Abstract
BACKGROUND
Novel therapeutic approaches for endometriosis based on molecular strategies may prove to be useful. Conditionally replicative adenoviruses (CRAds) are designed to exploit key differences between target and normal cells. The wild-type adenovirus (Adwt) promoter can be replaced by tissue-specific promoters, allowing viral replication only in target cells. Viral infectivity can be enhanced by altering Ad tropism via fiber modification. We investigated whether CRAds can be used to target endometriosis and determined the most efficient transcriptional- and transductional-targeting strategy.
METHODS
An in vitro study was carried out using human endometriotic cell lines, 11Z (epithelial) and 22B (stromal), normal human ovarian surface epithelial cell line (NOSE006) and primary human endometriosis cells. A total of 9 promoters and 12 Ad tropism modifications were screened by means of a luciferase reporter assay. From this screening data, three CRAds (CRAd-S-pK7, CRAd-S-RGD, CRAd-S-F5/3σ1, all incorporating the survivin promoter but with different fiber modifications) were selected to perform experiments using Adwt and a replication-deficient virus as controls. CRAds were constructed using a plasmid recombination system. Viral-binding capacity, rates of entry and DNA replication were evaluated by quantitative real-time PCR of viral genome copy. Cell-killing effects were determined by crystal violet staining and a cell viability assay for different concentrations of viral particles per cell.
RESULTS
Comparison of promoters demonstrated that the survivin promoter exhibited the highest induction in both endometriotic cell lines. Among the fiber-modified viruses, the polylysine modification (pK7) showed the best infection enhancement. CRAd-S-pK7 was validated as the optimal CRAd to target endometriosis in terms of binding ability, entry kinetics, DNA replication and cell-killing effect. CRAd-S-pK7 also exhibited a high level of DNA replication in primary endometriosis cells.
CONCLUSIONS
CRAd-S-pK7 has the best infection and cell-killing effect in the context of endometriosis. It could prove to be a useful novel method to target refractory cases of endometriosis.
Keywords: endometriosis, gene therapy, replicative adenovirus, survivin, targeting
Introduction
Endometriosis is a common chronic gynecological disorder associated with pelvic pain and infertility. It is characterized by the presence of uterine endometrial tissue outside the normal location. The prevalence of endometriosis is about 6–10% in the general female population, and in women with pain, infertility or both, the frequency is 35–50%. Medical treatments, such as oral contraceptive pills, progestagens, gonadotrophin-releasing hormone agonists and danazol, aim to lower circulating estrogen levels. In many cases, medical treatment does not provide adequate control of the disease, and in other cases, treatment has to be used only for a limited amount of time secondary to side effects and risk of osteoporosis. For women with pain, surgery commonly provides temporary relief, although symptoms recur in up to 75% of women within 2 years (Guidice and Kao, 2004). Endometriosis imposes a substantial burden on affected women in terms of their general well-being, the amount of money spent on expensive therapies and the amount of work days missed (Garry et al., 2000). Thus, new treatment modalities are needed to improve the management of endometriosis, especially in cases refractory to the current treatments available.
The etiology of endometriosis is not precisely understood. It is believed to arise from retrograde menstruation in the setting of unique immunologic and genetic background and environmental influences. Molecular processes involved include cell survival, cell adhesion, matrix degradation, matrix invasion, angiogenesis and cellular proliferation (Guidice, 2003). These processes are similar to those present in cancer. Although endometriosis is not considered a premalignant condition, it has been speculated that endometriosis holds malignant potential. In this regard, ovarian cancers and adjacent endometriotic lesions have shown common genetic alterations, such as PTEN, p53 and bcl gene mutations, suggesting a malignant genetic transition spectrum (Nezhat et al., 2008).
An emerging strategy in cancer therapy is the use of conditionally replicative adenoviruses (CRAds) that are designed to exploit key differences between tumor cells and normal cells to allow viral replication only in the former. Two strategies have been used to generate CRAds. One approach involves directly deleting a key adenovirus (Ad) gene, such as E1, to take advantage of the disordered cell cycle regulation in tumor cells with, for example, functionally deficient p53 or retinoblastoma signaling (Bischoff et al., 1996; Fueyo et al., 2000). Another approach involves replacement of wild-type Ad promoters with tumor-specific promoters to drive expression of genes essential for Ad replication, including E1. The utility of these agents is subservient to the identification of promoters which induce the appropriate inductivity and specificity profile. Intraperitoneal administration of CRAds in patients with recurrent ovarian cancer has already been shown to be safe in clinical trials (Vasey et al., 2002; Galanis et al., 2010).
However, the feasibility of the CRAd approach in the context of endometriosis is unknown. Indeed, this will be a novel approach as it has not been thoroughly investigated. Nevertheless, the need for improved management of patients with endometriosis makes this approach a worthwhile endeavor.
Targeting endometriosis at the molecular level is a promising approach. We have previously shown that a vascular endothelial growth factor (VEGF)-targeted CRAd has efficient viral replication in primary endometriotic cells (Rein et al., 2009). In this study, we explored the optimal transcriptional- and transductional-targeting strategy for endometriosis.
Transcriptional targeting seeks to exploit the unique transcriptional profile of the target cell by using tissue-specific promoters (TSP) to restrict transgene expression or viral replication to target cells (Glasgow et al., 2004). Thus, the replicative virus will be active only in the pathologic tissue and will exhibit minimal activity in normal tissues. One potentially useful TSP in the context of endometriosis is survivin. Survivin is an inhibitor of apoptosis protein. Several studies have shown that survivin expression is increased in endometriosis (Ueda et al., 2002; Fujino et al., 2006).
Transductional targeting involves genetic modifications of the Ad capsid proteins to reroute its entry via receptors that are expressed specifically on target cells. As an Ad-based therapeutic approach, CRAd is greatly dependent on vector-mediated tissue transduction. Ad cell entry is mediated via direct binding of the fiber knob domain to its primary cellular receptor, the coxsackievirus and adenovirus receptor (CAR) (Bergelson et al., 1997). Thus, a deficiency in CAR, as seen in several cancers, will limit the use of CRAd as a therapeutic agent. Therefore, it may be necessary to route the Ad vectors via CAR-independent pathways in the context of endometriosis and this strategy may also enhance transduction efficiency. One fiber modification involves the incorporation of a polylysine (pK7) peptide into the C-terminal end of the fiber knob domain. The peptide pK7 binds to heparan sulfate proteoglycans (Wickham et al., 1997). Incorporation of arginine–glycine–aspartate (RGD) peptide sequence into the HI loop of the fiber knob redirects binding to cellular integrin receptors (Dimitriev et al., 1998). Another strategy is knob switching to create chimeric fibers, as exemplified by AdF5/3, which contains the shaft of Ad serotype 5 (Ad5) and the knob of Ad serotype 3 (Ad3). The chimeric virus AdF5/3 effectively targets Ad3 receptors which include CD46, CD80 and CD86 cellular receptors (Kanerva et al., 2002; Ulasov et al., 2006, 2007a). AdF5/3σ1 is a mosaic fiber Ad5 vector encoding two fibers: a reovirus attachment protein sigma 1 (σ1) chimeric fiber and the chimeric AdF5/3 fiber. In addition to Ad3 receptors, AdF5/3σ1 also uses sialic acid and junction adhesion molecule 1 for cell entry (Tsuruta et al., 2007). More radical fiber modifications based on xenotype knob switching with non-human Ad have now been used. As an example, a recombinant Ad5 vector containing the canine Ad serotype 1 knob has shown efficient gene transfer in ovarian cancer (Stoff-Khalili et al., 2005).
In this study, we screened a panel of 9 Ads incorporating different TSPs and 12 fiber-modified Ads in order to identify the characteristics of a virus that would allow the highest level of gene expression in human endometriosis cells. From this screening data, infectivity-enhanced CRAds were used to verify this in human endometriotic cell lines as well as primary endometriosis cells. These data constitute a comprehensive preclinical evaluation of the feasibility and viability of a wide range of promoters as TSPs as well as a wide array of fiber modifications for the purpose of infectivity enhancement of Ad for endometriosis tissue. In the aggregate, this data set provides the basis for designing an optimal CRAd agent to treat endometriosis.
Materials and Methods
Cells lines
Endometriotic cell lines, 11Z (epithelial) and 22B (stromal), were maintained in Dulbecco's modified Eagle medium (Sigma-Aldrich, St Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), l-glutamine (200 µg/ml), penicillin (100 U/ml) and streptomycin (100 µg/ml). Cells were incubated at 37°C in a 5% CO2 environment under humidified conditions. These cell lines were established from active endometriotic lesions from women with endometriosis by in situ electroporation of endometriotic cells with a plasmid containing the SV40 virus (Zeitvogel et al., 2001). The characteristics of these cell lines have been described before and they have been found to be ideal models to study the molecular and cellular aspects of endometriosis in humans (Banu et al., 2008).
The control cell line, normal human ovarian surface epithelial cell line (NOSE006), (a kind gift from Dr A. Berchuck, Durham, NC, USA) was maintained in a 1:1 mixture MCDB 105 medium (Sigma-Aldrich) and Medium 199 (Sigma-Aldrich), supplemented with heat-inactivated 10% FBS, l-glutamine (200 µg/ml), penicillin (100 U/ml) and streptomycin (100 µg/ml) (Spillman et al., 2007). NOSE006 is a spontaneously immortalized normal human ovarian surface epithelial cell line.
The following cells were used in the generation of the recombinant Ads. Human lung carcinoma cell line A549 and human embryonic kidney HEK293 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). 911 cells were obtained with gratitude from Dr Van Der Eb (Leiden University, The Netherlands).
Primary endometriosis cells
All tissue samples were obtained after Institutional Review Board approval. Endometriotic implants and endometriomata were obtained at the time of surgery for diagnosis and treatment of endometriosis during the early proliferative phase of the menstrual cycle. At our institution, gynecologic surgery is performed during the early proliferative phase of the menstrual cycle to avoid potential adverse effects on an unsuspected pregnancy. This represents our usual standard of care. The tissue specimens were placed in RPMI 1640 medium (Sigma-Aldrich) containing 2% FBS, l-glutamine (300 µg/ml), penicillin (100 U/ml) and streptomycin (100 µg/ml). The time between the surgery and infection with viruses was kept to a minimum of 2 h. All procedures were performed under sterile condition. First, the specimen was centrifuged at 184g for 5 min, and the medium removed. The specimen was then cut as small as possible if it was large as in the case of an endometrioma. After adding 10 ml of phosphate-buffered saline (PBS), the tissue was transferred into a tissue grinder. Grinding was then carried out for up to 30 min. The cell suspension was filtered through a 100 µm Nylon cell strainer (Becton–Dickinson, Franklin Lakes, NJ, USA) under suction to remove cell debris. After a second centrifugation at 184g for 5 min, the PBS was removed and the pellet was resuspended in RPMI 1640 medium containing 2% FBS, l-glutamine (300 µg/ml), penicillin (100 U/ml) and streptomycin (100 µg/ml). Subsequently, the primary endometriotic cells were seeded at 1 × 105 cells per well onto 12-well plates followed by immediate infection with viruses at a multiplicity of infection (MOI) of 1000 viral particles per cell (vp/cell). Cells were incubated at 37°C in a 5% CO2 environment under humidified conditions.
Recombinant Ads
The names and characteristics of the viruses used are shown in Tables I–III. CRAd-Survivin (CRAd-S) constructs contain the human survivin promoter to drive E1 expression. To avoid non-specific viral replication, the native E1 promoter was deleted and the survivin-controlled E1 expression cassette was placed in the original E1 region (Van Houdt et al., 2006). Recombinant Ads were created by homologous recombination in 911 cells between a shuttle vector, pScs/PA/S, which carries a human survivin promoter, and a pVK700-based wild-type adenoviral 5 backbone containing a polylysine modification of the fiber knob (CRAd-S-pk7) (Wu et al., 2002), or an RGD motif incorporated into the HI loop of the adenoviral knob 5 protein (CRAd-S-RGD) (Dimitriev et al., 1998), or a mosaic fiber Ad5 vector encoding two fibers: a σ1 chimeric fiber and the chimeric AdF5/3 (CRAd-S-F5/3σ-1) (Tsuruta et al., 2007). A poly(A) signal was inserted between the inverted terminal repeat and the survivin promoter to retain the tissue specificity of the survivin promoter by stopping non-specific transcriptional activity of the inverted terminal repeat. The E3 gene was retained in order to increase the cytotoxic effect of the CRAd agents. Viruses were selected from single plaques on 911 cells, expanded in A549 cells and then purified by double cesium chloride gradient ultracentrifugation. This was followed by dialysis against PBS containing 10% glycerol. The viruses were titrated by plaque assay in HEK293 cells, and physical viral particle concentration (vp/ml) was determined spectrophotometrically based on absorbance at a wavelength of 260 nm. The viruses were stored at −80°C until use.
Table I.
Characteristics of transcriptionally targeted Ads.
| Virus name | Promoter | Reporter | Fiber modification | Replication | Reference |
|---|---|---|---|---|---|
| Ad5luc | CMV | Luciferase | No | No | Krasnykh et al. (1996) |
| AdSurvivinluc | Survivin | Luciferase | No | No | Van Houdt et al. (2006) |
| AdCOX2luc | COX2 | Luciferase | No | No | Yamamoto et al. (2001) |
| AdHeparanaseluc | Heparanase | Luciferase | No | No | Breidenbach et al. (2006) |
| AdSLPIluc | SLPI | Luciferase | No | No | Barker et al. (2003) |
| AdCXCR4luc | CXCR4 | Luciferase | No | No | Zhu et al. (2004a) |
| AdEGP-2luc | EGP-2 | Luciferase | No | No | Lu et al. (2005) |
| AdMesothelinluc | Mesothelin | Luciferase | No | No | Breidenbach et al. (2005) |
| AdMidkineluc | Midkine | Luciferase | No | No | Adachi et al. (2000) |
| Adroboluc | Robo4 | Luciferase | No | No | Unpublished |
Table II.
Characteristics of transductionally targeted Ads.
| Virus name | Promoter | Reporter | Fiber modification | Replication | Reference |
|---|---|---|---|---|---|
| AdRGDluc | CMV | Luciferase | RGD | No | Dimitriev et al. (1998) |
| AdF5/3luc | CMV | Luciferase | F5/3 | No | Kanerva et al. (2002) |
| Adσ1luc | CMV | Luciferase | σ1 | No | Tsuruta et al. (2005) |
| AdF5/3σ1luc | CMV | Luciferase | F5/3σ1 | No | Tsuruta et al. (2007) |
| AddoubleRGDluc | CMV | Luciferase | doubleRGD | No | Unpublished |
| AdpK7luc | CMV | Luciferase | pK7 | No | Wu et al. (2002) |
| AdRGDpK7luc | CMV | Luciferase | RGDpK7 | No | Wu et al. (2002) |
| AdCK1luc | CMV | Luciferase | CK1 (canine knob 1) | No | Stoff-Khalili et al. (2005) |
| AdCK2luc | CMV | Luciferase | CK2 (canine knob 2) | No | Stoff-Khalili et al. (2005) |
| AdOVF3/2luc | CMV | Luciferase | OVF3/2 (ovine fiber) | No | Unpublished |
| AdPF4/12luc | CMV | Luciferase | PF4/12 (porcine fiber) | No | Unpublished |
| AdPK2/3luc | CMV | Luciferase | PK2/3 (porcine knob) | No | Unpublished |
Table III.
Characteristics of CRAds.
| Virus name | Promoter | Reporter | Fiber modification | Replication | Reference |
|---|---|---|---|---|---|
| CRAd-S-pK7 | Survivin | No | pK7 | Yes | Ulasov et al. (2007b) |
| CRAd-S-RGD | Survivin | No | RGD | Yes | Ulasov et al. (2007b) |
| CRAd-S-F5/3σ1 | Survivin | No | F5/3σ1 | Yes | Unpublished |
Luciferase assay
Cells were seeded at 2 × 105 cells per well onto 48-well plates. The next day, the cells were infected with replication-deficient viruses at an MOI of 100 vp/cell. Forty-eight hours post-infection, the medium was removed and the cells were washed with PBS. The cells were then lysed with 50 µl of cell culture lysis reagent (Reporter Lysis Buffer; Promega Corp., Madison, WI, USA) for 10 min. The wells were scraped to collect the cell lysate. Twenty microliters of cell lysate from each well were mixed with 100 µl of luciferase assay reagent. Luciferase activity was then measured with a Berthold Lumat LB9501 (Wallac, Gaithersburg, MD, USA). Experiments were performed in triplicate and luciferase activities were standardized to the relative light unit values of Ad5luc [the cytomegalovirus (CMV) promoter activity was set as 100%].
Quantitative real-time PCR
DNA was isolated from infected cells according to a standard protocol, using a DNeasy tissue kit (Qiagen, Valencia, CA, USA). Duplexing real-time PCR (qPCR) was utilized. All primers and probes were designed by the Primer Express 1.5 software and synthesized by Sigma-Aldrich. To evaluate gene transfer, the number of Ad E4 gene copies contained in total DNA extracted from infected cells was determined by amplification of the E4 gene with forward primer 5′-GGAGTGCGCCGAGACAAC-3′, reverse primer 5′-ACTACGTCCGGCGTTCCAT-3′ and probe: 5′-6-FAM-TGGCATGACACTACGACCAA-CACGATCT- BHQ-1-3′. E4 copies were normalized to the house-keeping gene (human β-actin) amplified with forward primer 5′-CCAGCAGATGTGGATCAGCA-3′, reverse primer 5′-CTAGAAGCATTTGCGGTGGAC-3′ and probe 5′-6-HEX-AGGAGTATGACGAG-TCCGGCCCCTC-BHQ-1-3′.
With pre-optimized concentration of primers and probe, the components of qPCR mixture were designed to result in a master mix with a final volume of 9.0 µl per reaction containing 1× FastStart Taqman Probe Master (Roche Applied Science, Indianapolis, IN, USA), both target and house-keeping gene primers and probes in 100 nM. For the assay, known amount of E4 template DNA (108, 106, 104 and 102 copies/µl) was amplified to generate a standard curve for quantification of the E4 copy numbers of unknown samples. Known amount of human genomic DNA (50, 5.0, 0.5 and 0.05 ng/µl) was amplified to generate a standard curve for determination of the concentration of unknown samples. One microliter of sample will be added to 9.0 µl of PCR mixture in each well of Roche's 96-well plate. A no-template control received 1 µl of water. The plate was sealed by LightCycler 480 Sealing Foil (Roche Applied Science) and centrifuged to facilitate mixing.
All qPCR was carried out using a LightCyclerTM 480 (Roche Applied Science). Thermal cycling conditions were subjected to 10 min at 95°C and 45 cycles of 15 s at 95°C and 1 min at 60°C. Data were analyzed with LightCycler 480 software 1.5.0 SP1.
Binding assay
Twenty-four-well plates were seeded at 1 × 104 cells per well. The next day, the cells were infected with viruses at an MOI of 1000 vp/cell. The plates were then incubated at 4°C to allow viruses to bind to the cells while preventing them from entering into the cells (Morgan et al., 1969). After 3 h, the cells were harvested and washed with PBS three times at 4°C. DNA was isolated from cells according to a standard protocol using a DNeasy tissue kit and a qPCR assay for the E4 gene was performed. Ad E4 gene copy numbers detected in the samples were normalized to human β-actin. A higher MOI of 1000 vp/cell was used for the binding experiments since the plates were incubated at 4°C for a short period of only 3 h. This requires larger viral inocula in order to obtain accurate signal readouts.
Viral entry
Twelve-well plates were seeded at 1 × 105 cells per well. The next day, the cells were infected with viruses at an MOI of 1000 vp/cell. Plates were placed in an incubator at 37°C and timing was started. Cells were harvested at 1 and 2 h post-infection and were then washed with PBS. DNA was isolated from cells according to a standard protocol using a DNeasy tissue kit and a qPCR assay for E4 genes was performed. Ad E4 gene copy numbers were determined and normalized to human β-actin.
Quantitative analysis of viral replication
Twelve-well plates were seeded at 2 × 105 cells per well. The next day, the cells were infected with CRAd-S-pK7, CRAd-S-RGD, CRAd-S-F5/3σ-1, Adwt (positive control) or Ad5luc (negative control) at an MOI of 100 vp/cell. For replication analysis on Days 1, 3 and 6, infected cells were washed with PBS and were then subjected to DNA isolation using a DNeasy tissue kit. Subsequently, a qPCR assay for E4 genes was performed. Ad E4 gene copy numbers were determined and normalized to human β-actin. Data are reported as the ratio of E4 copy number per human β-actin copy.
Viral cytopathic assay
The cytotoxic effect of the CRAd agents was analyzed by determining the viability of cells by crystal violet staining after infection. Twenty-four-well plates were seeded at 2 × 104 cells per well. After 24 h, they were either mock-infected or infected with CRAds (CRAd-S-pK7, CRAd-S-RGD and CRAd-S-F5/3σ-1), Adwt (positive control) or Ad5luc (negative control) at MOI of 1, 10, 100 and 1000 vp/cell in 0.5 ml of media with 2% FBS. After 4 h, 1.5 ml of media with 10% FBS was added to each well. After 3 days, the medium was changed and fresh growth medium was added. After 6 days of infection, the medium was aspirated and the cells were fixed with 10% buffered formalin. After 10 min, the 10% buffered formalin was removed and the cells were stained with 1% crystal violet in 70% ethanol for 3 h, followed by washing with tap water and set to air dry. Pictures were taken with a Sony digital camera.
Cell viability assay (MTS assay)
Ninety-six-well plates were seeded at 5 × 103 cells per well. After 24 h, they were either mock-infected or infected with viruses at an MOI of 1, 10 and 100 vp/cell. The plates were incubated at 37°C and 5% CO2. After 7 days, 20 µl of 3-(4,5 dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) solution (Promega) was added to each well. The cells were then incubated for 4 h. The absorbance was recorded at 490 and 630 nm (background) with a Powerwave HT 340 (BioTek, Winooski, VT, USA) plate reader. The background reading at 630 nm was then subtracted from the reading obtained at 490 nm (OD). OD obtained from wells with infected cells were normalized to the values for uninfected cells in order to calculate the relative cell viability. Relative cell viability was plotted against vp/cell.
Statistics
Data are presented as mean ± SD. Statistical differences among groups were assessed with a two-tailed Student's t-test. A value of P < 0.05 was considered to be statistically significant.
Results
Evaluation of promoter activity for targeting endometriotic cell lines
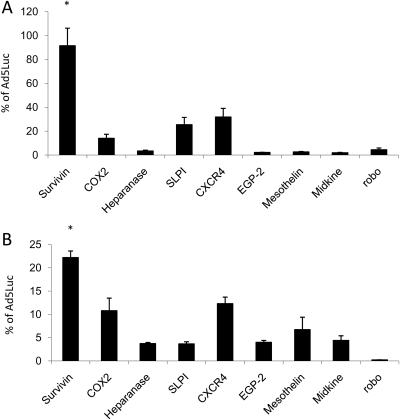
In order to investigate the optimal transcriptional-targeting strategy, we compared the transcriptional activity of nine Ads incorporating different TSPs to that of Ad5luc (Ad with the constitutive CMV promoter) at an MOI of 100 vp/cell. The TSPs were survivin, cyclooxygenase-2 (COX-2), heparanase, secretory leukocyte protease inhibitor (SLPI), CXC chemokine receptor 4 (CXCR4), epithelial glycoprotein-2 (EGP-2), mesothelin, midkine and roundabout (Table I). As shown in Fig. 1, AdSurvivinluc had the highest level of luciferase activity in the endometriotic epithelial (11Z) and stromal (22B) cell lines. Thus, the survivin promoter is the best candidate TSP for endometriosis.
Figure 1.
Transcriptional activity in endometriotic cell lines.
Luciferase activity by transcriptionally targeted Ads at an MOI of 100 vp/cell, expressed as a percent of Ad5luc activity in (A) 11Z and (B) 22B. Luciferase activities of the candidate promoters are expressed as a percentage of the CMV promoter activity. Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other viruses.
Evaluation of fiber modification for targeting endometriotic cell lines
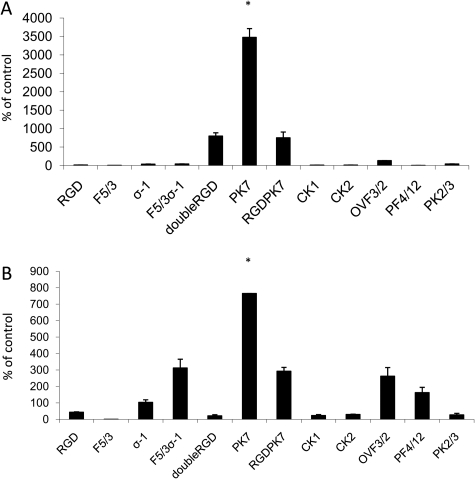
In order to investigate the optimal transductional-targeting strategy, we compared the transductional activity of 12 Ads incorporating different fiber modifications (Table II) in two endometriotic cell lines at an MOI of 100 vp/cell. Fiber modifications were tested independently of the promoter since all the Ads had the same CMV promoter. Figure 2 shows that the polylysine fiber modification (pK7) led to the highest level of luciferase activity in both endometriotic cell lines. Therefore, pK7 is the best fiber modification in the context of endometriosis.
Figure 2.
Transductional activity in endometriotic cell lines.
Luciferase activity by transductionally targeted Ads at an MOI of 100 vp/cell, expressed as a percent of control virus activity in (A) 11Z and (B) 22B. Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other viruses.
CRAd-S-pK7 shows superior binding to endometriotic cells
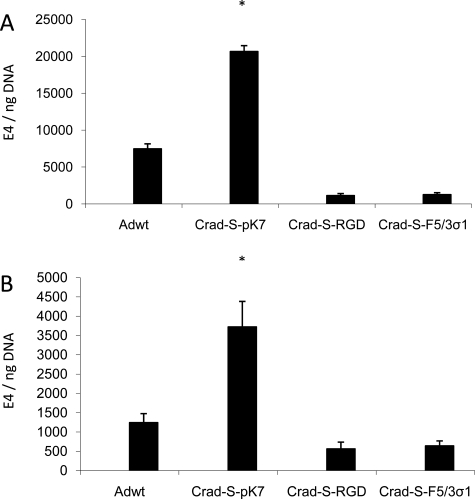
Having identified the optimal transcriptional- and transductional-targeting strategy using replication-deficient viruses, we proceeded to validate these findings in the context of CRAds. To this end, three CRAds were selected incorporating the desirable features suggested by the above experiments. They are all survivin promoter-based CRAds with different fiber modifications (Table III). Having identified the characteristics of the optimal CRAd (CRAd-S-pK7) from the initial screening experiments, we chose control CRAds with suboptimal characteristics for comparison (CRAd-S-RGD and CRAd-S-F5/3σ-1). Since the first interaction between a virus and the target cell is viral binding, the first logical step would be to investigate whether the selected CRAds bind to the endometriotic cell lines. Hence, binding capacities of the three selected CRAds were compared with that of Adwt. The cells were infected at an MOI of 1000 vp/cell. The cells were incubated at 4°C in order to prevent the viruses from entering the cells. As shown in Fig. 3, CRAd-S-pK7 had the greatest binding capacity to the cells for both endometriotic cell lines.
Figure 3.
Viral-binding ability in endometriotic cell lines.
Viral binding at an MOI of 1000 vp/cell, expressed as E4 gene copy numbers normalized to β-actin in (A) 11Z and (B) 22B. Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other viruses.
CRAd-S-pK7 shows superior entry kinetics into endometriotic cells
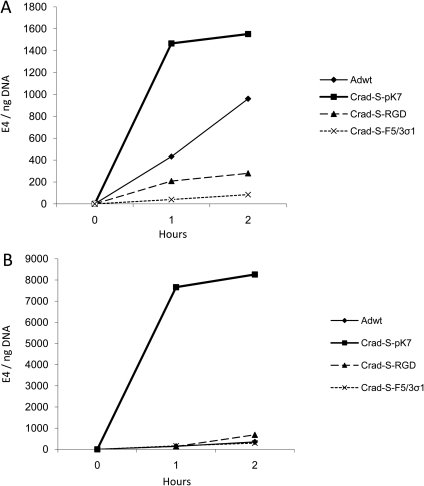
After binding to the cell, Ad enters the cell by endocytosis. We thus attempted to characterize the entry kinetics of the CRAds in the two cell lines. Entry kinetics of the three selected CRAds was compared with that of Adwt. The cells were infected at an MOI of 1000 vp/cell and were incubated at 37°C. Cells were harvested for qPCR after 1 and 2 h after infection. Figure 4 shows that CRAd-S-pK7 entered into the cells at a faster rate compared with the other viruses. This difference was even more prominent for the endometriotic stromal cell line, 22B, where CRAd-S-pK7 entered at a rate at least 47 times faster than any of the other viruses in the first hour after infection. Therefore, CRAd-S-pK7 enters into the endometriotic cells at a rate even faster than Adwt.
Figure 4.
Entry kinetics in endometriotic cell lines.
Viral entry rates at 1 and 2 h after infection at an MOI of 1000 vp/cell, expressed as E4 gene copy numbers normalized to β-actin in (A) 11Z and (B) 22B.
CRAd-S-pK7 has the best replication profile in endometriotic cell lines
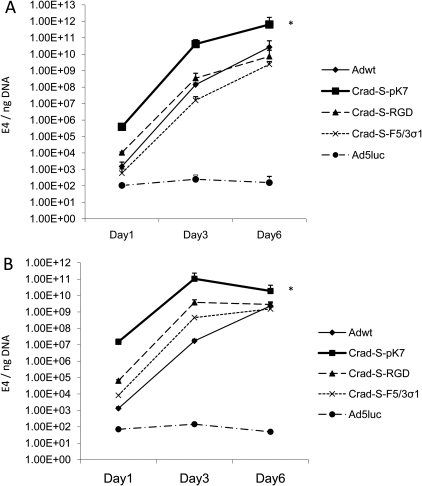
After achieving cell entry, Ad DNA starts to replicate in the nucleus. Thus, we compared the DNA replication rates of the viruses in both endometriotic cell lines. The cells were infected at an MOI of 100 vp/cell and the cells were harvested at the indicated time points in order to perform qPCR. E4 gene copy numbers were normalized to the human house-keeping gene, β-actin. The CRAd-S-pK7 genome had the highest level of replication in both endometriotic cell lines at all time points (Fig. 5). For 11Z, the CRAd-S-pK7 genome showed continuous replication for up to 6 days. Therefore, CRAd-S-pK7 possesses the best replication profile in endometriotic cells.
Figure 5.
Viral replication in endometriotic cell lines.
Cells were infected with CRAd-S-pK7, CRAd-S-RGD, CRAd-S-F5/3σ1, Adwt and Ad5luc at an MOI of 100 vp/cell. Cells and growth medium were harvested on Days 1, 3 and 6 after infection and then subjected to qPCR. E4 gene copy numbers were normalized to β-actin in (A) 11Z and (B) 22B. Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other viruses.
CRAd-S-pK7 selectively kills endometriotic cells
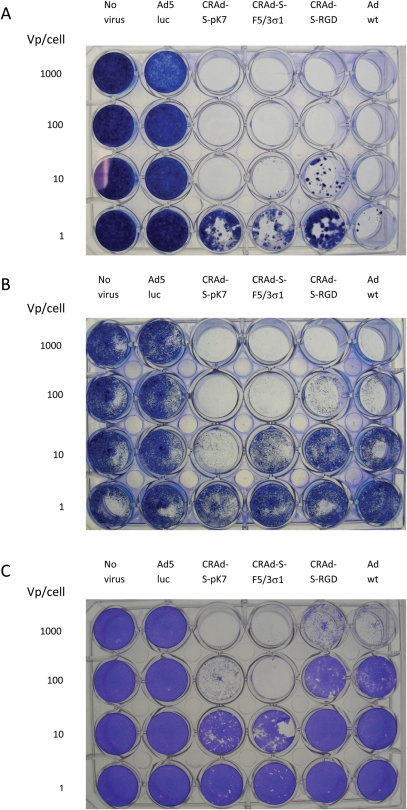
The definitive experiment that will demonstrate the efficacy of a CRAd as virotherapy is cell killing. Thus, all CRAds were tested for their cell-killing potential via cytolysis in the two endometriotic cell lines as well as the NOSE006 cell line (Fig. 6). NOSE006 is a normal human ovarian surface epithelial cell line which was used as a control. Cell lysis was evaluated after 6 days of incubation via crystal violet staining. CRAd-S-pK7 showed a dose-dependent cytopathic effect in both endometriotic cell lines. For 11Z, CRAd-S-pK7 was the only one with 100% cell lysis at an MOI of 10 vp/cell, among the three CRAds. For 22B, CRAd-S-pK7 showed the greatest cytotoxic potential, even superior to Adwt. In the case of NOSE006, CRAd-S-pK7 showed <5% cell lysis at 10 vp/cell. As expected, Ad5luc (replication-deficient virus) had no cytotoxic effect. This suggests that CRAd-S-pK7 would have a good therapeutic index if used intraperitoneally. This experiment also proves that CRAd-S-pK7 is the optimal CRAd for use in endometriosis.
Figure 6.
Viral cytopathic assay in endometriotic cell lines and control cell line (NOSE006).
Cells were infected at different vp/cell (1, 10, 100 and 1000). Cells were stained with crystal violet after 6 days of incubation. (A) 11Z, (B) 22B and (C) NOSE006.
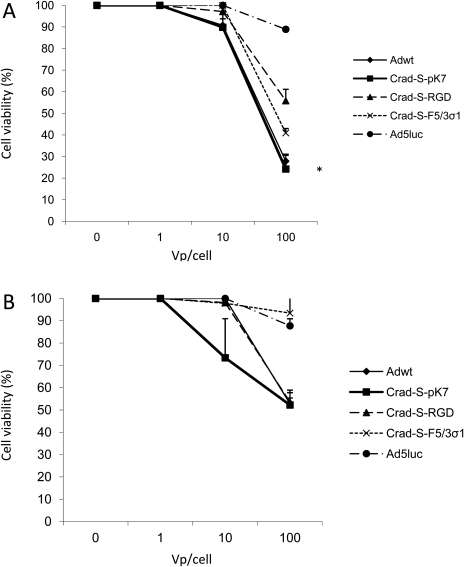
Another experiment that can be used to investigate cell killing is the MTS assay. Thus, the CRAds' cell-killing potential was further demonstrated by means of the MTS assay (Fig. 7). For 11Z, CRAd-S-pK7 had the highest cytotoxic effect at an MOI of 100 vp/cell, among the three CRAds. For 22B, CRAd-S-pK7 had the highest cytotoxic effect at an MOI of 10 vp/cell; however, the difference did not reach statistical significance (P = 0.075). This provides further evidence for the superior cytotoxic effect of CRAd-S-pK7 in endometriosis.
Figure 7.
MTS assay in endometriotic cell lines, (A) 11Z and (B) 22B.
OD is the difference between the reading obtained at 490 nm and the background reading at 630 nm. Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other CRAds.
CRAd-S-pK7 is the optimal CRAd to target primary human endometriosis cells
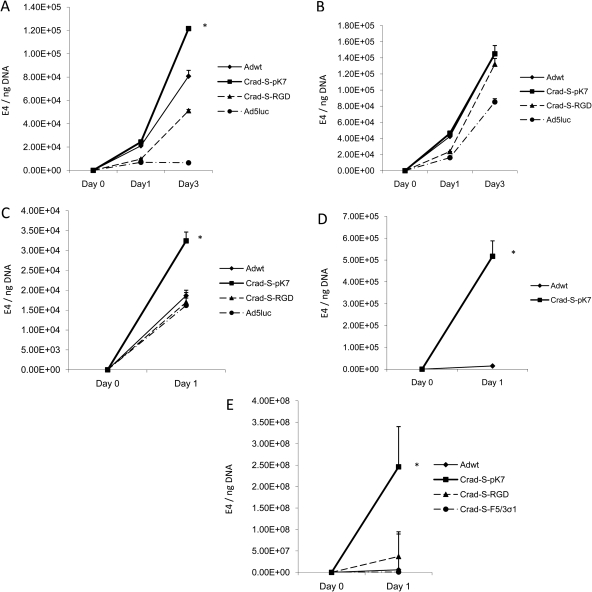
In order to verify the CRAd's potential in primary human tissue, we evaluated the viral replication rates in five primary human endometriosis samples from four patients (Fig. 8) with revised American Society for Reproductive Medicine (ASRM) Stages I–IV (American Society for Reproductive Medicine, 1997). One sample was an ovarian endometrioma (Fig. 8A), whereas the rest were peritoneal endometriotic implants. The cells were collected after the indicated time points after viral infection and then subjected to qPCR to calculate viral copy numbers. For all five samples, CRAd-S-pK7 showed the highest level of replication. However, in one sample, the replication rate of CRAd-S-pK7 was not statistically different from that of Adwt (Fig. 8B). Therefore, CRAd-S-pK7 was validated as the optimal CRAd to target primary human endometriosis cells.
Figure 8.
CRAds replicate in primary endometriosis cells. CRAd-S-pK7 has the best DNA replication profile.
Each bar represents the mean of three experiments ± SD. *P < 0.05 versus other viruses.
Discussion
We here report that the conditionally replicative adenovirus, CRAd-S-pK7, can target endometriosis with a cell-killing effect. This is the first study to evaluate an infectivity-enhanced CRAd for gene therapy in endometriosis. It proves that targeting endometriosis at the molecular level is a feasible and promising therapeutic approach.
The concept of gene therapy in the context of endometriosis was first applied in a mouse model which was produced by injecting endometrial fragments taken from donors into the peritoneal cavity of recipients (Dabrosin et al., 2002). Endometriotic lesions were eradicated in these mice by transient overexpression of angiostatin gene, a natural angiogenesis inhibitor, delivered to the mouse peritoneal cavity by an Ad vector.
Fortin et al. (2004) used ganciclovir to treat nude mice implanted with human endometrial fragments infected with a replication-defective Ad expressing thymidine kinase. This experimental model of endometriosis relies on the infection of human endometrial fragments by an Ad carrying green fluorescent protein. A significant decrease in lesion size was observed by in vivo imaging in ganciclovir-treated mice.
Another approach used dominant-negative mutants of estrogen receptor genes delivered to endometriosis cells via an Ad vector to abrogate estrogen action on these cells (Othman et al., 2007). This led to decreased cell proliferation and increased apoptosis of the endometriotic cells in vitro. Othman et al. (2008) concluded that an Ad under heparanase promoter was a promising vector for endometriosis gene therapy with a ‘endometriosis on, liver off’ phenotype. More recently, it has been shown that a VEGF-targeted CRAd showed efficient viral replication and induction of apoptosis in primary endometriotic cells (Rein et al., 2009).
Our study expands on the CRAd approach to a dual-targeting concept with combined transcriptional and transductional targeting. One key requirement for endeavoring molecular therapeutics for endometriosis is a high therapeutic index. Such a dual-targeting strategy has a great potential to increase the efficiency and specificity of targeted gene therapy for endometriosis.
Human immortalized endometriotic epithelial (11Z) and stromal (22B) cell lines were established from active endometriotic lesions from women with endometriosis by in situ electroporation of endometriotic cells with a plasmid containing the SV40 virus (Zeitvogel et al., 2001). These cells retained the phenotypic characteristics and several in vivo properties of endometriosis. It was subsequently shown that their gene expression patterns indicate their mitogenic, angiogenic, migrating and invading nature (Banu et al., 2007). Furthermore, these patterns closely match those in vivo from women with endometriosis as well as animal models. Therefore, these cell lines were selected for our experiments as they are ideal models to study the molecular aspects of endometriosis. The initial experiments involved screening a panel of transcriptionally targeted Ads and transductionally targeted Ads to identify the optimal promoter and fiber modification. Comparison of promoters demonstrated that the survivin promoter exhibited the highest induction in both endometriotic cell lines. Among the fiber-modified viruses, the polylysine modification (pK7) showed the best infection enhancement in both endometriotic cell lines. This information was then used to select appropriate CRAds for subsequent experiments. CRAd-S-pK7 was validated as the optimal CRAd to target endometriosis in terms of binding ability, entry kinetics, DNA replication and cell-killing effect.
Having shown that CRAd-S-pK7 is the optimal CRAd to target endometriosis in both endometriotic cell lines, we sought to prove the same in primary human endometriosis cells. One patient from each of the four ASRM stages was selected. Indeed, the DNA replication of CRAd-S-pK7 was even better than that of Adwt for four out of the five samples, whereas it was comparable to Adwt for the fifth sample. This suggests that CRAd-S-pK7 is superior to the VEGF-targeted CRAd that was investigated by Rein et al. (2009). In the latter report, the DNA replication of the VEGF-targeted CRAd was lower than that of Adwt for all three patients. In addition, we have shown that CRAd-S-pK7 can be used for patients with all stages of endometriosis. This also suggests that survivin is involved in the pathogenesis of all stages of the disease.
Survivin is one of the inhibitor of apoptosis proteins. Survivin gene expression was found to be closely associated with reduction of apoptotic cells and invasive phenotype in endometriosis (Ueda et al., 2002). Gene expression levels of survivin in ectopic endometriotic tissues were higher than those in eutopic endometrium obtained from patients with or without endometriosis over the whole cycle (Fujino et al., 2006). Survivin expression is increased in ovarian endometriomata where protection from apoptosis by survivin may be closely related to the growth of endometriomata (Goteri et al., 2005). Present during fetal development, survivin was undetectable in terminally differentiated adult tissues (Ambrosini et al., 1997). The fact that survivin expression is increased in endometriotic lesions while being undetectable in normal tissues indicates that survivin may be an excellent candidate TSP for targeting endometriosis. Our study shows that the survivin promoter exhibited the highest induction in both endometriotic epithelial (11Z) and stromal (22B) cell lines. This proves that the survivin promoter functions in different cell types. Therefore, our data lead to the incontrovertible conclusion that among the promoters that were screened, the survivin promoter is the best promoter in the context of endometriosis.
COX-2 is induced during inflammation and cell proliferation and differentiation. COX-2 expression in surface and glandular epithelia of normal women varies during the menstrual cycle, being lowest in the early proliferative phase and gradually increases thereafter (Ota et al., 2001). In patients with endometriosis, COX-2 expression was higher in eutopic endometrium and endometrioma. In addition, COX-2 expression in endometrioma remained pronounced throughout the menstrual cycle. Estradiol, COX-2, and aromatase exist in a positive feedback loop whereby estradiol stimulates COX-2 expression and the COX-2-prostaglandin E2 pathway induces aromatase expression leading to increased estradiol level (Bukulmez et al., 2007). The highest levels of COX-2 mRNA were found in red implants. This is consistent with our finding of fairly high activity of the COX2 promoter, although it was not the best promoter for endometriosis.
Heparanase can cleave heparan sulfate and degrade extracellular matrix and basement membrane. As a barrier, the ability of extracellular matrix and basement membrane is weakened and thus it becomes easier for endometriosis to invade. Heparanase was found to be highly expressed in the ectopic endometriotic lesions, regardless of the menstrual cycle phase (Xu et al., 2007). This was confirmed by Jingting et al. (2008) who reported higher expression of heparanase in the ectopic and eutopic endometrium of patients with endometriosis. In addition, heparanase expression correlated with clinical stage of endometriosis. Othman et al. (2008) concluded that an Ad under heparanase promoter was a promising vector for endometriosis gene therapy. However, our data suggest that heparanase is not highly expressed in all endometriotic implants and that the heparanase is not the optimal promoter in the context of endometriosis. In addition, survivin has a lower liver tropism compared with heparanase (Othman et al., 2008).
SLPI is an inhibitor of leukocyte proteases and plays a role in protecting mucosae from injury associated with inflammation. The amount of SLPI in the peritoneal fluid was elevated in patients with endometriosis and SLPI was localized in endometriotic lesions (Suzumori et al., 1999). It has been suggested that SLPI may act to suppress the progression of endometriosis by protease inhibition since ectopic growth of endometrium is dependent on proteolytic activity. However, our data suggest that SLPI is not highly expressed in all endometriotic implants as the SLPI promoter exhibited reasonably high activity only in the 11Z cell line.
Chemokines are a family of low molecular weight proteins that mediates cell migration. CXC chemokines are believed to be involved in the pathogenesis of endometriosis (Berkkanoglu and Arici, 2003). The ability of endometrial implants to survive in ectopic locations may be related to an aberrant immune response. CXCR4 is one receptor for the α-chemokine subfamily. Our results demonstrate that the CXCR4 promoter is transcriptionally active in endometriosis, although survivin is a better promoter. This is consistent with the findings of Furuya et al. (2007) who reported that CXCR4 was up-regulated in endometriosis.
EGP-2 is a transmembrane glycoprotein that functions as an epithelial cell adhesion molecule; hence, it is also known as Ep-CAM. It is expressed in a variety of epithelial tissue-derived cancers, including ovarian, endometrial and cervical carcinomas (Balzar et al., 1999). However, EGP-2 expression has not been evaluated in endometriosis. From our data, it can be concluded that EGP-2 is not highly expressed in endometriosis and thus it is unlikely to contribute to the pathogenesis of the disease.
Mesothelin is a glycosylphosphatidylinositol-linked cell surface glycoprotein which is expressed in different tumor types such as ovarian cancer, mesothelioma and different squamous cell cancers (Chang and Pastan, 1996). Mesothelin is not present in normal tissues except mesothelial cells. No studies have evaluated the expression of mesothelin in endometriosis. However, given the similarity between endometriosis and ovarian cancer, we thought that the mesothelin promoter was worth investigating in the context of endometriosis. Our data would suggest that mesothelin is not highly expressed in endometriosis.
Midkine is a member of the heparin-binding growth factor family and it has important roles in development. In spite of the fact that its expression is restricted to certain tissues in the adult, it is strongly induced in oncogenesis, inflammation and tissue repair. Midkine levels have been found to be higher in eutopic endometrium of patients with endometriosis, thus suggesting that increased midkine expression may be related to the initiation of ectopic endometrial implants and peritoneal invasion (Chung et al., 2002). Midkine concentrations in the peritoneal fluid of women with advanced endometriosis (Stages II–IV) were higher than those without endometriosis and with Stage I endometriosis (Hirota et al., 2005). Our data suggest that midkine is not highly expressed in ectopic endometriotic implants. This is also consistent with the finding that ectopic ovarian endometriosis tissue had a lower expression of midkine than eutopic endometrium from control and endometriosis patients (Chung et al., 2002).
Roundabout (robo) is a class of neural guidance receptors that binds slit ligands. Slit-robo signaling is involved in axonal repulsion as well as inhibition of leukocyte migration. The robo gene family consists of four members: robo1, robo2, robo3 and robo4. Robo4 has been described as endothelial-specific and it has been shown to be essential for angiogenesis in vivo in the zebrafish (Bedell et al., 2005). We hypothesized that robo4 may be involved in endometriotic angiogenesis and thus investigated the utility of robo4 as a promoter in endometriosis. However, our results would suggest that this is not the case given the fact that Adroboluc led to low luciferase expression in both cell lines (Fig. 1). In fact, very recently, Shen et al. (2009) found increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomata compared with normal endometrium. These investigators also suggested that SLIT/ROBO1-mediated angiogenesis may play a role in recurrence.
Among the different fiber modifications, the pK7 fiber modification, which involves the incorporation of a polylysine (pK7) peptide into the C-terminal end of the fiber knob domain, showed the best infectivity enhancement. The peptide pK7 binds to heparan sulfate proteoglycans (Wickham et al., 1997). This suggests that heparan sulfate is overexpressed in endometriosis and hence may play a role in the pathogenesis of the disease. A recent study analyzed the composition of sulfated glycosaminoglycans in deeply infiltrating endometriosis and found that dermatan sulfate predominated compared with heparan sulfate and chondroitin sulfate (Berardo et al., 2009). Thus, there is a paucity of studies that have evaluated heparan sulfate expression in endometriosis and this warrants further investigation.
The pathogenesis of endometriosis most likely involves retrograde menstruation through the fallopian tube into the peritoneal cavity. This is followed by attachment of the endometrial fragments onto the peritoneum through the interaction of integrins with the extracellular matrix molecules (Puy et al., 2002). Immunohistochemical analysis has shown that αvβ5 and αvβ6 integrins are expressed in ectopic tissue of patients with endometriosis. Incorporation of RGD peptide sequence into the HI loop of the fiber knob redirects binding to cellular integrin receptors (Dimitriev et al., 1998). There was significant infectivity enhancement with the doubleRGD fiber modification in the epithelial-like cell line, 11Z, consistent with increased expression of integrins in endometriotic implants (Fig. 2). However, this was not the case in the stromal-like cell line, 22B. Interestingly, the double modification RGDpK7 showed significant transductional activity in both endometriotic cell lines. Although its activity was better than RGD, this was nevertheless several fold lower than that of pK7. This suggests that there is not a synergistic effect between pK7 and RGD.
An interesting finding is that CRAd-S-F5/3σ-1 seems to have a major cytopathic effect on 11Z, even more than CRAd-S-pk7 at 1 vp/cell (Fig. 6A). This is unexpected since this particular fiber modification demonstrated minimal transduction in the 11Z cell line (Fig. 2A). A possible explanation is that this could represent a ‘fiber effect’. In this regard, certain fibers have toxicities independent of viral replication and this may explain this finding (Wang et al., 2010).
This study has several strengths and unique features. It is the most systematic and extensive investigation of the use of CRAds to target endometriosis with data on binding capacity, entry kinetics, DNA replication and cell-killing effects. This study examined a large number of viruses: 9 transcriptionally targeted Ads, 12 transductionally targeted Ads and 3 CRAds. It is the first study to introduce a dual-targeting concept with combined transcriptional and transductional targeting in the context of endometriosis. It is also the only study to use both endometriotic cell lines as well as primary human endometriosis cells. The primary human endometriosis samples include both peritoneal endometriotic implants and ovarian endometrioma in patients with endometriosis at all stages of the disease.
Our study is not without limitations. One limitation is the fact that due to lack of tissue material for the experiments, we did not obtain pathologic confirmation of endometriosis in three out of the five primary human samples (Fig. 8C–E). However, operative visualization of characteristic lesions is considered an acceptable surrogate for excision with histologic diagnosis of endometriosis (The Practice Committee of the American Society for Reproductive Medicine, 2008). There may be other cells in the primary human tissue material obtained at the time of laparoscopy. However, this reflects the treatment context in which the CRAd intervention is envisioned. In addition, more patient samples would have been desirable as our sample size of four patients was small. In validating a candidate CRAd agent, the Food and Drug Administration (FDA), in consideration of our applications, has exploited replication data in tissue samples from as few as three patients in order to give approval for clinical trials. This reflects the FDA's recognition as to the difficulty in obtaining data with primary human material and so far, these data have been adequate for obtaining FDA approval for a number of CRAd agents. Another limitation is that we did not demonstrate cell killing in primary endometriotic cells. In CRAd experiments, cell killing is best evaluated more than 5 days after viral infection. However, in our experience, we have noticed that primary endometriotic cells die before that, independent of the CRAd effect. We were thus unable to accurately measure the amount of cell killing attributable solely to the cytopathic effect of the CRAds. As replication is the predicate of cell killing, we used replication data as surrogate end-points to obtain quantitative data. On the other hand, the endometriotic cell lines are easier to culture for prolonged periods of time and hence they are more amenable to obtain quantitatively reproducible results. Lastly, our study was predicated upon the findings of previous studies showing increased survivin expression in ectopic endometriotic tissues. We thus focused on using this knowledge in our virotherapy approach to target our viruses accordingly. Our aim was not to investigate the expression of survivin since several studies have confirmed this finding.
The current study is a proof of principle study to assess the feasibility of using an infectivity-enhanced CRAd to target endometriosis. There are still many unanswered questions that will require further investigation and these unresolved issues will be the subject of future studies prior to translating the findings of this study into the clinical context. First, we do not know the degree of clinical efficacy of infectivity-enhanced CRAds in endometriosis. For example, it remains to be determined whether this approach will be effective in patients with deep infiltrating endometriosis, a patient population that will most likely benefit from such an approach given the major shortcomings of currently available treatment modalities. Furthermore, there are other strategies that can be explored in order to increase the therapeutic efficacy of CRAds. One such strategy is the use of armed CRAds whereby CRAds can be armed with a therapeutic transgene that enhances their cell-killing ability (Hermiston, 2000). In the context of endometriosis, comparison with anatomically adjacent cells is important given that an intraperitoneal approach for delivery of CRAds is envisioned. The rationale for choosing normal ovarian surface epithelial cells as a control is that the ovary is the commonest site for endometriosis. Although our data are reassuring that CRAd-S-pK7 has minimal cytopathic effect in normal ovarian surface epithelial cells, there are other potential targets in the peritoneal cavity. Safety is of paramount importance if such an approach were to be used in the clinical context. However, we do know that high intraperitoneal doses of CRAds have no significant clinical sequelae since clinical trials have demonstrated the feasibility of delivering relatively high dosages of conditionally replicative viruses intraperitoneally in patients with recurrent ovarian cancer with good tolerance (Vasey et al., 2002; Galanis et al., 2010). Furthermore, liver toxicity is a potential complication of Ad-based gene therapy as the liver is the predominant site of Ad vector localization after systemic administration. Therefore, a tissue on/liver off phenotype is critical for minimizing liver toxicity as well as optimizing the therapeutic benefit. However, this is unlikely to be a problem with CRAd-S-pK7 since survivin promoter-based CRAds have been shown to be inactive in a normal human liver organ culture (Van Houdt et al., 2006). In addition, Zhu et al. (2006) have shown that survivin has extremely low activity in human liver tissue. We have also analyzed survivin promoter activity in vivo where mice were injected with viruses via the tail vein (Zhu et al., 2004b). Survivin promoter activity was very low in major mouse organs including the liver, intestines, lungs, kidneys, spleen, heart and muscles. In addition, we have demonstrated that intravenous administration of pK7 fiber-modified viruses does not result in a higher concentration in several organs of the mouse (Rein et al., 2004). Therefore, these studies provide significant information about the expected safety profile of CRAd-S-pK7 in a clinical setting.
An important consideration for future translational application of this novel paradigm is the optimal route of administration of the CRAds. Possible routes include intraperitoneal and intravenous administration. Although it seems intuitive that the intraperitoneal approach is likely to give optimal results since endometriosis is usually disseminated within the pelvic cavity, this may not be the case. This is illustrated by one study where we have shown a higher tumor transduction following intravenous administration compared with intraperitoneal administration in an ovarian cancer mouse model (Breidenbach et al., 2004). Therefore, we do not know which route of administration is better in the context of endometriosis and this will require further investigation.
A gene therapy approach seems promising in targeting and efficiently killing endometriosis cells. In addition, as illustrated in this report, a gene therapy approach is useful to dissect disease processes and shed more light on the pathogenesis of endometriosis. The pathogenesis of endometriosis likely involves several factors and the contribution of each factor may be different in each patient. In the future, different vectors could be targeted for endometriosis in different patients depending on the distinct molecular signatures exhibited by their endometriotic implants. In this report, a CRAd with a survivin promoter and a polylysine fiber modification proved to have the optimal characteristics as a therapeutic agent for patients with all stages of endometriosis. In a clinical setting, survivin-targeted CRAds could be administered into the pelvic cavity during laparoscopy.
Funding
This study was financially supported by the Division of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, The University of Alabama at Birmingham and by a grant from the National Institutes of Health, 5R01CA121187, to D.T.C.
Acknowledgements
We thank Dr Hideyo Ugai for critically reading the manuscript.
References
- Adachi Y, Reynolds PN, Yamamoto M, Grizzle WE, Overturf K, Matsubara S, Muramatsu T, Curiel DT. Midkine promoter-based adenoviral vector gene delivery for pediatric solid tumors. Cancer Res. 2000;60:4305–4310. [PubMed] [Google Scholar]
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817–821. doi: 10.1016/s0015-0282(97)81391-x. [DOI] [PubMed] [Google Scholar]
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- Banu SK, Lee J, Starzinski-Powitz A, Arosh JA. Gene expression profiles and functional characterization of human immortalized endometriotic epithelial and stromal cells. Fertil Steril. 2008;90:972–987. doi: 10.1016/j.fertnstert.2007.07.1358. [DOI] [PubMed] [Google Scholar]
- Barker SD, Coolidge CJ, Kanerva A, Hakkarainen T, Yamamoto M, Liu B, Rivera AA, Bhoola SM, Barnes MN, Alvarez RD, et al. The secretory leukoprotease inhibitor (SLPI) promoter for ovarian cancer gene therapy. J Gene Med. 2003;5:300–310. doi: 10.1002/jgm.341. [DOI] [PubMed] [Google Scholar]
- Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA, et al. Roundabout4 is essential for angiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:6373–6378. doi: 10.1073/pnas.0408318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berardo PT, Abrao MS, Souza MLS, Machado DE, Silva LCF, Nasciutti LE. Composition of sulphated glycosaminoglycans and immunodistribution of chondroitin sulfate in deeply infiltrating endometriosis affecting the rectosigmoid. Micron. 2009;40:639–645. doi: 10.1016/j.micron.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Berkkanoglu M, Arici A. Immunology and endometriosis. Am J Reprod Immunol. 2003;50:48–59. doi: 10.1034/j.1600-0897.2003.00042.x. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- Breidenbach M, Rein DT, Wang M, Nettlebeck DM, Hemminki A, Ulasov I, Rivera AR, Everts M, Alvarez RD, Douglas JT, et al. Genetic replacement of the adenovirus shaft fibre reduces liver tropism in ovarian cancer gene therapy. Hum Gene Ther. 2004;15:509–518. doi: 10.1089/10430340460745829. [DOI] [PubMed] [Google Scholar]
- Breidenbach M, Rein DT, Everts M, Glasgow JN, Wang M, Passineau MJ, Alvarez RD, Korokhov N, Curiel DT. Mesothelin-mediated targeting of adenoviral vectors for ovarian cancer gene therapy. Gene Ther. 2005;12:187–193. doi: 10.1038/sj.gt.3302404. [DOI] [PubMed] [Google Scholar]
- Breidenbach M, Rein DT, Schondorf T, Khan KN, Herrmann I, Schmidt T, Reynolds PN, Vlodavsky I, Haviv YS, Curiel DT. A new targeting approach for breast cancer gene therapy using the heparanase promoter. Cancer Lett. 2006;240:114–122. doi: 10.1016/j.canlet.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2007;149:1190–1204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HW, Wen Y, Choi EA, Li H, Moon HS, Yu HK, Polan ML. Pleiotropin (PTN) and midkine (MK) mRNA expression in eutopic and ectopic endometrium in advanced stage endometriosis. Mol Hum Reprod. 2002;8:350–355. doi: 10.1093/molehr/8.4.350. [DOI] [PubMed] [Google Scholar]
- Dabrosin C, Gyorffy S, Margetts P, Ross C, Gauldie J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am J Pathol. 2002;161:909–918. doi: 10.1016/S0002-9440(10)64251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibres demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin M, Lepine M, Merlen Y, Thibeault I, Rancourt C, Gosselin D, Hugo P, Steff AM. Quantitative assessment of human endometriotic tissue maintenance and regression in a noninvasive mouse model of endometriosis. Mol Ther. 2004;9:540–547. doi: 10.1016/j.ymthe.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Fueyo J, Comez-Manzano C, Alemany R, Lee PSY, McDonell TJ, Mitlianga P, Shi YX, Levin VA, Yung WKA, Kyritsis AP. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- Fujino K, Ueda M, Takehara M, Futakuchi H, Kanda K, Yamashita Y, Terai Y, Ueki M. Transcriptional expression of survivin and its splice variants in endometriosis. Mol Hum Reprod. 2006;12:383–388. doi: 10.1093/molehr/gal042. [DOI] [PubMed] [Google Scholar]
- Furuya M, Suyama T, Usui H, Kasuya Y, Nishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38:1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barette BA, Kaur JS, Haluska PJ, Aderca I, Zman PJ, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R, Clayton R, Hawe J. The effect of endometriosis and its radical laparoscopic excision on quality of life indicators. Br J Obstet Gynaecol. 2000;107:44–54. doi: 10.1111/j.1471-0528.2000.tb11578.x. [DOI] [PubMed] [Google Scholar]
- Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4:1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- Goteri G, Lucarini G, Pieramici T, Filosa A, Pugnaloni A, Montik N, Biagini G, Tranquilli AL, Fabris G, Ciavattini A, et al. Endothelial cell survivin is involved in the growth of ovarian endometriotic cysts. Anticancer Res. 2005;25:4313–4318. [PubMed] [Google Scholar]
- Guidice LC. Genomics' role in understanding the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:119–124. doi: 10.1055/s-2003-41318. [DOI] [PubMed] [Google Scholar]
- Guidice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- Hermiston T. Gene delivery from replication-selective viruses: arming guided missiles in the war against cancer. J Clin Invest. 2000;105:1169–1172. doi: 10.1172/JCI9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y, Osuga Y, Koga K, Yoshino O, Hirata T, Harada M, Morimoto C, Yano T, Tsutsumi O, Sakuma S, et al. Possible implication of midkine in the development of endometriosis. Hum Reprod. 2005;20:1084–1089. doi: 10.1093/humrep/deh720. [DOI] [PubMed] [Google Scholar]
- Jingting C, Yangde Z, Yi Z, Mengxiong L, Rong Y, Yu Z, Guoqing P, Lixiu P. Expression of heparanase and angiopoietin-2 in patients with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2008;136:199–209. doi: 10.1016/j.ejogrb.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ, Barker SD, Staughn M, Barnes MN, Alvarez RD, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res. 2002;8:275–280. [PubMed] [Google Scholar]
- Krasnykh VN, Mikheeva GV, Douglas JT, Curiel DT. Generation of recombinant adenovirus vectors with modified fibres for altering viral tropism. J Virol. 1996;70:6839–6846. doi: 10.1128/jvi.70.10.6839-6846.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Makhija SK, Nettelbeck DM, Rivera AA, Wang M, Komarova S, Zhou F, Yamamoto M, Haisma HJ, Alvarez RD, et al. Evaluation of tumor-specific promoter activities in melanoma. Gene Ther. 2005;12:330–338. doi: 10.1038/sj.gt.3302385. [DOI] [PubMed] [Google Scholar]
- Morgan C, Rosenkranz HS, Mednis B. Structure and development of viruses as observed in the electron microscope. J Virol. 1969;4:777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, Nezhat C. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril. 2008;90:1559–1570. doi: 10.1016/j.fertnstert.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16:561–566. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- Othman EER, Salama S, Ismail N, Al-Hendy A. Toward gene therapy of endometriosis: adenovirus-mediated delivery of dominant negative estrogen receptor genes inhibits cell proliferation, reduces cytokine production, and induces apoptosis of endometriotic cells. Fertil Steril. 2007;88:462–471. doi: 10.1016/j.fertnstert.2006.11.046. [DOI] [PubMed] [Google Scholar]
- Othman EER, Zhu ZB, Curiel DT, Khatoon N, Salem HT, Khalifa EAM, Al-Hendy A. Toward gene therapy of endometriosis: transductional and transcriptional targeting of adenoviral vectors to endometriosis cells. Am J Obstet Gynecol. 2008;199:117.e1–117.e7. doi: 10.1016/j.ajog.2008.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puy LA, Pang C, Librach CL. Immunohistochemical analysis of αvβ5 and αvβ6 integrins in the endometrium and endometriosis. Int J Gynecol Pathol. 2002;21:167–177. doi: 10.1097/00004347-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Rein DT, Breidenbach M, Wu H, Han T, Haviv YS, Wang M, Kirby TO, Kawakami Y, Dall P, Alvarez RD, et al. Gene transfer to cervical cancer with fibre-modified adenoviruses. Int J Cancer. 2004;111:698–704. doi: 10.1002/ijc.20295. [DOI] [PubMed] [Google Scholar]
- Rein DT, Schmidt T, Bauerschmitz G, Hampl M, Beyer IM, Paupoo AAV, Curiel DT, Breidenbach M. Treatment of endometriosis with a VEGF-targeted conditionally replicative adenovirus. Fertil Steril. 2010;93:2687–2694. doi: 10.1016/j.fertnstert.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Liu X, Geng JG, Guo SW. Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas: a likely constituent biomarker for recurrence. Am J Pathol. 2009;175:479–488. doi: 10.2353/ajpath.2009.090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman MA, Lacy J, Murphy SK, Whitaker RS, Grace L, Teaberry V, Marks JR, Berchuck A. Regulation of the metastasis suppressor gene MKK4 in ovarian cancer. Gynecol Oncol. 2007;105:312–320. doi: 10.1016/j.ygyno.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoff-Khalili MA, Rivera AA, Glasgow JN, Le LP, Stoff A, Everts M, Tsuruta Y, Kawakami Y, Bauerschmitz GJ, Mathis JM, et al. A human adenoviral vector with a chimeric fibre from canine adenovirus type 1 results in novel expanded tropism for cancer gene therapy. Gene Ther. 2005;12:1696–1706. doi: 10.1038/sj.gt.3302588. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Sato M, Yoneda T, Ozaki Y, Takagi H, Suzumori K. Expression of secretory leukocyte protease inhibitor in women with endometriosis. Fertil Steril. 1999;72:857–867. doi: 10.1016/s0015-0282(99)00381-7. [DOI] [PubMed] [Google Scholar]
- The Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril. 2008;90:S260–S269. doi: 10.1016/j.fertnstert.2008.08.057. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Luongo CL, Komarova S, Kawakami Y, Curiel DT. Reovirus σ1 fibre incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem Biophys Res Commun. 2005;335:205–214. doi: 10.1016/j.bbrc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Pereboeva L, Glasgow JN, Rein DT, Kawakami Y, Alvarez RD, Rocconi RP, Siegal GP, Dent P, Fisher PB, et al. A mosaic fibre adenovirus serotype 5 vector containing reovirus σ1 and adenovirus serotype 3 knob fibres increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin Cancer Res. 2007;13:2777–2783. doi: 10.1158/1078-0432.CCR-06-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M, Yamashita Y, Takehara M, Terai Y, Kumagai K, Ueki K, Kanda K, Yamaguchi H, Akise D, Hung YC, et al. Survivin gene expression in endometriosis. J Clin Endocrinol Metab. 2002;87:3452–3459. doi: 10.1210/jcem.87.7.8682. [DOI] [PubMed] [Google Scholar]
- Ulasov IV, Tyler MA, Zheng S, Han Y, Lesniak MS. CD46 represents a target for adenoviral gene therapy of malignant glioma. Hum Gene Ther. 2006;17:556–564. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- Ulasov IV, Rivera AA, Han Y, Curiel DT, Zhu ZB, Lesniak MS. Targeting adenovirus to CD80 and CD86 receptors increases gene transfer efficiency to malignant glioma cells. J Neurosurg. 2007a;107:617–627. doi: 10.3171/JNS-07/09/0617. [DOI] [PubMed] [Google Scholar]
- Ulasov IV, Zhu ZB, Tyler MA, Han Y, Riviera AA, Khramtsov A, Curiel DT, Lesniak MS. Survivin-driven and fibre-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007b;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, Lamfers MLM, Rein D, Lesniak MS, Siegal GP, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006;104:583–592. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- Vasey PA, Schulman LN, Campos S, Davis J, Gore M, Johnston S, Kirn DH, O'Neill V, Siddiqui N, Seiden MV, et al. Phase I trial of intraperitoneal injection of the E1B-55kd-gene deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol. 2002;20:1562–1569. doi: 10.1200/JCO.2002.20.6.1562. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A. A recombinant adenovirus type 35 fibre knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115:592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Tzeng E, Shears LL, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fibre proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, Curiel DT. Double modification of adenovirus fibre with RGD and polylysine motifs improves coxsackie-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther. 2002;13:1647–1653. doi: 10.1089/10430340260201734. [DOI] [PubMed] [Google Scholar]
- Xu X, Ding J, Ding H, Shen J, Gattuso P, Prinz RA, Rana N, Dmowski WP. Immunohistochemical detection of heparanase-1 expression in eutopic and ectopic endometrium from women with endometriosis. Fertil Steril. 2007;88:1304–1310. doi: 10.1016/j.fertnstert.2006.12.081. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Alemany R, Adachi Y, Grizzle WE, Curiel DT. Characterization of the cyclooxygenase-2 promoter in an adenoviral vector and its application for the mitigation of toxicity in suicide gene therapy of gastrointestinal cancers. Mol Ther. 2001;3:385–394. doi: 10.1006/mthe.2001.0275. [DOI] [PubMed] [Google Scholar]
- Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159:1839–1852. doi: 10.1016/S0002-9440(10)63030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B, Rivera AA, Nettelbeck DM, Mahasreshti PJ, Leath CA, III, et al. Transcriptional targeting of adenoviral vector through the CXCR4 tumor-specific promoter. Gene Ther. 2004a;11:645–648. doi: 10.1038/sj.gt.3302089. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Makhija SK, Lu B, Wang M, Kaliberova L, Liu B, Rivera AA, Nettelbeck DM, Mahasreshti PJ, Leath CA, III, et al. Transcriptional targeting of tumors with a novel tumor-specific survivin promoter. Cancer Gene Ther. 2004b;11:256–262. doi: 10.1038/sj.cgt.7700679. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Chen Y, Makhija SK, Lu B, Wang M, Rivera AA, Yamamoto M, Wang S, Siegal GP, Curiel DT, et al. Survivin promoter-based conditionally replicative adenoviruses target cholangiocarcinoma. Int J Oncol. 2006;29:1319–1329. [PubMed] [Google Scholar]