Abstract
Our study investigates the effect of a highly selective cyclooxygenase-2 (COX-2) inhibitor, celecoxib, on the cytotoxicity of docetaxel in nude mice bearing A549 tumor xenografts and elucidates the molecular mechanisms of the antitumor effect of this combination. Female nu/nu mice, xenografted with s.c. A549 tumors were treated with either celecoxib (150 mg/kg/day), docetaxel (10 mg/kg) or a combination of both. The tumor tissues were quantified for the induction of apoptosis, intratumor levels/expressions of prostaglandin E2 (PGE2), 15 deoxy prostaglandin J2 (15-d PGJ2), microsomal prostaglandin E synthase (mPGES) and cytoplasmic phospholipase A2 (cPLA2). The combination of celecoxib with docetaxel significantly inhibited the tumor growth (p < 0.03) as compared to celecoxib or docetaxel alone, decreased the levels of PGE2 by 10-fold and increased the 15-d PGJ2 levels by 4-fold as compared to control. The combination also enhanced the peroxisome proliferator-activated receptor (PPAR)-γ expression, decreased the expression of cPLA2, mPGES and vascular endothelial growth factor (VEGF), but had no effect on the expression of COX-1 or COX-2 in tumor tissues. TUNEL staining of the tumor tissues showed a marked increase in the apoptosis in the combination group as compared to the celecoxib- or docetaxel-treated groups and this was associated with an increase in the intratumor p53 expression. In conclusion, the combination of celecoxib with docetaxel produces a greater antitumor effect in s.c. A549 tumors as compared to celecoxib or docetaxel alone and this effect is associated with concomitant alterations in the intratumor levels of PGE2 and 15-d PGJ2.
Keywords: COX-2, A549, PPAR-γ, PGE2
Lung cancer is the leading cause of cancer-related deaths in the United States. The strategies that have been employed in the treatment of cancer include radiation, chemotherapy or combination of radiotherapy with chemotherapy. Despite the recent advances in the treatment of lung cancer, the response and remission rates in non-small cell lung cancer (NSCLC) patients remain relatively low.1 Attention is now being directed to find novel combination anticancer agents with non-overlapping mechanisms of action to obtain enhanced anticancer efficacy with reduced adverse side effects. In this context, the use of selective cyclooxygenase (COX)-2 inhibitors in combination with cytotoxic drugs has been shown to have synergistic antitumor effect in colon2 and lung tumor models.3
Celecoxib is a highly selective COX-2 inhibitor and has been shown to have antitumor effect in human tumor xenograft models of colon,4,5 breast,6 prostate7 and Lewis lung carcinoma.4 The in vitro antiproliferative effect of celecoxib has been attributed to the induction of apoptosis.8 Docetaxel has been approved for the treatment of NSCLC patients and exhibits its cytotoxic effect due to decreased proliferation and induction of apoptosis by stabilization of microtubules. Thus, the combination of celecoxib with docetaxel might be beneficial and currently clinical trials are underway in NSCLC patients.9,10 The recent Phase II clinical data suggest that the combination of docetaxel and celecoxib can be safely given in NSCLC patients.11
The molecular mechanisms responsible for the antitumor effect of celecoxib alone and in combination with anticancer drugs have not been clearly elucidated yet. It has been reported that celecoxib decreases the intratumor prostaglandin E2 (PGE2) levels in head and neck xenograft tumors without affecting the COX-2 expression.12 The reduction of intratumor PGE2 levels has been thought to be mediated via enzymatic inhibition of COX-2 activity in the tumor milieu by celecoxib. Recent evidence also indicates that increased prostaglandin biosynthesis is associated with the expression and activity of cytoplasmic phospholipase A2 (cPLA2, an enzyme involved in the release of arachidonic acid from membrane lipids) and COX-2 in A549 cells.13 Further, COX-2 and microsomal prostaglandin E synthase (mPGES, an enzyme mediating the conversion of prostaglandin H2 to PGE2 and functionally linked to COX-2) have been shown to be overexpressed in lung cancer patients.14 In addition, other COX independent mechanisms have also been reported for selective COX-2 inhibitors.15,16 We have reported recently that increased expression of peroxisome proliferator activated receptor-γ (PPAR-γ) was associated with the in vitro cytotoxicity enhancement of doxorubicin by a selective COX-2 inhibitor nimesulide in human lung adenocarcinoma, A549 cells and the in vivo antitumor effect of nimesulide in s.c. A549 tumor xenografts.17,18 In the light of these studies, we hypothesize that the antitumor effect of celecoxib or its combination with docetaxel may be associated with reduced intratumor PGE2 levels along with alterations in the expression of key targets involved in the PGE2 biosynthesis such as cPLA2 and mPGE synthase. We also hypothesize that the combination of celecoxib with docetaxel may increase the expression of PPAR-γ17–19 and alter the 15-deoxy prostaglandin J2 (15-d PGJ2) levels.20
The objectives of our present investigation were to study: (i) the enhanced antitumor effect of the combination of celecoxib with docetaxel in vitro in human lung adenocarcinoma, A549 cells as well as its xenograft tumors in nude mice, (ii) the intratumor levels of PGE2 and 15-d PGJ2 and (iii) expression of cPLA2, mPGE synthase, PPAR-γ and vascular endothelial growth factor (VEGF) in tumor tissues harvested from mice. Our data indicate that celecoxib and its combination with docetaxel reduce the intratumor PGE2 levels. Our findings indicate that the combination of celecoxib with docetaxel has little effect on COX-2 expression, but decreases the expression of cPLA2 and mPGE synthase and enhances the expression of PPAR-γ and intratumor levels of 15-d PGJ2 levels.
Material and methods
Materials
Celecoxib and docetaxel were gift samples from Pfizer (Skokie, IL) and Aventis (Collegeville, PA), respectively. A549 cell line was obtained from American Type Culture Collection (Manassas, VA). Antibodies against COX-2, PPAR-γ were purchased from Cayman Chemical Company (Ann Arbor, MI). Antibodies against cPLA2, and mPGE synthase were purchased from Santa Cruz Bio-technology, Inc. (Santa Cruz, CA). All tissue culture chemicals and anti-β-actin antibody were obtained from Sigma Chemical Company (St. Louis, MO). PGE2 and 15-d PGJ2 colorimetric enzyme immunoassay kits were purchased from Assay Designs (Ann Arbor, MI). DeadEnd™ Colorimetric Apoptosis Detection kit was obtained from Promega Corporation (Madison, WI). All other chemicals were either reagent or tissue culture grade.
Enhancement of docetaxel activity by celecoxib in A549 cells
The tumor cells (A549) were plated into 96-well microtiter plates, at a concentration of 10,000 cells/well in F12K medium containing 5% serum and allowed to incubate overnight. Stock solutions of docetaxel and celecoxib were prepared in DMSO at 5.8 and 26.2 mM concentrations, respectively and subsequent dilutions were made in tissue culture medium. Various dilutions of docetaxel alone (0.00012–0.58 μM) and in combination with a fixed concentration of celecoxib (26.2 μM) were added to the cells and the plate was incubated for 72 hr at 37 ± 0.2°C in a 5% CO2-jacketed incubator. The cytotoxicity was assessed by crystal violet dye uptake assay,17,20 and quantified by measuring the absorbance of the dye at a wavelength of 540 nm, using an automated micro-plate reader (Biotek Instruments Inc.).
Enhancement of the antitumor effect of docetaxel by celecoxib in A549 tumors
Female Nu/Nu (6 weeks old, Harlan, Indianapolis, IN) were preselected for s.c. A549 tumors and xenografts were transplanted by s.c. administration of 5 × 106 A549 cells in the right hind leg.18 One-day after tumor implantation, the mice were treated with celecoxib (150 mg/kg/day) by oral gavage until the end of the study (28 days). Celecoxib was administered as a 2% w/v solution in ethanol: PEG 400: water (22.2:66.6:11.2, v/v). Docetaxel (10 mg/kg) was given by i.v. on Days 14, 18 and 22 after tumor implantation. The combination treatment group was given celecoxib and docetaxel essentially in the same way as administered for their respective individual treatment regimens. The control mice were administered with vehicle control. The tumor dimensions were measured twice weekly using a linear caliper and tumor volume was calculated using the equation V (mm3) = a × b2/2, where a is the largest diameter and b is the smallest diameter. The mice were fed with food and water ad lib.
Analysis of celecoxib in plasma samples
After the last dose of celecoxib was given on Day 28, blood samples were collected one hour after the oral dosing by cardiac puncture into heparinized tubes and analyzed subsequently by HPLC. Plasma levels of celecoxib were analyzed by reverse phase HPLC (C18 column 3.9 × 300 mm) using acetonitrile:water containing 0.05% v/v acetic acid) at 60:40. The eluted celecoxib was detected at 250 nm.
Western blotting of tumor tissues
Tumor tissues harvested at 28 days post-tumor implantation from control, celecoxib, docetaxel and docetaxel with celecoxib-treated mice were cut into small pieces and homogenized in PBS. The homogenate was centrifuged at top speed for 10 min to sediment the tissue fragments. Next, the lysis buffer (25 mM HEPES, Triton-X 0.1%, NaCl 300 mM, β-glycerophosphate 20 mM, MgCl2 1.5 mM, EDTA 0.2 mM, DTT 25 mM) with protease inhibitors (sodium orthovanadate 4 mM, sodium fluoride 400 mM, benzamidine 20 mM, leupeptin 2 μg/ml, aprotinine 4 μg/ml, PMSF 500 μM) were added. Samples were vortexed, incubated on ice for 30 min, centrifuged again and the supernatant was stored at −80°C. For Western blotting, equal amounts of supernatant protein (30 μg) from the control and different treatments were denatured by boiling for 5 min in SDS sample buffer, separated by 8% SDS-PAGE, and transferred to nitrocellulose membranes for immunoblotting. The membranes were blocked with 5% skim milk in Tris-buffered saline with Tween 20 [10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.5% Tween 20] and probed with COX-1, COX-2, cPLA2 and PPAR-γ antibodies (1:500). Bound antibodies were revealed with HRP conjugated secondary antibodies (1:2,000) using SuperSignal West pico chemiluminescent solution (Pierce, Rockford, IL).
Determination of PGE2 and 15-d PGJ2 levels in tumor tissues
The tumor tissue samples were homogenized in 70% ethanol and 30% of 0.1 M sodium phosphate (pH 4.0) and incubated on wet ice for 30 min. The samples were centrifuged and the supernatant was collected. A known volume of supernatant (Typically 250 μl) was dried under nitrogen and resuspended in assay bufferb and analyzed for PGE2 and 15-d PGJ2 using their respective colorimetric enzyme immunoassay kits as per the manufacturer’s recommendations (Assay Designs, Ann Arbor, MI).
Immunohistochemistry of mPGE synthase in tumor tissues
The tumor tissue samples collected at the end of the study (as described above) were frozen in cryomatrix and sectioned in a cryotome (Shandon Cryotome 0620, Thermo Shandon, Pittsburgh, PA) and immunostained for mPGE synthase. In brief, the 10 μm cryosections were washed 3× with PBS to remove the freezing matrix followed by the quenching of endogenous peroxidase activity by incubating the slides in 0.3% H2O2 solution. After this, the slides were washed again 3× with PBS and incubated with primary antibodies for mPGE synthase (1:50) for 1 hr at room temperature (RT) in a humidified chamber. Location of the primary antibodies was achieved by application of HRP conjugated secondary antibodies for 30 min at RT and DAB substrate. The slides were counter-stained with hematoxylin. Finally, the slides were observed with an Olympus BX40 light microscope equipped with computer controlled digital camera (QImaging, Burnaby, BC, Canada) and imaging software.
TUNEL assay of tumor tissues
DeadEnd colorimetric apoptosis detection system (Promega Corporation, Madison, WI) was used to detect apoptosis in tumor tissues harvested at 28 days post-tumor implantation. The tumor tissue cryosections as described above were washed in PBS and then fixed in 4% paraformaldehyde solution before incubation in 20 μg/ml proteinase K for 10 min. The sections were washed in PBS and incubated with TdT enzyme in a humidified chamber at 37°C for 60 min for incorporation of biotinylated nucleotides at the 3′-OH DNA ends. After endogenous peroxidase quenching, horseradish-peroxidase-labeled streptavidin was used to bound the biotinylated nucleotides, which are finally detected using hydrogen peroxide and stable chromogen DAB. Slides were observed with an Olympus BX40 microscope and analyzed as described earlier.
Effect of celecoxib, docetaxel and their combination on mRNA levels of COX-2, PPAR-γ, VEGF and mPGE synthase in A549 tumor tissues
The tumor tissues collected at the end of the study (28 days post-tumor implantation) were processed for total RNA using the Eppendorf Perfect RNA Mini Kit (Brinkman Instruments, Westbury, NY). Reverse transcription was carried out with Moloney-murine leukemia virus reverse transcriptase (MuLV-RT) (Applied Biosystem, CA) according to the manufacturer’s protocol with some modifications. The PCR reaction was carried out following the conditions: (94°C, 2 min) with COX-2, PPAR-γ, VEGF121, mPGE synthase and β-actin primer pairs and ATAQ DNA polymerase (Applied Biosystem) for 30 cycles of 94°C, 52°C, (60 °C for mPGES) and 72°C (1 min each), and then 10 min at 72 °C before holding at 4°C. The PCR products (COX-2, 226 bp; PPAR-γ, 360 bp; VEGF, 121 bp; mPGES, 459 bp and β-actin 390 bp) were separated in a 1.5% agarose gel and the band intensities were normalized with respect to β-actin using Scion Image Software (Beta 3b version, Scion Corporation, Frederick, MD).
Statistical analysis
Differences in tumor volume between treatment groups were analyzed using non-parametric Mann-Whitney test18 and the significance of the difference in the expression of PPAR-γ, cPLA2, p53, VEGF and the prostaglandin levels (PGE2 and 15-d PGJ2), among the different treatments was analyzed by one-way ANOVA followed by Tukey’s multiple comparison test using GraphPad PRISM version 2.0 software (San Diego, CA).
Results
Celecoxib potentiates the cytotoxicity of docetaxel in vitro and in vivo
We studied the effect of celecoxib on docetaxel cytotoxicity in vitro against A549 cells and as shown in Figure 1, the combination of celecoxib with docetaxel produced a greater cell growth inhibition as compared to docetaxel alone. The IC50 value for docetaxel alone was found to be 0.026 ± 0.0015 μM, whereas the combination of docetaxel with celecoxib showed an IC50 of 0.0145 ± 0.0034 μM against A549 cells, which was found to be statistically significant (p = 0.02). The concentration of celecoxib (26.2 μM) employed for enhancing the docetaxel activity was below its IC50 value (50.1 μM) against A549 cells and produced only 19.6% cell growth inhibition under the same experimental conditions.
Figure 1.
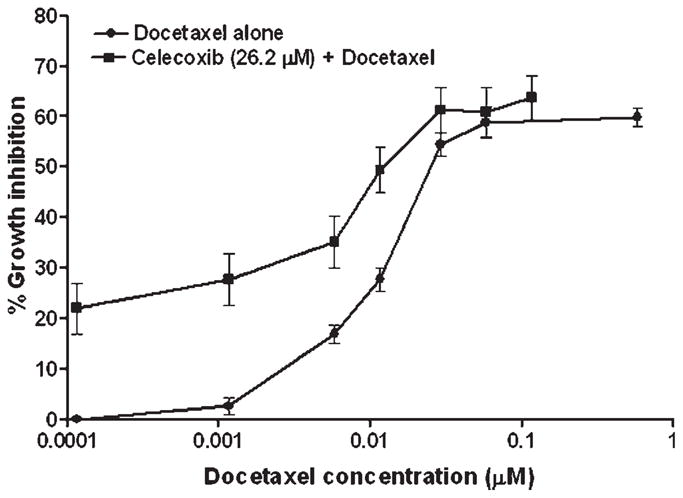
In vitro cell growth inhibitory effect of docetaxel alone and in combination with a fixed concentration of celecoxib (26.2 μM) against A549 cells. The cytotoxicity was assessed by crystal violet dye uptake assay and expressed with respect to the untreated control cells. Data was expressed as mean ± SEM of 4 independent experiments.
Figure 2 shows the tumor volume-time data profiles after the administration of vehicle control, celecoxib, docetaxel and the combination of celecoxib with docetaxel in mice xenografted with A549 tumors. It is evident from Figure 2 that the combination of celecoxib with docetaxel produced a greater antitumor effect as compared to celecoxib or docetaxel alone treatments. At 28 days post-tumor implantation, the tumor volumes were found to be 2,488 ± 330.6, 633.5 ± 110.7, 622.3 ± 113.1, 148.2 ± 39.38 mm3 (expressed as mean ± SEM) in vehicle, celecoxib, docetaxel and celecoxib with docetaxel-treated mice, respectively. These results indicate that there was 95.5% reduction in the tumor volume in celecoxib with docetaxel-treated mice as compared to 75% reduction in docetaxel-treated mice and 74.5% reduction in celecoxib-treated mice when expressed with respect to the tumor volume in vehicle-treated mice at 28 days post-tumor implantation. Mann-Whitney test on tumor volume data at 21–28 days in celecoxib with docetaxel-treated mice was found to be significantly lower (p < 0.03) as compared to vehicle, celecoxib- or docetaxel-treated mice. There was no significant difference in the plasma levels of celecoxib between celecoxib-treated mice (14.7 ± 4.6 μM) and celecoxib with docetaxel-treated mice (16.6 ± 3.7 μM).
Figure 2.
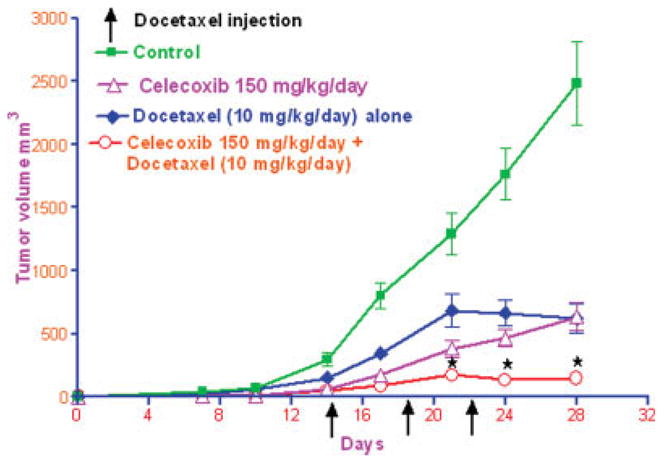
Antitumor activity of celecoxib, docetaxel and celecoxib with docetaxel combination against A549 lung tumors. The nude mice were s.c. implanted with 5 × 106 A549 cells in the right hind leg and randomly divided into 4 groups (10 per group). Two groups of mice were given celecoxib by oral gavage (150 mg/kg/day), initiated 1 day after tumor implantation and continued till the end of the experiment (28 days). One of the celecoxib fed group was further used for the administration of docetaxel, whereas the other group served as celecoxib only-treated group. Docetaxel was administered by i.v. via tail vein at 10 mg/kg dose on Days 14, 18 and 22 after tumor implantation. Docetaxel treatment was initiated on Day 14 in docetaxel alone and celecoxib with docetaxel-treated groups. Control mice were administered with vehicle used for docetaxel by i.v. in the same schedule as used for docetaxel treatment. The tumor dimensions (longest diameter and shortest diameter) were observed twice weekly. Tumor volume was calculated as the half the product of longest diameter and the square of the shortest diameter. Data presented was mean ± SEM. ⋆At 21–28 days post-tumor implantation, the tumor volumes observed in celecoxib with docetaxel combination-treated group were significantly lower (p < 0.03), as compared to control-, docetaxel-or celecoxib-treated mice as analyzed by non-parametric Mann-Whitney test.
Antitumor effect of celecoxib with docetaxel combination is associated with reduced intratumor PGE2 levels and cPLA2 expression
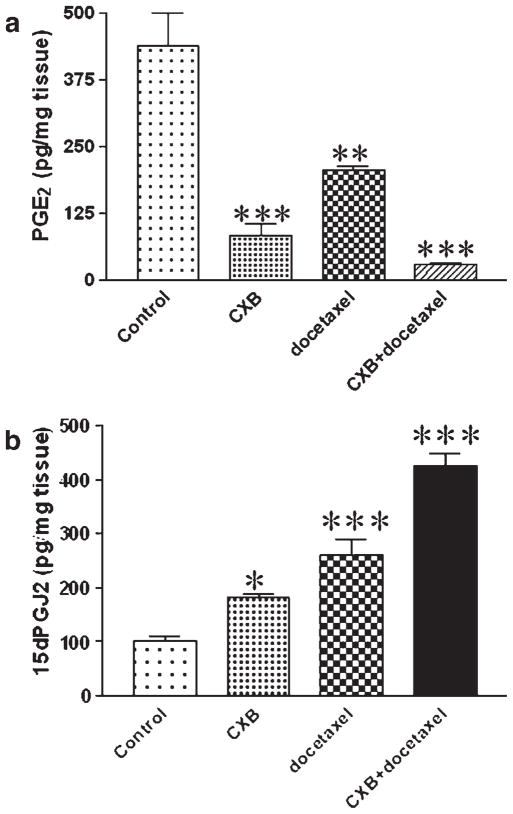
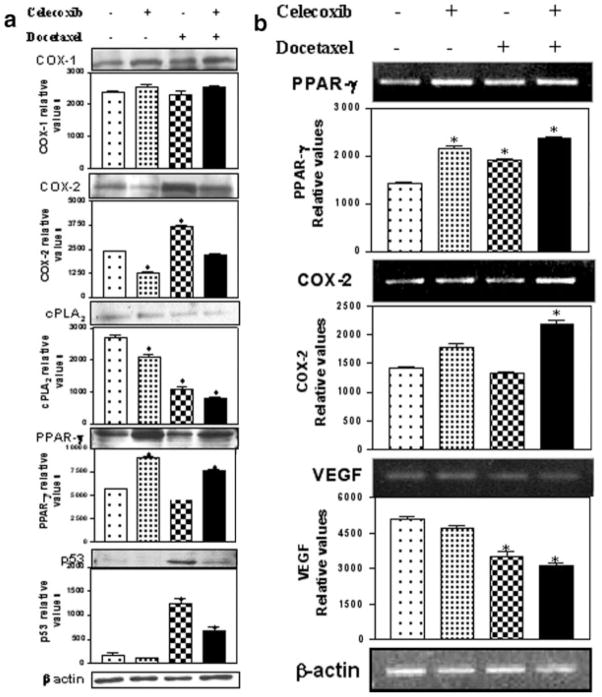
The intratumor PGE2 levels are presented in Figure 3a and it is evident from this figure that significant reduction in the PGE2 levels were observed in celecoxib, docetaxel and celecoxib with docetaxel-treated mice as compared to vehicle-treated control mice. The maximum reduction (93.3%) was observed in tumors from mice treated with celecoxib in combination with docetaxel, whereas celecoxib and docetaxel alone produced 81.2 and 52.9% reduction in PGE2, respectively. Western blot analysis of tumor tissues indicated that the expression of COX-1 and COX-2 was not altered in tumor tissues from celecoxib with docetaxel-treated mice as compared to vehicle (control)-treated mice (Fig. 4a). There was a 1.4-fold decrease in the COX-2 expression in tumor tissues from celecoxib-treated mice, whereas docetaxel treatment showed 1.5-fold elevated expression of COX-2 as compared to control (Fig. 4a). Further, we also observed that the expression of cPLA2 in tumor tissues from mice subjected to various treatments (celecoxib, docetaxel and celecoxib with docetaxel) was significantly (p < 0.001) lower by 1.3-, 2.4- and 3.3-fold respectively compared to the control mice (Fig. 4a).
Figure 3.
(a)-PGE2 and (b)-15-d PGJ2 levels as measured by enzyme immunoassay in tumors harvested at the end of the study (28 days post-tumor implantation). Data represented was mean ± SEM (n = 6). *Significant differences as compared to the vehicle control (p < 0.05). **Significant differences as compared to the vehicle control (p < 0.01). ***Significant differences as compared to the vehicle control (p < 0.001).
Figure 4.
(a) Western blotting of tumor tissue lysates for COX-1, COX-2, cPLA2, PPAR-γ, p53 and β-actin. The tumors were harvested 28 days post-tumor implantation and lysates were prepared as described in Material and Methods. Protein (30 μg) was loaded in each lane. (b) RT-PCR of COX-2, PPAR-γ and VEGF in tumor tissues. The PCR product sizes for COX-2 (226 bp), PPAR-γ (360 bp), VEGF (121 bp) and β-actin (390 bp) were determined using 100–2680 bp DNA ladder (Fisher Scientific Co., Atlanta, GA). *Significant differences as compared to the vehicle control (p < 0.001).
Celecoxib with docetaxel treatment enhances the expression of PPAR-γ and intratumor levels of 15-d PGJ2
We observed a significant (p < 0.001) increase in the expression of PPAR-γ in tumor tissues harvested from mice treated with celecoxib (1.6-fold) or celecoxib with docetaxel (1.5-fold) as compared to tumors from control mice by Western blotting (Fig. 4a) and RT-PCR techniques (Fig. 4b). Docetaxel treatment did not show any increase in the PPAR-γ expression by Western blotting, however, RT-PCR data showed a modest (1.3-fold) but significant increase in PPAR-γ. Enzyme immunoassay of 15-d PGJ2 (natural ligand for the PPAR-γ), in tumor tissues collected at 28 days post-tumor implantation showed 4-fold increase in the tumor tissues from celecoxib with docetaxel-treated mice as compared to control (Fig. 3b).
Inhibition of mPGE synthase by celecoxib with docetaxel
We have studied previously the localization of COX-2 in A549 tumor xenografts and found that treatment with a selective COX-2 inhibitor, nimesulide has no effect on COX-2 localization pattern between control and treated tumors. Nimesulide treatment resulted in a significant reduction in the intratumor PGE2 levels.18 In our present study, we observed that the combination of celecoxib with docetaxel resulted in maximum reduction in the intratumor PGE2 levels (Fig. 3a). We hypothesized that this reduction in the intratumor PGE2 levels, may be associated with alterations in the expression of mPGE synthase. We studied the effect of combination therapy of celecoxib with docetaxel on mPGE synthase expression by immunohistochemistry. We observed moderate immunostaining in the epithelial cells and low immunostaining in tumor associated blood vessels in specimens obtained from the control tumors (Fig. 5a). There was negligible staining in the blood vessels and weak immunostaining in epithelial cells of tumors obtained from mice treated with the combination of celecoxib with docetaxel (Fig. 5b). Our results are further confirmed by RT-PCR study, which showed that the combination of celecoxib with docetaxel produced greatest inhibition of mPGES mRNA levels (2.4-fold) as compared to tumors from untreated control mice (Fig. 6).
Figure 5.
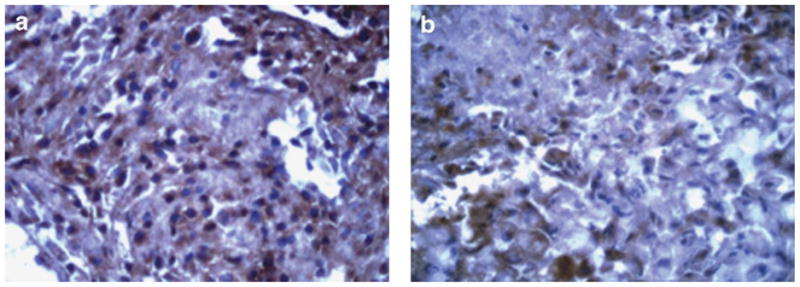
Immunohistochemical staining for mPGE synthase in tumors from (a) control- and (b) celecoxib with docetaxel combination-treated mice. The tumors were collected 28 days post-tumor implantation. Original magnification = 40×. It is evident that mPGE synthase staining in the combination-treated mice was weak as compared to the moderate staining observed in the vehicle-treated control mice.
Figure 6.
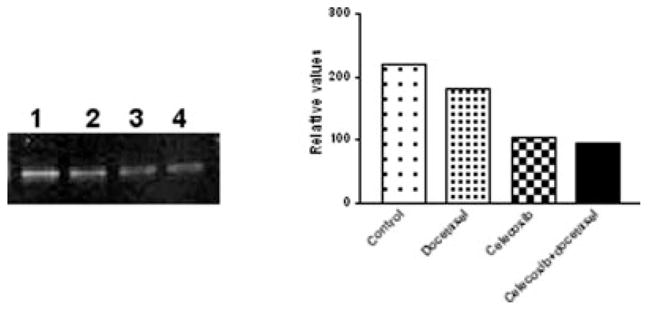
RT-PCR of mPGE synthase in tumor tissues. Lanes 1–4 represent control, docetaxel, celecoxib and celecoxib with docetaxel combination treatments, respectively. The tumor tissues were harvested 28 days post-tumor implantation and processed for RT-PCR as described in Material and Methods. The mPGE synthase product size (459 bp) was determined using a 100–2680 bp DNA ladder (Fisher Scientific Co., Atlanta, GA).
Celecoxib with docetaxel combination inhibits VEGF mRNA levels in tumors
The effect of celecoxib, docetaxel and celecoxib with docetaxel treatments on VEGF mRNA levels in tumor tissues is shown in Figure 4b. It is clear from Figure 4b that the combination of celecoxib with docetaxel treatment resulted in a significant (p < 0.001) reduction in the intratumor levels of VEGF mRNA as compared to the other treatments and control. The combination inhibited VEGF expression by 1.6-fold compared to control.
Induction of apoptosis in tumor tissues by the combination of celecoxib with docetaxel
As shown in Figure 7, celecoxib with docetaxel treatment showed a significant apoptotic response (50% increase) as compared to the control tumors. We also observed about 30–40% increase in apoptosis in celecoxib- or docetaxel-treated tumor tissues. The apoptotic response observed with docetaxel or celecoxib with docetaxel combination treatment group is associated with 5- and 3-fold increase in the expression of p53 in tumor tissues harvested from docetaxel or celecoxib with docetaxel combination, respectively; whereas its expression was not detected in tumor tissues from control- or celecoxib-treated mice (Fig. 4a).
Figure 7.
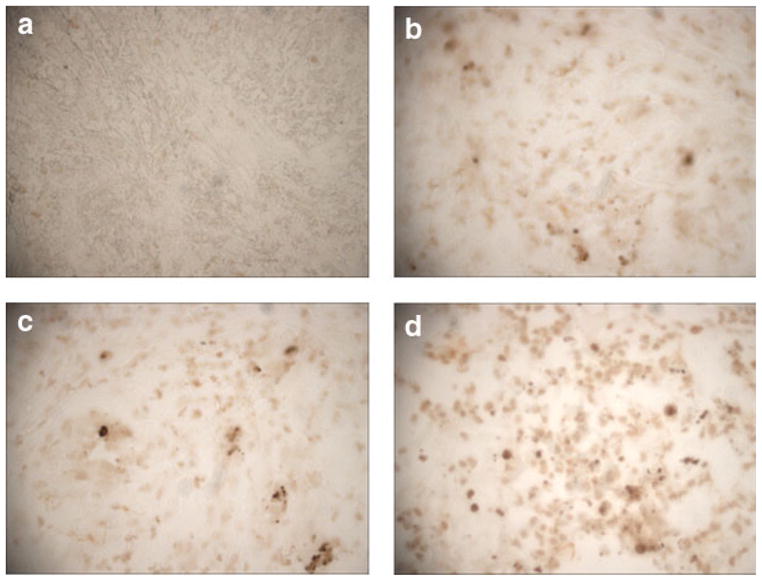
TUNEL staining of tumor tissue sections obtained from (a) control-, (b) celecoxib-, (c) docetaxel- and (d) celecoxib with docetaxel combination-treated mice. The tumors were harvested 28 days post-tumor implantation. Celecoxib treatment showed 30% increase in apoptosis over the control whereas docetaxel treatment showed 40% increase. The combination showed the highest increase of 50%. Original magnification = 40×.
Discussion
Extensive studies carried out in the last decade have shown that COX-2 is an important pharmacological target for the prevention and or treatment of lung and other cancers.21,22 These studies have unequivocally demonstrated that overexpression of COX-2 (a key enzyme involved in the prostaglandin biosynthesis) in human lung adenocarcinoma is associated with shortened patient survival.23–25 Further, selective COX-2 inhibitors such as nimesulide, JTE-522 and celecoxib have been shown to have antiproliferative effect against lung cancer cell lines and potentiated the in vitro cytotoxicity of anticancer drugs.3,8,17,26 It has been demonstrated that a COX inhibitor sulindac, increases the potential of antitumor activity of cytotoxic drugs in a murine LLC tumor model.27 Celecoxib has been shown to increase the cytotoxicity of CPT-11 in a colon tumor model2; and 5-fluorouracil and cyclophosphamide in murine LLC model.28 Even though clinical studies are in progress for the combination of celecoxib with docetaxel in NSCLC patients, preclinical data of this combination in lung tumor models has not been reported so far. There was a recent report dealing with the potentiation of tumor response to radiation involving docetaxel in human epidermoid carcinoma A431 tumor xenografts by selective COX-2 inhibitors, SC-236 and celecoxib.29 Our present study is aimed at determining the potential of celecoxib in increasing the docetaxel activity in vitro and in vivo in A549 lung tumors and to elucidate molecular mechanisms underlying its antitumor effect.
Our studies indicate that celecoxib significantly increases the in vitro cytotoxicity of docetaxel in A549 cells (Fig. 1) as well as the in vivo antitumor activity of docetaxel in s.c. A549 tumors (Fig. 2). The combination of celecoxib with docetaxel produced highest tumor growth inhibition as compared to celecoxib or docetaxel alone treatments. We used celecoxib at 150 mg/kg/day, which is below its maximum dose (250 mg/kg/day) employed in human head and neck squamous cell carcinoma, col26 murine carcinoma and HT-29 human colon carcinoma xenograft models.12,30 We followed the protocol of Dykes et al.31 for the administration of docetaxel to tumor bearing mice but at a lower dose (10 mg/kg, 3 times) than its effective dose (22 mg/kg, 3 times) in LX-1 lung tumor xenografts.31 In another study involving the efficacy of combination therapy using docetaxel, radiation and adenovirus-mediated p-53 gene transfer in A549 tumor xenografts, a single i.v. dose of docetaxel at 30 mg/kg (equivalent to the total dose of docetaxel use in our study) was employed.32 In our study, oral celecoxib was initiated 1 day after tumor implantation in accordance with our previous study.18 A recent study demonstrated potentiation of docetaxel and vinorelbine cytotoxicity in ACC-LC-319 lung tumor xenografts by the selective COX-2 inhibitor, JTE-522.3 In this study, Hida et al.3 initiated the COX-2 inhibitor treatment on the first day of tumor implantation and docetaxel was given by i.v. at a single dose of 5 mg/kg. We did not observe any significant difference in the tumor volumes between celecoxib (55.62 mm3) and celecoxib with docetaxel (47.81 mm3)-treated mice, at 14 days post-tumor implantation (i.e., when the first i.v. dose of docetaxel was administered in the combination group). As evident from Figure 2, the combination treatment produced a more pronounced inhibition of tumor growth as compared to individual treatments and thus indicates a cooperative tumor growth inhibitory effect between celecoxib and docetaxel in A549 tumor model.
Western blotting of tumor tissues indicated that the combination of celecoxib with docetaxel has no effect on COX-1 or COX-2 expression (Fig. 4a) but produced a maximum reduction in the intratumor PGE2 levels as compared to celecoxib or docetaxel (Fig. 3a). It has been shown that cytotoxic drugs like taxol and docetaxel stimulate COX-2 expression in human monocytes and mammary epithelial cell lines.33,34 Induction of COX-2 in tumor cells and in endothelial cells by taxanes may be a protective mechanism counterproductive to the antitumor effect of taxanes.35 It has also been reported that the addition of celecoxib to a regimen of paclitaxel and carboplatin abrogated the marked increase in PGE2 levels in primary tumors after treatment with paclitaxel and carboplatin in NSCLC patients.36 Reduction of intratumor PGE2 levels in tumor xenografts by COX-2 inhibitors such as nimesulide18 and celecoxib12,30 has been reported previously. Our data suggest that the pronounced tumor growth inhibition associated with the combination treatment is paralleled by the greatest inhibition of intratumor PGE2 levels indicating the role of tumor derived PGE2 in supporting the tumor growth. We hypothesize that celecoxib mediated reduction in the intratumor PGE2 levels may be associated with alterations in the expression of other enzymes involved in the PGE2 biosynthesis. We observed a 3.3-fold reduction in the expression of cPLA2 by Western blotting in tumor tissues obtained from celecoxib with docetaxel-treated mice. Cytosolic phospholipase A2 (cPLA2) is a rate-limiting key enzyme controlling the release of arachidonic acid from membrane phospholipids. The biosynthesis of PGE2 from arachidonic acid requires 2 enzymes that act sequentially. In the first step, arachidonic acid is converted to PGH2 catalyzed by COX, which exists in 2 forms designated as COX-1 (constitutive) and COX-2 (inducible). The COX-2 catalyzed PGH2 is subsequently converted to PGE2 by mPGE synthase. It has been reported previously that A549 cells express constitutively high expression of cPLA2 and COX-2 leading to increased PGE2 production.37 Further, a cPLA2 inhibitor has been found to inhibit the IL-1 stimulated PGE2 synthesis in human enterocytes.38 Similarly, IL-1β stimulation resulted in the induction of COX-2 and mPGE synthase in A549 cells.39 These observations suggest the importance of cPLA2 and mPGE synthase in PGE2 release from A549 cells. By immunohistochemistry and RT-PCR, we observed minimal staining and expression for mPGE synthase in celecoxib with docetaxel-treated tumor sections as compared to control tumors (Fig. 5b, 6). Our findings (reduction in the expression of cPLA2 and mPGE synthase in the combination-treated tumors) indicate that the reduction in the intratumor PGE2 levels is concomitantly associated with alterations in the expression of cPLA2 and mPGE synthase.
We have reported previously that nimesulide enhances the expression of PPAR-γ in s.c. A549 tumors.18 In our current study, we report that celecoxib alone and in combination with docetaxel enhances PPAR-γ (1.6- and 1.5-fold, respectively) may also contribute to the observed antitumor effect. It was observed recently that celecoxib and F-L-Leu (a PPAR-γ agonist), separately or in combination, increased the PPAR-γ expression in methylnitrosourea induced rat mammary tumors.40 Similarly, overexpression of PPAR-γ in oral squamous carcinoma cells and NSCLC cells by sulindac sulfide has also been reported.41,42 Our results are in agreement with these reported findings and suggest that PPAR-γ overexpression may contribute to the antitumor effect of celecoxib or celecoxib with docetaxel treatments as observed in our study.
Our results also indicate that the lowering of intratumor PGE2 levels in tumor tissues from the combination of celecoxib with docetaxel-treated mice is associated with an increase in the intra- tumor levels of 15-d PGJ2. As hypothesized by Badawi et al.,40 COX-2 inhibition and PPAR-γ activation inhibit PGE2 synthesis and switch the cells back to PGD2 and PGJ series dominated state. This is helpful in maintaining a normal physiological state. This argument is further supported by the observation that 15-d PGJ2 suppressed the IL-1β induced PGE2 synthesis in rheumatoid synovial fibroblasts via inhibition of COX-2 and cPLA2.43
Increased expression of COX-2 has been associated with increased levels of VEGF mRNA and protein levels44,45 and selective COX-2 inhibitors have been shown to inhibit VEGF expression in tumor tissues.46,47 Further, the role of specific COX-2 derived PGE2 in mediating the VEGF activity in tumor cells has been described recently.45 Our data shown in Figures 2 and 4 suggest that the superior antitumor effect of celecoxib and docetaxel combination is associated with reduced VEGF mRNA levels as compared to control tumors. It is known that VEGF is an important angiogenic factor and celecoxib has been shown to have antiangiogenic properties.48 It may be speculated, therefore, that celecoxib-mediated inhibition of intratumor PGE2 levels and VEGF expression, may contribute to the potentiation of docetaxel activity in tumors via inhibition of angiogenesis (Fig. 8).
Figure 8.
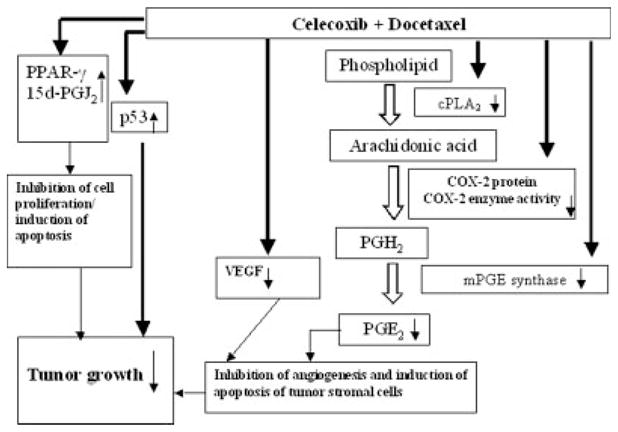
This is a hypothetical model describing the possible mechanism(s) involved in the in vivo antitumor effect of the combination of celecoxib with docetaxel The combination of celecoxib with docetaxel significantly decreases the intratumor PGE2 levels (by inhibition of COX-2 enzyme activity of tumor stromal cells and possibly via reduced expression of mPGE synthase), which is associated with a concomitant increase in the intratumor levels of 15-d PGJ2. The inhibition of intratumor PGE2 levels may contribute to the reduction in the tumor growth by inhibition of angiogenesis (supported by the reduced VEGF expression in tumor tissues obtained from the celecoxib with docetaxel-treated-mice as observed in our study and as reported elsewhere46) and induction of stromal cell apoptosis. The combination treatment has an inhibitory effect on cPLA2 expression, which may inhibit the release of arachidonic acid from membrane lipids and also contribute to the apoptosis as observed by Yu et al.50 The combination of celecoxib with docetaxel treatment resulted in an increase in the intratumor expression of PPAR-γ and p53 protein. As hypothesized of Badawi et al.40 that inhibition of PGE2 synthesis and activation of PPAR-γ may switch the cells back to PGD2 and PGJ series dominated state, which may contribute to the inhibition of tumor growth. The upregulation of p53 is one of the important modes of apoptosis induction by taxanes51 and in combination with other cytotoxic drugs.52,53 Recent evidence suggests that COX-2 and p53 exhibit physiologically relevant interactions in A549 cells, where it has been shown that endogenous p53 co-precipitating with endogenous COX-2 using COX-2 specific antibodies. It has also been shown that a COX-2 selective inhibitor potentiates p53-induced apoptosis. It has been hypothesized that overexpression of COX-2 in various tumors negatively affect p53 function that harbor wild-type p53 and further the antitumor activity of COX-2 inhibitors may be attributed to the inhibition of COX-2 activity, thereby protecting wild-type p53 from negative effects of COX-2 resulting in tumor growth inhibition.49 The direct effect of the combination of celecoxib with docetaxel is indicated by bold arrow. The arachidonic acid pathway of prostaglandin biosynthesis is indicated by open arrow.
We observed that the combination treatment of celecoxib with docetaxel produced maximum apoptosis, which is associated with a 3-fold increase in the intratumor p53 expression. Our data do not support a quantitative relationship between p53 expression and apoptosis, however, a recent report indicates a physiologically relevant interaction between COX-2 and p53 in A549 cells. It has been shown that p53 upregulates COX-2 expression and COX-2 in turn inhibits p53-dependent transcription. It has also been shown that a selective COX-2 inhibitor, SC-236 potentiates p53-mediated apoptosis.49 We speculate, therefore, that the apoptosis induced by celecoxib or celecoxib with docetaxel may be attributed in part through the COX-2 and p53 interactions and the corresponding modulation of p53 dependent transcription by the inhibition of COX-2 activity. Further studies are warranted to understand the relationship between COX-2 and p53 and its role in tumor growth inhibition and apoptosis.
In conclusion, the combination of celecoxib with docetaxel enhances the in vitro and in vivo antitumor effect of docetaxel and this effect is associated with modulation of intratumor PGE2 and 15-d PGJ2 levels, decreased expression of cPLA2, mPGE synthase and VEGF and increased expression of PPAR-γ.
Acknowledgments
The authors acknowledge financial support provided by an RCMI award from NIH. We also thank Pfizer (Skokie, IL) and Aventis (Collegeville, PA) for the gift samples of celecoxib and docetaxel, respectively.
References
- 1.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, Zhao TLM, Piazza G, Klein-Szanto AJP, Pamukcu R, Alila H, Baunn PA, Jr, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–8. [PubMed] [Google Scholar]
- 2.Trifan OC, Durham WF, Salazar VS, Horton J, Levine BD, Zweifel BS, Davis TW, Masferrer JL. Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res. 2002;62:5778–84. [PubMed] [Google Scholar]
- 3.Hida T, Kozaki K, Ito H, Miyaishi O, Tatematsu Y, Suzuki T, Matsuo K, Sugiura T, Ogawa M, Takahasi T, Takahashi T. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase 2 inhibitor, JTE-522 and conventional anticancer drugs. Clin Cancer Res. 2002;8:2443–7. [PubMed] [Google Scholar]
- 4.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–11. [PubMed] [Google Scholar]
- 5.Williams CS, Watson AJM, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–51. [PubMed] [Google Scholar]
- 6.Blumenthal RD, Waskewich C, Goldenberg DM, Lew W, Flefleh C, Burton J. Chronotherapy and chronotoxicity of the cyclooxygenase-2 inhibitor, celecoxib in athymic mice bearing human breast cancer xenografts. Clin Cancer Res. 2001;7:3178–85. [PubMed] [Google Scholar]
- 7.Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC. Inhibition of cyclooxgenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol. 2000;164:820–5. doi: 10.1097/00005392-200009010-00056. [DOI] [PubMed] [Google Scholar]
- 8.Waskewich C, Blumenthal RD, Li H, Stein R, Goldenberg DM, Burton J. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2 negative hematopoietic and epithelial cell lines. Cancer Res. 2002;62:2029–33. [PubMed] [Google Scholar]
- 9.Gadgeel SM, Thatai L, Kraut M, Wozniak A, Worden F, Ward D, Belzer K, Hodges C, Kalemkerian GP. Phase II study of celecoxib and docetaxel in non-small cell lung cancer (NSCLC) patients with progression after platinum-based therapy. ASCO; 2003. Abstract 2749. [Google Scholar]
- 10.Shehadeh NJ, Kalemkerian GP, Wozniak A, Kruat M, Belzer K, Ward D, Hodges C, Gadgeel SM. Preliminary results of a phase II study of celecoxib and weekly docetaxel in elderly (?70 yrs) or PS2 patients with advanced non-small cell lung cancer (NSCLC) ASCO; 2003. Abstract 2758. [Google Scholar]
- 11.Nugent FW, Graziano S, Levitan N, Collea R, Gajra A, Mertens WC, O’Connell C, Sirard J, Marshall J, McCann J. Docetaxel and COX-2 inhibition with celecoxib in relapsed/refractory non-small cell lung cancer (NSCLC): promising progression-free survival in a phase II study. ASCO; 2003. Abstract 2697. [Google Scholar]
- 12.Zweifel BS, Davis TW, Ornberg RL, Masferrer JL. Direct evidence for a role of cyclooxygenase 2-derived prostaglandin E2 in human head and neck xenograft tumors. Cancer Res. 2002;62:6706–11. [PubMed] [Google Scholar]
- 13.Blaine SA, Wick M, Dessev C, Nemenoff RA. Induction of cPLA2 in lung epithelial cells and non-small cell lung cancer is mediated by Sp1 and c-Jun. J Biol Chem. 2001;276:42737–43. doi: 10.1074/jbc.M107773200. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimatsu K, Altorki NK, Golijanin D, Zhang F, Jakobsson P-J, Dannenberg AJ, Subbaramaiah K. Inducible prostaglandin E synthase is overexpressed in non-small cell lung cancer. Clin Cancer Res. 2001;7:2669–74. [PubMed] [Google Scholar]
- 15.Minter HA, Eveson JW, Huntley S, Elder DJ, Hague A. The cyclooxygenase 2-selective inhibitor NS398 inhibits proliferation of oral carcinoma cell lines by mechanisms dependent and independent of reduced prostaglandin E2 synthesis. Clin Cancer Res. 2003;9:1885–97. [PubMed] [Google Scholar]
- 16.Han C, Leng J, Demetris AJ, Wu T. Cyclooxygenase-2 promotes human cholangiocarcinoma growth: evidence for cyclooxygenase-2-independent mechanism in celecoxib-mediated induction of p21waf1/cip1 and p27kip1 and cell cycle arrest. Cancer Res. 2004;64:1369–76. doi: 10.1158/0008-5472.can-03-1086. [DOI] [PubMed] [Google Scholar]
- 17.Haynes A, Shaik MS, Chatterjee A, Singh M. Evaluation of an aerosolized selective COX-2 inhibitor as a potentiator of doxorubicin in non-small cell lung cancer cell line. Pharmacol Res. 2003;20:1485–95. doi: 10.1023/a:1025774630993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaik MS, Chatterjee A, Singh M. Effect of a selective cyclooxygenase (COX)-2 inhibitor, nimesulide on the growth of lung tumors and their expression of COX-2 and peroxisome proliferator-activated receptor-γ. Clin Cancer Res. 2004;10:1521–9. doi: 10.1158/1078-0432.ccr-0902-03. [DOI] [PubMed] [Google Scholar]
- 19.Haynes A, Shaik MS, Chatterjee A, Singh M. Formulation and evaluation of aerosolized celecoxib for the treatment of lung cancer. Pharmacol Res. 2005;22:427–39. doi: 10.1007/s11095-004-1881-z. [DOI] [PubMed] [Google Scholar]
- 20.Badawi AF, Badr MZ. Expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-gamma and levels of prostaglandin E2 and 15-deoxy-delta12, 14-prostaglandin J2 in human breast cancer and metastasis. Int J Cancer. 2003;103:84–90. doi: 10.1002/ijc.10770. [DOI] [PubMed] [Google Scholar]
- 21.Dannenberg AJ, Altorki NK, Boyle JO, Lin DT, Subbaramaiah K. Inhibition of cyclooxygenase-2: an approach to preventing cancer of the upper aerodigestive tract. Ann N Y Acad Sci. 2001;952:109–15. doi: 10.1111/j.1749-6632.2001.tb02731.x. [DOI] [PubMed] [Google Scholar]
- 22.Subongkot S, Frame D, Leslie W, Drajer D. Selective cyclooxygenase-2 inhibition: A target in cancer prevention and treatment. Pharmacotherapy. 2003;23:9–28. doi: 10.1592/phco.23.1.9.31916. [DOI] [PubMed] [Google Scholar]
- 23.Wolf H, Saukkonen K, Karjalainen A, Vainio H, Ristimaki A. Expression of cyclooxygenase -2 in human lung carcinoma. Cancer Res. 1998;58:4997–5001. [PubMed] [Google Scholar]
- 24.Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahasi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–4. [PubMed] [Google Scholar]
- 25.Achiwa H, Yatabe Y, Hida T, Kuroish T, Kozaki K-I, Nakamura T, Ogawa M, Sugiura T, Mitsudomi T, Takahashi T. Prognostic significance of elevated cyclooxygenase 2 expression in primary, resected lung adenocarcinomas. Clin Cancer Res. 1999;5:1001–5. [PubMed] [Google Scholar]
- 26.Hida T, Kozaki KI, Muramatusu H, Masuda A, Shimizu S, Mitsudomi T, Sugiura T, Ogawa M, Takahashi T. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–11. [PubMed] [Google Scholar]
- 27.Teicher BA, Korbut TT, Menon K, Holden SA, Ara G. Cyclooxygenase and lipoxygenase inhibitors as modulators of cancer therapies. Cancer Chemother Pharmacol. 1994;33:515–22. doi: 10.1007/BF00686511. [DOI] [PubMed] [Google Scholar]
- 28.Moore RJ, Zwelfi BS, Heuvelman DM, Leahy KM, Edwards DA, Woerner BM, Ornberg RL, Seibert K, Koki AT, Masferrer JL. Enhanced antitumor activity by co-administration of celecoxib and the chemotherapeutic agents cyclophosphamide and 5-FU. Proc Am Assoc Cancer Res. 2000;41:409. [Google Scholar]
- 29.Nakata E, Mason KA, Hunter N, Husain A, Raju U, Liao Z, Ang KK, Milas L. Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol Biol Phys. 2004;58:369–75. doi: 10.1016/j.ijrobp.2003.09.061. [DOI] [PubMed] [Google Scholar]
- 30.Davis TW, O’Neal JM, Pagel MD, Zweifel BS, Mehta PP, Heuvelman DM, Masferrer JL. Synergy between celecoxib and radiotherapy results from inhibition of cyclooxygenase-2 derived prostaglandin E2, a survival factor for tumor and associated vasculature. Cancer Res. 2004;64:279–85. doi: 10.1158/0008-5472.can-03-1168. [DOI] [PubMed] [Google Scholar]
- 31.Dykes DJ, Bissery MC, Harrison SD, Jr, Waud WR. Response of human tumor xenografts in athymic nude mice to docetaxel (RP56976, Taxotere) Invest New Drugs. 1995;13:1–11. doi: 10.1007/BF02614214. [DOI] [PubMed] [Google Scholar]
- 32.Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, Ji L. Synergistic inhibition of human lung cancer cell growth by adenovirus-mediated wild-type p53 gene transfer in combination with docetaxel and radiation therapeutics in vitro and in vivo. Clin Cancer Res. 2001;7:2887–97. [PubMed] [Google Scholar]
- 33.Cassidy PB, Moos PJ, Kelly RC, Fitzpatrick FA. Cyclooxygenase-2 induction by paclitaxel, docetaxel and taxane analogues in human monocytes and murine macrophages: structure-activity relationships and their implications. Clin Cancer Res. 2002;8:846–55. [PubMed] [Google Scholar]
- 34.Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. J Biol Chem. 2000;275:14838–45. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 35.Merchan JR, Yayaram DR, Supko JG, He X, Bubley GJ, Sukhatme VP. Increased endothelial uptake of paclitaxel as a potential mechanism for its antiangiogenic effects: potentiation by cox-2 inhibition. Int J Cancer. 2005;113:490–8. doi: 10.1002/ijc.20595. [DOI] [PubMed] [Google Scholar]
- 36.Altorki NK, Keresztes RS, Port JL, Libby DM, Korst RJ, Flieder DB, Ferrara CA, Yankelevitz DF, Subbaramaiah K, Pasmantier MW, Dannenberg AJ. Celecoxib, a selective cyclooxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small cell lung cancer. J Clin Oncol. 2003;21:2645–50. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 37.Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997;272:14501–4. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 38.Grossman EM, Longo WE, Mazuski JE, Panesar N, Kaminski DL. Role of cytoplasmic and secretory phospholipase A2 in intestinal epithelial cell prostaglandin E2 formation. Int J Surg Investig. 2000;1:467–76. [PubMed] [Google Scholar]
- 39.Catley MC, Chivers JE, Cambridge LM, Holden N, Slater DM, Staples KJ, Bergmann MW, Loser P, Barnes PJ, Newton R. IL-1β-dependent activation of NF-κB mediates PGE2 release via the expression of cyclooxygenase-2 and microsomal prostaglandin E synthase. FEBS Lett. 2003;547:75–9. doi: 10.1016/s0014-5793(03)00672-0. [DOI] [PubMed] [Google Scholar]
- 40.Badawi AF, Eldeen M, Liu Y, Ross EA, Badr MZ. Inhibition of rat mammary gland carcinogenesis by simultaneous targeting of cyclooxygenase-2 and peroxisome proliferator-activated receptor γ. Cancer Res. 2004;64:1181–9. doi: 10.1158/0008-5472.can-03-2556. [DOI] [PubMed] [Google Scholar]
- 41.Nikitakis NG, Hebert C, Lopes MA, Reynolds MA, Sauk JJ. PPAR gamma-mediated antineoplastic effect of NSAID sulindac on human oral squamous carcinoma cells. Int J Cancer. 2002;98:817–23. doi: 10.1002/ijc.10278. [DOI] [PubMed] [Google Scholar]
- 42.Wick M, Hurteau G, Dessev C, Chan D, Geraci ME, Winni RA, Heasley LE, Nemenoff RA. Peroxisome proliferator-activated receptor-γ is a target of nonsteroidal in-inflammatory drugs mediating cyclooxygenase-independent inhibition of lung cancer cell growth. Mol Pharmacol. 2002;62:1207–14. doi: 10.1124/mol.62.5.1207. [DOI] [PubMed] [Google Scholar]
- 43.Tsubouchi Y, Kawahito Y, Kohno M, Inoue K-I, Hla T, Sano H. Feedback control of the arachidonate cascade in rheumatoid synoviocytes by 15-deoxy-delta12, 14-Prostaglandin J2. Biochem Biophys Res Commun. 2001;283:750–55. doi: 10.1006/bbrc.2001.4847. [DOI] [PubMed] [Google Scholar]
- 44.Gallo C, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddi V, Chiarugi V, Masini E. Cycloxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3:53–61. doi: 10.1038/sj.neo.7900127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eibl G, Bruemmer D, Okada Y, Duffy JP, Law RE, Reber HA, Hines OJ. PGE(2) is generated by specific COX-2 activity and increases VEGF production in COX-2 expressing human pancreatic cancer cells. Biochem Biophys Res Commun. 2003;306:887–97. doi: 10.1016/s0006-291x(03)01079-9. [DOI] [PubMed] [Google Scholar]
- 46.Kim KY, Lee JW, Ahn BW, Ryu PD, Nam MJ. Loss of endogenous TGF-β effect induces mouse hepatoma malignancy by correlation with cyclooxygenase-2 and VEGF. Hepatol Res. 2003;26:302–10. doi: 10.1016/s1386-6346(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 47.Tortora G, Caputo R, Damiano V, Melisi D, Bianco R, Fontanini G, Veneziani BM, De Placido S, Bianco AR, Ciardiello F. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res. 2003;9:1566–72. [PubMed] [Google Scholar]
- 48.Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–31. [PubMed] [Google Scholar]
- 49.Corcoran CA, He Q, Huang Y, Sheikh MS. Cycloxygenase-2 interacts with p53 and interferes with p53-dependent transcription and apoptosis. Oncogene. 2005;24:1634–40. doi: 10.1038/sj.onc.1208353. [DOI] [PubMed] [Google Scholar]
- 50.Yu HG, Huang JA, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Inhibition of cytosolic phospholipase A2 mRNA expression: a novel mechanism for acetylsalicylic acid-mediated growth inhibition and apoptosis in colon cancer cells. Regul Pept. 2003;114:101–107. doi: 10.1016/s0167-0115(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 51.Ganansia-Leymarie V, Bischoff P, Bergerat JP, Holl V. Signal transduction pathways of taxanes-induced apoptosis. Curr Med Chem Anti-Canc Agents. 2003;3:291–306. doi: 10.2174/1568011033482422. [DOI] [PubMed] [Google Scholar]
- 52.Pohl G, Rudas M, Taucher S, Stranzl T, Steger GG, Jakesz R, Pirker R, Filipits M. Expression of cell cycle regulatory proteins in breast cancer carcinomas before and after preoperative chemotherapy. Breast Cancer Res Treat. 2003;78:97–103. doi: 10.1023/a:1022165715043. [DOI] [PubMed] [Google Scholar]
- 53.Avramis VI, Nandy P, Kwock R, Solorzano MM, Mukherjee SK, Dannenberg P, Cohen LJ. Increased p21/WAF-1 and p53 protein levels following sequential three drug combination regimen of fludarabine, cytarabine and docetaxel induces apoptosis in human leukemia cells. Anticancer Res. 1998;18:2327–38. [PubMed] [Google Scholar]