Abstract
Background
Glaucoma is a group of diseases characterised by retinal ganglion cell dysfunction and death. Detection of glaucoma and its progression are based on identification of abnormalities or changes in the optic nerve head (ONH) or the retinal nerve fibre layer (RNFL), either functional or structural. This review will focus on the identification of structural abnormalities in the RNFL associated with glaucoma.
Discussion
A variety of new techniques have been created and developed to move beyond photography, which generally requires subjective interpretation, to quantitative retinal imaging to measure RNFL loss. Scanning laser polarimetry uses polarised light to measure the RNFL birefringence to estimate tissue thickness. Optical coherence tomography (OCT) uses low-coherence light to create high-resolution tomographic images of the retina from backscattered light in order to measure the tissue thickness of the retinal layers and intraretinal structures. Segmentation algorithms are used to measure the thickness of the retinal nerve fibre layer directly from the OCT images. In addition to these clinically available technologies, new techniques are in the research stages. Polarisation-sensitive OCT has been developed that combines the strengths of scanning laser polarimetry with those of OCT. Ultra-fast techniques for OCT have been created for research devices. The continued utilisation of imaging devices into the clinic is refining glaucoma assessment. In the past 20 years glaucoma has gone from a disease diagnosed and followed using highly subjective techniques to one measured quantitatively and increasingly objectively.
Glaucoma is a disease that is identified biologically by the death of retinal ganglion cells. The axons of these cells make up the retinal nerve fibres that gather at the optic nerve head and form the anterior-most layer of the retina. As retinal ganglion cells are lost, through processes not yet fully understood, the retinal nerve fibres are also lost, and this layer thins. These defects can often be observed during fundus examination of patients, or in red-free fundus photographs of the optic disc and surrounding retina. Areas of retinal nerve fibre layer (RNFL) loss are less brightly reflective, and can show up as dark bands radiating out from the disc, following the typical arcuate paths of the nerve fibre bundles. However, imaging of the en face view of the retinal nerve fibre layer (RNFL) can only provide information primarily about location and width of the defects, but not specific quantitative information about the depth or extent of the tissue loss. Additionally, diffuse loss leading to overall RNFL thinning can be much harder to detect on a red-free view, and identification of defects on standard colour photographs can be challenging. Fundus photography can provide good qualitative information about glaucomatous damage indicators such as ONH cupping and the presence of optic disc haemorrhages, but more recent advances in ocular imaging technology promise to provide much more objective and quantitative information about the health and thickness of the retinal never fibre layer. This review aims to cover the technologies currently available clinically for imaging of the RNFL, as well as new techniques that show great potential for future clinical utility.
SCANNING LASER POLARIMETRY
This imaging technique, demonstrated ex vivo in 1990 and demonstrated in vivo in glaucoma in 1995,1 2 takes advantage of the optical properties of the parallel bundled retinal nerve fibres. The neural axons are essentially a series of thin cylinders with diameters smaller than the wavelength of near-infrared visible light. Because of their size, and perhaps also related to the intracellular arrangement of structures such as microtubules and mitochondria, the tissue is birefringent, such that reflected polarised light undergoes a phase shift corresponding to the amount of birefringent tissue through which it passed. In the eye this is proportional to the thickness of the RNFL. This phase shift can be mapped across the retina by scanning the light across the area of interest, in this case the region centred on the ONH. Measurements of the retinal birefringence can be mapped as estimated RNFL thickness based on a conversion from 0.67 nm of retardation equal to 1 µm of fibre.2 Compensation is made for the birefringence elsewhere in the eye, such as that inherent in the cornea, lens, retinal pigment epithelium and sclera, so that the confounding influence of these structures on the retinal measurements is minimised.
Numerous papers have been published establishing the relationship between the phase shift and true RNFL thickness in both healthy patients and patients with glaucomatous loss,3 4 and the measurements were found to be reproducible within a time period when structural change has not occurred.5 6 Since these basics of the technology were established, many studies have tested the ability of both the early clinical device, the Nerve Fibre Analyzer (Laser Diagnostic Technologies, San Diego, California), and the current device used most predominantly clinically, the GDx (Carl Zeiss Meditec, Vista, California).7–11 The GDx compensates for corneal birefringence with either the Variable Cornea Compensation (GDx VCC) or the more advanced Enhanced Corneal Compensation (GDx ECC).12 The newer GDx ECC has been shown to produce more reproducible results and allow for more accurate discrimination of glaucoma.13–15 The GDx provides a variety of parameters derived from the RNFL measurements, but Nerve Fibre Indicator, a support vector–machine-derived algorithm, has been reported as providing the best discrimination for glaucoma.8 10
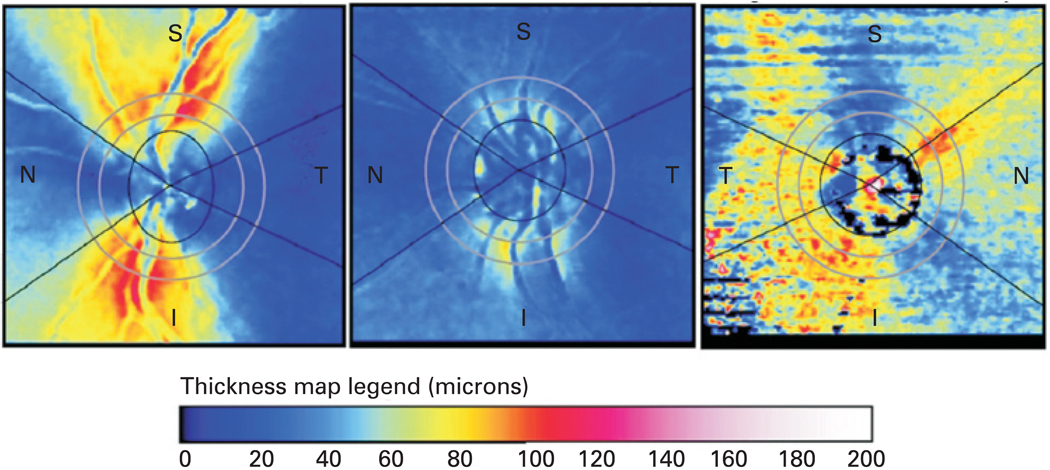
Scanning laser polarimetry has been somewhat less commonly used compared with other ocular imaging techniques, in large part to the fact that it is measuring RNFL thickness indirectly, making it susceptible to confounding by non-RNFL birefringence. Corneal compensation is a very important factor to consider, and while the new GDx-ECC method has improved image acquisition and better compensation of non-RNFL birefringence,12 14 15 issues may still arise. Opacities in the ocular media can greatly affect the ability to create meaningful measurements, resulting in abnormal retardation patterns.16 Because of these limitations, scanning laser polarimetry has not received the same level of attention and development for retinal nerve fibre measurement as optical coherence tomography (OCT). An example of typical scans with the GDx-ECC, as well as a scan resulting in abnormal retardation patterns can be seen in fig 1.
Figure 1.
Scanning laser polarimetry (SLP) birefringence maps. The map on the left represents a typical healthy patient’s SLP scan (GDx, Carl Zeiss Meditec, Dublin, California), showing bright thick superior and inferior nerve bundles. The centre map represents a patient with significant nerve fibre loss due to glaucoma. The map on the right displays an abnormal birefringence pattern due to error caused by ocular opacity resulting in poor image quality. The retinal nerve fibre layer thickness colour scale is included. I, inferior; N, nasal; S, superior; T, temporal.
CONFOCAL SCANNING LASER OPHTHALMOSCOPY
Confocal scanning laser ophthalmoscopy (CSLO) is a technique used primarily for topographic mapping of the retinal surface, in particular, the ONH. CSLO does also provide an estimation of RNFL thickness around the ONH. This is estimated by measuring the distance between the reference plane and the surface along the contour line. Because this measurement is based on an arbitrarily designated reference plane, rather than true RNFL measurement, its value is limited. Glaucoma discrimination has been shown to be poorer for this parameter than the more commonly used ONH parameters from the Heidelberg Retina Tomograph (HRT, Heidelberg Engineering GmBH; Heidelberg, Germany), the CSLO clinically employed most often.17 18 The RNFL information is available on some of the standard clinical printouts of the Heidelberg Retina Tomograph, including comparison with a normative database; however, because the measurement is made very differently than with GDx or OCT, interdevice comparison is difficult.19 20
OPTICAL COHERENCE TOMOGRAPHY
OCT is a technology frequently employed for clinical imaging of the RNFL. The first iteration of the technology, time domain OCT (TD-OCT), was published in 1991,21 and from that one article the OCT literature has blossomed to over 1100 publications using OCT in 2007 alone. More than 10 000 ophthalmic OCT devices have been sold globally to date (C Ritter, Carl Zeiss Meditec, Inc, personal communication). This technique uses low-coherence interferometry to examine light backscattered from the layers of the retina, or other tissues of interest. By evaluating the interference pattern of light back-reflected from the sample interacting with light reflected from a mirror mounted on a reference arm that traverses a known distance corresponding to the scanning distance through the retina, the position and intensity of the retinal light reflection can be mapped along a line through a single point through the depth of the retina. This single line is known as an A-scan. Many of these A-scans can be acquired side by side along a line scanned transversely across the retina, providing a cross-sectional view or a slice through the layers of the retina, from which the tissue layer thicknesses can then be measured directly. The RNFL is one of the layers with the greatest reflectance, due to the structure of the fibres being perpendicular to the direction of the light beam, and it being the anterior most layer of the retina. This allows it to be automatically segmented and measured by accurately computer algorithms.22
To quantify the total amount of retinal nerve fibre entering the optic nerve head, a protocol of a circular scan centred on the optic nerve head with a diameter of 3.4 mm is performed. This diameter is now known to fortuitously place the circle in an area where the retinal nerve fibre thickness is most stable. Originally this circle size was arbitrarily chosen to be outside the disc margin, even in the case of large discs, and to avoid peripapillary atrophy but close enough to allow sampling in the thick peripapillary RNFL close to the ONH.23 From this, the thickness of the RNFL can be measured directly and compared over time to look for thickness defects and changes. The visualisation of the RNFL by OCT can be seen in fig 2. Comparison with a normative database can aid in identifying overall thinning of the layer, as well as identification of focal defects. Additionally, thickness values can be averaged both globally, 360° around the circle, and sectorally, in quadrants or other smaller sectors such as clock hours. The global thickness values are found to have good reproducibility within single visits or a few days, and the sectoral measurements, while being somewhat less reproducible than the global RNFL thickness, are still sufficiently reproducible to be used diagnostically, and provide valuable information about localised defects.24 These averaged measurements have been tested in a multitude of studies across many populations of subjects and have been well established as one of the best predictors of glaucomatous disease.25–31
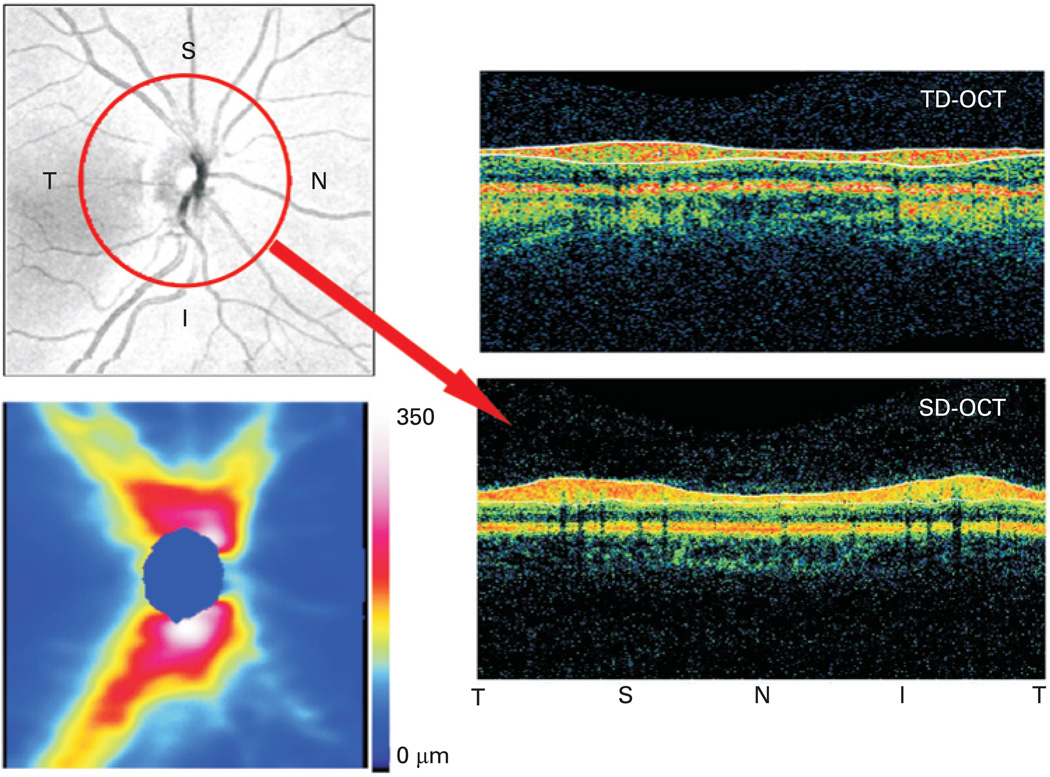
Figure 2.
Optical coherence tomography retinal nerve fibre layer (RNFL) dataset visualisation. The upper left is an en-face image of a spectral domain optical coherence tomography (SD-OCT) 3D data cube, created by averaging the reflectance along each A-scan. The lower left is the RNFL thickness map around the optic nerve (the blue spot in the centre) along with the colour scale. The two B-scans on the right are circular scans generated in two different ways. The time domain optical coherence tomography (TD-OCT) is gathered solely as a single circular scan shown. The SD-OCT image is sampled in postprocessing from the 3D data set along the red circle shown on the en-face image. Note the improvement in image clarity with SD-OCT’s increased resolution. The location along the circle is labelled below the B-scans. I, inferior; N, nasal; S, superior; T, temporal.
TD-OCT, which measures the time of flight of backscatterd light in order to assess tissue thickness, is somewhat limited in its speed, as data are collected pixel by pixel for each A-scan. TD-OCT acquires 400 A-scans/s, so it can be used to collect no more than one to three circular OCT scans with 256 A-scans/OCT at a time, because otherwise movement artefacts make it too difficult to acquire accurate data. Additionally, with TD-OCT there is no clear way to definitively know how accurate the centration of the circumpapillary scanning circle is once the scan has been acquired. A photograph of the circle placement is made after the OCT scans are acquired, but movement can affect the accuracy of that location, since the scan is not acquired simultaneously. Variability in circle placement is known to affect global, and especially sectoral retinal thickness measurements.32
The newest clinical iteration of OCT technology is spectral domain OCT (SD-OCT), which permits much faster scanning than TD-OCT. SD-OCT commercially available devices produce 24 000–55 000 A-scans/s.33 SD-OCT may also provide a better axial resolution than TD-OCT, at 3–6 µm compared with 10–15 µm for TD-OCT, as many SD-OCT devices use a broad bandwidth light source to achieve low coherence. SD-OCT works by collecting all backscattered light frequencies simultaneously (hence “spectral domain”). Each frequency of light represents a different tissue depth, so the frequencies of reflections can be mapped to the various retinal layers. Because of this, each A-scan can be acquired nearly instantaneously, rather than requiring mechanical scanning of a reference arm to map through the depth of the retina as is done for TD-OCT. This is the source of the large increase in speed that SD-OCT is able to achieve. This decreased acquisition time allows for rapid scanning which can provide high-density OCT tomograms, as well as complete 3D data sets (3D OCT). 3D OCT may be useful for imaging in of the macula and optic nerve. In the case of the ONH and surrounding peripapillary area, postprocessing and the creation of a virtual 3.4 mm circle can be performed. The 3D dataset was resampled and segmented to provide precise placement around the centre of the optic nerve head. This also allows more consistent placement over time, which produces more reproducible measurements and may enable finer assessment of progressive RNFL thinning. Individual circles can still be scanned around the optic nerve head similarly to TD-OCT using SD-OCT, and the higher resolution and speed will allow for faster circle acquisition, which may minimise motion artefacts compared with TD-OCT.
Segmentation across the 3D data set can also provide a RNFL thickness map across the scanned area, similar to the map provided by scanning laser polarimetry; however, OCT measures thickness more directly and is subject to less confounding and fewer artefacts. This thickness map can be used to identify overall thinning or wedge-shaped focal defects qualitatively and quantitatively, and their progression can be monitored objectively. The map may also provide identification of small defects that might be missed because they only become statistically significant beyond the 3.4 mm circle. A comparison of the information from a data circle from TD-OCT and a 3D data set from SD-OCT can be seen in fig 2. New technologies for visualisation of the 3D data set may help the ability of OCT to visualise defects qualitatively as well. For example, 3D data sets can be sliced in planes parallel to the surface of the retina, creating what is known as C-scans or c-mode images.34 Software is available to select data in flat planes but also those contoured for the retina (or other tissue under study), to allow for more accurate selection of specific layers. This allows visualisation of the tissue layer of interest. The technology for processing SD-OCT datasets is still in development, so many new methods of visualising and analysing the 3D OCT data sets are yet to come; the clearest analogy is to the early days of MRI.
FUTURE TECHNOLOGY
A technology that is beginning to generate interest in glaucoma imaging research but has not yet been adapted to the clinic is polarisation-sensitive OCT.35 36 This technique combines some of the benefits of both scanning laser polarimetry and OCT. It is believed that because the RNFL is the most birefringent layer in the retina, the combination of the high axial resolution of the OCT coupled with OCT measurement of tissue birefringence could allow for a greater contrast between the RNFL and the other layers of the retina, potentially enabling more precise measures of the RNFL thickness. An additional benefit is the retinal surface can be used as a reference, so corneal compensation is not an issue using PS-OCT. 35 The total amount of phase retardation measured is represented by RNFL birefringence×RNFL thickness×2, since the light performs a double pass through the RNFL.37–39 The amount of birefringence has not been found to vary as a function of circular scan radius, meaning that the number of nerve fibres is what affects the polarisation, not just the thickness. However, birefringence has been shown to be a function of position around the ONH, with high birefringence in the superior and inferior.37–39 This means that direct conversion of phase retardation to RNFL thickness may induce error if birefringence is assumed to be constant across the retina. Much greater knowledge of typical birefringence patterns will need to be understood before this technology can have clinical relevance.
A next-generation OCT technology known as swept-source OCT (SS-OCT) is currently of great interest in the ocular imaging research community but has not yet been implemented clinically. Swept-source OCT is similar to SD-OCT, in that the location of the reflection is encoded in the frequency of the light. However, rather than detecting a single broadband reflection, a swept-source laser is used to cover the range of frequencies one at a time. This method, while currently providing axial resolution more on the level of TD-OCT, is even faster than SD-OCT, acquiring at a rate of >200 000 A-scans/s.40 41 This allows for the acquisition of 3D data sets with virtually no motion artefacts, which may be present in SD-OCT, and may allow more averaging of images, which can increase the signal-to-noise ratio. SS-OCT may provide the ability to image over greater areas of the retina, and still have fewer motion artefacts than SD-OCT. Currently, swept-source laser technology is not technically or financially viable for clinical use, but optoelectronic advances may make this more clinically useful.
RNFL imaging is performed using a relatively new set of technologies and is of great relevance to the field of glaucoma. The ability to make objective, quantitative measurements of the RNFL is an important technique for assessing glaucomatous damage and progression. OCT, SLP and CSLO techniques are available for clinical use, and continue to be developed and investigated intensively for improved glaucoma diagnostic utility. Imaging of the retina does not render fundus photography and clinical examination obsolete, but instead provides a valuable tool for gaining objective, quantitative knowledge of the structural damage for each glaucoma patient.
Acknowledgments
Funding: Supported in part by National Institutes of Health contracts R01-EY13178-09, R01-EY11289-23 and P30-EY08098-20 (Bethesda, Maryland), The Eye and Ear Foundation (Pittsburgh, Pennsylvania) and unrestricted grants from Research to Prevent Blindness, Inc. (New York).
Footnotes
Competing interests: JSS receives royalties for intellectual property licensed by Massachusetts Institute of Technology to Carl Zeiss Meditec. GW received research funding from Carl Zeiss Meditec and Optovue. JSS received honoraria from Carl Zeiss Meditec, Heidelberg Engineering and Optovue. KAT reports no conflicts.
REFERENCES
- 1.Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fibre layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119:627–636. doi: 10.1016/s0002-9394(14)70221-1. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Dreher AW, Coleman A, et al. Histopathologic validation of Fourier-ellipsometry measurements of retinal nerve fiber layer thickness. Arch Ophthalmol. 1990;108:557–560. doi: 10.1001/archopht.1990.01070060105058. [DOI] [PubMed] [Google Scholar]
- 3.Morgan JE, Waldock A, Jeffery G, et al. Retinal nerve fibre layer polarimetry: histological and clinical comparison. Br J Ophthalmol. 1998;82:684–690. doi: 10.1136/bjo.82.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuulonen A, Airaksinen PJ. Polarimetry of the retinal nerve fiber layer. Curr Opin Ophthalmol. 1996;7:34–38. doi: 10.1097/00055735-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Junghardt A, Schmid MK, Schipper I, et al. Reproducibility of the data determined by scanning laser polarimetry. Graefes Arch Clin Exp Ophthalmol. 1996;234:628–632. doi: 10.1007/BF00185296. [DOI] [PubMed] [Google Scholar]
- 6.Leung CK, Cheung CY, Lin DS, et al. Longitudinal variability of optic disc and retinal nerve fiber layer measurements. Invest Ophthalmol Vis Sci. 2008;49:4886–4892. doi: 10.1167/iovs.07-1187. [DOI] [PubMed] [Google Scholar]
- 7.Brusini P, Salvetat ML, Parisi L, et al. Discrimination between normal and early glaucomatous eyes with scanning laser polarimeter with fixed and variable corneal compensator settings. Eur J Ophthalmol. 2005;15:468–476. doi: 10.1177/112067210501500409. [DOI] [PubMed] [Google Scholar]
- 8.Da Pozzo S, Iacono P, Marchesan R, et al. Scanning laser polarimetry with variable corneal compensation and detection of glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2005;243:774–779. doi: 10.1007/s00417-004-1118-1. [DOI] [PubMed] [Google Scholar]
- 9.Nicolela MT, Martinez-Bello C, Morrison CA, et al. Scanning laser polarimetry in a selected group of patients with glaucoma and normal controls. Am J Ophthalmol. 2001;132:845–854. doi: 10.1016/s0002-9394(01)01215-6. [DOI] [PubMed] [Google Scholar]
- 10.Reus NJ, Lemij HG. Diagnostic accuracy of the GDx VCC for glaucoma. Ophthalmology. 2004;111:1860–1865. doi: 10.1016/j.ophtha.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb RN, Zangwill L, Berry CC, et al. Detection of glaucoma with scanning laser polarimetry. Arch Ophthalmol. 1998;116:1583–1589. doi: 10.1001/archopht.116.12.1583. [DOI] [PubMed] [Google Scholar]
- 12.Saito H, Tomidokoro A, Yanagisawa M, et al. Scanning laser polarimetry with enhanced corneal compensation in patients with open-angle glaucoma. J Glaucoma. 2008;17:24–29. doi: 10.1097/IJG.0b013e318133fb47. [DOI] [PubMed] [Google Scholar]
- 13.Bowd C, Tavares IM, Medeiros FA, et al. Retinal nerve fiber layer thickness and visual sensitivity using scanning laser polarimetry with variable and enhanced corneal compensation. Ophthalmology. 2007;114:1259–1265. doi: 10.1016/j.ophtha.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 14.Mai TA, Reus NJ, Lemij HG. Retinal nerve fiber layer measurement repeatability in scanning laser polarimetry with enhanced corneal compensation. J Glaucoma. 2008;17:269–274. doi: 10.1097/IJG.0b013e31815c3a6b. [DOI] [PubMed] [Google Scholar]
- 15.Reus NJ, Zhou Q, Lemij HG. Enhanced imaging algorithm for scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2006;47:3870–3877. doi: 10.1167/iovs.05-0067. [DOI] [PubMed] [Google Scholar]
- 16.Hoh ST, Greenfield DS, Liebmann JM, et al. Factors affecting image acquisition during scanning laser polarimetry. Ophthalmic Surg Lasers. 1998;29:545–551. [PubMed] [Google Scholar]
- 17.Burgansky-Eliash Z, Wollstein G, Bilonick RA, et al. Glaucoma detection with the Heidelberg retina tomograph 3. Ophthalmology. 2007;114:466–471. doi: 10.1016/j.ophtha.2006.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deleon-Ortega JE, Arthur SN, McGwin G, Jr, et al. Discrimination between glaucomatous and nonglaucomatous eyes using quantitative imaging devices and subjective optic nerve head assessment. Invest Ophthalmol Vis Sci. 2006;47:3374–3380. doi: 10.1167/iovs.05-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iester M, Mermoud A. Retinal nerve fiber layer measured by Heidelberg retina tomograph and nerve fiber analyzer. Eur J Ophthalmol. 2005;15:246–254. doi: 10.1177/112067210501500212. [DOI] [PubMed] [Google Scholar]
- 20.Nagai-Kusuhara A, Nakamura M, Fujioka M, et al. Association of retinal nerve fibre layer thickness measured by confocal scanning laser ophthalmoscopy and optical coherence tomography with disc size and axial length. Br J Ophthalmol. 2008;92:186–190. doi: 10.1136/bjo.2007.127480. [DOI] [PubMed] [Google Scholar]
- 21.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa H, Piette S, Liebmann JM, et al. Detecting the inner and outer borders of the retinal nerve fiber layer using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2002;240:362–371. doi: 10.1007/s00417-002-0461-3. [DOI] [PubMed] [Google Scholar]
- 23.Gabriele ML, Ishikawa H, Wollstein G, et al. Peripapillary nerve fiber layer thickness profile determined with high speed, ultrahigh resolution optical coherence tomography high-density scanning. Invest Ophthalmol Vis Sci. 2007;48:3154–3160. doi: 10.1167/iovs.06-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budenz DL, Fredette MJ, Feuer WJ, et al. Reproducibility of peripapillary retinal nerve fiber thickness measurements with Stratus OCT in glaucomatous eyes. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Badala F, Nouri-Mahdavi K, Raoof DA, et al. Optic disk and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144:724–732. doi: 10.1016/j.ajo.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hougaard JL, Heijl A, Bengtsson B. Glaucoma detection by Stratus OCT. J Glaucoma. 2007;16:302–306. doi: 10.1097/IJG.0b013e318032e4d4. [DOI] [PubMed] [Google Scholar]
- 27.Manassakorn A, Nouri-Mahdavi K, Caprioli J. Comparison of retinal nerve fiber layer thickness and optic disk algorithms with optical coherence tomography to detect glaucoma. Am J Ophthalmol. 2006;141:105–115. doi: 10.1016/j.ajo.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Schuman JS, Hee MR, Arya AV, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Wollstein G, Ishikawa H, Wang J, et al. Comparison of three optical coherence tomography scanning areas for detection of glaucomatous damage. Am J Ophthalmol. 2005;139:39–43. doi: 10.1016/j.ajo.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Wollstein G, Paunescu LA, Ko TH, et al. Ultrahigh-resolution optical coherence tomography in glaucoma. Ophthalmology. 2005;112:229–237. doi: 10.1016/j.ophtha.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–470. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabriele ML, Ishikawa H, Wollstein G, et al. Optical coherence tomography scan circle location and mean retinal nerve fiber layer measurement variability. Invest Ophthalmol Vis Sci. 2008;49:2315–2321. doi: 10.1167/iovs.07-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. doi: 10.1016/j.preteyeres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa H, Kim J, Friberg TR, et al. Three dimensional optical coherence tomography (3D-OCT) image enhancement with segmentation free contour modeling C-mode. Invest Ophthalmol Vis Sci. doi: 10.1167/iovs.08-2703. Published Online First: 24 October 2008. doi:10.1167/iovs.08-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cense B, Chen TC, Park BH, et al. In vivo depth-resolved birefringence measurements of the human retinal nerve fiber layer by polarization-sensitive optical coherence tomography. Opt Lett. 2002;27:1610–1612. doi: 10.1364/ol.27.001610. [DOI] [PubMed] [Google Scholar]
- 36.Yamanari M, Miura M, Makita S, et al. Phase retardation measurement of retinal nerve fiber layer by polarization-sensitive spectral-domain optical coherence tomography and scanning laser polarimetry. J Biomed Opt. 2008;13:014013. doi: 10.1117/1.2841024. [DOI] [PubMed] [Google Scholar]
- 37.Cense B, Chen TC, de Boer JF. In vivo thickness and birefringence determination of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. Bull Soc Belge Ophtalmol. 2006:109–121. [PubMed] [Google Scholar]
- 38.Cense B, Chen TC, Park BH, et al. Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography. Invest Ophthalmol Vis Sci. 2004;45:2606–2612. doi: 10.1167/iovs.03-1160. [DOI] [PubMed] [Google Scholar]
- 39.Cense B, Chen TC, Park BH, et al. In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. J Biomed Opt. 2004;9:121–125. doi: 10.1117/1.1627774. [DOI] [PubMed] [Google Scholar]
- 40.Choma MA, Hsu K, Izatt JA. Swept source optical coherence tomography using an all-fiber 1300-nm ring laser source. J Biomed Opt. 2005;10:44009. doi: 10.1117/1.1961474. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan VJ, Huber R, Gorczynska I, et al. High-speed, high-resolution optical coherence tomography retinal imaging with a frequency-swept laser at 850 nm. Opt Lett. 2007;32:361–363. doi: 10.1364/ol.32.000361. [DOI] [PubMed] [Google Scholar]