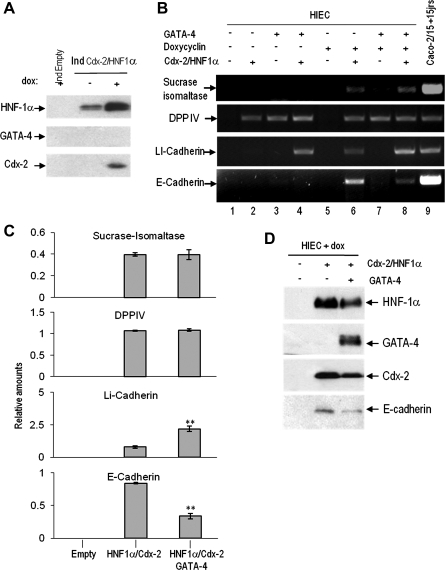
The effect of the expression of the individual transcription factors on differentiation was tested by evaluating mRNA levels of well-established intestinal differentiation markers by RT-PCR analysis. In doxycycline-induced cells expressing Cdx2, we observed a weak increase in DPPIV relative to control HIECIndEmpty cells (Fig. 4A). However, doxycycline-induced cells expressing HNF-1α showed high levels of DPPIV and the appearance of LI-cadherin and E-cadherin transcripts (Fig. 4A). It is of note that basal levels of HNF-1α expressed in uninduced HIECIndHNF-1α (Fig. 2A) were sufficient to induce DPPIV expression (Fig. 4A). The sucrase-isomaltase transcript was not detected under these conditions.
Fig. 4.
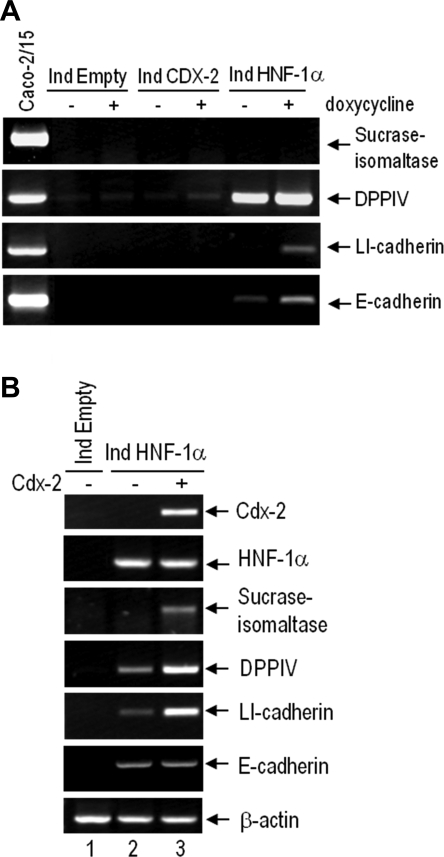
Representative RT-PCR analysis for the characterization of the HNF-1α- and Cdx2-expressing HIEC inducible cell lines. A: detection at the transcript level of differentiation markers in inducible cell populations under uninduced (−) or induced (+) conditions. Postconfluent Caco-2/15 cells were used as differentiated cell control. Cdx2 has little or no effect on HIEC cell differentiation markers when expressed alone (lane 5) as opposed to HNF-1α that induces expression of LI- and E-cadherin as well as DPPIV (lane 7). DPPIV was found to be expressed under a HNF-1α dosage-independent manner since it was similarly detected under +dox conditions (lane 6), which only allow basal HNF-1α expression. B: detection at the transcript level of the differentiation markers in the induced HIECIndHNF-1α cells reinfected with an empty virus (lane 2) or virus coding for the constitutive expression of Cdx2 (lane 3). HIECIndEmpty cells + doxycycline were used as control (lane 1). Expression of transcription factors was verified in all samples. Primers for β-actin were used for normalization. Combination of HNF-1α with Cdx2 induces expression of the terminal differentiation marker sucrase-isomaltase.
Because of the important initiating role of HNF-1α in differentiation marker expression, we further investigated the impact of Cdx2 in HIECIndHNF-1α cells using retroviral strategies. HIECIndHNF-1α cells were reinfected allowing constitutive Cdx2 expression and analyzed by RT-PCR 4 days after infection. As shown in Fig. 3B, the HNF-1α/Cdx2 combination led to the induction of detectable levels of sucrase-isomaltase expression. We also observed that coexpression of Cdx2 in HIECIndHNF-1α cells increased the transcript levels of DPPIV and LI-cadherin compared with cells coinfected with the empty virus. It is noteworthy that coexpressing Cdx2 constitutively in HIECIndHNF-1α cells had no additive effect on E-cadherin expression (Fig. 4B, lane 3).
GATA-4 as another key player in the human enterocytic differentiation process.
The GATA-binding zinc finger family member GATA-4 has been extensively studied in a cell differentiation context for its ability to regulate intestinal specific genes alone or in a cooperative way with Cdx and/or HNF transcription factors (13, 22, 67) and has been linked to physiological processes such as proximal-distal specification, gene activation/repression and cell fate (9, 11). GATA-4 immunostaining in the human proximal ileum revealed a relatively strong and uniform distribution of this factor in cells of the crypt and villus regions (Fig. 5, A and B). To analyze the role of GATA-4 in human intestinal crypt cells, we used the same viral strategy as above to establish stable HIEC cell populations expressing the transcription factor in an inducible manner. The stable inducible HIEC cell line expressed significant amounts of GATA-4 under a doxycycline-dependent manner (Fig. 5C), and here again indirect immunofluorescence revealed expression in 85–90% of the cells after doxycycline induction (not shown). Expression of GATA-4 had no influence on the expression of Cdx2 or HNF-1α. Weak expression of GATA-4 was detected in the absence of doxycycline treatment leading to a basal level of transcription factor expression in uninduced cells (Fig. 5C), a phenomenon not observed under control conditions (IndEmpty). Proliferation assays with HIECIndGATA-4 showed that GATA-4 has no effect on intestinal cell growth compared with uninduced and control cells (Fig. 5D).
Fig. 5.
Intestinal distribution of GATA-4 and its effect on HIEC cell growth. Representative immunofluorescent staining on frozen sections of adult proximal ileum mucosa for the detection of GATA-4 in the villus (A) and crypt (B) regions (bars = 100 μm). Nuclear staining of GATA-4 was ubiquitously detected throughout the epithelium from the lower part of the crypts to the tip of the villi. C: Western blot analysis of GATA-4 expression in inducible HIEC cell lines. GATA-4 was not detectable in control cells (HIECIndEmpty) or in Cdx2- or HNF-1α-expressing cells. GATA-4 was expressed at significant levels in doxycycline-induced HIECIndGATA-4 cells. D: proliferation assay with the GATA-4-inducible HIEC cell line demonstrates no significant effect on growth. HIECIndEmpty cells (presence or absence of doxycycline) were grown in parallel with HIECIndGATA-4 cell populations.
Generation and characterization of double inducible HIECIndHNF-1α/Cdx2 cells ± GATA-4.
Considering the promoting effect of the HNF-1α/Cdx2 combination on the expression of functional differentiation markers, we further examined the model by establishing cell lines expressing both HNF-1α and Cdx2 in an inducible manner. This perspective promotes a model in which it would be possible to control the expression of two factors that impair cell growth (HNF-1α and Cdx2) in the presence/absence of constitutive GATA-4 expression to study the cooperative effect of these three factors. This cell line, named HIECIndHNF-1α/Cdx2, was found to express low amounts of HNF-1α and no detectable Cdx2 under basal conditions, which were both found to be expressed in significant amounts upon doxycycline induction (Fig. 6A).
Fig. 6.
Characterization of the double-inducible HIECIndHNF-1α/Cdx2 cell line and impact of the HNF-1α/Cdx2/GATA-4 combination on the expression of intestinal epithelial differentiation markers. A: Western blot analysis exposing leaky expression of HNF-1α, but not of Cdx2, in the absence (−) of doxycycline in HIECTeton B: representative RT-PCR analysis for the detection of sucrase-isomaltase, DPPIV, LI-cadherin, and E-cadherin transcripts in the presence (+) or absence (−) of doxycycline in control (IndEmpty) (−) and Hnf-1α/Cdx2 (+) cells. Postconfluent Caco-2/15 cells were used as a well-differentiated control. Combined HNF-1α/Cdx2 expression induced the 4 tested markers (lane 6), whereas the addition of GATA-4 further increased LI-cadherin and reduced E-cadherin expression (lane 8) at significant levels (C), an observation further confirmed by real-time PCR (**P < 0.01). D: representative Western blot confirming the expression of the 3 factors at the protein level. A reduction of E-cadherin in response to the constitutive expression of GATA-4 in the induced HIECIndHNF-1α/Cdx2 cells was also apparent.
As expected, some effects related to the leakiness of the system were observed in HIECIndHNF-1α/Cdx2 in the absence of doxycycline such as an induction of DPPIV (Fig. 6B, lane 2) and the triggering of LI-cadherin expression (Fig. 6A, lane 4), the latter being observed upon coexpression with GATA-4. It is also noteworthy that GATA-4 alone induced DPPIV expression (Fig. 6B, lanes 3 and 7). In the presence of doxycycline, HNF-1α/Cdx2 expression was sufficient to trigger LI-cadherin and E-cadherin as well as sucrase-isomaltase expression at detectable levels (Fig. 6B, lane 6; Fig. 6C). Subsequent infection for the forced expression of GATA-4 in induced HIECIndHNF-1α/Cdx2 cells led to a further increase in LI-cadherin expression (Fig. 6B, lane 8; Fig. 6C; P < 0.01) but had no further effect on sucrase-isomaltase or DPPIV. Surprisingly, a sustained reduction of E-cadherin expression at both mRNA (Fig. 6B, lane 8; Fig. 6C; P < 0.005) and protein (Fig. 6D) levels was noted, suggesting a complex role for GATA-4 in the transcriptional regulation of differentiation-related genes. Real-time PCR confirmed these effects of GATA-4 on LI and E-cadherin expression in induced HIECIndHNF-1α/Cdx2 cells. The data showed that GATA-4 led to an 8.6× increase of LI-cadherin (P < 0.04) and a 2.4× decrease in E-cadherin expression (P < 0.005). However, it is noteworthy that cells constitutively expressing GATA-4 exhibited decreased expression of HNF-1α and Cdx2 (Fig. 6D).
Influence of GATA-4 on acquisition of morphological features.
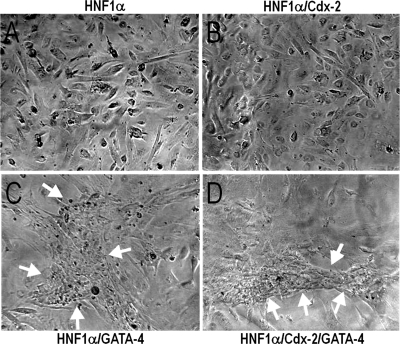
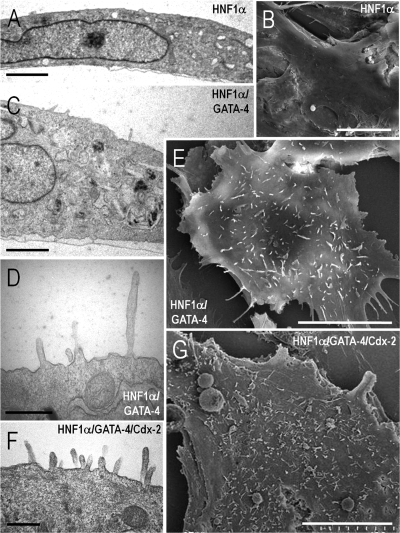
Because of the effect of HNF-1α and Cdx2 on differentiation marker expression, the potential role of these factors in enterocytic cell polarization onset was assessed. By phase contrast microscopy, no major morphological changes were observed in doxycycline-induced HIECIndHNF-1α cells (Fig. 7A) or in doxycycline-induced HIECIndHNF-1α/Cdx2 (Fig. 7B) both infected with empty virus. However, significant morphological changes were observed in both HIECIndHNF-1α cells and HIECIndHNF-1α/Cdx2 cells infected with the GATA-4 encoding virus (Fig. 7, C and D, respectively), namely the reorganization of the monolayer by generation of multicellular structures arranged in a latticelike configuration. Consistent with these observations, electron microscopy analysis revealed additional features resulting from the expression of the HNF-1α/Cdx2 and GATA-4 combination (Fig. 8). Indeed, as in wild-type HIEC cells, induced HIECIndHNF-1α + empty virus (Fig. 8, A and B) or induced HIECIndHNF-1α/Cdx2 + empty virus, as well as the GATA-4-expressing parental HIEC cells (not shown), displayed no obvious differentiation-related features, as illustrated by a poorly organized cytoplasm and the flat surface of their plasma membrane. In contrast, induced HIECIndHNF-1α cells expressing GATA-4 displayed a better organized cytoplasmic compartment (Fig. 8C) and the presence of microvilli at their luminal membrane (Fig. 8, D and E). A higher number and organization of microvilli were consistently observed in cells expressing the three factors as illustrated for induced HIECIndHNF-1α/Cdx2 cells + GATA-4 (Fig. 8, F and G).
Fig. 7.
Phase-contrast microscopy of induced HIECIndHNF-1α cells infected with either empty (A) or GATA-4 expressing vector (C) or doxycycline-induced HIECIndHNF-1α/Cdx2 cells infected with either empty (B) or GATA-4 expressing vector (D). GATA-4 expression induces the reorganization of the monolayer into latticelike structures (arrows in C and D).
Fig. 8.
Ultrastructural characterization of the effects of HNF-1α, Cdx2, and GATA-4 expression in HIEC cells by transmission (A, C, D, F) and scanning (B, E, G) electron microscopy. Doxycycline-induced HIECIndHNF-1α cells infected with either empty (A and B) or GATA-4 expressing vector (C–E) or doxycycline-induced HIECIndHNF-1α/Cdx2 cells infected with the GATA-4 expressing vector (F and G) were characterized. Cells showed poorly differentiated features typical to the original HIEC cells except upon forced expression of GATA-4. Bars = 2 μm in A and C; 10 μm in B, E, and G; 500 nm in D and F.
Analysis of gene expression profiles in transcription factor-expressing cells.
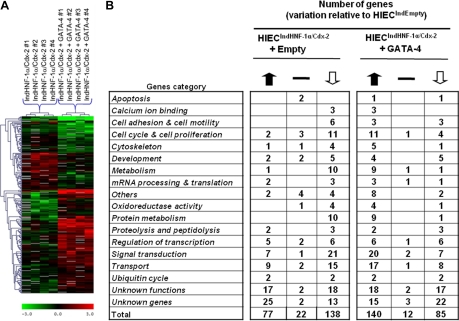
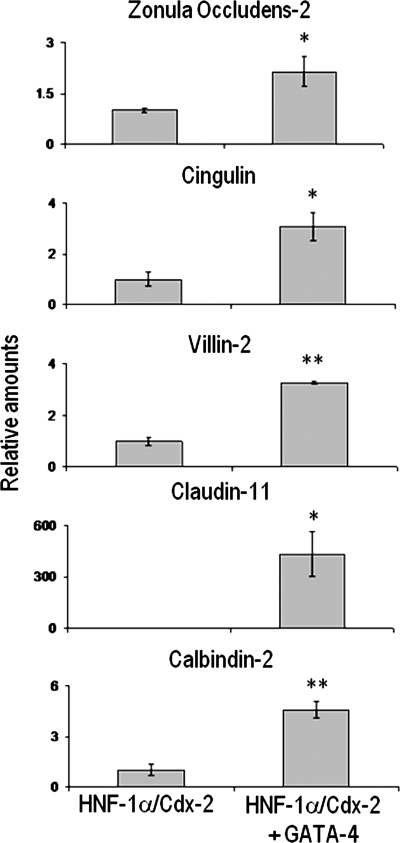
The effects of HNF-1α, Cdx2, and GATA-4 on gene expression in human intestinal crypt cells were further investigated by DNA microarray analysis. Using HIECIndEmpty cells as a control, we focused on HNF-1α/Cdx2 expressing cells (HIECIndHNF-1α/Cdx2) with or without subsequent infection by the GATA-4-encoding virus since these two combinations were found to be the most efficient to trigger complementary aspects of intestinal cell differentiation. Among the 19,200 cDNA clones present on the microarrays, data for 12,858 genes, collected in at least three of the four experiments, were included in the statistical analysis. Overall, a total of 237 genes belonging to various functional categories were found to be significantly modulated in both models. Analysis of genes expressed-based clustering of the two groups allowed distinguishing each of the two cell models (Fig. 9A). Interestingly, as shown in Fig. 9B, 215 genes were found to be significantly modified in HNF-1α/Cdx2 expressing HIEC cells, the majority of genes for transport (15), signal transduction (21) and cell cycle and cell proliferation (11) were downregulated. In induced HIECIndHNF-1α/Cdx2 expressing GATA-4, the expression of 225 genes was significantly altered, 140 genes were upregulated, many of which belonged to categories involved with transport (17), signal transduction (20), cell cycle and cell proliferation (11) and metabolism (9). Consequently with the morphological features observed when GATA-4 is conjointly expressed with HNF-1α and Cdx2 in HIEC, we performed gene ontology and functional classification analysis from microarray data by comparing induced HIECIndHNF-1α/Cdx2 with induced HIECIndHNF-1α/Cdx2 + GATA-4 cells. Interestingly, five morphology/polarity-associated genes were found to be upregulated in the presence of GATA-4 (Table 2). Real-time PCR assays confirmed that the tight-junction proteins zonula occludens-2, cingulin, and claudin-11 (14, 29, 38), as well as the calcium transport-associated calbindin-2 (26) and the microvilli actin/membrane linker villin-2 (43) mRNAs, were all significantly upregulated in induced HIECIndHNF-1α/Cdx2 + GATA-4 compared with induced HIECIndHNF-1α/Cdx2 without GATA-4 (Fig. 10). These observations reinforce the specific role for GATA-4 in cell polarity onset. The original data discussed in this publication have been deposited in the NCBI's Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE11421.
Fig. 9.
Hierarchical clustering of the genes differentially expressed in response to HNF-1α/Cdx2 expression in the absence or presence of GATA-4. A: common expression patterns were determined by nonbiased clustering analysis, revealing several groups of genes whose expression was modulated in response to HNF-1α/Cdx2 and HNF-1α/Cdx2+GATA-4 in HIEC cells. B: modulation of the differentially expressed genes in HIECIndHNF-1α/Cdx2+empty vector or HIECIndHNF-1α/Cdx2+GATA-4 cells, compared with HIECIndEmpty cells, was determined for each main biological process (P < 0.05).
Table 2.
Combined effect of GATA-4 with HNF-1α and Cdx2 gives rise to cell polarity functional markers in HIEC cells
| Gene | Fold Increase (P < 0.05) | Localization/Function |
|---|---|---|
| Zonula occludens-2 | 1.58 | Tight junction integrity |
| Claudin-11 | 3.11 | Tight junction integrity |
| Cingulin | 1.93 | Tight junction integrity |
| Calbindin-2 | 2.88 | Calcium transport |
| Villin-2 | 3.40 | Actin/membrane linker (microvilli) |
Gene ontology analysis and functional classification from microarray data from HIECIndHNF-1α/Cdx2+GATA-4 vs. HIECIndHNF-1α/Cdx2+empty vector.
Fig. 10.
Combined effects of GATA-4 with HNF-1α and Cdx2 on cell polarity marker expression in HIEC cells. Increased expression of zonula occludens-2, cingulin, villin-2, claudin-11, and calbindin-2 detected in microarrays (Table 2) was confirmed by real-time PCR in HIECIndHNF-1α/Cdx2+GATA-4 compared with HIECIndHNF-1α/Cdx2+empty vector (*P < 0.05; **P < 0.005).
Initiation of the enterocytic differentiation program influences the expression of intestinal stem/progenitor cell markers in HIEC.
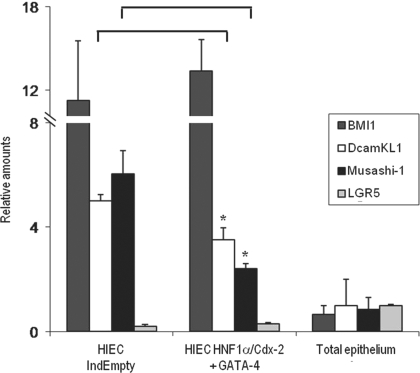
The analysis of microarray data also revealed a significant alteration in the levels of the intestinal stem cell marker DcamKL1 in HNF-1α/Cdx2/GATA-4 expressing cells compared with control HIECIndEmpty. Considering the undifferentiated status of HIEC cells, we analyzed the expression levels of the well-characterized intestinal stem/progenitor markers DcamKL1, Bmi1, Lgr5, and Musashi-1 (4, 40, 42, 44, 50, 56, 57) in HIECIndEmpty and cells that express the three factors by real-time PCR to evaluate whether the triggering of differentiation could affect the expression of such markers (Fig. 11). A total epithelial fraction was used as the control. Although detectable, only low relative amounts of Lgr5 were observed in HIECIndEmpty, whereas the three other markers were strongly expressed compared with the epithelial fraction. Bmi1 expression levels were unaffected by the expression of HNF-1α, Cdx2, and GATA-4, whereas the expression of DcamKL1 and Musashi-1 was significantly impaired by the initiation of the differentiation process (30 and 58%, respectively).
Fig. 11.
Effect of prodifferentiation transcription factors on the expression of intestinal stem/progenitor cell markers in HIEC. Expression levels of Bmi1, DcamKL1, Musashi-1, and Lgr5 were quantified in HIECIndEmpty and HNF-1α/Cdx2/GATA-4 expression HIEC by real-time PCR. Total epithelial fractions were used as control. Significant decreases of DcamKL1 and Musashi-1 were observed in differentiating HIEC compared with HIECIndEmpty (*P < 0.05).
DISCUSSION
In the present study, the effects of ectopic expression of the transcription factors HNF-1α, Cdx2, and GATA-4, alone and in combination, in human intestinal crypt cells were evaluated on cell proliferation and differentiation-related gene expression and cell morphology. Our results suggest that, although HNF-1α acts as a key factor, Cdx2 and GATA-4 exert cooperative effects, leading to the initiation of the differentiation program both morphologically and functionally.
Cdx2 has been previously described as an important regulator of intestinal differentiation, mainly on the basis of its effects in the immortalized rat intestinal epithelial cells IEC-6 (61) and of gene expression in various colon carcinoma cell lines (32, 37, 55). However, as shown herein, expression of Cdx2 in normal human epithelial ileal crypt cells does not lead to morphological or differentiation-linked gene expression changes, although alteration of cell proliferation has been noted (23). The absence of endogenous HNF-1α and GATA-4 in HIEC cells may provide a clue to the lack of effect of Cdx2 on differentiation. Indeed, IEC-6 cells express endogenous levels of GATA-4 and can be induced to express HNF-1α under specific culture conditions (39), a phenomenon never observed with HIEC cells. Moreover, recent studies in rodent models revealed the key involvement of HNF-4α in the enterocytic differentiation cascade (2, 39). However, in the human model, ectopic expression of HNF-4α, even combined with Cdx2, failed to induce HNF-1α, sucrase-isomaltase and the LI- and E-cadherins whereas it provided induction of DPPIV and the differentiated epithelial cell-specific extracellular matrix glycoprotein nephronectin (8), suggesting that HNF-1α and HNF-4α have complementary roles in human intestinal mucosa. Taken together, these data strengthen the idea that other transcription factors, such as HNF-1α and GATA-4, are required to cooperate with Cdx2 (13) to trigger intestine specific gene expression in crypt epithelial cells. Indeed, as shown herein, we observed that these factors can exert regulatory influences on distinct cell functions, namely proliferation, the onset of polarization, and tissue-specific gene expression.
HNF-1α expression, as previously shown for Cdx2 (23), resulted in a significant reduction in human crypt cell proliferation. Interestingly, the effects were found to be additive in cells coinfected for the expression of HNF-1α and Cdx2, suggesting that they can regulate proliferation via different pathways. Further experimentation revealed that both factors impaired cell-cycle progression through the G1 phase and, whereas Cdx2 expression reduced cyclinD1 levels, HNF-1α appeared to regulate expression of the cyclin-dependent kinase inhibitor p27Kip1.
Among the differentiation markers of intestinal epithelial cells, E-cadherin was undetectable in normal HIEC cells, a phenomenon consistent with its observed repression in the crypt progenitor cell population of the human intestine (24). The lack of LI-cadherin, another intestinal cadherin, as well as the absence of typical cell-cell junctions the weak expression of the brush border membrane enzyme DPPIV and the presence of the intestinal progenitor/stem cell markers such as Bmi1, DcamKL1, and Musashi-1 reinforced the idea that HIEC cells are blocked in an undifferentiated state. The expression of these intestinal prodifferentiation transcription factors allows progression toward intestinal specification and, interestingly, also leads to a corresponding downregulation of DcamKL1 and Musashi-1. However, Bmi1 expression, which has been reported to be closely associated with self-renewal in undetermined cell types, was not affected by the initiation of enterocytic differentiation in HIEC cells. This observation suggests that Bmi1 is involved in chromatin regulatory mechanisms which most likely occur upstream of HNF-1α, Cdx2, and GATA-4.
Cdx2 is considered to be a master regulator of intestinal identity acting through the repression of anteriorization-associated factors like Sox2 (27) and of pluripotency genes such as Oct4 and NANOG in early mouse embryonic development (60). Thus the specific expression of this parahox factor, together with its interacting partners, could be the cause of the observed repression of some of these intestinal stem/progenitor markers in our cell model of ileal origin. Still, the barely detectable levels of Lgr5 expression in HIEC further complicate the determination of the accurate status that could be conferred to these cells. This human cell model exhibits many typical characteristics of intestinal stem/progenitor cells such as undifferentiated state and specific marker expression, but clearly further experiments on self-renewal and multipotence regulatory mechanisms will be needed to clarify their exact nature.
Our results have shown that forced expression of HNF-1α was sufficient to trigger the expression of E-cadherin, LI-cadherin, and DPPIV. Little is known about the functional relationship between LI-cadherin expression and HNF-1α, whereas a modulatory influence of HNF-1α has been previously reported on DPPIV (20, 21) and E-cadherin (71) in various systems. As demonstrated, GATA-4, as well as Cdx2, had only a slight impact on enterocyte differentiation when expressed alone. However, GATA-4 expression in HIECIndHNF-1α cells triggered the formation of multicellular structures reminiscent of those observed during differentiation of the Cdx2-expressing IEC-6 cell model (61). Electron microscopic analysis showed the presence of microvilli-like structures at the luminal surface, a feature never seen in GATA-4-deficient HIEC cells. In addition, GATA-4 has recently been shown to downregulate integrin α8β1, a newly identified crypt cell integrin, which in turn affects microfilament organization through RhoA/ROCK downregulation (7). RhoA/ROCK downregulation has also been reported in differentiating HT-29 cells (30). Furthermore, our data suggest a specific cooperative role for GATA-4 with the two other factors promoting morphological changes and the expression of genes associated with tight junctions. The apparent inhibitory effect of GATA-4 on E-cadherin expression remains unclear, although consistently observed at both the transcript and protein levels. Since the constitutive expression of GATA-4 led to a decrease in HNF-1α and Cdx2 protein levels in induced HIECIndHNF-1α/Cdx2, GATA-4 could be involved in the fine tuning surrounding the stabilization of these two transcription factors consequently affecting E-cadherin expression indirectly. One may speculate that this is in some way related to the complex transcriptional role of GATA-4 in the control of intestinal crypt cell fate as previously described (11). Moreover, considering the number of repressed or upregulated genes following GATA-4 expression in HIEC observed herein by gene array analysis, it seems clear that this transcription factor exerts a dual role in regulating gene expression.
The combination of HNF-1α with Cdx2 triggered an almost complete growth arrest and induced the expression of E-cadherin, LI-cadherin, and DPPIV, as well as that of the terminal enterocyte differentiation marker sucrase-isomaltase. Thus the cooperative influence of HNF-1α with other factors, such as Cdx2, on HIEC cells appears critical for the initiation of functional differentiation. Likewise, cooperation with GATA-4 promoted the initiation of morphological changes and expression of genes associated with cell junctions. Thus significant changes in the repertoire of gene expression must be analyzed considering the multiple roles of HNF-1α in gene expression, from the control of chromatin structure to the direct regulation of promoter activities (53). Indeed, HNF-1α can tissue specifically regulate histone hyperacetylation linked to transcriptional activation of genes (47) and physically interact with chromatin remodeling enzymes such as the histone acetyltransferases (58). These interactions are thus likely to be involved in the transcriptional regulation of genes important for the intestinal differentiation process. HNF-1α can also act as an activator of gene expression in intestinal cells that do not require chromatin remodeling (12). From a functional point of view, HNF-1α, Cdx2, and GATA-4 appear to cooperate in the regulation of the expression of several enterocyte-related genes such as sucrase-isomaltase (13, 31), lactase-phlorizin hydrolase (12, 36, 41, 64, 67), liver fatty acid binding protein (16), adenosine deaminase (18), and claudin-2 (22), as well as those identified herein. This suggests that these three cooperating factors are involved in the overall regulatory mechanism of differentiation-related gene expression of the enterocytic lineage. The precise mechanisms by which HNF-1α acts on intestinal crypt cell proliferation and differentiation remain to be elucidated as well as the specific involvement of Cdx2 and GATA-4 in these HNF-1α-dependent processes. In addition to their potential activator or repressor activity, depending on the target gene, and their possible role in chromatin remodeling mentioned above, a variety of additional mechanisms are also likely to be involved in intestinal crypt cells. For instance, it has been suggested that there is an equilibrium between GATA-4 transcriptional activation and CCAAT displacement protein-mediated repression of the sucrase-isomaltase gene (13). Furthermore, it is clear that other factors, such as HNF-4α (35, 39, 59) and GATA-5 and GATA-6 (28, 36, 68), can also participate in the regulation of various aspects of intestinal gene expression (69). Finally, considering the nonterminal differentiated state of our model, it would also be interesting to investigate the involvement of other factors such as Id2, the PAX, Nkx, and HOX families, and the polycomb groups in the global enterocytic differentiation process (45, 54, 70).
In summary, the present work has led to the identification of the cooperative effects of HNF-1α with Cdx2 and GATA-4 in the modulation of proliferation, acquisition of morphological features, and generation of important changes in gene expression in human intestinal crypt cells, resulting in the initiation of the enterocyte differentiation program.
GRANTS
This work was supported by a Canadian Institutes of Health Research Grant MOP 57727 (to J.-F. Beaulieu). J.-F. Beaulieu is the recipient of the Canada Research Chair in Intestinal Physiopathology. F. Boudreau is a Chercheur-boursier du Fonds de la recherche en santé du Québec (FRSQ). J.-F. Beaulieu and F. Boudreau are members of the FRSQ-funded Centre de Recherche Clinique Étienne Le Bel of the Centre Hospitalier Universitaire de Sherbrooke. J.-F. Beaulieu is member of the Reseau de thérapie cellulaire et tissulaire of the FRSQ.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Nuria Basora and Elizabeth Herring for reviewing the manuscript and Manon Lepage for cryosections. We also acknowledge the excellent collaboration of Québec Transplant in obtaining fresh specimens of normal adult small intestine. Electron microscopy was performed at the Electron Microscopy Facility of the Faculty of Medicine and Health Sciences and the Centre de caractérisation des matériaux of the Université de Sherbrooke. Laser scanning cytometry was performed at the Cell Imaging Facility located in the Genetics Division/Department of Pediatrics of the Faculty of Medicine and Health Sciences of the Université de Sherbrooke, Sherbrooke, Canada. This facility is funded by grants from the Canadian Foundation for Innovation and from the Centre de Recherche Clinique Étienne Le Bel of the Centre Hospitalier Universitaire de Sherbrooke.
REFERENCES
- 1.Auclair BA, Benoit YD, Rivard N, Mishina Y, Perreault N. Bone morphogenetic protein signaling is essential for terminal differentiation of the intestinal secretory cell lineage. Gastroenterology 133: 887–896, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Babeu JP, Darsigny M, Lussier CR, Boudreau F. Hepatocyte nuclear factor 4α contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. Am J Physiol Gastrointest Liver Physiol 297: G124–G134, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Babyatsky MW, Podolsky DK. Growth and development of the gastrointestinal tract. In: Textbook of Gastroenterology, edited by Yamada T.Philadelphia, PA: Lippincott, 1999, p. 547–584 [Google Scholar]
- 4.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Basora N, Herring-Gillam FE, Boudreau F, Perreault N, Pageot LP, Simoneau M, Bouatrouss Y, Beaulieu JF. Expression of functionally distinct variants of the beta(4)A integrin subunit in relation to the differentiation state in human intestinal cells. J Biol Chem 274: 29819–29825, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JF, Nichols B, Quaroni A. Posttranslational regulation of sucrase-isomaltase expression in intestinal crypt and villus cells. J Biol Chem 264: 20000–20011, 1989 [PubMed] [Google Scholar]
- 7.Benoit YD, Lussier C, Ducharme PA, Sivret S, Schnapp LM, Basora N, Beaulieu JF. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK dependent mechanism. Biol Cell 101: 695–708, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit YD, Teller IC, Herring E, Beaulieu JF. Characterization of the integrin alpha8 beta1-nephronectin interaction in the human intestinal mucosa (Abstract). Gastroenterology 132: A631, 2007. [Google Scholar]
- 9.Beuling E, Bosse T, aan de Kerk DJ, Piaseckyj CM, Fujiwara Y, Katz SG, Orkin SH, Grand RJ, Krasinski SD. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev Biol 322: 179–189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289: G381–G387, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Bosse T, Piaseckyj CM, Burghard E, Fialkovich JJ, Rajagopal S, Pu WT, Krasinski SD. Gata4 is essential for the maintenance of jejunal-ileal identities in the adult mouse small intestine. Mol Cell Biol 26: 9060–9070, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosse T, van Wering HM, Gielen M, Dowling LN, Fialkovich JJ, Piaseckyj CM, Gonzalez FJ, Akiyama TE, Montgomery RK, Grand RJ, Krasinski SD. Hepatocyte nuclear factor-1α is required for expression but dispensable for histone acetylation of the lactase-phlorizin hydrolase gene in vivo. Am J Physiol Gastrointest Liver Physiol 290: G1016–G1024, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem 277: 31909–31917, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Citi S, Paschoud S, Pulimeno P, Timolati F, De Robertis F, Jond L, Guillemot L. The tight junction protein cingulin regulates gene expression and RhoA signaling. Ann NY Acad Sci 1165: 88–98, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Desloges N, Basora N, Perreault N, Bouatrouss Y, Sheppard D, Beaulieu JF. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: relation with cell proliferation. J Cell Biochem 71: 536–545, 1998 [PubMed] [Google Scholar]
- 16.Divine JK, McCaul SP, Simon TC. HNF-1alpha and endodermal transcription factors cooperatively activate Fabpl: MODY3 mutations abrogate cooperativity. Am J Physiol Gastrointest Liver Physiol 285: G62–G72, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Duprey P, Chowdhury K, Dressler GR, Balling R, Simon D, Guenet JL, Gruss P. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine. Genes Dev 2: 1647–1654, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Dusing MR, Brickner AG, Lowe SY, Cohen MB, Wiginton DA. A duodenum-specific enhancer regulates expression along three axes in the small intestine. Am J Physiol Gastrointest Liver Physiol 279: G1080–G1093, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Dydensborg AB, Herring E, Auclair J, Tremblay E, Beaulieu JF. Normalizing genes for quantitative RT-PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am J Physiol Gastrointest Liver Physiol 290: G1067–G1074, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Erickson RH, Gum JR, Lotterman CD, Hicks JW, Lai RS, Kim YS. Regulation of the gene for human dipeptidyl peptidase IV by hepatocyte nuclear factor 1 alpha. Biochem J 338: 91–97, 1999 [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson RH, Lai RS, Lotterman CD, Kim YS. Identification of upstream stimulatory factor as an activator of the human dipeptidyl peptidase IV gene in Caco-2 cells. Gene 258: 77–84, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 203: 15–26, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Escaffit F, Pare F, Gauthier R, Rivard N, Boudreau F, Beaulieu JF. Cdx2 modulates proliferation in normal human intestinal epithelial crypt cells. Biochem Biophys Res Commun 342: 66–72, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Escaffit F, Perreault N, Jean D, Francoeur C, Herring E, Rancourt C, Rivard N, Vachon PH, Pare F, Boucher MP, Auclair J, Beaulieu JF. Repressed E-cadherin expression in the lower crypt of human small intestine: a cell marker of functional relevance. Exp Cell Res 302: 206–220, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Fang R, Olds LC, Sibley E. Spatio-temporal patterns of intestine-specific transcription factor expression during postnatal mouse gut development. Gene Expr Patterns 6: 426–432, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Feher JJ, Fullmer CS, Wasserman RH. Role of facilitated diffusion of calcium by calbindin in intestinal calcium absorption. Am J Physiol Cell Physiol 262: C517–C526, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial-mesenchymal signaling by Cdx2. Dev Cell 16: 588–599, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X, Sedgwick T, Shi YB, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol 18: 2901–2911, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Mariscal L, Tapia R, Huerta M, Lopez-Bayghen E. The tight junction protein ZO-2 blocks cell cycle progression and inhibits cyclin D1 expression. Ann NY Acad Sci 1165: 121–125, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Gout SP, Jacquier-Sarlin MR, Rouard-Talbot L, Rousselle P, Block MR. RhoA-dependent switch between alpha2beta1 and alpha3beta1 integrins is induced by laminin-5 during early stage of HT-29 cell differentiation. Mol Biol Cell 12: 3268–3281, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu N, Adachi T, Matsunaga T, Tsujimoto G, Ishihara A, Yasuda K, Tsuda K. HNF-1alpha participates in glucose regulation of sucrase-isomaltase gene expression in epithelial intestinal cells. Biochem Biophys Res Commun 353: 617–622, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Hinoi T, Lucas PC, Kuick R, Hanash S, Cho KR, Fearon ER. CDX2 regulates liver intestine-cadherin expression in normal and malignant colon epithelium and intestinal metaplasia. Gastroenterology 123: 1565–1577, 2002 [DOI] [PubMed] [Google Scholar]
- 33.James R, Kazenwadel J. Homeobox gene expression in the intestinal epithelium of adult mice. J Biol Chem 266: 3246–3251, 1991 [PubMed] [Google Scholar]
- 34.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129: 2619–2628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojima K, Kishimoto T, Nagai Y, Tanizawa T, Nakatani Y, Miyazaki M, Ishikura H. The expression of hepatocyte nuclear factor-4alpha, a developmental regulator of visceral endoderm, correlates with the intestinal phenotype of gastric adenocarcinomas. Pathology 38: 548–554, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Krasinski SD, Van Wering HM, Tannemaat MR, Grand RJ. Differential activation of intestinal gene promoters: functional interactions between GATA-5 and HNF-1α. Am J Physiol Gastrointest Liver Physiol 281: G69–G84, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Lorentz O, Duluc I, Arcangelis AD, Simon-Assmann P, Kedinger M, Freund JN. Key role of the Cdx2 homeobox gene in extracellular matrix-mediated intestinal cell differentiation. J Cell Biol 139: 1553–1565, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lui WY, Wong EW, Guan Y, Lee WM. Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A. J Cell Physiol 211: 638–648, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Lussier CR, Babeu JP, Auclair BA, Perreault N, Boudreau F. Hepatocyte nuclear factor-4α promotes differentiation of intestinal epithelial cells in a coculture system. Am J Physiol Gastrointest Liver Physiol 294: G418–G428, 2008 [DOI] [PubMed] [Google Scholar]
- 40.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Mitchelmore C, Troelsen JT, Spodsberg N, Sjostrom H, Noren O. Interaction between the homeodomain proteins Cdx2 and HNF1alpha mediates expression of the lactase-phlorizin hydrolase gene. Biochem J 346: 529–535, 2000 [PMC free article] [PubMed] [Google Scholar]
- 42.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat 213: 52–58, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci USA 101: 17705–17710, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murayama M, Okamoto R, Tsuchiya K, Akiyama J, Nakamura T, Sakamoto N, Kanai T, Watanabe M. Musashi-1 suppresses expression of Paneth cell-specific genes in human intestinal epithelial cells. J Gastroenterol 44: 173–182, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Ng RK, Gurdon JB. Epigenetic inheritance of cell differentiation status. Cell Cycle 7: 1173–1177, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Pageot LP, Perreault N, Basora N, Francoeur C, Magny P, Beaulieu JF. Human cell models to study small intestinal functions: recapitulation of the crypt-villus axis. Microsc Res Tech 49: 394–406, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Parrizas M, Maestro MA, Boj SF, Paniagua A, Casamitjana R, Gomis R, Rivera F, Ferrer J. Hepatic nuclear factor 1-alpha directs nucleosomal hyperacetylation to its tissue-specific transcriptional targets. Mol Cell Biol 21: 3234–3243, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perreault N, Beaulieu JF. Primary cultures of fully differentiated and pure human intestinal epithelial cells. Exp Cell Res 245: 34–42, 1998 [DOI] [PubMed] [Google Scholar]
- 49.Perreault N, Beaulieu JF. Use of the dissociating enzyme thermolysin to generate viable human normal intestinal epithelial cell cultures. Exp Cell Res 224: 354–364, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Potten CS, Booth C, Tudor GL, Booth D, Brady G, Hurley P, Ashton G, Clarke R, Sakakibara S, Okano H. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71: 28–41, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Quaroni A, Beaulieu JF. Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology 113: 1198–1213, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol 80: 248–265, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rollini P, Fournier RE. The HNF-4/HNF-1alpha transactivation cascade regulates gene activity and chromatin structure of the human serine protease inhibitor gene cluster at 14q32.1. Proc Natl Acad Sci USA 96: 10308–10313, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell RG, Lasorella A, Dettin LE, Iavarone A. Id2 drives differentiation and suppresses tumor formation in the intestinal epithelium. Cancer Res 64: 7220–7225, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5′ -flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 277: 21361–21370, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA 106: 7101–7106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology 134: 849–864, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Soutoglou E, Viollet B, Vaxillaire M, Yaniv M, Pontoglio M, Talianidis I. Transcription factor-dependent regulation of CBP and P/CAF histone acetyltransferase activity. EMBO J 20: 1984–1992, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stegmann A, Hansen M, Wang Y, Larsen JB, Lund LR, Ritie L, Nicholson JK, Quistorff B, Simon-Assmann P, Troelsen JT, Olsen J. Metabolome, transcriptome, and bioinformatic cis-element analyses point to HNF-4 as a central regulator of gene expression during enterocyte differentiation. Physiol Genomics 27: 141–155, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132: 2093–2102, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol 16: 619–625, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teller IC, Auclair J, Herring E, Gauthier R, Menard D, Beaulieu JF. Laminins in the developing and adult human small intestine: relation with the functional absorptive unit. Dev Dyn 236: 1980–1990, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Tremblay E, Auclair J, Delvin E, Levy E, Menard D, Pshezhetsky AV, Rivard N, Seidman EG, Sinnett D, Vachon PH, Beaulieu JF. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. J Cell Biochem 99: 1175–1186, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Troelsen JT. Adult-type hypolactasia and regulation of lactase expression. Biochim Biophys Acta 1723: 19–32, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Uesaka T, Kageyama N, Watanabe H. Identifying target genes regulated downstream of Cdx2 by microarray analysis. J Mol Biol 337: 647–660, 2004 [DOI] [PubMed] [Google Scholar]
- 66.Vachon PH, Beaulieu JF. Transient mosaic patterns of morphological and functional differentiation in the Caco-2 cell line. Gastroenterology 103: 414–423, 1992 [DOI] [PubMed] [Google Scholar]
- 67.Van Wering HM, Bosse T, Musters A, de Jong E, de Jong N, Hogen Esch CE, Boudreau F, Swain GP, Dowling LN, Montgomery RK, Grand RJ, Krasinski SD. Complex regulation of the lactase-phlorizin hydrolase promoter by GATA-4. Am J Physiol Gastrointest Liver Physiol 287: G899–G909, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Van Wering HM, Huibregtse IL, van der Zwan SM, de Bie MS, Dowling LN, Boudreau F, Rings EH, Grand RJ, Krasinski SD. Physical interaction between GATA-5 and hepatocyte nuclear factor-1alpha results in synergistic activation of the human lactase-phlorizin hydrolase promoter. J Biol Chem 277: 27659–27667, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Walters JR. Recent findings in the cell and molecular biology of the small intestine. Curr Opin Gastroenterol 21: 135–140, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Westerman BA, Murre C, Oudejans CB. The cellular Pax-Hox-helix connection. Biochim Biophys Acta 1629: 1–7, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Yamagata K, Nammo T, Moriwaki M, Ihara A, Iizuka K, Yang Q, Satoh T, Li M, Uenaka R, Okita K, Iwahashi H, Zhu Q, Cao Y, Imagawa A, Tochino Y, Hanafusa T, Miyagawa J, Matsuzawa Y. Overexpression of dominant-negative mutant hepatocyte nuclear factor-1 alpha in pancreatic beta-cells causes abnormal islet architecture with decreased expression of E-cadherin, reduced beta-cell proliferation, and diabetes. Diabetes 51: 114–123, 2002. [DOI] [PubMed] [Google Scholar]