Abstract
Purpose
To evaluate the impact of a multidisciplinary clinic on the clinical care recommendations of patients with pancreatic cancer compared with the recommendations the patients received prior to review by the multidisciplinary tumor board.
Methods
The records of 203 consecutive patients referred to the Johns Hopkins pancreatic multidisciplinary clinic were prospectively collected from November 2006 to October 2007. Cross-sectional imaging, pathology, and medical history were evaluated by a panel of medical/radiation oncologists, surgical oncologists, pathologists, diagnostic radiologists, and geneticists. Alterations in treatment recommendations between the outside institution and the multidisciplinary clinic were recorded and compared.
Results
On presentation, the outside computed tomography (CT) report described locally advanced/unresectable disease (34.9%), metastatic disease (17.7%), and locally advanced disease with metastasis (1.1%). On review of submitted imaging and imaging performed at Hopkins, 38 out of 203 (18.7%) patients had a change in the status of their clinical stage. Review of the histological slides by dedicated pancreatic pathologists resulted in changes in the interpretation for 7 of 203 patients (3.4%). Overall, 48 out of 203 (23.6%) patients had a change in their recommended management based on clinical review of their case by the multidisciplinary tumor board. Enrollment into the National Familial Pancreas Tumor Registry increased from 52 out of 106 (49.2%) patients in 2005 to 158 out of 203 (77.8%) with initiation of the multidisciplinary clinic.
Conclusion
The single-day pancreatic multidisciplinary clinic provided a comprehensive and coordinated evaluation of patients that led to changes in therapeutic recommendations in close to one-quarter of patients.
Keywords: Multidisciplinary, Pancreas, Cancer, Outcome
As the care of patients with cancer has become more complex, fewer patients are being treated with single-modality therapy. Rather, most patients with cancer are now cared for using a combination of surgery, chemotherapy, and/or radiation therapy. As such, multidisciplinary cancer clinics have become increasingly prevalent in the management of patients with malignancies. Multidisciplinary clinics allow specialists to work together to develop consensus recommendations in accordance with guidelines and protocols endorsed by the clinical team.1 Over time, multidisciplinary care has become accepted as the optimal mechanism for delivering care in oncology. 1–3 In the USA, both the Commission on Cancer and the American College of Surgeons require multidisciplinary cancer conferences for the accreditation of health centers delivering multidisciplinary care.3–5
Although multidisciplinary care has widely been endorsed and accepted, the impact of formal multidisciplinary clinics has yet to be established. In fact, little qualitative and quantitative research exists to determine the impact of multidisciplinary clinics on patient outcomes.6 Data on the effect of multidisciplinary clinics on patient outcomes are clearly important, as these clinics require a substantial amount of clinical and institutional resources. While improved outcomes in breast cancer patients treated at multidisciplinary clinics have been documented,7,8 no such data exist for patients with gastrointestinal malignancies. Specifically, the impact of multidisciplinary clinics to treat patients with pancreatic cancer has not been previously documented. The use of multidisciplinary clinics to assess patients with pancreatic carcinoma may be particularly of benefit. Multidisciplinary input can establish the correct diagnosis, and can help in determining resectability (e.g., clearly resectable versus borderline resectable versus unresectable),9,10 timing/sequence of therapy (e.g., neoadjuvant versus adjuvant), 11 and appropriateness for clinical trial accrual.12
The Pancreatic Cancer Center at Johns Hopkins Hospital offers a comprehensive multimodality program that provides diagnostic and consultation services for patients with newly diagnosed pancreatic cancer in a one-site one-visit format. The pancreatic multidisciplinary clinic offers an opportunity for patients and physicians to review comprehensively all details of the clinical case, after which therapeutic recommendations are offered. The objective of the current study was to evaluate the impact of the multidisciplinary clinic on the clinical care recommendations of patients compared with those patients received prior to review by the multidisciplinary tumor board.
METHODS AND MATERIALS
In 2006, the Pancreatic Cancer Center at Johns Hopkins Hospital established the pancreatic cancer multidisciplinary clinic. The purpose of the clinic was to provide a comprehensive multispecialty evaluation for patients with pancreatic carcinoma. The multidisciplinary clinic was conducted on a weekly basis and involved mostly new, but also routine follow-up, patient consultations. For the purposes of this study, only new consultations were included in the analyses. Patient referrals were screened by a clinical nurse coordinator (JC) and further triaged by the clinical director (JH). The coordinator was responsible for obtaining all pertinent previous medical records, pathology slides and reports, and cross-sectional imaging as well as official readings of prior scans. All imaging was submitted to the radiologists for interpretation and the pathology slides were submitted to the pathologists for review. Patients were initially triaged as having localized resectable disease, metastatic disease, or borderline resectable disease.
On the morning of clinic, routine laboratory blood work was performed and the patients underwent a pancreatic protocol three-dimensional (3D) computed tomography (CT) scan (Table 1). All 3D CT studies were performed with a Definition Source CT scanner (Siemens Medical Solutions, Malvern, NJ) according to a standard protocol.13,14 Mid-morning, the patients assembled for an overview of support services including briefings by nutrition, nursing, social work and the research coordinators from the National Familial Pancreas Tumor Registry.15 Late in the morning, patients were then seen by fellows, residents, nurse practitioners, and/or physician assistants, who performed a complete history and physical exam. All cases were subsequently presented at a multidisciplinary conference attended by at least one pathologist, radiologist, radiation oncologist, medical oncologist, and surgical oncologist. On average, every clinic was attended by two or more physicians from each discipline. All patient information, pathology findings, and radiology images were reviewed and discussed. The multidisciplinary group then discussed the case and agreed on a consensus recommendation that was based on the collective judgment of the physicians in attendance. Full details of the tumor board recommendations were then communicated back to the patients that afternoon by appropriate staff members of the multidisciplinary team.
TABLE 1.
Pancreatic multidisciplinary clinic: patient schedule
| Time period | Objective |
|---|---|
| 07:00–09:00 | Necessary imaging and laboratory studies obtained |
| 09:00–10:00 | Patients given overview of support services; 10–15 min briefings: |
|
|
| 10:00–12:00 | Patients seen by physician extenders including nurse practitioners, physician assistants, residents, and fellows for complete history and physical exam |
| 12:00–14:00 | Formal case review by multidiscipline tumor board |
|
|
| 14:00–16:00 | Full details of the tumor board recommendations discussed with patient by staff physicians. Note dictated to referring physician |
For the purposes of this study, patient age, race, distance from the institution, as well as clinicopathologic and treatment-related factors were recorded. The records of 203 consecutive patients referred to the multidisciplinary tumor board from November 11, 2006 to October 9, 2007 were reviewed. Significant alterations in pathologic, surgical, radiologic, or oncologic treatment recommendations between the outside institution and the multidisciplinary clinic were recorded. Specifically, the outside recommendations were determined by a systematic review of the outside institution’s records, including outside hospital clinic notes. A significant alteration was defined as a change that had the potential to alter the patient’s clinical therapy or outcome. How the alterations/differences in clinical assessment and therapeutic recommendations affected the management of patients with pancreatic cancer were then assessed.
RESULTS
The study population consisted of 203 consecutive patients seen in the multidisciplinary clinic. Table 2 shows the clinicopathologic features of the patients. There were 97 (47.8%) men and 106 (52.2%) women. The median patient age was 64 years (range, 31–90 years). The majority of patients (n = 159; 78.3%) were from out of state; the distance traveled for consultation varied greatly with a median of 186 miles (range, 5–2794 miles). Most patients (n = 180; 88.7%) were self-referred or referred from a hospital not affiliated with Johns Hopkins Hospital. Twenty-nine patients had a history of familial pancreatic cancer as defined by the presence of two or more first-degree relatives with a diagnosis of pancreatic cancer in the family.
TABLE 2.
Clinical and pathologic characteristics of patients (n = 203)
| Variable | Number of patients (%) |
|---|---|
| Age (years) | |
| ≤49 | 26 (12.8) |
| 50–59 | 43 (21.1) |
| 60–69 | 61 (30.0) |
| ≥70 | 73 (36.1) |
| Male | 97 (47.8) |
| Self-referred | 180 (88.7) |
| History of familial pancreatic cancer | 29 (14.3) |
| Distance traveled (miles) | |
| ≤50 | 43 (21.2) |
| 50.1–100 | 30 (14.8) |
| 100.1–500 | 75 (36.9) |
| >500 | 55 (27.1) |
Patients presented with a spectrum of clinical scenarios. The majority of patients presented with either a diagnosis of infiltrating ductal adenocarcinoma of the pancreas (n = 106; 52.2%) or a “suspicious” pancreatic mass presumed to be adenocarcinoma (n = 49; 24.1%). A minority of patients were referred with presumed distal cholangiocarcinoma (n = 13; 6.4%), well-differentiated endocrine neoplasm (n = 7; 3.4%), ampullary adenocarcinoma (n = 6; 3.0%), intraductal papillary mucinous neoplasm (n = 4; 2.0%), duodenal adenocarcinoma (n = 2; 1.0%) or for other reasons (n = 16; 7.9%). The majority of patients presented prior to any surgical resection (n = 174; 85.7%), while a minority presented following surgical resection (n = 28; 13.8%) or palliative double bypass (n = 1; 0.5%). The median time between surgery at the other institution and presentation to our clinic was 4 weeks (range, 2–88 weeks). Only 2 of the 29 patients who underwent surgery at another institution had received some form of additional therapy prior to matriculation to the multidisciplinary clinic (both patients had received chemoradiation therapy).
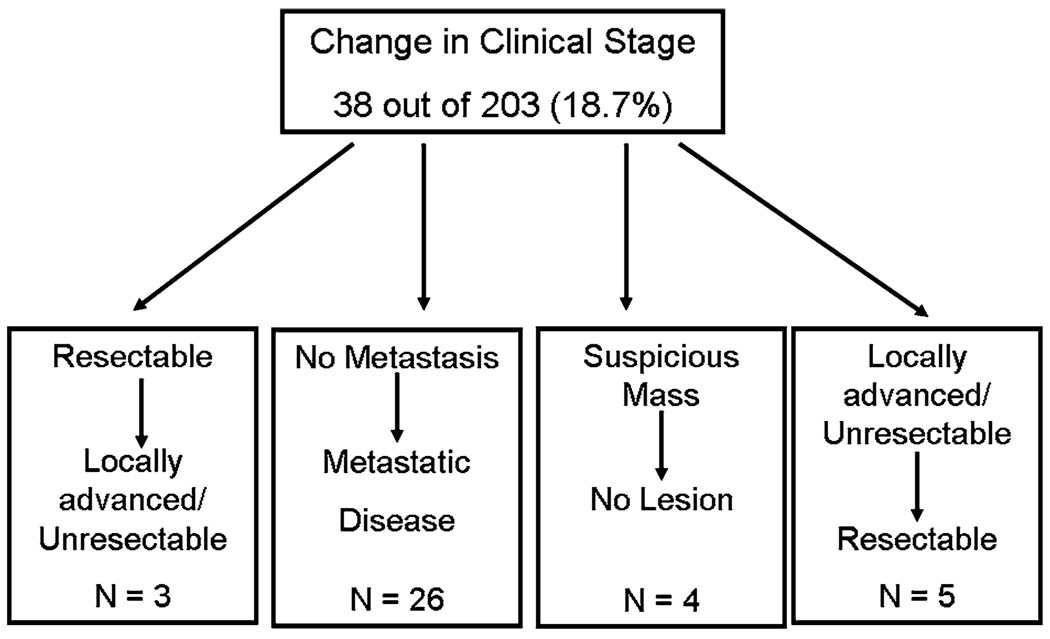
While all patients (n = 203, 100%) who presented to the multidisciplinary clinic had prior outside cross-sectional imaging, most patients (n = 148, 72.9%) underwent repeat pancreatic protocol 3D CT scanning at Johns Hopkins Hospital. Among those patients who had their pancreatic lesion still in situ (n = 175; 85.7%), the official CT report from the other institution described locally advanced/unresectable disease in 61 (34.9%) patients, metastatic disease in 31 (17.7%), and locally advanced disease with metastasis in 2 (1.1%). On review of both the outside as well as repeat CT scan imaging obtained at the time of the multidisciplinary clinic, 38 out of 203 (18.7%) patients had a change in the status of their clinical stage (Fig. 1). Specifically, 3 of 38 (7.9%) patients who presented with presumed resectable disease were found to have locally advanced/unresectable disease. In addition, 26 of 38 (68.4%) patients were found to have previously undetected metastases, thereby upstaging them to stage IV disease. Four patients (10.5%) who presented with a “suspicious” mass in the pancreas were ultimately determined not to have a true pancreatic mass on cross-sectional imaging. For example, a tortuous vessel was found mimicking a pancreatic mass. Of note, 5 out of 38 patients (13.2%) initially presented with a diagnosis of locally advanced/unresectable disease, but were subsequently deemed to be surgical candidates following re-review of the imaging.
FIG. 1.
On review of both the outside as well as repeat CT scan imaging obtained at the time of the multidisciplinary clinic, 38 out of 203 (18.7%) patients had a change in the status of their clinical stage based on re-review of cross-sectional imaging.
Review of the histologic slides by dedicated pancreatic pathologists resulted in changes in the interpretation for 7 of 203 patients (3.4%). Of those 7 patients originally diagnosed with pancreatic adenocarcinoma, review of the pathology led to a change in diagnosis to well-differentiated endocrine neoplasm (n = 2), breast carcinoma metastatic to the pancreas (n = 1), gastrointestinal stromal tumor (n = 1), gall-bladder cancer (n = 1), benign inflammatory process (n = 1), and serous cystadenoma (n = 1) (Fig. 2).
FIG. 2.
Example case of change in diagnosis following review of the histologic slides by dedicated pancreatic pathologist. Although the patient initially presented with a diagnosis of pancreatic adenocarcinoma, the diagnosis was changed to microcystic (serous) cystadenoma. Note the multiple small back-to-back cysts lined by uniform flat or short cuboidal cells with clear cytoplasm on both low-(A) and high-power (B) views of the core-needle biopsy specimen of the pancreas.
Seven patients were admitted to the hospital directly from the multidisciplinary clinic. Reasons for admission included: cholangitis (n = 3), newly discovered pulmonary embolism on CT scan (n = 2), dehydration (n = 1), and newly discovered carcinomatosis with failure to thrive (n = 1).
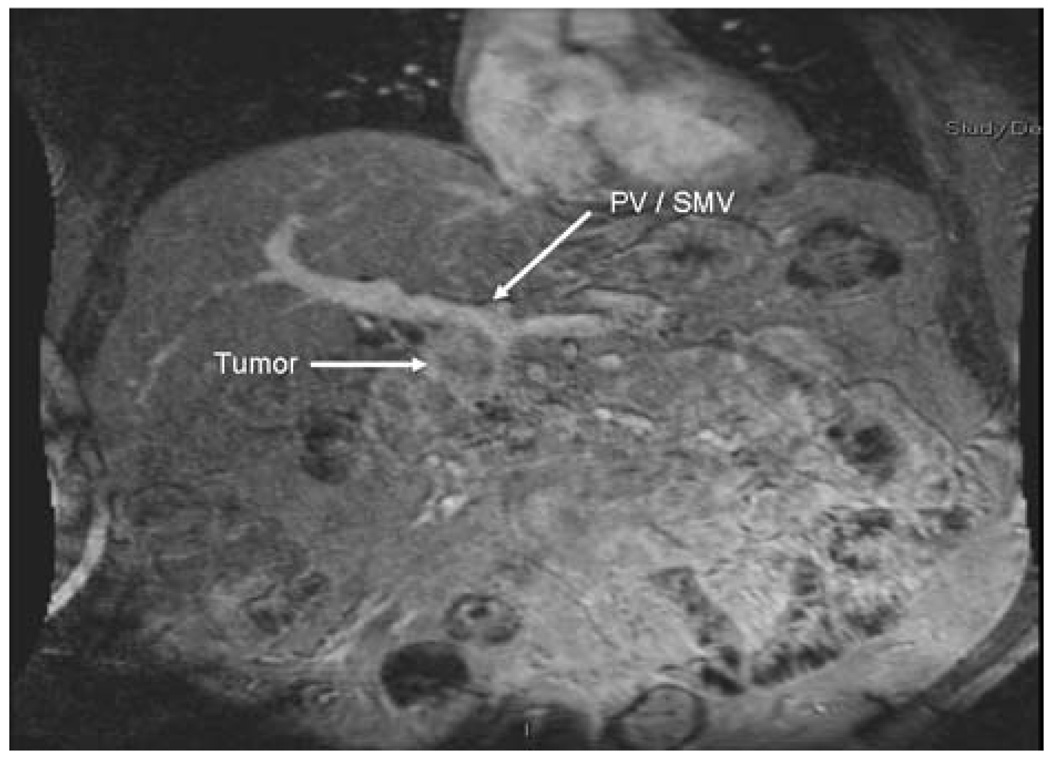
Overall, 48 out of 203 (23.6%) patients had a change in their recommended management based on clinical review of their case by the multidisciplinary tumor board (Table 3). In some cases, the changes were made based on the approach to the treatment of pancreatic cancer adopted by the multidisciplinary clinic. For example, several patients (n = 3) were declared unresectable at the outside institution because of tumor abutment to the portal/superior mesenteric vein (Fig. 3). At our institution, as with other major pancreatic cancer centers,16–18 resection and reconstruction of the portal–superior mesenteric vein is routinely considered and performed in this clinical setting. In other instances, well-differentiated endocrine neoplasms (n = 2) were deemed unresectable because of their size as well as involvement with local visceral structures. On review of these cases, the endocrine mass did not involve critical vasculature structures; therefore, these neoplasms were reclassified as resectable. In other cases, the identification of previously unsuspected metastatic disease had important therapeutic implications (n = 26; 12.8%). The recognition of previously undetected metastasis not only aborted previous plans for surgery, but also altered recommendations for the use of chemotherapy and radiation therapy. In general, patients with metastatic disease were offered systemic chemotherapy while patients with locally advanced unresectable disease would be offered definitive chemoradiation.
TABLE 3.
Reason for the recommended management change based on clinical review by the multidisciplinary tumor board (48 out of 203 patients; 23.6%)
| Reason for change in recommended management | Number of patients (n = 48) |
|---|---|
| Change in findings of cross-sectional imaging | |
| Previously unrecognized locally unresectable disease |
3 |
| Previously unrecognized metastatic disease | 26 |
| No lesion seen on repeat imaging | 4 |
| Disease deemed to be resectable | 5* |
| Change in diagnosis based on pathologic review | 7 |
| Change in surgical recommendation | 5* |
Two patients had a change in their recommended management based on a combination of both repeat cross-sectional imaging and surgical case review.
FIG. 3.
Example case of change in resectability status of pancreatic lesion. The patient was deemed unresectable at the outside institution because of tumor abutment to the portal/superior mesenteric vein. However, the patient was reclassified as resectable with planned concomitant resection and reconstruction of the portal–superior mesenteric vein following review at multidisciplinary conference.
Another important effect of the multidisciplinary clinic was that it increased patient access to clinical trials, as well as the National Familial Pancreas Tumor Registry. Specifically, of the 203 patients seen in multidisciplinary clinic 51 patients were offered participation in a clinical trial and 10 patients were actively enrolled. Enrollment into the National Familial Pancreas Tumor Registry increased from 52 out of 106 (49.2%) in 2005 to 158 out of 203 (77.8%) with initiation of the multidisciplinary clinic. This represented a near doubling in the number of patients who joined the registry as compared with a similar time period from the previous year.
DISCUSSION
While the use of weekly multidisciplinary tumor boards to review select oncologic cases has been adopted at many institutions, formal single-site, single-day multidisciplinary clinics have not been common. It is also important to note that many other major cancer centers – even those without a formal single-day clinic – employ a similar multidisciplinary approach to that espoused here. The reasons for the lack of single-day multidisciplinary clinics are, however, multifactorial. Multidisciplinary clinics require dedicated institutional resources, as well as significant time commitments from the various subspecialty physicians. In addition, the throughput in multidisciplinary clinics has been criticized as inefficient and slow, as only a limited number of patients can be seen by the relatively large number of providers present at any given clinic. However, with the advent of the Internet and increased patient knowledge, patients are increasingly seeking care from cancer centers that offer a single-center specialized multidisciplinary setting. While the multidisciplinary clinic can be an educational and reassuring environment, actual data on the therapeutic effect of multidisciplinary clinics are essential to establish empirically the impact of these clinics on patient care. Although improved outcomes in breast cancer patients treated at multidisciplinary clinics have been reported,7,8 the current study is the first to report such data for patients with gastrointestinal malignancies. Data from the pancreas multidisciplinary cancer clinic at Johns Hopkins Hospital revealed that the clinical care recommendations of the multidisciplinary team compared with those received prior to review at the clinic led to changes in therapeutic recommendations in up to one-quarter of patients with pancreatic cancer. Such data obviously have wide-reaching implications for both patients and physicians.
For patients presenting to our multidisciplinary pancreatic clinic, the leading reason for a recommended change in the therapeutic plan was a new finding on cross-sectional imaging. Specifically, 38 out of 203 (18.7%) patients had a change in the status of their clinical stage following re-review of all available CT scan imaging (i.e., both at other centers and at Johns Hopkins). Of note, the overwhelming majority (68.4%) of these patients were found to have previously undetected metastases, thereby upstaging them to stage IV disease. Reliable preoperative staging of pancreatic cancer is critical in selecting those patients without metastatic disease, as only these patients are likely to benefit from surgical resection. Small hepatic metastases (<1cm) often cannot be reliably identified on preoperative CT imaging.19 In fact, the sensitivity of CT to detect metastases ranges from 38% to 73%.20–23 Weg et al.24 and Kopka and Grabbe25 have noted that a slice thickness of 2–4 mm is superior to 5–10 mm in the detection of small liver metastases. The introduction of multidetector CT imaging has allowed the acquisition of these thinner slices in liver imaging, resulting in improved detection rates of liver metastases.26 In the current study, the majority of patients (72.9%) underwent a repeat CT scan at Johns Hopkins. At that time, scan slices 0.75 mm thick were acquired and scanning data were reconstructed in a 3D format. All images were reviewed by senior radiologists with extensive experience in pancreatic cross-sectional imaging. As a result, 26 patients were identified to have previously unsuspected metastases. These data emphasize the importance of high-quality imaging and expert review in assessing patients for distant metastatic disease prior to consideration of surgery.
Accurate CT imaging is also critical in assessing locoregional resectability.19,27,28 Recently, 3D CT scan has been reported to enhance the assessment of the tumor–vascular interface,14 as the 3D format allows for the viewing of oblique orientations of the pancreas within the retroperitoneum.13,29 Accurate information concerning the relation of the tumor with the superior mesenteric artery and superior mesenteric–portal vein are particularly critical as more and more centers no longer consider venous involvement a contraindication to surgical resection. 16,30,31 In the current study, three patients with adenocarcinoma of the pancreas were initially deemed to be unresectable at the other institution solely due to tumor abutment/involvement with the portal vein. Pancreaticoduodenectomy with vascular resection in properly selected patients, however, can be associated with a median survival of approximately 2 years, which is not different from those who undergo standard pancreaticoduodenectomy.16,30 As such, similar to other high-volume pancreatic centers, 16–18 surgeons at Johns Hopkins will routinely consider patients for resection if the tumor can be completely removed (R0) with planned vein resection and reconstruction. Similarly, it has been our philosophy to pursue aggressively resection of locally advanced primary well-differentiated pancreatic endocrine neoplasms. While there is some controversy about the treatment of patients with advance endocrine neoplasms,32,33 several studies have reported that aggressive surgery can be done with acceptable morbidity and can be associated with durable survival.34,35 In the current series, two patients who were originally designated as unresectable at other institutions were deemed to be operable. Both patients underwent a successful R0 resection, although resection of adjacent visceral structures (e.g., stomach, colon, adrenal gland, small bowel) and/or portal vein was sometimes required.
Second opinion surgical pathology can result in major therapeutic and prognostic modifications for patients. Krontz et al.36 reported that second opinion surgical pathology resulted in a change in diagnosis in 1.4% of cases reviewed. In that study, the authors36 conclude that, although the overall percentage of affected cases was not large, the consistent rate of discrepant diagnoses uncovered by second opinion surgical pathology had a significant therapeutic, legal, and financial impact. Similarly, we herein report that review of the histologic slides by dedicated pancreatic pathologists resulted in changes in the interpretation for 7 of 203 patients (3.4%). Importantly, in two patients the diagnosis changed from a malignant to a benign process (benign inflammatory process n = 1 and serous cystadenoma n = 1). In the other patients, the change in diagnosis had important implications with regard to the therapeutic planning (e.g., in one case the misinterpretation of a bile duct adenoma for a metastasis led to the abortion of surgery) or prognosis (e.g., endocrine versus adenocarcinoma). Despite the controversies regarding costs, a routine second pathologic opinion before a major therapeutic intervention has been advocated as an important element to improve patient care.36,37 Data from the current study further corroborate how a multidisciplinary approach that incorporates routine review of outside pathology can lead to small, but meaningful, changes in diagnosis, therapy, and prognosis.
Another significant advantage of a multidisciplinary clinic is its ability to disseminate knowledge more effectively about local/national support groups, pertinent familial registries, as well as potential clinical trials. Enrollment in cancer trials is low for all patient groups, but is particularly poor amongst certain racial minorities and the elderly.38 Although the reasons for low trial accrual is clearly multifactorial, lack of adequate screening and presentation of clinical trials to patients for consideration contributes to lack of participation.39,40 Given that traditional therapy for pancreatic cancer has yielded poor long-term results, investigation of novel protocol-based therapies is particularly important and should be offered to those interested patients who meet study eligibility criteria. Adoption of the multidisciplinary clinic resulted in 25.1% of patients being offered participation in a clinical trial. Unfortunately, probably secondary to the long distances that patients traveled, active accrual into a trial was lower (4.9%). Enrollment into our familial research register, the National Familial Pancreas Tumor Registry, however, nearly doubled with initiation of the multidisciplinary clinic.
The current study had several limitations. Clinicians at our multidisciplinary clinic were not blinded to the previous recommendations of the other institution. As such, clinicians had the benefit of both the outside as well as the Johns Hopkins data on which to base their clinical recommendations. A blinded comparison of the other institution’s versus the multidisciplinary clinic’s recommendation would not have been ethically feasible. Blinding was not necessary, however, to meet the primary objective of the current study, which was to assess the relative value added of the multidisciplinary clinic. In addition, the current study lacked information on patient evaluation and satisfaction with the multidisciplinary clinic. These data are currently being prospectively collected and will be subsequently reported.
In conclusion, the pancreatic multidisciplinary clinic review of cross-sectional imaging resulted in a change in clinical stage in 18.7% of patients. Re-review of pathology resulted in a 3.4% change in diagnosis. Overall, the multidisciplinary clinic had a significant impact on the clinical care recommendations of patients with pancreatic cancer. Specifically, multidisciplinary case review resulted in an overall 23.6% change in the therapeutic plan of patients with presumed pancreatic cancer. The pancreatic multidisciplinary clinic was an efficient and effective means to assess patients with presumed pancreatic cancer. The clinic facilitated consensus recommendations and less confusion regarding the therapeutic plan. In addition, the single-day format improved patient education and permitted greater interaction with support staff (social work, nutrition, etc.). Results of the current study support the efficacy of a pancreatic cancer multidisciplinary clinic to provide an important expert opinion, as it led to dramatic changes in the care of a significant subset of patients.
ACKNOWLEDGEMENTS
Support: Dr. Pawlik is supported by Grant Number 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of the publication are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
Presented at the American Pancreatic Association’s Annual Meeting, Chicago, Illinois, November 1, 2007.
The Johns Hopkins Multidisciplinary Pancreas Clinic Team: Ranh Voong, BA, Marian Raben, PA, Barish Edil, MD, Fariba Asrari, MD, Cathy Stanfield, PA, Karen Horton, MD, Marty A. Makary, MD, MPH, Ross Donehower, MD, Luis A. Diaz, Jr., MD.
REFERENCES
- 1.Back MF, Ang EL, Ng WH, et al. Improvements in quality of care resulting from a formal multidisciplinary tumour clinic in the management of high-grade glioma. Ann Acad Med Singapore. 2007;36:347–351. [PubMed] [Google Scholar]
- 2.Gabel M, Hilton NE, Nathanson SD. Multidisciplinary breast cancer clinics. Do they work? Cancer. 1997;79:2380–2384. [PubMed] [Google Scholar]
- 3.Petty JK, Vetto JT. Beyond doughnuts: tumor board recommendations influence patient care. J Cancer Educ. 2002;17:97–100. doi: 10.1080/08858190209528807. [DOI] [PubMed] [Google Scholar]
- 4.Nyquist JG, Radecki SE, Gates JD, et al. An educational intervention to improve hospital tumor conferences. J Cancer Educ. 1995;10:71–77. doi: 10.1080/08858199509528338. [DOI] [PubMed] [Google Scholar]
- 5.Radecki SE, Nyquist JG, Gates JD, et al. Educational characteristics of tumor conferences in teaching and non-teaching hospitals. J Cancer Educ. 1995;9:204–216. [PubMed] [Google Scholar]
- 6.Wright FC, De Vito C, Langer B, et al. Multidisciplinary cancer conferences: a systematic review and development of practice standards. Eur J Cancer. 2007;43:1002–1010. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Newman EA, Guest AB, Helvie MA, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107:2346–2351. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 8.Chang JH, Vines E, Bertsch H, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001;91:1231–1237. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Ujiki MB, Talamonti MS. Guidelines for the surgical management of pancreatic adenocarcinoma. Semin Oncol. 2007;34:311–320. doi: 10.1053/j.seminoncol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Crane CH, Varadhachary G, Wolff RA, et al. The argument for pre-operative chemoradiation for localized, radiographically resectable pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:365–382. doi: 10.1016/j.bpg.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Varadhachary GR, Tamm EP, Crane C, et al. Borderline resectable pancreatic cancer. Curr Treat Options Gastroenterol. 2005;8:377–384. doi: 10.1007/s11938-005-0040-x. [DOI] [PubMed] [Google Scholar]
- 13.Novick SL, Fishman EK. Three-dimensional CT angiography of pancreatic carcinoma: role in staging extent of disease. Am J Roentgenol. 1998;170:139–143. doi: 10.2214/ajr.170.1.9423619. [DOI] [PubMed] [Google Scholar]
- 14.House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8:280–288. doi: 10.1016/j.gassur.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 16.Tseng JF, Raut CP, Lee JE, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–949. doi: 10.1016/j.gassur.2004.09.046. discussion 949-50. [DOI] [PubMed] [Google Scholar]
- 17.Poon RT, Fan ST, Lo CM, et al. Pancreaticoduodenectomy with en bloc portal vein resection for pancreatic carcinoma with suspected portal vein involvement. World J Surg. 2004;28:602–608. doi: 10.1007/s00268-004-7250-6. [DOI] [PubMed] [Google Scholar]
- 18.Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg. 1996;224:342–347. doi: 10.1097/00000658-199609000-00010. discussion 347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemke DA, Cameron JL, Hruban RH, et al. Potentially resectable pancreatic adenocarcinoma: spiral CT assessment with surgical and pathologic correlation. Radiology. 1995;197:381–385. doi: 10.1148/radiology.197.2.7480681. [DOI] [PubMed] [Google Scholar]
- 20.Fishman EK, Horton KM. Imaging pancreatic cancer: the role of multidetector CT with three-dimensional CT angiography. Pancreatology. 2001;1:610–624. doi: 10.1159/000055871. [DOI] [PubMed] [Google Scholar]
- 21.Schima W, Ba-Ssalamah A, Kolblinger C, et al. Pancreatic adenocarcinoma. Eur Radiol. 2007;17:638–649. doi: 10.1007/s00330-006-0435-7. [DOI] [PubMed] [Google Scholar]
- 22.Bartolozzi C, Donati F, Cioni D, et al. Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol. 2004;14:14–20. doi: 10.1007/s00330-003-1966-9. [DOI] [PubMed] [Google Scholar]
- 23.Balci NC, Semelka RC. Radiologic diagnosis and staging of pancreatic ductal adenocarcinoma. Eur J Radiol. 2001;38:105–112. doi: 10.1016/s0720-048x(01)00295-9. [DOI] [PubMed] [Google Scholar]
- 24.Weg N, Scheer MR, Gabor MP. Liver lesions: improved detection with dual-detector-array CT and routine 2.5-mm thin collimation. Radiology. 1998;209:417–426. doi: 10.1148/radiology.209.2.9807568. [DOI] [PubMed] [Google Scholar]
- 25.Kopka L, Grabbe E. Biphasic liver diagnosis with multiplanar-detector spiral CT. Radiologe. 1999;39:971–978. doi: 10.1007/s001170050590. [DOI] [PubMed] [Google Scholar]
- 26.Kopp AF, Heuschmid M, Claussen CD. Multidetector helical CT of the liver for tumor detection and characterization. Eur Radiol. 2002;12:745–752. doi: 10.1007/s00330-001-1177-1. [DOI] [PubMed] [Google Scholar]
- 27.Graf O, Boland GW, Warshaw AL, et al. Arterial versus portal venous helical CT for revealing pancreatic adenocarcinoma: conspicuity of tumor and critical vascular anatomy. Am J Roentgenol. 1997;169:119–123. doi: 10.2214/ajr.169.1.9207510. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy MJ, Evans J, Sagar G, et al. Prediction of resectability of pancreatic malignancy by computed tomography. Br J Surg. 1998;85:320–325. doi: 10.1046/j.1365-2168.1998.00584.x. [DOI] [PubMed] [Google Scholar]
- 29.Fishman EK, Wyatt SH, Ney DR, et al. Spiral CT of the pancreas with multiplanar display. Am J Roentgenol. 1992;159:1209–1215. doi: 10.2214/ajr.159.6.1442384. [DOI] [PubMed] [Google Scholar]
- 30.Nakagohri T, Kinoshita T, Konishi M, et al. Survival benefits of portal vein resection for pancreatic cancer. Am J Surg. 2003;2003:149–153. doi: 10.1016/s0002-9610(03)00173-9. [DOI] [PubMed] [Google Scholar]
- 31.Weitz J, Kienle P, Schmidt J, et al. Portal vein resection for advanced pancreatic head cancer. J Am Coll Surg. 2007;204:712–716. doi: 10.1016/j.jamcollsurg.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Metz DC. Diagnosis and treatment of pancreatic neuroendocrine tumors. Semin Gastrointest Dis. 1995;6:67–78. [PubMed] [Google Scholar]
- 33.Norton JA. Neuroendocrine tumors of the pancreas and duodenum. Curr Probl Surg. 1994;31:77–156. doi: 10.1016/0011-3840(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 34.Teh SH, Deveney C, Sheppard BC. Aggressive pancreatic resection for primary pancreatic neuroendocrine tumor: is it justifiable? Am J Surg. 2007;193:610–613. doi: 10.1016/j.amjsurg.2007.01.014. discussion 613. [DOI] [PubMed] [Google Scholar]
- 35.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 36.Kronz JD, Westra WH, Epstein JI. Mandatory second opinion surgical pathology at a large referral hospital. Cancer. 1999;86:2426–2435. [PubMed] [Google Scholar]
- 37.Tomaszewski JE, LiVolsi VA. Mandatory second opinion of pathologic slides: is it necessary? Cancer. 1999;86:2198–2200. doi: 10.1002/(sici)1097-0142(19991201)86:11<2198::aid-cncr4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 38.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 39.Ford BM, Evans JS, Stoffel EM, et al. Factors associated with enrollment in cancer genetics research. Cancer Epidemiol Biomarkers Prev. 2006;15:1355–1359. doi: 10.1158/1055-9965.EPI-05-0816. [DOI] [PubMed] [Google Scholar]
- 40.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]