Abstract
Purpose of review
In diabetic nephropathy (DN), insulin resistance and hyperinsulinemia correlate with the development of albuminuria. The possibility that altered insulin signaling in glomerular cells and particularly podocytes contributes to the development of DN will be discussed.
Recent findings
While normal podocytes uptake glucose in response to insulin, diabetic podocytes become insulin resistant in experimental DN prior to the development of significant albuminuria. Both clinical and experimental data suggest that insulin sensitizers may be renoprotective independently of their systemic effects on the metabolic control of diabetes.
Summary
We will review the clinical and experimental evidence that altered insulin signaling correlates with the development of DN in both type 1 and type 2 diabetes, and that insulin sensitizers may be superior to other hypoglycemic agents in the prevention of DN. We will then review potential mechanisms by which altered podocyte insulin signaling may contribute to the development of DN. Understanding the role of podocyte in glucose metabolism is important because it may lead to the discovery of novel pathogenetic mechanisms of DN, it may affect current strategies for prevention and treatment of DN, and it may allow for the identification of novel therapeutic targets.
Keywords: diabetic nephropathy, podocytes, insulin signaling
INTRODUCTION
Diabetic nephropathy (DN) is the most common single cause of end stage renal disease in the USA, and affects patients with both type 1 and type 2 diabetes [1]. Mesangial matrix expansion and glomerular basement membrane thickening have been recognized as the key histological features of DN [2]. However, loss of podocyte structure and function has been described as one of the early feature of DN in both patients with type 1 and type 2 diabetes [3–10], and represents an independent predictor of disease progression in patients at high risk for DN [4, 5]. Loss of podocyte number has also been described in experimental models of DN [11, 12]. In these models, podocytopenia is independent of the hemodynamic derangement that characterizes DN including significant increases in blood pressure [13], suggesting an important role of glucose metabolism in the loss of podocyte structure and function.
The notion that deranged glucose metabolism plays pivotal role in podocyte malfunction has advanced but remains to be established. Although the primary defect in type 1 diabetes is diminished insulin secretion and in type 2 diabetes is diminished insulin sensitivity, it is widely recognized that insulin resistance (IR) occurs in type 1 diabetes [14, 15] and that beta cell failure develops in type 2 diabetes [16–18]. Beside the traditional target organs of insulin action (liver and skeletal muscle), the cardiovascular system and the kidney have been also recognized to be insulin targets [19]. Although IR may certainly plays a role in the timing of the clinical onset of type 1 and 2 diabetes, it also may effect both microvascular and macrovascular outcomes of diabetes [20]. Thus, it is possible that disruption of normal insulin signaling (hyperinsulinemia, insulin resistence or absolute insulin deficiency) may play a significant role in the pathogenesis of diabetic complications. We will review herein the clinical and experimental evidence linking IR and/or hyperinsulinemia to DN. We will particularly focus attention on experimental studies aimed at the analysis of the effect of insulin and insulin signaling on podocyte function.
INSULIN RESISTANCE AND MICROALBUMINURIA
Microalbuminuria (MA) is the earliest clinical manifestation of DN [21] and represents a major risk for cardiovascular events and for progression to end stage renal disease [21–23]. Multiple studies have shown that IR correlates with the onset of MA in patients with type 2 diabetes [18, 20, 24, 25], as well as in nondiabetic subjects [26]. Interestingly, the correlation between IR and MA is independent of blood pressure, since it has been reported also in normotensive patients with type 2 diabetes [20]. Impaired insulin sensitivity in diabetic patients may be associated with altered renal cell glucose metabolism, contributing to progressive disease independently of hyperglycemia [25]. The correlation of MA with IR in normotensive and normoglycemic patients suggests that IR alone may have a causative role in the development of MA.
Diabetic nephropathy develops with similar features in type 1 and type 2 diabetes [2]. Since IR is associated with MA in type 2 diabetes, the question arises of how IR may contribute to DN in type 1 diabetes. IR has indeed been reported in type 1 diabetes [14, 15, 25]. The EUROPDIAB study, which analyzed 1134 normotensive patients with type 1 diabetes, has clearly shown that surrogate markers of IR (such as elevated tryglycerides and increased waist to hip ratio) are risk factors for the development of MA [27]. IR has indeed been shown to precede MA in 16 uncomplicated patients with type 1 diabetes [28]. Seven out of those 16 patients developed MA at 3 years of follow-up, and development of MA was associated with significantly lower glucose disposal rate (p<0.01) than those patients without MA. In a separate study, the total body glucose disposal rate was compared in 14 patients with type 1 diabetes and normoalbuminuria and 14 patients with MA. A lower glucose disposal rate reflective of IR was observed in patients with MA [29]. A similar correlation between IR and MA was reported in euglycemic family members of type 1 diabetic patients with MA [30]. However, one study comparing normolabuminuric and microalbuminuric patients failed to identify a correlation between IR and MA [31]. Supporting a correlation between IR and the development of microvascular complications are experimental data on diabetic retinopathy. In fact, insulin-receptor signaling is highly compromised in the retina of a model of streptozotocin-induced diabetes in rats, suggesting that a disrupted insulin-signaling pathway may play a key role for the development of microvascular complications of diabetes [32]. Furthermore, renal disease similar to DN can be observed in patients with a genetic mutation of the insulin receptor, which would again suggest that disruption of normal insulin signaling is a part of the disease process in DN [33].
HYPERINSULINEMIA AND MICROALBUMINURIA
Hyperinsulinemia is often utilized as a surrogate marker of IR. However, there is not sufficient evidence to distinguish whether IR or hyperinsulinemia is the significant risk factor for the development of MA. A summary of relevant studies that have investigated an association of IR or hyperinsulinemia with MA is provided in Table 1. Such important gap of knowledge requires further investigation, since it may strongly influence the decision to utilize insulin sensitizers and/or insulin in a different manner, particularly in those patients at risk for the development of MA. An elegant study performed in 1980 showed that insulin infusion during euglycemic clamps in patients with type 1 diabetes resulted in the development of transient albuminuria 45 minutes after initiation of insulin infusion [19]. A recent report in 175 patients with type 1 diabetes from the Natural History of Diabetes Study has shown correlation between MA and fasting insulin but not MA and IR [34]. The role of hyperinsulinemia alone as a risk factor for MA has been studied in type 2 diabetes as well [35, 36]. It is interesting to note that insulin can influence glomerular permeability to albumin in patients with type 2 diabetes but not in healthy subjects [35], suggesting that a disruption of the insulin signaling cascade may be sufficient for insulin to result in MA in type 2 diabetes.
Table 1. Relevant studies that show the relation of insulin resistance and hyperinsulinemia with MA.
A summary of 9 studies addressing the role of hyperinsulinemia and/or insulin resistance in the development of MA is provided. For each study, the first author, publication year, study design, patient population and number of patients are shown. “Yes” and “No” in the latter two columns indicate whether hyperinsulinemia and/or insulin resistance correlate with the development of MA
| STUDY | DESIGN | PATIENT | No OF PATIENTS |
Role of hyperinsulinemiain DN |
Role of IR in DN |
|---|---|---|---|---|---|
| 1. Groop. L 1993 | prospective | Type 2 | 71 | Yes | - |
| 2.Parvanova.A.I 2006 |
Cross-sectional Case I |
Type 2 | 158 | - | Yes |
| 3.Chaturvedi. N. EUROPDIA 2001 |
Prospective | Type 1 | 1134 | - | Yes |
| 4. Ekstrand 1998 |
prospective | Type 1 | 16 | No | Yes |
| 5. Yip. J 1993 | Prospective | Type 1 | 28 | - | Yes |
| 6. Catalano. C 1997 |
Prospective | Type 2 | 24 | yes | No |
| 7. Kohler 2000 | Cross secitonal | Type 2 | 1167 | yes | - |
| 8. Mogensen. C.E. 1980 |
Cross secitonal | Type1 | 5 | yes | - |
| 9.Mykkanen. L. 1998 |
Cross sectional | Non diabetic | 982 | yes | - |
RENOPROTECTIVE EFFECTS OF INSULIN SENSITIZERS IN DIABETES
Whether hyperinsulinemia and/or IR play a causative role in the development of MA can be addressed by the analysis of renal outcomes in intervention trials aimed at the improvement of IR, particularly those trials designed to compare the effect of insulin sensitizers to other hypoglycemic agents and or insulin. A recent double-blind, randomized, placebo controlled study, examined the change in urinary albumin excretion (UAE), the improvement in insulin sensitivity and circulating adipocytokine levels in T2DM patients treated with rosiglitazone when compared to other oral hypoglycemic. Despite a similar hemoglobin A1c (A1c), patients receiving rosiglitazone had a significant reduction in UAE at 3 months of follow up. Such reduction was associated with increased adiponectin concentrations [37*]. This is interesting, since among the multiple pleiotropic functions of adiponectin, a direct role in the prevention of proteinuria and podocyte damage has been recently recognized in experimental animals [38]. Another multicentered randomized controlled trial compared the effect of metformin and pioglitazone in 1200 patients with type 2 diabetes. After 52 weeks of treatment, patients had equal A1c and fasting plasma glucose. However, patients randomized to pioglitazone had an 18% reduction in albumin/creatinine ratio when compared to 2% in the metformin treated group [39]. Those trials indeed suggest that the agent used to achieve a target A1c may have a highly significant impact on renal outcome. If fact, a post hoc analysis of the UKPDS trial focusing on 1704 overweight patients (UKPDS 34) was designed to investigate whether intensive glucose control with metformin had any specific advantage or disadvantage [40]. Among patients randomized to intensive blood-glucose control, metformin that enhance insulin sensitivity also showed a greater effect than chlorpropamide, glibenclamide, or insulin for any diabetes-related endpoint, including albuminuria.
A potential causative role of insulin in the development of MA is suggested by insulin infusion studies type 1 diabetic patients [19] as well as in a very provocative cross sectional study in 890 patients with type 2 diabetes. In the latter, patients receiving insulin versus other hypoglycemic agents were compared [41]. After correction for diabetes duration, gender, A1c, those patients treated with insulin had a higher incidence of MA [41]. Thus, studies specifically designed to address if insulin per se may be deleterious to renal outcome are needed. These findings, combined with the recently reported increased cardiovascular mortality in 10,251 patients with type 2 diabetes receiving intensive treatment for diabetes (mainly with insulin) [42], offer a strong rationale to compare outcomes when different hypoglycemic agents are utilized. Although large clinical trials of the thiazolidinedione (TZDs) have been curtailed due to the increased cardiovascular mortality reported with rosiglitazone [43], a post hoc analysis of the PROactive trial performed in a selected population of patients with type 2 diabetes and glomerular filtration rate less than 60 ml/min has demonstrated that pioglitazone utilization is safe in this patient population and that it may even confer protection from major cardiovascular events [44].
Among experimental studies supporting a favorable role of insulin sensitizers versus insulin in renoprotection, S Ohtomo et al. randomized diabetic rats to receive either pioglitazone (PGZ) or insulin [45**]. This study demonstrated that PGZ but not insulin reduced proteinuria, preserved renal function, and improved diabetic glomerular and tubular lesions. This effect was independent of the metabolic control, since fasting glycemia and A1C were improved but more so with insulin than with PGZ [45**]. Furthermore, very provocative experimental data does exist suggesting a role for peroxisome- proliferators-receptor-(PPR)-alpha agonist in the improvement of IR and albuminuria [46]. Fenofibrates act as PPAR-alpha ligands and improve insulin sensitivity and glycemic control. In the kidney, fenofibrates decreased UAE and attenuate glomerular mesangial matrix accumulation in db/db mice, a known model of type 2 diabetes [46]. Whether any mean to improve peripheral insulin sensitivity may translate into prevention of DN remains to be established. While aspirin has been recognized to have insulin sensitizing properties [47], no data have suggested so far that it can improve UAE in either type 2 [48] or type 1 diabetic patients [49]. In addition, we have recently reported that db/db mice treated with a JNK inhibitor as well as JNK knock out mice rendered diabetic by streptozotocin injection (STZ) experience improved insulin sensitivity concomitant with worsened albuminuria [50*]. Thus, not all insulin sensitizing agents may be renoprotective.
PODOCYTES AND INSULIN SIGNALING
The glomerular filtration barrier has three important components: endothelial cells, the glomerular basement membrane and the podocytes [51]. Recently, the role of podocytes in DN has become the subject of intense research effort, since podocytes foot process effacement and decreased podocyte number have been reported in patient with either type 1 or type 2 diabetes in the early phases of DN [3, 5–10, 52].
Podocytes express all the elements of the insulin signaling cascade as well as glucose transporters such as GLUT-1 and GLUT-4 [53], and are capable of increasing their glucose uptake when they are stimulated with insulin. Furthermore, we have recently demonstrated that podocytes isolated from diabetic db/db mice are unable to respond to insulin even when isolated from mice prior to the onset of MA [54]. However, the role of insulin signaling as well as the molecular mechanisms of podocyte specific IR remains to be established. One possibility is that the local inflammatory phenotype associated with IR may contribute to podocyte malfunction. This is suggested by experimental intervention studies that have demonstrated how TZDs may ameliorate the progression of podocytes injury associated with puromucin aminonucleoside, a model to evaluate renal injury in rats [55]. In fact, TZDs directly affect the secretion of pro-inflammatory mediators by macrophages (macrophage colony stimulated factor, TNF-α, IL-6, PAI-1 and IL-1β) and modulate podocyte apoptosis (decreased activated caspase 3, restoration of p27 and Bcl-xl) [55]. Thus, a potential role for TZDs as therapeutic strategy in podocyte related diseases is warranted [56]. Among other potential mechanisms of podocyte specific IR, we have shown that podocyte inability to respond to insulin may result from an increased phosphorylation of c-jun N terminal kinase (JNK) [50] a mediator of inflammation, apoptosis and IR. JNK activation resulted in increased susceptibility to cell death through the inability to properly phosphorylate AKT [54]. However, as discussed earlier, while treatment of db/db mice with JNK inhibitors resulted in improved peripheral insulin sensitivity, worsening of albuminuria was also observed [50]. Interestingly, JNK inhibition resulted in a down-regulation of nephrin mRNA in both db/+ and db/db glomeruli [50]. Nephrin is a transmembrane protein of the inmunoglobulin superfamily; mutations of the genes that code for this protein cause a severe proteinuric syndrome in children with congenital nephrotic syndrome of the Finnish type [57]. Nephrin is thought to prevent protein loss through the maintenance of the slit diaphragm between adjacent podocyte foot processes, serving as a signal molecule and regulating the actin cytoskeleton of the podocyte through Nck adaptor proteins [58–60], which could in turn affect proper trafficking of receptors and glucose transporter to the cell membrane. In fact, nephrin is essential to allow insulin dependent GLUT-4 vesicles translocation to the plasma membrane in podocytes [61**], and regulation of nephrin expression in the early phases of DN has been described [62]. Thus, the potential role of nephrin in the development of podocyte specific IR should also be further investigated. Among other potential mechanisms to be explored, the modulation of adiponectin production observed in diabetes may also directly affect podocyte function [38]. Although it has been demonstrated that adiponectin protects podocytes via stimulation of the AMPK pathway and reduced oxidative stress, it is also possible that adiponectin facilitates proper glucose uptake in podocytes as described in other peripheral tissues [63]. Furthermore, adiponectin resistance may occur in podocytes and contribute to cell malfunction; in this respect, we have generated preliminary data suggesting a down-regulation of adiponectin receptors 1 and 2 in podocytes isolated from db/db mice when compared to db/+ (data not shown). The role of Cathepsin L in the development of podocyte IR should also be considered. Cathepsin L has been shown to be markedly up-regulated in diabetic glomeruli [64], and increased Cathepsin L is associated to degradation of insulin receptor β subunits and GLUT-4 in adipocytes [65]. Thus, it is possible that podocyte specific IR is related to a modulation of cathepsin L expression and function in podocytes.
One additional possibility is that insulin per se, particularly in the setting of compromised insulin receptor signaling, may signal through alternative pathways and be deleterious to podocyte function. It is possible, for instance, that insulin directly affects in a PI3K/PIP3 dependent manner [66] the activity of transient receptor potential canonical channel 6 (TRPC6), which have been demonstrated to play an important role in several proteinuric diseases [67–69]. This is particularly important, since calcium influx and calcineurin activation in podocytes have been characterized as key determinant of podocyte function [70, 71] and calcineurin isoforms are upregulated in the experimental DN [72]. Furthermore, since we have demonstrated podocyte specific IR in early diabetes [54], and our prior observations demonstrated a strong autocrine up-regulation of the IGF-1 receptor pathway in experimental models of DN [73–75], it is possible that insulin, in the presence of IR, acts through alternative pathways such as the IGF-1 pathway, that we have shown to be deleterious to glomerular cells [73–75].
SUMMARY AND CONCLUSIONS
In summary, several studies have suggested a contribution of IR and hyperinsulinemia to the development of MA. Clinical and experimental studies have also suggested that improvement of IR in diabetes may result in improvement of albuminuria. Whether this translates into improvement of glomerular filtration rate and decreased progression to end stage renal disease remains to be established. While long term follow-up study are being performed to address this latter question, understanding the direct role of insulin and insulin receptor signaling in podocytes may lead to the development of novel therapeutic strategies for the prevention and the cure of DN.
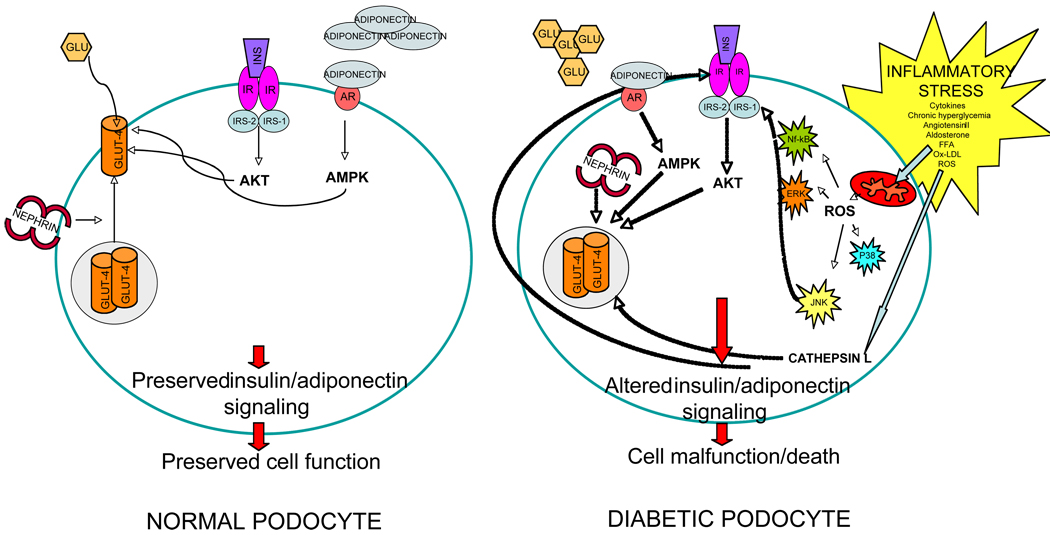
Figure 1. Proposed model for altered metabolic function in normal and diabetic podocytes.
Shown is a schematic and simplified representation of glucose metabolism in normal and diabetic podocytes. In NORMAL PODOCYTES, glucose uptake is granted by normal insulin receptor signaling and glucose transporters expression and function (mainly GLUT-4). GLUT4 is maintained functional by proper nephrin expression and GLUT-4 vesicle trafficking to the plasma membrane. Furthermore, adiponectin may contribute to normal glucose uptake via AMPK. Normal insulin and adiponectin signaling will facilitate cell survival through proper phosphorylation of AKT. In the DIABETIC PODOCYTE, the local inflammatory stress may lead to dysruption of insulin receptor signaling via ROS generation and activation of stress activated protein kinases such as NF-kB and JNK, responsible for cell malfunction and cell death. Furthermore, suppression of nephrin expression and decreased adiponectin signaling will result in GLUT4 intracytoplasmic sequestration and lack of function, while up-regulation of Cathepsin L may contribute to degradation of insulin receptor and GLUT-4.
ACKNOWLEDGMENTS
Alessia Fornoni is supported by NIH DK082636. Peter Mundel is supported by NIH R01DK57683 and R01DK62472.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.USRDS. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. 2007
- 2.Fioretto P, Caramori ML, Mauer M. The kidney in diabetes: dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia. 2008;51:1347–1355. doi: 10.1007/s00125-008-1051-7. [DOI] [PubMed] [Google Scholar]
- 3.Dalla Vestra M, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–1035. doi: 10.2337/diabetes.52.4.1031. [DOI] [PubMed] [Google Scholar]
- 4.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 5.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy GR, Kotlyarevska K, Ransom RF, et al. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17:32–36. doi: 10.1097/MNH.0b013e3282f2904d. [DOI] [PubMed] [Google Scholar]
- 7.Steffes MW, Schmidt D, McCrery R, et al. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Verzola D, Gandolfo MT, Ferrario F, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007 doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 9.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 10.White KE, Bilous RW. Structural alterations to the podocyte are related to proteinuria in type 2 diabetic patients. Nephrol Dial Transplant. 2004;19:1437–1440. doi: 10.1093/ndt/gfh129. [DOI] [PubMed] [Google Scholar]
- 11.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 12.Koya D, Haneda M, Nakagawa H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. Faseb J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 13.Fornoni A, Ijaz A, Tejada T, et al. Role of inflammation in diabetic nephropathy. Curr Diabetes Rev. 2008;4:10–17. doi: 10.2174/157339908783502361. [DOI] [PubMed] [Google Scholar]
- 14.Greenbaum CJ. Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev. 2002;18:192–200. doi: 10.1002/dmrr.291. [DOI] [PubMed] [Google Scholar]
- 15.Leslie RD, Taylor R, Pozzilli P. The role of insulin resistance in the natural history of type 1 diabetes. Diabet Med. 1997;14:327–331. doi: 10.1002/(SICI)1096-9136(199704)14:4<327::AID-DIA315>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Kasuga M. Insulin resistance and pancreatic beta cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. ix. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen CE, Christensen NJ, Gundersen HJ. The acute effect of insulin on heart rate, blood pressure, plasma noradrenaline and urinary albumin excretion. The role of changes in blood glucose. Diabetologia. 1980;18:453–457. doi: 10.1007/BF00261700. [DOI] [PubMed] [Google Scholar]
- 20.Groop L, Ekstrand A, Forsblom C, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:642–647. doi: 10.1007/BF00404074. [DOI] [PubMed] [Google Scholar]
- 21.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N Engl J Med. 1984;310:356–360. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Stampfer MJ, Castelli WP, et al. The prognostic significance of proteinuria: the Framingham study. Am Heart J. 1984;108:1347–1352. doi: 10.1016/0002-8703(84)90763-4. [DOI] [PubMed] [Google Scholar]
- 23.Yuyun MF, Dinneen SF, Edwards OM, et al. Absolute level and rate of change of albuminuria over 1 year independently predict mortality and cardiovascular events in patients with diabetic nephropathy. Diabet Med. 2003;20:277–282. doi: 10.1046/j.1464-5491.2003.00940.x. [DOI] [PubMed] [Google Scholar]
- 24.Forsblom CM, Eriksson JG, Ekstrand AV, et al. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia. 1995;38:363–369. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 25.Parvanova AI, Trevisan R, Iliev IP, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 26.Mykkanen L, Zaccaro DJ, Wagenknecht LE, et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 27.Chaturvedi N, Bandinelli S, Mangili R, et al. Microalbuminuria in type 1 diabetes: rates, risk factors and glycemic threshold. Kidney Int. 2001;60:219–227. doi: 10.1046/j.1523-1755.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 28.Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 29.Yip J, Mattock MB, Morocutti A, et al. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 30.Yip J, Mattock M, Sethi M, et al. Insulin resistance in family members of insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;341:369–370. doi: 10.1016/0140-6736(93)90167-f. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Schmitz O, Orskov H, et al. Similar insulin sensitivity in NIDDM patients with normo- and microalbuminuria. Diabetes Care. 1995;18:834–842. doi: 10.2337/diacare.18.6.834. [DOI] [PubMed] [Google Scholar]
- 32.Reiter CE, Wu X, Sandirasegarane L, et al. Diabetes reduces basal retinal insulin receptor signaling: reversal with systemic and local insulin. Diabetes. 2006;55:1148–1156. doi: 10.2337/diabetes.55.04.06.db05-0744. [DOI] [PubMed] [Google Scholar]
- 33.Musso C, Javor E, Cochran E, et al. Spectrum of renal diseases associated with extreme forms of insulin resistance. Clin J Am Soc Nephrol. 2006;1:616–622. doi: 10.2215/CJN.01271005. [DOI] [PubMed] [Google Scholar]
- 34.Rademacher E, Mauer M, Jacobs DR, Jr, et al. Albumin excretion rate in normal adolescents: relation to insulin resistance and cardiovascular risk factors and comparisons to type 1 diabetes mellitus patients. Clin J Am Soc Nephrol. 2008;3:998–1005. doi: 10.2215/CJN.04631007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Catalano C, Muscelli E, Quinones Galvan A, et al. Effect of insulin on systemic and renal handling of albumin in nondiabetic and NIDDM subjects. Diabetes. 1997;46:868–875. doi: 10.2337/diab.46.5.868. [DOI] [PubMed] [Google Scholar]
- 36.Kohler KA, McClellan WM, Ziemer DC, et al. Risk factors for microalbuminuria in black americans with newly diagnosed type 2 diabetes. Am J Kidney Dis. 2000;36:903–913. doi: 10.1053/ajkd.2000.19080. [DOI] [PubMed] [Google Scholar]
- 37. Miyazaki Y, Cersosimo E, Triplitt C, et al. Rosiglitazone decreases albuminuria in type 2 diabetic patients. Kidney Int. 2007;72:1367–1373. doi: 10.1038/sj.ki.5002516. This is among the best small clinical studies that addresses the specific role of TZDs in the reduction of albuminuria in diabetes.
- 38.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schernthaner G, Matthews DR, Charbonnel B, et al. Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab. 2004;89:6068–6076. doi: 10.1210/jc.2003-030861. [DOI] [PubMed] [Google Scholar]
- 40.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 41.Savage S, Estacio RO, Jeffers B, et al. Increased complications in noninsulin-dependent diabetic patients treated with insulin versus oral hypoglycemic agents: a population study. Proc Assoc Am Physicians. 1997;109:181–189. [PubMed] [Google Scholar]
- 42.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 44.Schneider CA, Ferrannini E, Defronzo R, et al. Effect of pioglitazone on cardiovascular outcome in diabetes and chronic kidney disease. J Am Soc Nephrol. 2008;19:182–187. doi: 10.1681/ASN.2007060678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohtomo S, Izuhara Y, Takizawa S, et al. Thiazolidinediones provide better renoprotection than insulin in an obese, hypertensive type II diabetic rat model. Kidney Int. 2007;72:1512–1519. doi: 10.1038/sj.ki.5002570. This is the best experimental evidence that TZDs may be more renoprotective than insulin in DN, independently of the achieved metabolic control of diabetes. Interestingly, both albuminuria and hard outcomes (histological lesions and creatinine clearance) are utilized.
- 46.Park CW, Zhang Y, Zhang X, et al. PPARalpha agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69:1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 47.Yuan M, Konstantopoulos N, Lee J, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 48.Gaede P, Hansen HP, Parving HH, et al. Impact of low-dose acetylsalicylic acid on kidney function in type 2 diabetic patients with elevated urinary albumin excretion rate. Nephrol Dial Transplant. 2003;18:539–542. doi: 10.1093/ndt/18.3.539. [DOI] [PubMed] [Google Scholar]
- 49.Hansen HP, Gaede PH, Jensen BR, et al. Lack of impact of low-dose acetylsalicylic acid on kidney function in type 1 diabetic patients with microalbuminuria. Diabetes Care. 2000;23:1742–1745. doi: 10.2337/diacare.23.12.1742. [DOI] [PubMed] [Google Scholar]
- 50. Ijaz A, Tejada T, Catanuto P, et al. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney Int. 2009;75:381–388. doi: 10.1038/ki.2008.559. This study utilizes a novel insulin sensitizer (JNK inhibitor) to demonstrate a discordant association between systemic insulin resistance and albuminuria in experimental DN, suggesting that more studies are needed to understand the role of glomerular insulin signaling in the pathogenesis of DN.
- 51.Mundel P, Shankland SJ. Glomerular podocytes and adhesive interaction with glomerular basement membrane. Exp Nephrol. 1999;7:160–166. doi: 10.1159/000020596. [DOI] [PubMed] [Google Scholar]
- 52.Meyer TN, Thaiss F, Stahl RA. Immunoadsorbtion and rituximab therapy in a second living-related kidney transplant patient with recurrent focal segmental glomerulosclerosis. Transpl Int. 2007;20:1066–1071. doi: 10.1111/j.1432-2277.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 53.Coward RJ, Welsh GI, Yang J, et al. The human glomerular podocyte is a novel target for insulin action. Diabetes. 2005;54:3095–3102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 54.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 55.Yang HC, Ma LJ, Ma J, et al. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 2006;69:1756–1764. doi: 10.1038/sj.ki.5000336. [DOI] [PubMed] [Google Scholar]
- 56.Kanjanabuch T, Ma LJ, Chen J, et al. PPAR-gamma agonist protects podocytes from injury. Kidney Int. 2007;71:1232–1239. doi: 10.1038/sj.ki.5002248. [DOI] [PubMed] [Google Scholar]
- 57.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 58.Jones N, Blasutig IM, Eremina V, et al. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- 59.Tryggvason K, Pikkarainen T, Patrakka J. Nck links nephrin to actin in kidney podocytes. Cell. 2006;125:221–224. doi: 10.1016/j.cell.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Verma R, Kovari I, Soofi A, et al. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Coward RJ, Welsh GI, Koziell A, et al. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. This study clearly demonstrated that human podocytes are insulin sensitive cells expressing both GLUT4 and GLUT1 that can uptake glucose in response to insulin. Furthermore, this study highlight the connection between insulin sensitivity and nephrin, and important protein involved in the pathogenesis of DN.
- 62.Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 63.Kadowaki T, Yamauchi T, Kubota N, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sever S, Altintas MM, Nankoe SR, et al. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Invest. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang M, Zhang Y, Pan J, et al. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat Cell Biol. 2007;9:970–977. doi: 10.1038/ncb1623. [DOI] [PubMed] [Google Scholar]
- 66.Tseng PH, Lin HP, Hu H, et al. The canonical transient receptor potential 6 channel as a putative phosphatidylinositol 3,4,5-trisphosphate-sensitive calcium entry system. Biochemistry. 2004;43:11701–11708. doi: 10.1021/bi049349f. [DOI] [PubMed] [Google Scholar]
- 67.Moller CC, Wei C, Altintas MM, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 68.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 70.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathieson PW. Proteinuria and immunity--an overstated relationship? N Engl J Med. 2008;359:2492–2494. doi: 10.1056/NEJMcibr0806881. [DOI] [PubMed] [Google Scholar]
- 72.Gooch JL, Pergola PE, Guler RL, et al. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol. 2004;15:1421–1429. doi: 10.1097/01.asn.0000128076.91545.bb. [DOI] [PubMed] [Google Scholar]
- 73.Fornoni A, Rosenzweig SA, Lenz O, et al. Low insulin-like growth factor binding protein-2 expression is responsible for increased insulin receptor substrate-1 phosphorylation in mesangial cells from mice susceptible to glomerulosclerosis. Endocrinology. 2006;147:3547–3554. doi: 10.1210/en.2006-0066. [DOI] [PubMed] [Google Scholar]
- 74.Karl M, Potier M, Schulman IH, et al. Autocrine activation of the local insulin-like growth factor I system is up-regulated by estrogen receptor (ER)-independent estrogen actions and accounts for decreased ER expression in type 2 diabetic mesangial cells. Endocrinology. 2005;146:889–900. doi: 10.1210/en.2004-1121. [DOI] [PubMed] [Google Scholar]
- 75.Tack I, Elliot SJ, Potier M, et al. Autocrine activation of the IGF-I signaling pathway in mesangial cells isolated from diabetic NOD mice. Diabetes. 2002;51:182–188. doi: 10.2337/diabetes.51.1.182. [DOI] [PubMed] [Google Scholar]