Abstract
BACKGROUND
The validity of studies on fecundability in Western countries has been questioned. The complexity of societal and cultural factors makes it difficult to dissect pure biological impact. Our aim was to assess couple fecundability in a population which to a large degree is unaffected by the same socio-cultural influences.
METHODS
We conducted a prospective study on time-to-pregnancy (TTP), with a complete follow-up between 2005 and 2007, among 205 newly married couples in two Palestinian agricultural villages. The couples had never had premarital sex and all planned to become pregnant. We followed the couples from the date of marriage until pregnancy was recognized by a pregnancy test, or at maximum 12 months.
RESULTS
Overall fecundability was 0.17. Unexpectedly, cycle fecundability increased during the first cycles from 0.16 (cycle 1) to 0.25 (cycle 5), after which the expected decline started. The initial increase in fecundability was restricted to couples with teenage brides. A total of 70.7% of the couples conceived within 6 cycles, 13.4% did not conceive during follow-up. Prolonged TTP was associated with the oldest age category for both genders. Educated women appeared to be highly fecund.
CONCLUSIONS
The fecundability result is probably uninfluenced by the societal and cultural factors seen in Western populations, because premarital sex is a taboo in this Muslim population. The increase in fecundability during the first months following marriage is difficult to interpret, but could be due to either behavioural or biological influences.
Keywords: fecundability, time-to-pregnancy, prospective cohort, Palestine
Introduction
Fecundity, i.e. the capacity to reproduce is fundamental to maintaining the human species. It is well known that fertility rates have been declining worldwide as reviewed by Skakkebaek et al., 2006, and in developed countries fertility rates are well below 2.1, which is necessary to sustain a population at its current level. Furthermore, there has been increasing concern amongst both the public and the scientific community that fecundity may be declining at least in developed countries (Carlsen et al., 1992). However, studies on time trends in fecundability have shown both increasing (Joffe, 2000; Jensen et al., 2005; Scheike et al., 2008) and decreasing fecundability (Notkola, 1995) over time.
Human reproductive capacity can be measured at the population level using a time-to-pregnancy (TTP) approach, i.e. measuring the number of months or menstrual cycles it takes a couple to conceive. TTP can be studied prospectively or retrospectively, both designs having their weaknesses and strengths (Joffe et al., 2005). Prospective studies, in particular, provide an estimate of population fecundability that is the probability of conception in a menstrual cycle (Baird et al., 1986). Recognizable fecundability is the probability of a conception which is recognized at the end of the conception cycle by the non-occurrence of menstruation. A large fraction of all conceptions fails to implant or aborts before the beginning of the next cycle (Bongaarts, 1975).
Thus far, all prospective studies assessing population fecundability have been conducted in Western societies with a wide range (from 0.15 to 0.35) in the observed recognizable fecundability (de Mouzon et al., 1988; Wilcox et al., 1988; Ellish et al., 1996; Zinaman et al., 1996; Bonde et al., 1998). In general, prospective studies have been criticized because of problems in the representativeness of the data due lack of good sampling frame and frequently a low participation rate (Joffe et al., 2005). Also, prospective studies have adopted different study decisions on eligibility and in verifying the first menstrual cycle at risk of conception, making it difficult to compare their findings. Consequently, the question arises whether this apparent variability in human fecundability reflects true differences in population fecundability or rather is related to methodological issues.
Family sizes are still high in the Palestinian population, 5.9 in the West Bank and 7.0 on the Gaza Strip (Khawaja, 2003; Palestinian Central Bureau of Statistics, 2007). Premarital sex is a cultural and religious taboo in such a Muslim population (Khawaja, 2003; DeJong et al., 2005). Also, for cultural reasons, couples regularly want to become pregnant immediately after marriage (Khawaja, 2000, 2003; DeJong et al., 2005; Rashad et al., 2005).
Studying newly married couples in the contemporary Palestinian population provides a good opportunity to get a minimally biased estimate of couple fecundability with a prospective design. The objective of this study was describe the cohort, to assess fecundability among newly married Palestinian couples living in agricultural villages, and to discuss the comparability of prospective studies on fecundability.
Materials and Methods
Study population
We conducted a prospective cohort study on TTP among inhabitants of two agricultural Palestinian villages of Hebron district, Beit-U'mmar and Halhul. The total population in Beit-U'mmar is about 14 000 inhabitants and that of nearby Halhul about 22 000 inhabitants (Palestinian Central Bureau of Statistics, 2008).
All the couples planning to marry are obliged to register at the Thalassaemia Centre in Hebron. We identified 207 newly married couples registered during May 2005–August 2007 in the two villages. We provided all couples with a written informed consent explaining the objectives of the study. Also, the consent included information on the voluntary participation and the possibility to withdraw at any point of the study. The study was conducted in accordance with the current revision of the Helsinki Declaration and ethical approval was obtained from the University of Oslo and Hebron University.
All the 207 couples who were identified in this procedure were willing to participate and took part in a baseline interview 2–4 weeks after marriage. We excluded two couples because the wife had been previously married, and 205 couples remained for follow-up, 94 from Beit-U'mmar and 111 from Halhul. All participating couples confirmed in the baseline interview that they were planning to get pregnant (i.e. were not using any contraception to avoid pregnancy).
Collection and handling of the data
Two trained female nurses from the same villages conducted two types of structured face-to-face interviews (baseline and follow-up) in the homes of the participants. The baseline interview took place between 2 and 4 weeks after the wedding day. The baseline questionnaire included questions on socio-demographic factors (male and female age, education, height and weight). Both questionnaires for the wife focused on the most recent menstrual cycle, and included questions on the length and regularity of menstrual cycle, menstrual bleeding intensity and premenstrual tension. Frequency of intercourse was reported by the wife through the following question: ‘At the beginning of marriage /during the last month: how many times per week did you have sexual intercourse?’.
We further interviewed the wives monthly using a follow-up questionnaire until pregnancy was confirmed or 12 months after baseline interview. Questions on physical, environmental and occupational exposure were included. A simultaneous follow-up questionnaire for the men focused on occupational exposure.
Pregnancies were verified with a pregnancy test (ordinary pregnancy test strip, HK1HCG2-100) performed by the interviewer at home if the wife had missed a period before the baseline interview or after the previous interview. The woman was asked to take another test in the village clinic if the home test was positive.
The outcome variable was TTP, assessed as the number of menstrual cycles with presumable ovulation occurring after marriage until recognized pregnancy. We assumed that ovulation occurs 14 days after a menstrual period. Therefore, we considered the cycle when marrying as the first cycle at risk, if the last period before the marriage was within 14 days of marriage. Accordingly, 86 couples were at risk in the cycle of marriage, and 13 (0.151) of those became pregnant immediately. Otherwise, the first full cycle after marriage was considered as the first cycle at risk resulting 119 couples with 20 (0.168) first cycle pregnancies.
TTP was censored for 27 non-pregnant couples after 1 year of follow-up at cycle 12 or 13. We also censored four couples before cycle 12: two couples divorced after cycles 7 and 11, respectively, one husband was arrested after cycle 3, and one wife started medication after cycle 9.
Three categories for education of the man and the wife were adopted: basic school (1–10 years), secondary (11–12) and college or university (>12). For frequency of intercourse, we used four categories: one to six, seven and more than seven times/week, and unknown (23 couples refused to answer this question). Also, we used three categories for age and menstrual duration, the other independent variables were dichotomized (Table I).
Table I.
Characteristics of 205 newly married Palestinian couples and distribution of TTP.
| Characteristics | No. | % | Mean fecundability (95% CI) | Pregnant in first cycle (n = 33)% | Pregnant in ≤6 cycles (n = 145)% | Mean cycles to pregnancy (SD)a | Percent not pregnant in 12 cyclesb (n = 27) % |
|---|---|---|---|---|---|---|---|
| Total | 205 | 100 | 0.17 ( 0.14,0.19) | 16.1 | 70.7 | 5.1 (3.8) | 13.4 |
| Characteristics of the wife | |||||||
| Age (years) | |||||||
| 14–19 | 76 | 37.1 | 0.17 (0.13,0.22) | 13.2 | 77.6 | 5.0 (3.7) | 13.2 |
| 20–24 | 95 | 46.3 | 0.18 (0.15,0.23) | 18.9 | 71.6 | 4.9 (3.6) | 9.5 |
| >24 | 34 | 16.6 | 0.11 (0.08,0.17) | 14.7 | 52.9 | 6.2 (4.5) | 23.5 |
| Education | |||||||
| 1–10 years (basic school) | 25 | 12.2 | 0.13 (0.08,0.20) | 12.0 | 64.0 | 6.0 (4.4) | 24.0 |
| 11–12 years (secondary school) | 66 | 32.2 | 0.15 (0.12,0.20) | 18.2 | 69.7 | 5.2 (4.1) | 16.7 |
| >12 years (college/university) | 114 | 55.6 | 0.18 (0.15,0.22) | 15.8 | 72.8 | 4.8 (3.4) | 8.8 |
| Regularity of menstrual cycle | |||||||
| Regular | 165 | 80.5 | 0.17 (0.14,0.20) | 15.8 | 70.3 | 5.1 (3.7) | 12.1 |
| Irregular | 40 | 19.5 | 0.16 (0.11,0.22) | 17.5 | 62.5 | 5.3 (4.1) | 17.5 |
| Length of menstrual cycle (days) | |||||||
| 15–25 | 57 | 27.8 | 0.16 (0.12,0.21) | 21.1 | 70.2 | 5.2 (3.7) | 14.0 |
| 26–28 | 126 | 61.5 | 0.16 (0.13,0.22) | 12.7 | 69.0 | 5.3 (3.7) | 12.7 |
| >28 | 22 | 10.7 | 0.21 (0.13,0.33) | 22.7 | 81.8 | 4.1 (3.7) | 13.6 |
| Age at menarche (years) | |||||||
| 11–13 | 87 | 42.4 | 0.15 (0.12,0.18) | 11.5 | 69.0 | 5.5 (4.0) | 18.4 |
| >13 | 118 | 57.6 | 0.18 (0.15,0.22) | 19.5 | 72.0 | 4.9 (3.6) | 9.3 |
| Premenstrual tension | |||||||
| Yes | 81 | 39.5 | 0.14 (0.11,0.18) | 12.3 | 64.2 | 5.7 (3.9) | 17.3 |
| No | 124 | 60.5 | 0.18 (0.15,0.22) | 18.5 | 75.0 | 4.7 (3.6) | 10.5 |
| Menstrual bleeding (days) | |||||||
| ≤5 | 133 | 64.8 | 0.16 (0.13,0.19) | 15.8 | 68.0 | 5.3 (3.9) | 14.3 |
| >5 | 72 | 35.1 | 0.18 (0.14,0.23) | 16.7 | 76.4 | 4.8 (3.5) | 11.1 |
| Characteristics of the husband | |||||||
| Age (years) | |||||||
| 16–24 | 58 | 28.3 | 0.18 (0.13, 0.23) | 19.0 | 69.0 | 5.1 (3.6) | 10.3 |
| 25–29 | 98 | 47.8 | 0.18 (0.15, 0.22) | 16.3 | 76.0 | 4.8 (3.6) | 10.2 |
| >29 | 49 | 23.9 | 0.13 (0.10, 0.18) | 12.2 | 63.3 | 5.8 (4.4) | 22.4 |
| Education | |||||||
| 1–10 years (basic school) | 58 | 28.3 | 0.19 (0.14, 0.25) | 29.3 | 76.0 | 4.5 (4.0) | 13.8 |
| 11–12 years (secondary school) | 79 | 38.5 | 0.15 (0.12, 0.19) | 14.0 | 68.4 | 5.5 (3.9) | 15.2 |
| >12 years (college/university) | 68 | 33.2 | 0.16 (0.13, 0.21) | 7.4 | 69.1 | 5.3 (3.5) | 10.3 |
| Frequency of intercourse/week | |||||||
| One to six times | 32 | 16.0 | 0.10 (0.06, 0.15) | 9.4 | 53.1 | 6.5 (4.5) | 31.2 |
| Seven times | 64 | 31.2 | 0.16 (0.12, 0.21) | 12.5 | 71.2 | 5.2 (3.8) | 14.1 |
| More than seven times | 86 | 42.0 | 0.20 (0.16, 0.25) | 22.1 | 74.4 | 4.6 (3.5) | 6.1 |
| Missing | 23 | 11.2 | 0.17 (0.11, 0.27) | 13.0 | 78.3 | 5.0 (3.5) | 8.7 |
aAmong 174 couples who got pregnant during the follow-up.
bA total of 27 women were censored after 1 year of follow-up, 20 of them after 12 cycles and 7 after 13 cycles without getting pregnant.
Statistical analysis
We conducted all analysis by using STATA SE® v. 10 (Stata Statistical Software, 2007). We estimated the mean fecundability as a number of cycles leading to pregnancy divided by the total number of cycles by using the STRATE command. We also did tabular analysis to assess relations between different factors and compared mean TTPs across cycles and other categories, as well as corresponding standard deviations and 95% confidence intervals (95% CI).
Results
The mean age of men was 27.8 years (range 16–62) and that of wives 21.7 years (14–42). Teenage wives accounted for over a third of our participants, and only 16.6% of the wives were older than 24 years. Women were more educated on average (13.2 years) than their husbands (12.1 years). None of the wives smoked. Additional characteristics of the study population and crude fecundability distributions are presented in Table I. The overall cycle fecundability was 0.17 (95% CI: 0.14, 0.19). Altogether, 16.1% of the wives got pregnant in the first cycle and 70.7% within six cycles. Twenty-seven couples (13.4%) did not conceive within 1 year (i.e. sub-fecund couples). Female age >24 years as well as male age >29 years were related to prolonged TTP whereas highly educated women appeared to be highly fertile. Also fecundability was increasing along with increasing coital frequency. The 76 teenage brides were more likely than the others to have a coital frequency below seven times a week (23.3 versus 14.8%) as well as to refrain from answering the question on coital frequency (16/76 versus 7/129).
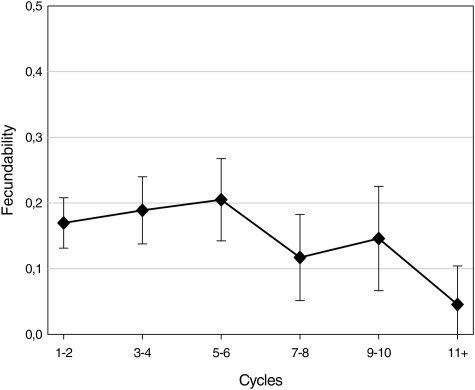
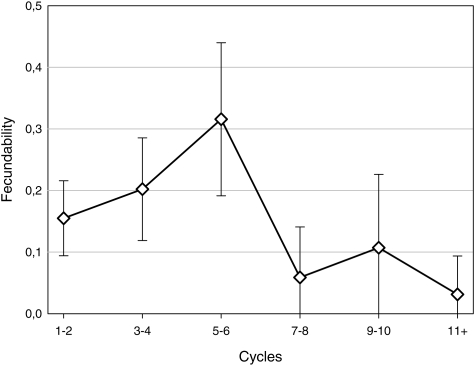
Figure 1 shows fecundability across cycle categories, with low values (0.16) in the first cycle, a maximum (0.25) in cycle 5 and a decline thereafter. The pattern with increasing fecundability during the first cycles was examined in more detail. This initial increase seemed to be restricted to the 76 teenage wives (Fig. 2), with fecundabilities being 0.13, 0.18 and 0.26 in the first three cycles. A similar pattern was not found for older wives (0.18, 0.18 and 0.17). The average coital frequency per week decreased marginally from the first cycle to the fifth cycle in both age groups (from 8.7 to 8.4 in teenage wives and from 9.1 to 8.7 in older wives).
Figure 1.
Fecundability and 95% CI across categories of menstrual cycles among 205 newly married Palestinian couples.
Figure 2.
Fecundability and 95% CI across categories of menstrual cycles among 76 newly married Palestinian couples where the bride was <20 years.
Discussion
We found an overall fecundability of 0.17 among newly married couples in rural Palestinians, which is low compared with findings in Western countries. Contrary to reported figures (Weinberg and Gladen, 1986), cycle-specific fecundability increased initially during the first five cycles, and declined only thereafter. The proportion of sub-fecund couples, defined as no pregnancy within the 1-year follow-up, was 13.4%.
Comparison with other studies
It is well known that pregnancy-based retrospective studies on TTP tend to overestimate population fecundability (Baird et al., 1986; Olsen et al., 1998; Juul et al., 2000; Joffe et al., 2005; Bonde et al., 2006), and this is particularly true for older female age (Jensen et al., 2000). Retrospective studies are often restricted to couples who eventually have got pregnant. This implies that sterile couples are excluded and subfertile couples are underrepresented. However, it is now recognized as good practice that also retrospective studies on TTP need to include information on unprotected intercourse not leading to pregnancy (Bonde et al., 2006).
Prospective studies are free of some problems related to bias in retrospective studies such as sterility exclusion and varying persistence of trying (Jensen et al., 2000). On the other hand, prospective studies have been criticized because of questionable representativeness of the data (Joffe et al., 2005).
We consider our study population exceptionally suitable for a study on couple fecundability. This consideration is built on two assumptions. First, premarital sex is a cultural taboo in the contemporary Palestinian community, and although there may be differences between a taboo and its adherence, we found no evidence of premarital pregnancy nor non-married co-habitation. Second, the study population started unprotected sexual intercourse at the date of marriage while trying to get their first child. Consequently, the study population is most likely not influenced by the main societal and cultural factors (e.g. premarital sex, unprotected sex without intention to become pregnant, induced abortions) that could question the validity and comparability of prospective studies on fecundability conducted in Western populations (Bonde et al., 2006).
In previous prospective studies, we see a wide range in observed recognizable fecundability (0.15–0.35) in healthy populations (de Mouzon et al., 1988; Wilcox et al., 1988; Ellish et al., 1996; Zinaman et al., 1996; Bonde et al., 1998). In the following, we compare our findings with selected prospective studies as regards to societal factors, eligibility and study decisions.
We found similar recognizable fecundability as the observed 0.16 in the cohort of Danish first-pregnancy planners (Bonde et al., 1998), and in the population-based US study (Ellish et al., 1996). Despite the apparent similarity of the findings, there are fundamental differences between these studies. The Danish study started with more than 52 000 trade union members, which after strict eligibility criteria and low participation finally came up with 430 participants (Bonde et al., 1998). Highly fecund couples were likely to be underrepresented in the Danish study (Bonde et al., 1998). By contrast, we were able to recruit all the first-time-married couples in the two villages, making it possible to conduct the study with a relatively small source population. In the US study (Ellish et al., 1996), in turn, women who had been trying to become pregnant ≤12 months or were planning to stop contraception within 6 months were eligible. Contrary to the current study, a drop in fecundability was observed after the first cycle (Ellish et al., 1996).
Because of the high prevalence of premarital sexual activity in most Western communities, studies may have suffered from less precise verification of the first cycle at risk compared with the present study (de Mouzon et al., 1988; Ellish et al., 1996). This lack of early verification clearly underestimates true fecundability because the most fecund couples could have conceived during the recruitment period, and thus having made themselves ineligible for the study. This underestimation of fecundability, due to inclusion of couples with one or more cycles at risk before follow-up, was demonstrated by de Mouzon et al. (1988). Recognizable fecundability was 0.24 among non-smokers in the whole material (n = 1500). The corresponding fecundability was 0.34 in the 468 couples with the first at risk cycle included in the study (de Mouzon et al., 1988).
Some studies have come up with higher recognizable fecundability estimates than we do in this study. Wilcox et al. (1988) studied women who planned to stop using birth-control and observed a recognizable fecundability of about 0.25 for each of the first three cycles. However, women with a history of fertility problems were excluded, and this decision could in part explain the higher fecundability compared with the present study. Also, couples presenting obvious signs of infertility were excluded from the study by de Mouzon et al. (1988). This study decision could in part explain the high observed fecundability of about 0.30 among non-smokers (de Mouzon et al., 1988). On the other hand, earlier demographic studies also suggest a population fecundability of about 0.25 (Leridon, 1977), implying a low overall fecundability among the Palestinians.
Using retrospective approaches, regional differences in waiting TTP have been observed in European countries (Juul et al., 1999; Karmaus and Juul, 1999), as well as between Thai and European regions (Tuntiseranee et al., 1998). These studies and an earlier letter by Sallmén (1996) point out the importance of using a common protocol and standardized questionnaires in comparative studies. Despite the common protocol, cultural differences that include different contraceptive practices and different concepts of pregnancy planning make comparisons difficult (Sallmén, 1996). We believe that prospective studies on TTP also face similar comparability problems. It is hard to say whether the apparent similarity in observed fecundability indicates resemblance in population fecundity, or whether differences reflect true disparity in human fecundity across studies.
We unexpectedly found fecundability increasing rather than decreasing in the first menstrual cycles. A decrease along with menstrual cycles is expected as the most fecund get pregnant and leave follow-up (Weinberg and Gladen, 1986; Khawaja, 2000). Such decrease was seen in some (Wilcox et al., 1988; Ellish et al., 1996; Zinaman et al., 1996), but not all (de Mouzon et al., 1988; Bonde et al., 1998), prospective settings. Recent use of oral contraceptives was common in one study (de Mouzon et al., 1988), and could therefore have caused the low initial fecundability. This explanation is excluded in the current study. The initial increase was restricted to teenage brides. More frequent anovulatory cycles in teenage wives could be a biologically plausible explanation for the initial increase, but irregular cycles, premenstrual tension and cycle duration were comparable to those of older wives. Also, we consider it unlikely that modest differences in sexual activity between younger and older wives could explain this age-related pattern. This is because we observed only a very small reduction rather than an increase in sexual activity during the first months of follow-up both in younger and older wives. Alternatively, the explanation could be socio-cultural or behavioural among those inexperienced young couples. The data at hand are not well suited to distinguish between the two.
Study validity
We used a prospective design and followed the occurrence of pregnancy among all couples who married in two consecutive years in two agricultural villages. Participation was complete and follow-up close to complete. Thus, we consider the data to be representative and also free of many common shortcomings in prospective designs. Workshops in the two villages before start of data collection, explaining the study importance, and using two local female nurses as interviewers may have helped in reaching the present high participation rate.
Moreover, we also successfully verified the first cycle at risk. This assumption is based on two observations: first, we found no premarital conceptions. Second, we observed reasonably similar first cycle fecundabilities in the two groups distinguished by the assessed occurrence of ovulation before or at or after the date of marriage. Previous prospective studies may have failed to verify the first cycle adequately, and in some studies the requirement to start the follow-up from the first cycle at risk after stopping any use of contraception has been relaxed (Ellish et al., 1996). Therefore, we consider our findings valid for young newly married couples in the Palestinian rural population.
Some weaknesses of the present study must be addressed. First, we used a clinical pregnancy, as it was not a feasible option to use daily hormone measurements to identify implantation or preclinical pregnancy. Hence, the results should be interpreted as recognizable fecundability, and we have compared our findings with recognized fecundability in other studies. Second, we used rather crude variable indicators, and even lacked data on some factors that could be important in explaining our findings. Examples are data on couple mental stress, or biological measures to differentiate between ovulatory and anovulatory cycles. Also, the number of subjects was modest though comparable with some of the referred studies (Wilcox et al., 1988; Ellish et al., 1996; Zinaman et al., 1996).
Inferences
Several behavioural, biological or societal explanations for the finding of a rather low overall fecundability could be possible. Low coital frequency in the first months of marriage could explain the finding. However, we saw no evidence of low coital frequency during the very first months of marriage or on an increase thereafter. Another explanation could be that this population endures difficult life conditions, being influenced by the political situation and the occupation. This is likely to create mental stress and lack of predictability that could influence hormonal balance reduce fecundability (Henriksen, 1999; Hjollund et al., 1999). However, this explanation is speculative since we do not have individual or couple data on mental stress. Also, the teen brides could be stressed due to the traditional values in Arab population related to having children and have a first child quickly, as well as new responsibilities and commitments in their new family (DeJong et al., 2005; Palestinian Central Bureau of Statistics, 2007). It remains to be seen whether fecundability increases in this type of population and in younger wives in particular, along with increasing experience on marital living.
Conclusion
We found an overall fecundability of 0.17 among rural Palestinians, which is low compared with findings in Western countries. Contrary to studies previously published, we found fecundability to be increasing in the first five cycles of follow-up. Biological or behavioural factors might explain these findings.
Authors' roles
All authors participated in the conceptualisation and writing of the report, and have seen, reviewed and approved the final version. Y.I., K.N., E.B. and P.K. designed the study. Y.I. and K.N. participated in data collection. Y.I., K.N. and M.S. wrote the first draft and participated in the preliminary analysis. Y.I., M.S., K.N. and P.K. participated in data analysis and interpretation.
Funding
The original study was supported by the NUFU pro X1 50/2002. The NUFU pro X1 50/2002 had no control over the study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. All authors have no conflict of interest.
Acknowledgements
Thanks to Mrs Naseem Talhami, Hebron University, for technical help in data entering and cleaning. Also thanks to the two nurses who had done a great work in all data collection phases, Mrs Yousra Abu-A'reesh and Mrs Kefaya Khlayiel. Also we would like to thank all the participated couples for their time and cooperation.
References
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Hjollund NH, Jensen TK, Ernst E, Kolstad H, Henriksen TB, Giwercman A, Skakkebaek NE, Andersson AM, Olsen J. A follow-up study of environmental and biologic determinants of fertility among 430 Danish first-pregnancy planners: design and methods. Reprod Toxicol. 1998;12:19–27. doi: 10.1016/s0890-6238(97)00096-8. doi:10.1016/S0890-6238(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Joffe M, Sallmén M, Kristensen P, Olsen J, Roeleveld N, Wilcox A. Validity issues relating to time-to-pregnancy studies of fertility. Epidemiology. 2006;17:347–349. doi: 10.1097/01.ede.0000210239.80406.46. doi:10.1097/01.ede.0000210239.80406.46. [DOI] [PubMed] [Google Scholar]
- Bongaarts J. A method for estimation of fecundability. Demography. 1975;12:645–660. doi:10.2307/2060719. [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. doi:10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong J, Jawad R, Mortagy I, Shepard B. The sexual and reproductive health of young people in the Arab countries and Iran. Reprod Health Matters. 2005;13:49–59. doi: 10.1016/s0968-8080(05)25181-9. doi:10.1016/S0968-8080(05)25181-9. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Spira A, Schwatz D. A prospective study of the relation between smoking and fertility. Int J Epidemiol. 1988;17:378–384. doi: 10.1093/ije/17.2.378. doi:10.1093/ije/17.2.378. [DOI] [PubMed] [Google Scholar]
- Ellish NJ, Saboda K, O'Connor J, Nasca PC, Stanek EJ, Boyle C. A prospective study of early pregnancy loss. Hum Reprod. 1996;11:406–412. doi: 10.1093/humrep/11.2.406. [DOI] [PubMed] [Google Scholar]
- Henriksen TB. General psychosocial and work-related stress and reduced fertility. Scand J Work Environ Health. 1999;25:38–39. [PubMed] [Google Scholar]
- Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, Ernst E, Giwercman A, Skakkebaek NE, Olsen J. Distress and reduced fertility: a follow-up study of first-pregnancy planners. Fertil Steril. 1999;72:47–53. doi: 10.1016/s0015-0282(99)00186-7. doi:10.1016/S0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Selection bias in determining the age dependence of waiting time to pregnancy. Am J Epidemiol. 2000;152:565–572. doi: 10.1093/aje/152.6.565. doi:10.1093/aje/152.6.565. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Christensen K. Time trends in waiting time to pregnancy among Danish twins. Hum Reprod. 2005;20:955–964. doi: 10.1093/humrep/deh723. doi:10.1093/humrep/deh723. [DOI] [PubMed] [Google Scholar]
- Joffe M. Time trends in biological fertility in Britain. Lancet. 2000;355:1961–1965. doi: 10.1016/S0140-6736(00)02328-X. doi:10.1016/S0140-6736(00)02328-X. [DOI] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;15:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- Juul S, Karmaus W, Olsen J and the European Infertility, Subfecundity Study Group. Regional differences in waiting time to pregnancy: pregnancy-based surveys from Denmark, France, Germany, Italy and Sweden. Hum Reprod. 1999;14:1250–1254. doi: 10.1093/humrep/14.5.1250. doi:10.1093/humrep/14.5.1250. [DOI] [PubMed] [Google Scholar]
- Juul S, Keiding N, Tvede M. Retrospectively sampled time-to-pregnancy data may make age-decreasing fecundity look increasing. European Infertility and Subfecundity Study Group. Epidemiology. 2000;11:717–719. doi: 10.1097/00001648-200011000-00019. doi:10.1097/00001648-200011000-00019. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Juul S. Infertility and subfecundity in population-based samples from Denmark, Germany, Italy, Poland and Spain. Eur J Public Health. 1999;9:229–235. doi:10.1093/eurpub/9.3.229. [Google Scholar]
- Khawaja M. The recent rise in the Palestinian fertility: permanent or transient? Popul Stud. 2000;54:331–346. doi: 10.1080/713779091. doi:10.1080/713779091. [DOI] [PubMed] [Google Scholar]
- Khawaja M. The fertility of Palestinian women in Gaza, the West Bank, Jordan and Lebanon. Population. 2003;58:273–302. [Google Scholar]
- Leridon H. Human Fertility. Chicago: University of Chicago Press; 1977. [Google Scholar]
- Notkola I. New information on the prevalence of infertility. Uutta tietoa hedelmättömyyden yleisyydestä. Suom Lääkäril. 1995;50:865–870. (in Finnish) [Google Scholar]
- Olsen J, Juul S, Basso O. Measuring time to pregnancy: methodological issues to consider. Hum Reprod. 1998;13:1751–1753. doi: 10.1093/humrep/13.7.1751. doi:10.1093/humrep/13.7.1751. [DOI] [PubMed] [Google Scholar]
- Palestinian Central Bureau of Statistics. Palestinian Family Health Survey, 2006: Final Report. Ramallah: Palestinian Central Bureau of Statistics (PCBS); 2007. [Google Scholar]
- Palestinian Central Bureau of Statistics. Population, Housing and Establishment Census 2007. Census Final Results in the West Bank-Summary (Population and Housing) Ramallah: Palestinian Central Bureau of Statistics (PCBS); 2008. Palestine. [Google Scholar]
- Rashad H, Osman M, Roudi-Fahimi F. Marriage in the Arab World. Washington, DC: Population Reference Bureau Policy Brief (PRB); 2005. [Google Scholar]
- Sallmén M. Fertility in UK compared with Finland. Lancet. 1996;348:616. doi: 10.1016/S0140-6736(05)64833-7. Comment on: Joffe M. Lancet. 1996; 347, 1519–1522. doi:10.1016/S0140-6736(05)64833-7. [DOI] [PubMed] [Google Scholar]
- Scheike TH, Rylander L, Carstensen L, Keiding N, Jensen TK, Strömberg U, Joffe M, Akre O. Time trends in human fecundability in Sweden. Epidemiology. 2008;19:191–196. doi: 10.1097/EDE.0b013e31816334ad. doi:10.1097/EDE.0b013e31816334ad. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Jørgensen N, Main KM, de Meyts ER, Leffers H, Andersson AM, Juul A, Carlsen E, Mortensen GK, Jensen TK, et al. Is human fecundity declining? Int J Androl. 2006;29:2–11. doi: 10.1111/j.1365-2605.2005.00573.x. doi:10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Tuntiseranee P, Olsen J, Chongsuvivatwong V, Limburata S. Fecundity in Thai and European regions: results based on waiting time to pregnancy. Hum Reprod. 1998;13:471–477. doi: 10.1093/humrep/13.2.471. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Gladen BC. The beta-geometric distribution applied to comparative fecundability studies. Biometrics. 1986;42:547–560. doi:10.2307/2531205. [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Zinaman MJ, Clegg ED, Brown CC, O'Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996;65:503–509. [PubMed] [Google Scholar]