Abstract
BACKGROUND
The objective of this study was to identify baseline predictors of live birth in anovulatory patients undergoing ovulation induction, and based on these predictors, develop nomograms for estimation of the probability of live birth in a single cycle.
METHODS
Univariate and multivariate logistic regression were used for retrospective analysis of clinical, sonographic and endocrinological parameters collected prior to the start of ovarian stimulation in a cohort of anovulatory World Health Organization (WHO) Group II patients (n = 335), who were resistant to clomiphene citrate (CC) and therefore stimulated with gonadotrophins using a low-dose step-up protocol.
RESULTS
The univariate analysis identified age [OR = 0.91 (95% CI: 0.84–0.98), P = 0.015], duration of infertility [OR = 0.71 (95% CI: 0.56–0.91), P = 0.007], serum follicle stimulating hormone (FSH) concentration at the start of stimulation [OR = 0.83 (95% CI: 0.69–0.99), P = 0.034] and menstrual cycle pattern (P = 0.022) as significant predictors of live birth. Baseline concentrations of luteinizing hormone, androgens, glucose and insulin, as well as body mass index, were not predictors of live birth. In the multivariate analysis, duration of infertility, FSH and menstrual cycle pattern were independent predictors, and nomograms were designed with these three parameters for individual prediction of the probability of live birth.
CONCLUSIONS
The chances of live birth in women with WHO Group II anovulatory infertility resistant to CC undergoing ovulation induction with gonadotrophins is highly influenced by the menstrual cycle pattern. Increases in duration of infertility and concentration of FSH (within the normal range) before the start of stimulation have negative influences on the likelihood of achieving a live birth.
Keywords: anovulation, gonadotrophin, live birth, nomogram, prediction model
Introduction
Chronic anovulation is a frequent cause of infertility in women. Most of these women have irregular menstrual cycles but serum concentrations of follicle stimulating hormone (FSH) and estradiol (E2) within the normal range, and are classified as World Health Organization (WHO) Group II anovulatory patients (World Health Organization, 1993). For these normogonadotrophic anovulatory infertile patients wishing to conceive, administration of the anti-estrogen clomiphene citrate (CC) to induce ovulation is the first-line pharmacological treatment, due to low costs and few complications (Practice Committee, ASRM, 2006; Thessaloniki ESHRE/ASRM-sponsored PCOS consensus workshop group, 2008). However, this treatment does not always lead to pregnancy. In fact, 15–25% of the anovulatory women are resistant to CC therapy and about 40–50% of the women ovulating after CC-treatment fails to conceive following six ovulatory cycles (Kousta et al., 1997; Messinis and Milingos, 1997; Homburg, 2005). Low-dose gonadotrophin regimens have so far primarily been used to increase treatment success in CC-resistant women (Homburg, 2005), with reported pregnancy rates per ovulatory cycle of 15–20% and cumulative pregnancy rates of 40–75% in three to six cycles (Thessaloniki ESHRE/ASRM-sponsored PCOS consensus workshop group, 2008; Li et al., 2010).
Efforts have been made to identify baseline characteristics that may predict ovarian response and outcome of ovulation induction in WHO II anovulatory infertile women (for review, see Fauser et al., 2008). Female age, body mass index (BMI), menstrual cycle history (oligomenorrhea versus amenorrhea), free androgen index, hirsutism score and duration of infertility have been shown to correlate with various outcome measures in CC cycles (Imani et al., 1998, 1999; Rausch et al., 2009). A number of baseline parameters (BMI, response to previous CC-treatment, the number of follicles, serum concentrations of FSH, testosterone and androstenedione) have also been suggested to be predictive of ovulation and somewhat differ from those predictive of conception (woman's age, testosterone and insulin-like-growth-factor-I) in women with gonadotrophin-induced ovulation (Imani et al., 2002; Mulders et al., 2003a).
The main objective of the present investigation was to identify baseline predictors of live birth in CC-resistant normogonadotropic anovulatory patients undergoing ovarian stimulation with gonadotrophins. A secondary objective was to develop nomograms based on these predictors for straightforward prediction of the probability of live birth in individual patients.
Materials and Methods
Study population
Data collected from two randomized controlled trials in anovulatory WHO Group II women (Platteau et al., 2006; Balen et al., 2007) were combined to create a large study cohort. These trials had ovulation rate as a primary outcome measure after stimulation with different gonadotrophin preparations using low-dose step-up protocols of uniform pattern. The ovulation, pregnancy and live birth rates were similar in the two trials. A total of 335 patients were recruited at 36 European fertility centres (13 in Belgium, 9 in Denmark, 5 in Sweden and 9 in the UK). All patients met the following inclusion criteria: chronic anovulation (amenorrhoea or oligomenorrhea, or anovulation based on progesterone concentrations in women with cycles of 21–35 days duration); failure to ovulate with CC doses of at least 100 mg/day for at least 5 days or failure to conceive after three cycles of ovulation induction with CC; infertility for ≥1 year before randomization; age 18–39 years; BMI 19–35 kg/m2; at least one patent tube documented within 3 years prior to screening; a normal pelvis documented by a transvaginal ultrasound scan with respect to uterus, fallopian tubes and ovaries within 3 months prior to screening; early follicular serum FSH concentrations between 1 and 12 IU/l, concentrations of prolactin and total testosterone not suggestive of hyperprolactinaemia or androgen-secreting tumours; and a male partner with a normal semen analysis or semen from a donor.
Study protocol
Stimulation treatment was started 2–5 days after a spontaneous or progesterone-induced menstrual bleed with either highly purified urinary menotrophin (MENOPUR; Ferring Pharmaceuticals A/S, Copenhagen, Denmark), highly purified urinary FSH (BRAVELLE; Ferring Pharmaceuticals A/S, Copenhagen, Denmark) or recombinant FSH (rFSH; follitropin alfa, Gonal-F; Merck Sereno, Geneva, Switzerland). The starting dose of gonadotrophin was 75 IU daily, which was maintained for 7 days. After the first 7 days, the dose was either maintained or increased by 37.5 IU increments according to individual response. All subjects were maintained on their specific dose level for at least 7 days. The maximum permitted daily dose was 225 IU and the women were treated with the gonadotrophin for a maximum of 6 weeks. A single dose of 5000 IU of human chorionic gonadotrophin (hCG) (PROFASI, Merck Serono, Geneva, Switzerland) was given to trigger ovulation when one follicle of ≥17 mm or two to three follicles of ≥15 mm was achieved. Subjects were not given hCG in either of the following situations: no follicular response after 6 weeks of gonadotrophin treatment, or greater than or equal to four follicles with diameters of ≥15 mm. Any medication for luteal support (e.g. progesterone or hCG) was prohibited. Serum progesterone was measured in the mid-luteal phase (6–9 days after hCG) and ovulation was defined as a progesterone concentration of ≥7.9 ng/ml (25 nmol/l).
Data analysis
All clinical, sonographic and endocrinological parameters assessed prior to the start of stimulation was included in the univariate logistic regression analysis with the dependent variable, live birth. Regarding menstrual pattern, patients were analysed in three groups: amenorrhoea (absence of bleeding for >6 months), oligomenorrhea (irregular bleedings with intervals of >35 days but ≤6 months) and anovulatory cycles based on progesterone concentrations in women with cycles of 21–35 days duration. The predictive value of each variable was summarized as an odds ratio (OR) with 95% confidence interval (95% CI). All variables with a P-value of <0.1 in the univariate regression analysis were included in the secondary multivariate regression analysis. The multivariate model was reduced stepwise and variables with a P-value of <0.1 were kept in the final model. Model fit was checked by the Hosmer and Lemeshow goodness-of-fit test. Internal validation in the form of bootstrapping was performed to reduce the risk of over-fitting (Steyerberg et al., 2001). A linear shrinkage factor was estimated in 1000 bootstrap runs. In each run, the model was selected in a stepwise selection with all variables entered. The shrinkage factor was applied to adjust the ORs and 95% CIs in the final model. The predictive ability of the model was assessed by determining the area under the receiver-operating characteristics (ROC) curve. A similar bootstrap approach to the above was used to derive the bias-adjusted ROC area. The relationship between infertility duration, FSH and menstrual cycle pattern was evaluated by analysis of variance (one-way ANOVA). Nomograms were designed on the basis of patient characteristics for prediction of the individual chances of achieving a live birth for CC-resistant patients with oligomenorrhea, amenorrhea or anovulatory cycles of 21–35 days duration. To avoid over-fitting, the nomograms were adjusted by the same shrinkage factor as for the ORs in the final model.
Results
There were 335 patients included in the present analysis and of them 283 (84.5%) ovulated. The clinical and ongoing pregnancy rates were 18.2 and 17.3%, respectively, ending in a live birth rate of 17.3% (14.9% singleton). Patient characteristics, sonographic data and endocrine profile prior to start of ovarian stimulation are shown in Table I.
Table I.
Clinical, sonographic and endocrinological parameters prior to start of ovarian stimulation of the entire group of normogonadotropic anovulatory patients (n = 335).
| Parameter | Valuea | Q1b | Q3c |
|---|---|---|---|
| Clinical | |||
| Female age (years) | 29 | 26 | 32 |
| BMI (kg/m2) | 24.5 | 21.3 | 29.3 |
| <25 | 53% | ||
| 25–29.9 | 27% | ||
| ≥30 | 20% | ||
| Waist-to-hip ratio | 0.81 | 0.76 | 0.87 |
| <0.85 | 68% | ||
| ≥0.85 | 32% | ||
| Menstrual cycle status | |||
| Oligomenorrhea | 54% | ||
| Amenorrhea | 18% | ||
| Anovulatory cycles of 21–35 days | 28% | ||
| Duration of infertility (years) | 2.5 | 1.7 | 3.5 |
| Previous pregnancy | 38% | ||
| Failure to ovulate on CC | 40% | ||
| Failure to conceive on CC | 60% | ||
| Sonographicd | |||
| Antral follicle count | 17 | 8 | 28 |
| Mean ovarian volume (cm3) | 6.9 | 4.9 | 9.9 |
| Endocrinologicald | |||
| LH (IU/l) | 6.1 | 4.4 | 9.3 |
| FSH (IU/l) | 5.0 | 4.2 | 6.2 |
| Estradiol (pmol/l) | 147 | 110 | 195 |
| Prolactin (μg/l) | 11 | 8.0 | 15 |
| Androstenedione (nmol/l) | 6.4 | 4.6 | 8.6 |
| Total testosterone (nmol/l) | 1.7 | 1.3 | 2.1 |
| SHBG (nmol/l) | 48 | 30 | 79 |
| Glucose, normale | 90% | ||
| Insulin, normale | 71% | ||
| Insulin-to-glucose ratio | 2.1 | 1.3 | 3.5 |
aData are median or percentage of patients.
b25th percentile.
c75th percentile.
dAt start of stimulation.
eAs defined by central laboratory reference ranges.
Table II shows the results of the univariate analyses of the association between the prestimulation characteristics and live birth in an ovulation induction cycle with gonadotrophins. The age of the woman [OR = 0.91 (95% CI: 0.84–0.98), P = 0.015], duration of infertility [OR = 0.71 (95% CI: 0.56–0.91), P = 0.007] and serum FSH concentration at start of stimulation [OR = 0.83 (95% CI: 0.69–0.99), P = 0.034] were found to be negatively associated with live birth. The menstrual cycle history also had a significant impact on the probability of live birth (P = 0.022); compared with women with oligomenorrhea, the lowest OR was obtained for women with anovulatory cycles of 21–35 days [OR = 0.33 (95% CI: 0.15–0.73)].
Table II.
Univariate and multivariate analysis of the association between live birth and clinical, sonographic and endocrinological parameters prior to start of ovarian stimulation, and type of gonadotrophin treatment in the entire group of normogonadotropic anovulatory patients (n = 335).
| Parameter | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Clinical | ||||||
| Female age (years) | 0.91 | 0.84–0.98 | 0.015 | |||
| BMI (kg/m2) | 1.01 | 0.95–1.08 | 0.673 | |||
| Waist-to-hip ratioa | 1.15 | 0.90–1.48 | 0.271 | |||
| Menstrual cycle pattern | 0.022 | 0.040 | ||||
| Oligomenorrhea (reference) | 1.00 | 1.00 | ||||
| Amenorrhea | 0.69 | 0.32–1.47 | 0.63 | 0.32–1.22 | ||
| Anovulatory cycles | 0.33 | 0.15–0.73 | 0.45 | 0.23–0.88 | ||
| Duration of infertility (years) | 0.71 | 0.56–0.91 | 0.007 | 0.78 | 0.64–0.95 | 0.013 |
| Previous pregnancy | 0.77 | 0.43–1.41 | 0.402 | |||
| Failure to conceive on CC | 0.87 | 0.49–1.54 | 0.632 | |||
| Failure to ovulate on CC | 1.15 | 0.65–2.04 | 0.632 | |||
| Sonographicb | ||||||
| Antral follicle count | 1.01 | 0.99–1.03 | 0.209 | |||
| Mean ovarian volume (cm3) | 1.00 | 0.93–1.07 | 0.966 | |||
| Endocrinologicalb | ||||||
| LH (IU/l) | 1.00 | 0.94–1.07 | 0.895 | |||
| FSH (IU/l) | 0.83 | 0.69–0.99 | 0.034 | 0.86 | 0.74–1.01 | 0.062 |
| Estradiol (pmol/l)c | 0.92 | 0.83–1.02 | 0.111 | |||
| Prolactin (μg/l) | 1.01 | 0.98–1.03 | 0.696 | |||
| Androstenedione (nmol/l) | 0.99 | 0.92–1.07 | 0.818 | |||
| Total testosterone (nmol/l) | 0.91 | 0.57–1.44 | 0.678 | |||
| SHBG (nmol/l) | 1.01 | 1.00–1.01 | 0.142 | |||
| Glucose, normald | 2.16e | 0.63–7.35e | 0.219e | |||
| Insulin, normald | 1.11e | 0.59–2.08e | 0.756e | |||
| Insulin-to-glucose ratio | 0.94 | 0.83–1.07 | 0.331 | |||
| Type of gonadotrophin | ||||||
| HP-FSH versus rFSHf | 0.84 | 0.37–1.89 | 0.674 | |||
| HP-hMG versus rFSHg | 0.80 | 0.36–1.78 | 0.587 | |||
aSteps of 0.1.
bAt start of stimulation.
cSteps of 25 pmol/l.
dAs defined by central laboratory reference ranges.
eNormal versus abnormal.
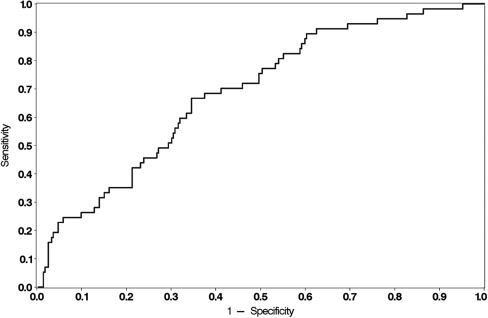
The pretreatment parameters that yielded P-values of <0.1 in the univariate analysis were entered into a multivariate logistic regression analysis to develop a model for prediction of the chances of live birth in individual patients. The parameters that were kept in the final model were menstrual cycle pattern (P = 0.040), duration of infertility (P = 0.013) and serum FSH (P = 0.062) (Table II). After internal validation by bootstrapping, the ORs and 95% CIs in the multivariate model were adjusted for over-fitting by a shrinkage factor of 19%. The Hosmer and Lemeshow test gave an acceptable fit (P = 0.244). The unadjusted ROC-AUC was found to be 0.69, and the bias-adjusted ROC-AUC found by bootstrap re-sampling was 0.66 (95% CI: 0.59–0.73). A graphical presentation of the model is shown in Fig. 1.
Figure 1.
ROC curve displaying sensitivity and specificity of the predictive model in the whole study cohort (n = 335).
Table III shows the parameters that were predictors of live birth in the multivariate logistic regression model, in relation to the type of menstrual cycle pattern. The live birth rates ranged from 8.5% in women with anovulatory cycles of 21–35 days duration to 16.4% in women with amenorrhea and to 22.2% in oligomenorrheic women. Duration of infertility was comparable between the three menstrual cycle groups, but the serum FSH concentration was significantly higher in patients with anovulatory cycles of 21–35 days duration (5.9 ± 2.7 IU/l) than in patients with oligomenorrhea (5.2 ± 1.9 IU/l) or amenorrhea (4.6 ± 1.8 IU/l).
Table III.
Relationship between menstrual cycle pattern, live birth and the parameters that were predictors of live birth in the multivariate logistic regression model.
| Parameter | All patients n = 335 | Menstrual cycle pattern |
P-valuea | ||
|---|---|---|---|---|---|
| Oligomenorrhea (n = 180) (54%) | Amenorrhea (n = 61) (18%) | Anovulatory cycles of 21–35 days (n = 94) (28%) | |||
| Live birth | 17.3% | 22.2% | 16.4% | 8.5% | 0.022 |
| Duration of infertility (years) | 2.9 ± 1.8 | 2.7 ± 1.6 | 3.1 ± 2.8 | 3.0 ± 1.4 | 0.390 |
| FSH (IU/l) | 5.3 ± 2.2 | 5.2 ± 1.9 | 4.6 ± 1.8b | 5.9 ± 2.7c,d | 0.001 |
aThe relationship between menstrual cycle pattern and live birth was evaluated by logistic regression. The relationships between menstrual cycle pattern and duration of infertility and FSH were evaluated by one-way ANOVA, and individual groups were compared with Student's t-test.
bP < 0.07 versus oligomenorrhea.
cP < 0.01 versus oligomenorrhea.
dP < 0.001 versus amenorrhea.
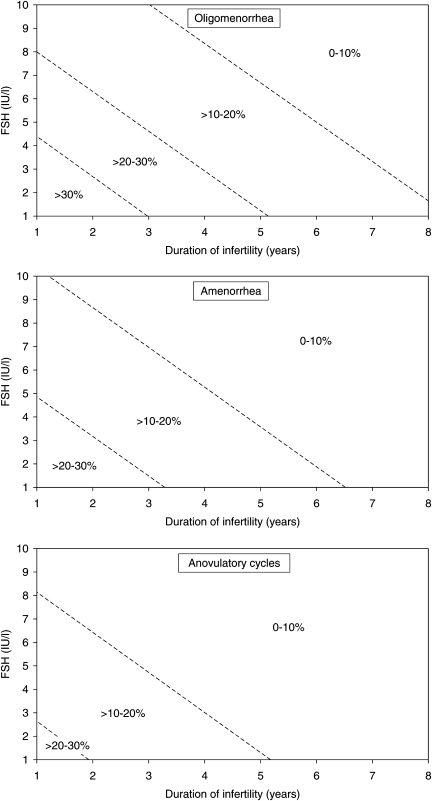
The multivariate logistic regression model was used to design nomograms for prediction of the chances of achieving a live birth following gonadotrophin stimulation (categorized by every 10% points) for a given anovulatory patient. Separate nomograms were developed for the three menstrual cycle pattern categories (Fig. 2). The estimated chance of live birth is reduced by increased duration of infertility and an increasing concentration of FSH (within the normal range) before start of stimulation for all three categories.
Figure 2.
Nomograms for estimation of the probability of live birth in a single cycle after ovulation induction with gonadotrophins in normogonadotropic patients with oligomenorrhea, amenorrhea or anovulatory menstrual cycles of 21–35 days duration.
Discussion
This large multicentre study of 335 patients treated with strictly defined and monitored protocols showed that the three simple pretreatment parameters (i) duration of infertility, (ii) basal serum FSH concentration and (iii) the menstrual cycle status were predictive of live birth after ovulation induction with gonadotrophins in normogonadotropic anovulation. Since a P-value threshold of 0.1 was used in the multivariate logistic regression model to minimize erroneous exclusion of relevant predictors, FSH was included in the final model although this predictor was not statistically significant at the 5% level (P = 0.062). Age, on the other hand, was not a predictor in the multivariate analysis (P > 0.1), which could be explained by the fact that age could not add much to the model after considering the duration of infertility. Neither the sonographic assessments such as antral follicle counts and ovarian volume, patients characteristics such as BMI and waist-to-hip ratio or several endocrine parameters including LH, androgen concentrations nor those related to presence or absence of hyperinsulinaemia were predictive of a successful treatment.
As also found in other studies, models for prediction of pregnancy or live birth have a modest discriminatory power. In the predictive model developed by van Wely et al. (2005), who studied 85 patients with polycystic ovary syndrome treated for 272 cycles, the area under the ROC curve was 0.72, similar to the value of 0.69 (or 0.66 when bias-adjusted) in the present study.
As mentioned above, the present study analysed a single ovulation induction cycle. Another, clinically relevant approach, is to study the cumulative effects of repetitive treatment cycles, as shown by Eijkemanns et al. (2003) who used a treatment algorithm with clomiphene followed by gonadotrophins to predict live birth in 240 patients, or by van Wely et al. (2005) who studied ongoing pregnancies within 1 year after the start of gonadotrophin treatment in 85 patients. Similar to what is found in the present study, van Wely et al. (2005) showed that the presence of oligomenorrhea and short duration of infertility were associated with higher chances of an ongoing pregnancy within 12 months. Thus, it is likely that the prediction based on results from a single cycle can be extrapolated to the cumulative effects of treatments over time. In relation to the endocrine predictors, a lower free androgen index was associated with higher chances of an ongoing pregnancy within 12 months (van Wely et al., 2005). The study by Eijkemanns et al. (2003) suggested that older age, longer duration of infertility and higher insulin-to-glucose ratio may be used to identify poor prognosis patients.
In the meta-analysis of 13 studies of the outcome of ovulation induction with gonadotrophins by Mulders et al. (2003b), the main end-point was pregnancy. This meta-analysis found that the only two parameters predictive of pregnancy were insulin resistance (presence or absence of hyperinsulinaemia) with an OR of 0.29 (95% CI: 0.10–0.80) and serum LH concentration with a pooled OR of 1.04 (95% CI: 1.01–1.07). However, the prediction of live birth may be different, as this meta-analysis indicated that the miscarriage rate was different in obese versus non-obese patients (OR 3.05; 95% CI: 1.45–6.44). In this context, it should be noted that neither the prediction models by van Wely et al. (2005), Eijkemanns et al. (2003) and Mulders et al. (2003b) nor the present model identified obesity (or BMI) as a predictor of pregnancy or live birth rate in normogonadotropic anovulatory women. An earlier analysis of the present study cohort evaluated several treatment outcome parameters following ovulation induction with gonadotrophins in non-obese versus obese women and found no differences in pregnancy rates (Balen et al., 2006). It thus seems consistent that BMI is not a predictor of either pregnancy or live birth. Regarding other proposed predictors, hyperinsulinaemia may be a factor (Mulders et al., 2003b), even though well-conducted large trials with a rather homogeneous patient population with polycystic ovarian syndrome and oligo- or amenorrhea (van Wely et al., 2005) failed to show any association to live birth rates. Therefore, our finding in the present large series does challenge the importance of hyperinsulinaemia, at least in patients within a certain BMI range. Since obesity is related to hyperinsulinaemia, the BMI range may be important, as our study was restricted to patients with BMI below 35 kg/m2.
In the present study, 28% of the patients were classified as having normogonadotrophic anovulatory infertility with preserved menstrual cyclicity of <35 days. These patients had normal serum concentrations of FSH at baseline, and were included based on local assessments of progesterone suggesting non-ovulatory cycles. However, as evident from Table III, such patients had a significantly higher FSH concentration and lower live birth rate (8.5%) than oligomenorrheic (22.2%) and amenorrheic patients (16.4%), even though it could be anticipated that the degree of ovulatory disturbance would be less severe in this group of patients. Given the less stringent criteria for including these patients based on progesterone assessments in terms of timing and cut-off, it may be speculated that some of these patients with preserved cycles had additional but concealed infertility factors that may explain part of the poorer prognosis. Thus, patient heterogeneity may be present and may explain the differences regarding FSH as a predictor. However, the meta-analysis of available studies by Mulders et al. (2003b) also included studies with patients who had anovulatory cycles as well as some with patients who had earlier ovarian electrocautery. Furthermore, the possible predictive value of basal FSH was also analysed by Eijkemanns et al. (2003) in patients treated with a classical ovulation induction protocol; first with CC followed by gonadotrophins. In that study, basal FSH tended to be a significant predictor of pregnancy (P = 0.07), but basal FSH was higher in the pregnant than in the non-pregnant group. Another possibility is that the slightly higher FSH concentrations observed in our patients with no live birth reflect that some of these patients may have a minor increase in ovarian age. As reviewed by Hendriks et al. (2005), increased FSH concentrations do have some, even though low, predictive value for achievement of pregnancy following assisted reproductive technology (ART). The same may be true for ovulation induction.
In order to increase the clinical usefulness of the findings in the multivariate model, prediction nomograms were developed in the present study (Fig. 2). As three independent predictors were found, two-dimensional nomograms were constructed showing the prediction in relation to duration of infertility and basal FSH for each menstrual cycle pattern. To illustrate how the nomogram may be used an oligomenorrheic patient with 3 years of infertility and a basal FSH of 3 IU/l will have a predicted chance of live birth between 20 and 30% for a single cycle. In contrast, an anovulatory patient with 21–35 days cycle duration with 4 years of infertility and a basal FSH of 6 IU/l is predicted to have a low chance of live birth of <10%.
On the basis of our nomograms, the predicted live birth rates may thus vary 3-fold between patients with different characteristics. On the basis of registry data of in vitro fertilization (Templeton et al., 1996), similar or even larger differences in prognosis are present among patients treated with IVF. Our data should be used to encourage couples with a good prognosis, such as short duration of infertility and oligomenorrhea, to undergo ovulation induction treatment. Additionally, it is likely that such couples would benefit from several treatments cycles (Eijkemanns et al., 2003) if the first few do not provide a pregnancy. The concern is how to advice a couple who according to our data are predicted to have a chance of live birth of <10% per attempt, as patients with characteristics such as a high FSH, long duration of infertility and perhaps minor ovulatory disturbances also have a low chance of live birth with ART (Templeton et al., 1996). Irrespective of such considerations, our nomogram may allow couples to be appropriately informed about their prognosis, and thus make a fully informed decision on treatment.
In summary, extensive endocrine testing in anovulatory WHO Group II patients undergoing ovulation induction with gonadotrophins was of little use in relation to prediction of live birth, as only basal FSH concentration was an independent endocrine predictor. In contrast, the simple clinical parameters relating to duration of infertility and menstrual cycle pattern were important determinants of successful outcomes.
Funding
The study was sponsored by Ferring Pharmaceuticals A/S, Copenhagen, Denmark.
Acknowledgements
The authors thank Klaus Juel Olsen, PhD, Larix Aps, Denmark, for statistical assistance. We would also like to thank all the centres from which the study cohort was derived: Belgium: AZ-VUB, Brussels; Virga Jesse Ziekenhuis, Hasselt; AZ Groeninge, Kortrijk; CHR Citadelle, Liège; Hôpital Erasme, Brussels; Hôpital Saint Vincent, Rocourt; ZOL Campus St Jan, Genk; Centre Hospitalier Notre Dame, Charleroi; AZ St Lucas, Gent; Private Practice, Aalter; AZ Jan Portaels Campus Zuid, Vilvoorde; UZ Gasthuisberg, Leuven and Universitair Ziekenhuis, Gent. Denmark: Copenhagen University Hospital; Brædstrup Hospital; Randers Hospital; Skive Hospital; Holbæk Hospital; Herlev Hospital; Hvidovre Hospital; Odense University Hospital and Skejby Hospital. Sweden: Uppsala University Hospital; Sahlgrenska University Hospital, Gothenburg; Lund University Hospital; Karlstad Hospital and Helsingborg Hospital. UK: Leeds General Infirmary, Leeds; Birmingham Women's Hospital; St Michael's Hospital, Bristol; Ninewells Hospital, Dundee; Glasgow Royal Infirmary; The Jessop Wing, Sheffield; Liverpool Women's Hospital; Princess Anne Hospital, Southampton and Guy's Hospital, London.
References
- Balen AH, Platteau P, Andersen AN, Devroey P, Sørensen P, Helmgaard L, Arce J-C. The influence of body weight on response to ovulation induction with gonadotrophins in 335 women with World Health Organization group II anovulatory infertility. BJOG. 2006;113:1195–1202. doi: 10.1111/j.1471-0528.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- Balen A, Platteau P, Nyboe Andersen A, Devroey P, Helmgaard L, Arce J-C for the Bravelle Ovulation Induction (BOI) Study Group. Highly purified FSH is as efficacious as recombinant FSH for ovulation induction in women with WHO Group II anovulatory infertility: a randomized controlled non-inferiority trial. Hum Reprod. 2007;22:1816–1823. doi: 10.1093/humrep/dem075. doi:10.1093/humrep/dem075. [DOI] [PubMed] [Google Scholar]
- Eijkemanns MJC, Imani B, Mulders AGMGJ, Habbema JDF, Fauser BCJM. High singleton live birth rate following classical ovulation induction in normogonadotrophic anovulatory infertility (WHO 2) Hum Reprod. 2003;18:2357–2362. doi: 10.1093/humrep/deg459. doi:10.1093/humrep/deg459. [DOI] [PubMed] [Google Scholar]
- Fauser BCJM, Diedrich K, Devroey P on behalf of the Evian Annual Reproduction (EVAR) Workshop Group 2007. Predictors of ovarian response: progress towards individualized treatment in ovulation induction and ovarian stimulation. Hum Reprod Update. 2008;14:1–14. doi: 10.1093/humupd/dmm034. doi:10.1093/humupd/dmm034. [DOI] [PubMed] [Google Scholar]
- Hendriks DJ, Mol BW, Bancsi LF, Te Velde ER, Broekmans FJ. Antral follicle count in the prediction of poor ovarian response and pregnancy after in vitro fertilization: a meta-analysis and comparison with basal follicle-stimulating hormone level. Fertil Steril. 2005;83:291–301. doi: 10.1016/j.fertnstert.2004.10.011. doi:10.1016/j.fertnstert.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Homburg R. Clomiphene citrate—end of an era? A mini-review. Hum Reprod. 2005;20:2043–2051. doi: 10.1093/humrep/dei042. doi:10.1093/humrep/dei042. [DOI] [PubMed] [Google Scholar]
- Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of patients remaining anovulatory during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 1998;83:2361–2365. doi: 10.1210/jcem.83.7.4919. doi:10.1210/jc.83.7.2361. [DOI] [PubMed] [Google Scholar]
- Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617–1622. doi: 10.1210/jcem.84.5.5705. doi:10.1210/jc.84.5.1617. [DOI] [PubMed] [Google Scholar]
- Imani B, Eijkemans MJ, Faessen GH, Bouchard P, Giudice LC, Fauser BC. Prediction of the individual follicle-stimulating hormone threshold for gonadotropin induction of ovulation in normogonadotropic anovulatory infertility: an approach to increase safety and effciency. Fertil Steril. 2002;77:83–90. doi: 10.1016/s0015-0282(01)02928-4. doi:10.1016/S0015-0282(01)02928-4. [DOI] [PubMed] [Google Scholar]
- Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3:359–365. doi: 10.1093/humupd/3.4.359. doi:10.1093/humupd/3.4.359. [DOI] [PubMed] [Google Scholar]
- Li RHW, Van Esch M, De Vries J, Colin Duncan W, Anderson RA. Gonadotrophin ovulation induction is a successful treatment for World Health Organisation Group II anovulatory subfertility in women aged up to 40 and with body mass index up to 34. Hum Fertil. 2010;13:35–40. doi: 10.3109/14647270903490765. [DOI] [PubMed] [Google Scholar]
- Messinis IE, Milingos SD. Current and future status of ovulation induction in polycystic ovary syndrome. Hum Reprod Update. 1997;3:235–253. doi: 10.1093/humupd/3.3.235. doi:10.1093/humupd/3.3.235. [DOI] [PubMed] [Google Scholar]
- Mulders AG, Eijkemans MJ, Imani B, Fauser BCJM. Prediction of chances for success or complications in gonadotrophin ovulation induction in normogonadotrophic anovulatory infertility. Reprod Biomed Online. 2003a;7:48–56. doi: 10.1016/s1472-6483(10)61747-6. [DOI] [PubMed] [Google Scholar]
- Mulders AGMGJ, Laven JSE, Eijkemans MJC, Hughes EG, Fauser BCJM. Patient predictors for outcome with gonadotropin ovulation induction in women with normogonadotrophic anovulatory infertility: a metaanalysis. Hum Reprod Update. 2003b;9:429–449. doi: 10.1093/humupd/dmg035. doi:10.1093/humupd/dmg035. [DOI] [PubMed] [Google Scholar]
- Platteau P, Nyboe Andersen A, Balen A, Devroey P, Sørensen P, Helmgaard L, Arce J-C for the Menopur Ovulation Induction (MOI) Study Group. Similar ovulation rates, but different follicular development with highly purified menotrophin compared with recombinant FSH in WHO Group II anovulatory infertility: a randomized controlled study. Hum Reprod. 2006;21:1798–1804. doi: 10.1093/humrep/del085. doi:10.1093/humrep/del085. [DOI] [PubMed] [Google Scholar]
- Practice Committee, ASRM. Use of clomiphene citrate in women. Fertil Steril. 2006;86:S187–S193. doi: 10.1016/j.fertnstert.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Rausch ME, Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, McGovern PG, Cataldo NA, et al. Reproductive Medicine Network. Predictors of pregnancy in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:3458–3466. doi: 10.1210/jc.2009-0545. doi:10.1210/jc.2009-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW, Eijkemans MJC, Habbema JDF. Application of shrinkage techniques in logistic regression analysis: a case study. Stat Neerl. 2001;55:76–88. doi:10.1111/1467-9574.00157. [Google Scholar]
- Templeton A, Morris JK, Parslow W. Factors that affect outcome of in-vitro fertilisation treatment. Lancet. 1996;348:1402–1406. doi: 10.1016/S0140-6736(96)05291-9. doi:10.1016/S0140-6736(96)05291-9. [DOI] [PubMed] [Google Scholar]
- Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23:462–477. doi: 10.1093/humrep/dem426. doi:10.1093/humrep/dem426. [DOI] [PubMed] [Google Scholar]
- van Wely M, Bayram N, van der Veen F, Bossuyet PMM. Predicting ongoing pregnancy following ovulation induction with recombinant FSH in women with polycystic ovarian syndrome. Hum Reprod. 2005;20:1827–1832. doi: 10.1093/humrep/deh891. doi:10.1093/humrep/deh891. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Manual for the Standardized Investigation and Diagnosis of the Infertile Couple. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]