Abstract
Background
Hypertrophic cardiomyopathy (HCM) is diagnosed clinically by the presence of left ventricular hypertrophy (LVH). However, LVH is absent in a significant number of genotype-positive patients. Because myocyte dysfunction and disarray are the primary abnormalities in HCM, we reasoned that tissue Doppler imaging could identify contraction and relaxation abnormalities, irrespective of hypertrophy, in a transgenic rabbit model of human HCM.
Methods and Results
M-mode, 2D, Doppler echocardiography and tissue Doppler imaging were performed in nontransgenic (n=24), wild-type β-myosin heavy chain-arginine403 (n=14), and mutant β-myosin heavy chain-glutamic acid403 (n=24) transgenic rabbits. Mean septal thicknesses were 2.0±0.3, 2.0±0.25, and 2.75±0.3 mm in the 3 groups, respectively (P=0.001). LVH was absent in 9 of the 24 mutant rabbits. Left ventricular dimensions, systolic function, heart rate, mitral inflow velocities, and time intervals were similar in the groups. However, the difference between atrial reversal and transmitral A wave duration was increased in the mutant rabbits (P<0.001). More importantly, systolic and early diastolic tissue Doppler velocities were significantly lower in all mutant rabbits (7.45±2.2 versus 10.8±2.3 cm/s in nontransgenic and 9.0±0.76 cm/s in wild-type; P<0.001), including the 9 without LVH. A systolic velocity <8.5 cm/s had an 86% sensitivity and 100% specificity in identifying the mutant transgenic rabbits.
Conclusions
Myocardial contraction and relaxation were reduced in the mutant β-myosin heavy chain-glutamic acid403 transgenic rabbit model of human HCM, irrespective of the presence or absence of LVH. In addition, tissue Doppler imaging is more sensitive than conventional echocardiography for HCM screening.
Keywords: cardiomyopathy, hypertrophy, genetics, echocardiography, imaging
Hypertrophic cardiomyopathy (HCM), a genetic disease caused by mutations in contractile sarcomeric proteins,1 is diagnosed clinically by left ventricular hypertrophy (LVH) in the absence of increased external load.1 However, LVH is absent in a significant number of mutation carriers because of the variable penetrance of the mutations2–4 and the confounding effects of modifier genes,5 sex,6 and environmental factors. Overall, LVH is neither very sensitive nor very specific in HCM diagnosis.2–4
Experimental data suggest that cardiac myocyte contractile function in HCM is reduced and that the hypertrophy is compensatory.7–10 These data, in conjunction with myocyte disarray, the characteristic hallmark of HCM,1 led us to reason that tissue Doppler imaging (TDI) could identify contraction and relaxation abnormalities in HCM, irrespective of LVH. Similarly, we expected TDI to be more sensitive for the diagnosis of HCM than conventional echocardiography. Thus, we analyzed myocardial structure and function using M-mode, 2D, Doppler echocardiography and TDI in a transgenic rabbit model of human HCM,11 which gave us the opportunity to assess cardiac phenotype without the confounding effects of genetic heterogeneity.
Methods
The Institutional Review Board of Baylor College of Medicine approved the study protocol.
β-Myosin Heavy Chain Transgenic Rabbits
The methods of generating wild-type β-myosin heavy chain-arginine403 (β-MyHC-R403) and mutant (MyHC-glutamic acid403 [β-MyHC-Q403]) transgenic rabbits and confirming the expression of the transgene proteins, as well as relevant histological studies, have been published previously.11 A total of 24 nontransgenic, 14 β-MyHC-R403, and 24 β-MyHC-Q403 rabbits were examined.
Echocardiographic Studies
Adult rabbits were anesthetized11 and imaged randomly, without knowledge of the genotype. Two-dimensional and Doppler echocardiography were performed with a Sequoia ultrasound system (Mountain View) equipped with a 7-MHz transducer and TDI capability. After acquiring parasternal and apical views, pulse-Doppler was used to record transmitral and pulmonary venous flow in the apical 4-chamber view. A 5-mm sample volume was placed at the levels of the mitral annulus and tips to record 10 to 15 cardiac cycles at each level. Pulmonary venous flow (10 to 15 cycles) was recorded from the right paraseptal vein with the guidance of color Doppler. Isovolumic relaxation time was obtained by placing the sample volume midway between the left ventricular (LV) outflow tract and the mitral valve tips.
TDI was applied in the pulse-Doppler mode to allow for a spectral display of the mitral annulus velocities at its septal and lateral corners. Gains and filters were adjusted to acquire a clear tissue signal without background noise. Studies were recorded for playback and analysis.
Echocardiographic Analysis
A single observer blinded to the genotypes performed the analysis. Two-dimensional measurements included LV end-diastolic (EDD) and end-systolic (ESD) dimensions, aortic root size, left atrial anteroposterior diameter, and septal and posterior wall thickness. Fractional shortening was computed as follows: [(LVEDD−LVESD)/LVEDD] × 100.
All Doppler values represent the average of 5 beats. Mitral inflow was analyzed for peak E and peak A velocities, E/A ratio, acceleration and deceleration times of E velocity, and isovolumic relaxation time. A wave duration was measured at the mitral annulus level. The peak, duration, and velocity-time integral of pulmonary venous flow velocities were also measured. The pulmonary venous flow systolic filling fraction was computed by dividing the systolic velocity-time integral by the total forward velocity-time integral. The difference between atrial reversal and transmitral A wave duration was calculated. Systolic, early diastolic, and late diastolic tissue Doppler (TD) velocities at both corners of the mitral annulus were measured. The early/late diastolic ratio and the dimensionless parameter E/early diastolic velocity were computed at both corners. The latter parameter is an index that corrects for the influence of LV relaxation on mitral peak E velocity and provides a good estimate of LV filling pressures in HCM.12
Statistical Analysis
Variables among the 3 groups were compared by ANOVA. The Bonferroni t test was used for pairwise multiple comparisons. Statistical significance was defined by P≤0.05.
Results
All animals had M-mode, 2D, Doppler echocardiography and TDI studies that were satisfactory for analysis. There were 7 females in each group, and no significant difference existed in the mean age of the rabbits among the groups.
Reproducibility
Seven animals (3 nontransgenic, 2 β-MyHC-R403, and 2 β-MyHC-Q403) were imaged at a later date. Interobserver and intraobserver differences were as follows: septal thickness, 0.15±0.1 and 0.12±0.1 mm; LV end-diastolic dimension, 3±0.8 and 2±0.8 mm; E velocity, 4±1.5 and 3±1 cm/s; isovolumetric relaxation time, 4±4 and 3±1 ms; TD systolic velocity, 0.4±0.1 and 0.3±0.1 cm/s; and early diastolic velocity, 0.32±0.1 and 0.26±0.1 cm/s, respectively.
2D Measurements
Consistent with our previous report,11 mean septal and posterior wall thicknesses were significantly higher in the β-MyHC-Q403 animals compared with β-MyHC-R403 and nontransgenic animals. However, 9 of the 24 mutant transgenic rabbits (38%) had normal wall thicknesses. No significant differences existed in the mean age and the male/female ratio in those with and without LVH. LV and left atrial dimensions, fractional shortening, and aortic root size were not significantly different among the groups, with the exception of LV end-diastolic dimension, which was smaller in the β-MyHC-Q403 rabbits compared with β-MyHC-R403 and non-transgenic rabbits (Table).
Table.
Two-Dimensional and Doppler Echocardiographic Measurements
| Nontransgenic (n = 24) | β-MyHC-R403 (n = 14) | β-MyHC-Q403 (n = 24) | |
|---|---|---|---|
| Heart rate, beats/min | 148±25 | 145±24 | 145±26 |
| 2D Echocardiography | |||
| LVEDD, mm | 14.1±0.19 | 13.5±0.28 | 12.8±0.24* |
| LVESD, mm | 8.8±0.16 | 8±0.22 | 8.2±0.2 |
| FS, % | 37.6±7.6 | 37.4±7.9 | 36.2±8 |
| Septal thickness, mm | 2.0±0.3 | 2.0±0.25 | 2.75±0.3† |
| Posterior wall thickness, mm | 2.2±0.23 | 2.0±0.3 | 2.9±0.25‡ |
| Aortic root, mm | 7.5±0.2 | 7±0.3 | 8±0.2 |
| LA AP dimension, mm | 7±0.14 | 7.1±0.18 | 7.4±0.16 |
| Mitral inflow | |||
| Peak E velocity, cm/s | 48±6.7 | 50±6.4 | 46±9.4 |
| Peak A velocity, cm/s | 26.5±6 | 28±5.5 | 28±7.4 |
| E/A ratio | 1.8±0.3 | 1.8±0.35 | 1.6±0.4 |
| IVRT, ms | 40±6 | 40±7 | 45±8 |
| Acceleration time, ms | 44±10 | 45±13 | 48±13 |
| Deceleration time, ms | 87±20 | 70±24 | 84±27 |
| AFF | 0.2±0.04 | 0.2±0.05 | 0.2±0.03 |
| A duration, ms | 64±14 | 62±19 | 58±12 |
| Pulmonary vein measurements | |||
| Peak systolic velocity, cm/s | 23±8 | 24±7 | 21±8 |
| Peak diastolic velocity, cm/s | 39±9 | 38±9 | 36±10.5 |
| Peak atrial velocity, cm/s | 13±3 | 13±4 | 13±4 |
| Systolic TVI, cm | 3.3±1.06 | 2.9±0.9 | 3.2±1.2 |
| Diastolic TVI, cm | 4.8±1.2 | 4.4±1.6 | 4.5±1.9 |
| Atrial TVI, cm | 0.4±0.07 | 0.35±0.1 | 0.4±0.06 |
| SFF | 0.39±0.07 | 0.4±0.12 | 0.42±0.08 |
| Ar duration (ms) | 58±10 | 57±17 | 65±17 |
| Ar-A duration (ms) | −6.3±10.5 | −10.2±19 | 9.4±16.6§ |
| TD measurements | |||
| Lateral Sa, cm/s | 10.8±2.3 | 9±0.76 | 7.45±2.2|| |
| Lateral Ea, cm/s | 10.2±1.7 | 9.6±0.9 | 6.8±1.5|| |
| Lateral Aa, cm/s | 4±1.3 | 4±0.8 | 4±1.5 |
| Lateral Ea/Aa | 2.2±0.3 | 2.5±0.4 | 1.6±0.4|| |
| Lateral E/Ea | 4.7±0.7 | 5±0.4 | 6.8±0.4|| |
| Septal Sa, cm/s | 8±2 | 7±1 | 5.9±1.5¶ |
| Septal Ea, cm/s | 8±1.5 | 7.5±1.5 | 5±1.3|| |
| Septal Aa, cm/s | 3.3±0.4 | 2.8±0.7 | 3.5±0.4 |
| Septal Ea/Aa | 2.13±0.61 | 2.7±0.9 | 1.5±0.61|| |
| Septal E/Ea | 6.1±0.8 | 6.9±1.4 | 10.7±1.2|| |
Values are mean±SD. LVEDD indicates left ventricular end-diastolic dimension; LVESD, left ventricular end systolic dimension; FS, fractional shortening; Ar, atrial reversal; AP, anteroposterior; IVRT, isovolumic relaxation time; AFF, atrial filling fraction; TVI, time-velocity integral; SFF, systolic filling fraction; Sa, systolic velocity; Ea, early diastolic velocity; and Aa, late diastolic velocity.
F=2.8, P=0.07;
F=4.0, P=0.001;
F=4.2, P=0.001;
P=0.002;
P<0.001;
P=0.003.
Mitral and Pulmonary Venous Flow
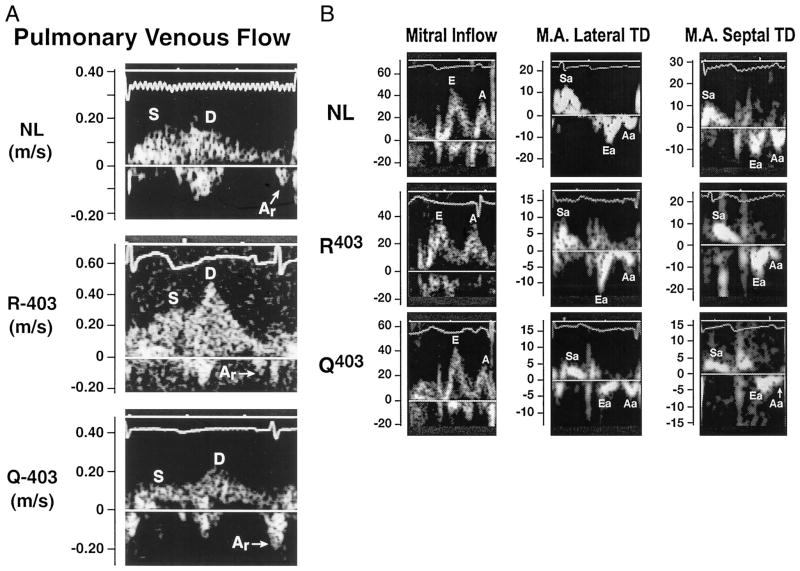
Heart rate, mitral and pulmonary venous velocities, and time intervals were not significantly different among the groups (Table). However, the isovolumic relaxation time was slightly longer and the difference between atrial reversal and transmitral A wave duration (a measure of LV end-diastolic pressure)12 was significantly increased in the mutant group.
TD Velocities
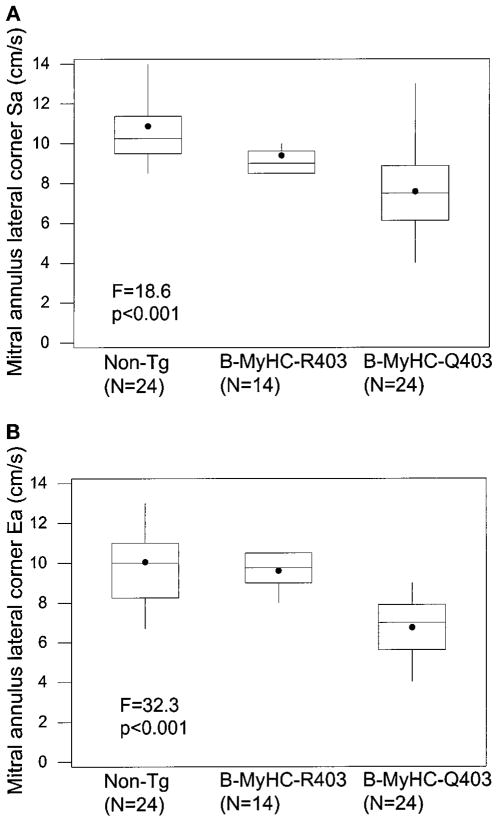
Systolic and early diastolic velocities at both corners of the mitral annulus were significantly lower in the β-MyHC-Q403 rabbits (Figures 1 and 2 and the Table). All 24 mutant transgenic animals, including the 9 with normal wall thicknesses, had reduced TD velocities. Systolic and early diastolic velocities were reduced in 9 of the 9 and in 8 of the 9 mutant transgenic rabbits without LVH, respectively (septal systolic velocity, 5.8±1.6 cm/s; septal diastolic velocity, 4.7±1.1 cm/s; lateral systolic velocity, 7.2±1.2 cm/s; and lateral diastolic velocity, 6.5±0.9 cm/s). Unlike the mitral E/A ratio, the early/late diastolic ratio was significantly lower in the mutant transgenic rabbits, and the E/early diastolic velocity ratio (at both corners of the mitral annulus) was significantly higher (Table), suggesting an increase in LV filling pressure.
Figure 1.
Pulmonary venous flow (A) and mitral inflow (cm/s) and TD velocities (cm/s) at both corners of the mitral annulus (B) in each of the 3 animal groups. Note the prominent atrial reversal (Ar) velocity and the lower TD velocities in the mutant β-MyHC-Q403 rabbits. NL indicates nontransgenic; S and Sa, systolic; D, diastolic; M.A., mitral annulus; Ea, early diastolic; and Aa, late diastolic.
Figure 2.
Box plot showing mean (●), median (center line), first and third quartiles (top and bottom of the box), and lowest and highest values (vertical lines) of mitral annulus lateral systolic (Sa; A) and early diastolic (Ea; B) velocities in the 3 groups. Non-TG indicates nontransgenic.
Accuracy of Doppler Parameters in Identifying Mutant Transgenic Rabbits
Conventional mitral and pulmonary venous flow indices did not distinguish between the groups, with the exception of the difference between atrial reversal and transmitral A wave duration (≥10 ms), which identified the mutant transgenic rabbits with a 96% specificity and a 63% sensitivity. In contrast, TD velocities were highly accurate in identifying the mutant transgenic rabbits. A systolic velocity <8.5 cm/s at the lateral corner of the annulus had a sensitivity of 86% and a specificity of 100%. A lateral annular early diastolic velocity <8 cm/s had 96% sensitivity and 85% specificity, and an early/late diastolic ratio of <1.8 had a sensitivity of 88% and a specificity of 87%. Similarly, a systolic velocity <7 cm/s, an early diastolic velocity <7 cm/s, and an early/late diastolic velocity ratio <1.6 at the septal corner of the mitral annulus had high sensitivities (83%, 96%, and 81%, respectively) and specificities (87%, 91%, and 89%, respectively).
Discussion
These results show that myocardial contraction and relaxation are consistently reduced in the mutant β-MyHC-Q403 transgenic rabbit model of human HCM, including those without LVH or mitral inflow abnormalities. In the absence of LVH, TDI detects mutant rabbits on the basis of myocardial dysfunction, and it is more sensitive than conventional echocardiography.
Echocardiography is currently used to screen for HCM based on the presence of LVH,2–6 which is absent in a significant number of mutation carriers.2–4 Similarly, one-third of the mutant transgenic rabbits (9 of 24) had no delectable LVH. However, TDI was reduced in all mutant transgenic rabbits, irrespective of the presence or absence of LVH. Although the clinical significance of reduced TD velocities remains to be established, these findings suggest TDI could diagnose HCM at a stage before and independent of LVH. Early and accurate phenotypic diagnosis of mutation carriers by TDI could provide an opportunity to prevent or modify the clinical manifestations of HCM and would complement genetic testing, which is complicated by allelic and nonallelic heterogeneity. We caution that a number of factors, such as blood pressure and ventricular volumes, could potentially affect systolic and early diastolic velocities and limit the utility of TDI in the diagnosis of HCM in human patients without LVH. Nevertheless, TDI could be useful in identifying mutation carriers in HCM families without concomitant diseases. The prognostic significance and pathological correlates of reduced TD velocities are unknown. The β-MyHC-Q403 mutation, expressed in transgenic rabbits, is associated with a high incidence of sudden cardiac death,3 myocyte and myofibrillar disarray, and increased interstitial collagen.11 Whether reduced TD velocities carry prognostic significance and whether they are reflective of early histological changes in the myocardium or impaired myocyte mechanical function, as shown during in vitro studies,7–9 remains to be established.
Reduced systolic velocities in the β-MyHC-Q403 transgenic rabbits supports the hypothesis that myocardial contractility in HCM is reduced.10 All 9 mutant rabbits without LVH had reduced systolic velocities. Many factors, including genetic and biological variances, can modulate the hypertrophic response, which could explain the absence of LVH, despite a reduction of TD velocities. Subsequent evolution of LVH in these rabbits will support the notion that LVH is compensatory. Overall, no significant differences existed in TD velocities between mutant rabbits with and without LVH. A few mutant rabbits with LVH had normal Sa or Ea velocities. An increased wall thickness led to reduced midsystolic wall stress (afterload), which favorably affected systolic velocities in a few rabbits with LVH. Likewise, a smaller end-systolic volume led to a higher diastolic suction force and an apparently preserved early diastolic velocity. Our findings are also in accord with the results of previous studies in HCM patients showing impaired LV diastolic function.13 However, the previous studies were performed in patients with classic HCM who had cardiac hypertrophy and, thus, they cannot address the potential application of TDI in the diagnosis of HCM or assessment of LV function in the absence of LVH. In addition, these previous studies were subject to the confounding effects of genetic and phenotypic heterogeneity.
In summary, myocardial systolic and diastolic function were reduced in the mutant β-MyHC transgenic rabbit model of human HCM. TDI accurately identified the mutant transgenic rabbits, even in the absence of LVH, and thus, it was more sensitive than conventional echocardiography. These findings, if confirmed in humans, not only provide insight into the pathogenesis of HCM, but also would establish a new and highly accurate technique of screening for HCM.
Acknowledgments
This study was supported by grants from the National Heart, Lung, and Blood Institute’s Specialized Centers of Research (P50-HL42267–01) and an Established Investigator Award (9640133N) from the American Heart Association, National Center, Dallas, Tex.
References
- 1.Seidman CE, Seidman JG. Molecular genetics of inherited cardiomyopathies. In: Chien KR, editor. Molecular Basis of Cardiovascular Disease. Philadelphia, Pa: Saunders; 1999. pp. 251–263. [Google Scholar]
- 2.Niimura H, Bachinski LL, Sangwatanaroj S, et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N Engl J Med. 1998;338:1248–1257. doi: 10.1056/NEJM199804303381802. [DOI] [PubMed] [Google Scholar]
- 3.Watkins H, Rosenzweig A, Hwang D, et al. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992;326:1108–1114. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- 4.Fananapazir L, Epstein ND. Prevalence of hypertrophic cardiomyopathy and limitations of screening methods. Circulation. 1995;92:700–704. doi: 10.1161/01.cir.92.4.700. [DOI] [PubMed] [Google Scholar]
- 5.Brugada R, Kelsey W, Lechin M, et al. Role of candidate modifier genes on the phenotypic expression of hypertrophy in patients with hypertrophic cardiomyopathy. J Investig Med. 1997;45:542–551. [PubMed] [Google Scholar]
- 6.Abchee A, Marian AJ. Prognostic significance of beta-myosin heavy chain mutations is reflective of their hypertrophic expressivity in patients with hypertrophic cardiomyopathy. J Investig Med. 1997;45:191–196. [PubMed] [Google Scholar]
- 7.Marian AJ, Zhao G, Seta Y, et al. Expression of a mutant (Arg92Gln) human cardiac troponin T, known to cause hypertrophic cardiomyopathy, impairs adult cardiac myocytes contractility. Circ Res. 1997;81:76–85. doi: 10.1161/01.res.81.1.76. [DOI] [PubMed] [Google Scholar]
- 8.Rust EM, Albayya FP, Metzger JM. Identification of a contractile deficit in adult cardiac myocytes expressing hypertrophic cardiomyopathy-associated mutant troponin T proteins. J Clin Invest. 1999;103:1459–1467. doi: 10.1172/JCI6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Iizuka K, Kelly RA, et al. An α-cardiac myosin heavy chain gene mutation impairs contraction and relaxation function of cardiac myocytes. Am J Physiol. 1999;276:H1780–H1787. doi: 10.1152/ajpheart.1999.276.5.H1780. [DOI] [PubMed] [Google Scholar]
- 10.Marian AJ. Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet. 2000;355:58–60. doi: 10.1016/s0140-6736(99)06187-5. [DOI] [PubMed] [Google Scholar]
- 11.Marian AJ, Wu Y, Lim DS, et al. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest. 1999;104:1683–1692. doi: 10.1172/JCI7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 13.Severino S, Caso P, Galderisi M, et al. Use of pulsed Doppler tissue imaging to assess regional left ventricular diastolic dysfunction in hypertrophic cardiomyopathy. Am J Cardiol. 1998;82:1394–1398. doi: 10.1016/s0002-9149(98)00648-1. [DOI] [PubMed] [Google Scholar]