Abstract
What is the potential for recovery of locomotor ability after a spinal cord injury? Both human and animal studies show that the spinal cord has the potential to reorganize and/or readjust to the loss of supraspinal input and utilize the remaining peripheral input to actually control stepping and standing. Motor training can be used to provide sensory ensembles within the spinal circuitry that are task-specific, i.e., step training improves stepping and stand training improves standing. A large component of this learning is a function of improved coordination of motor pools within and among limbs. The most successful type of training includes variability in the performed task, i.e., monotonous repetition of the exact same sensorimotor pattern results in “learned disuse”. The use of robotics for training specific motor tasks has become more prevalent recently and we report here that using an “assist-as-needed” approach for step training after a severe spinal cord injury provides a high probability of successful rehabilitation. The “assist-as-needed” paradigm allows variability in the step trajectory within specific boundaries such that the robotic arms constrain the deviations in a manner mimicking that observed under normal, intact conditions. Another critical feature of robotic devices or step training seems to be the ability to integrate normal hip and leg motion as occurs during normal stepping. These types of robotic devices have the potential to aid therapists in the clinical setting and to enhance the ability of spinal cord injured individuals to regain the maximum locomotor ability possible.
Keywords: spinal cord injury, rehabilitation, robotic motor training, sensory input, central pattern generation, spinal cord plasticity
1. Introduction
1.1. General concepts of spinal plasticity after a spinal cord injury
The spinal cord normally is continuously interacting with brain and peripheral sensory input during postural and locomotor activities. The relative importance of each of these sources of information and control are undoubtedly changing constantly, i.e., within a brief timeframe (milliseconds and seconds) and over more prolonged periods (even weeks, months or years). After a spinal cord injury, in almost all cases the dependence on these components change dramatically and rapidly. In those cases where the change is gradual, e.g., with some progressive disease such as a spinal tumor, animal studies suggest that there can be remarkable levels of adaptation and recovery of function to the gradual loss of sources of control from supraspinal centers (7). In most cases when there is a traumatic accident involving the spinal cord, however, the immediate loss of specific sources of information and neural control provides no opportunity for this remarkable adaptive potential to be manifested. But even in the case of a sudden traumatic accident involving a severe spinal cord injury, we are beginning to learn that specific and aggressive rehabilitative methods can enable one to regain at least some of this adaptive potential. The resources, knowledge, and skill required to accomplish this, however, are not widely available.
One strategy that can partially alleviate this limitation is the development of effective robotic devices. Such devices should be able to provide a means to employ activity-dependent interventions to enhance the recovery of neuromotor function after a wide range of neuromotor disorders. These devices can be an advantage, if properly designed, because they can be used as a means of enhancing motivation of the subject, collecting quantitative data that the subject and the therapist can interpret with respect to rate of progress, and eventually reducing the cost of therapy by minimizing therapist time. Another practical advantage of properly designed robotic devices is that they can be used safely and readily in the home as well as in the clinic. Thus it is more likely that an individual will practice more often while being motivated to perform at a higher level when the practice is embedded in a game-like environment and when access to such a resource is readily available, i.e., in the home.
1.2. Plasticity of the intact versus the injured nervous system
Although it would be difficult to document, it is the perception of the present authors that the general perspective of therapists, clinicians, and scientists is that the potential, and even the mechanisms, for adaptation differ in individuals with an intact versus a severely injured neuromuscular system. It appears, however, that the kinds of adaptations to activity-related events are essentially the same in the spinal cord of injured and uninjured humans and animals. In fact, it appears that the magnitude and rate of these adaptations are enhanced markedly immediately after an injury and as the level of adaptation approaches normal properties, the rate of improvement decreases substantially. An example of this phenomenon is evident when looking at the rate of improvement of stepping in an individual with a severe spinal cord injury who had no stepping ability prior to locomotor training. In such a case it is highly likely that significant improvement will occur at the onset of training. In contrast a less dramatic rate of improvement is likely to occur in less severely injured individuals (14). In both cases the improvements can be highly significant to the individual, but the difference in relative improvement would be orders of magnitude greater for the more severely injured individual. This difference is analogous to what would be expected with a comparison of an uninjured unskilled versus an uninjured skilled individual. In both cases, the mechanisms involved in the adaptive events are likely to be essentially the same for the two groups being compared.
2. The sensory system becomes a critical source of control after a severe spinal cord injury
It is generally assumed that the sensory information projecting to the spinal cord and brain serves to correct errors in movement, i.e., provides corrective feedback in response to the activation of sensory receptors from a wide range of tissues and tissue environments that change in a predictable way. There now is considerable evidence, however, that the sensory system plays a much more fundamental role in modulating and perhaps even controlling locomotion after a complete spinal cord injury (16, 19, 24, 35, 41). In the case of a complete spinal cord injury there is no sharing of sources of control and modulation by the brain and the periphery since only the periphery continues to have access to the spinal circuitry. Given this lone source of control other than the intrinsic control within the spinal circuitry, the importance of the peripheral sensory system is enhanced greatly.
In cases in which the spinal cord injury is incomplete, however, there appears to be extensive adaptations in the interaction of multiple supraspinal centers having access directly or indirectly to the more restricted descending systems remaining to the spinal circuitry. For example, a dramatic level of plasticity that can occur after severe, but incomplete, spinal cord injuries in humans that results in recovery of reasonably successful locomotion (14, 15). Similarly, after a mid-thoracic spinal cord hemisection, a rat or mouse can recover near normal locomotor capability within a matter of weeks and this recovery can simply occur spontaneously (7). When a second hemisection is made on the opposite side and at a higher thoracic level 10 weeks after the first lesion, again the animals are able to recover very effective bilateral weight-bearing locomotion. When the two lesions were administered simultaneously or when a single, complete spinal cord transection was made, however, the animals were not able to recover the ability to generate any successful weight-bearing steps. These results demonstrate that an extensive level of reorganization of the interaction of supraspinal input to the intraspinal circuitries along the thoracic and lumbar segments occurred when some supraspinal input was present. Therefore, it is not essential to have long descending direct control of all motor pools that control locomotion, but some supraspinal input can be effective in facilitating recovery by activating the lumbosacral motor pools via propriospinal neurons.
The importance and effectiveness of sensory input in generating stepping has been demonstrated in a wide range of experiments. The critical experiments addressing the role of sensory information are those performed in vivo and without anesthetics. In essence, an experimental model in which the spinal circuitry and the sensory input remain intact while all communication with the brain is eliminated can be used to assess the functional interaction of sensory input and the circuitry intrinsic to the lumbosacral spinal cord. This circuitry presumably also can be recognized as being responsible for central pattern generation. When the hindlimbs of a complete mid-thoracic spinal cat or rat are placed on a moving treadmill belt, the stepping rate will be proportional to the speed of the belt (10, 18, 19, 23). In addition the level of activation of the motor pools will be modulated directly with the level of loading placed on the limbs during stepping (17,42, 43). Similar responses have been reported for human subjects with a complete thoracic spinal lesion (3, 12, 28). A further illustration of the importance of sensory information in the spinal animal is the observation that the hindlimbs step backwards when the treadmill belt direction is reversed (27) and adjusts the kinematics of the swing phase of a step in response to an obstacle placed in its path (22).
Further evidence of the importance of the sensory information in modulating locomotion after a complete spinal cord injury is demonstrated by the finding that chronic complete spinal rats can regain stepping in the presence of epidural stimulation as long as the dorsal root afferents are intact (34). When there is extensive unilateral deafferentation of the lumbosacral segments, effective stepping facilitated by epidural stimulation occurs only on the non-deafferented side. A few rapid movements can be observed occasionally on the deafferented side when the rat's hindlimbs are placed on a moving treadmill belt weeks after deafferentation. Thus while the intact limb stepped with reasonable consistency, rapid spastic-like movements occurred only occasionally on the deafferented side. This finding was somewhat surprising given the extensive evidence for the presence of commissural interneurons (33) that theoretically could drive the contralateral locomotor circuitry that is not receiving any sensory input, i.e., the deafferented side.
The capability of the spinal circuitry to learn has been demonstrated in a number of experiments. The spinal cord can learn to step if it is trained to step (10) and it can learn to stand if it is trained to stand (9). In all of these experiments learning could have been a result of having access to sensory information that directly activated specific portions of the spinal networks that generate locomotion. The “Horridge” preparation is a simpler model of spinal learning that consists of shocking the paw of a spinal rat or mouse when the limb extends beyond a threshold level (26). The spinal cord can learn to withdrawal the paw to avoid the shock presented to that limb.
All of these experiments demonstrate that the spinal cord does not only respond in a stereotypical reflex manner characteristic of a corrective response, but it can interpret complex patterns of sensory information and generate a motor response that is congruent with the sensory information that it receives. Essentially these experiments demonstrate that the sensory system can serve as a control system for the spinal circuitry. Furthermore, these experiments demonstrate an impressive level of precision with which the spinal circuitry can interpret sensory information in a highly dynamic environment and then use these complex patterns of sensory information to generate successful stepping over a range of speeds, loads, and even directions. These results raise the issue as to whether the importance of sensory information as a control system has been markedly underestimated for the intact neuromotor system. Is the sensory system of such great importance only after a severe injury?
3. Using robotic devices to assess the quality and amount of sensory information projecting to the spinal cord
Mechanical devices have been developed for achieving very precisely controlled passive stepping, cycling, and standing. In each instance, however, the likelihood of a significant level of relearning of a motor task and the probability of realizing significant levels of muscle adaptation, e.g., recovery of some muscle mass, is quite small. To realize gains in neuromotor skill and improving muscle function with the aid of robotic devices, the neural and muscular elements must be intricately involved in the generation of the movement. For example, the spinal circuitry that controls locomotion must be actively engaged during robotic training for spinal learning of stepping to occur.
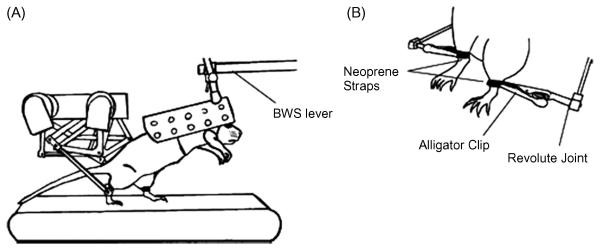
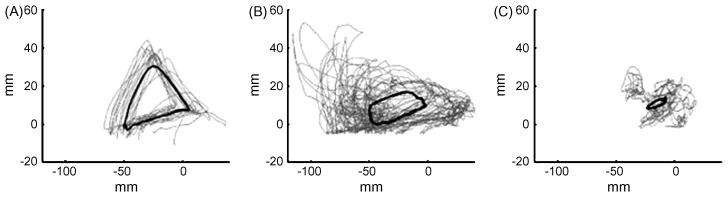
An example of a first generation robotic device designed for assessing and training a locomotor task in spinal rats is shown in Figure 1. The device is designed so that the rat steps bipedally while being supported via the trunk (39). The body weight support system can be automated to control the level of weight bearing performed by the rat. The device only controls the position of the ankle of each leg: our experience suggests that this single point of control is more efficient than trying to control each joint of each limb, a technical task that would be very difficult in rats or humans. The utility of using this robotic device as an assessment tool is shown in Figure 2. Normal uninjured rats and rats that have received a moderate or severe contusion are tested with the robotic device in a passive mode, i.e., used only to record the trajectory of the ankle during a series of steps. It is readily apparent that the shape and consistency of the trajectory of bipedal stepping is affected more severely in the rat that received the more serious injury.
Fig. 1.
(A) Rat is placed in a cloth harness and attached to the end of the body weight support device. Orientation of the rat's torso is adjustable with a lockableball joint and the amount of weight support delivered to the animal is precisely controlled. Rat steps bipedally in the device and the small robotic arms attach to each hindlimb with neoprene straps. (B) Robotic linkages attach to the rat's hindlimb by holding together both ends of a neoprene strap with an alligator clip. Alligator clip is allowed to rotate within the parasagittal plane of the animal. (From [40])
Fig. 2.
Mean step trajectory (bold lines) and total movement trajectories (gray lines) for a (A) control (no injury), (B) moderately injured (200 kdyne impact to midthoracic spinal cord), and (C) a severely injured (250 kdyne impact) animal. Animals stepped bipedally on a motorized treadmill at 12 cm/s, while the attached robots recorded the movement trajectories of their ankles. From these overall movement trajectories, good steps were identified, and the mean of those steps was calculated. Number of steps identified and used to calculate the mean trajectory was 19 for the control animal, 65 for the moderately injured animal, and 23 for the severely impaired animal. (From [40])
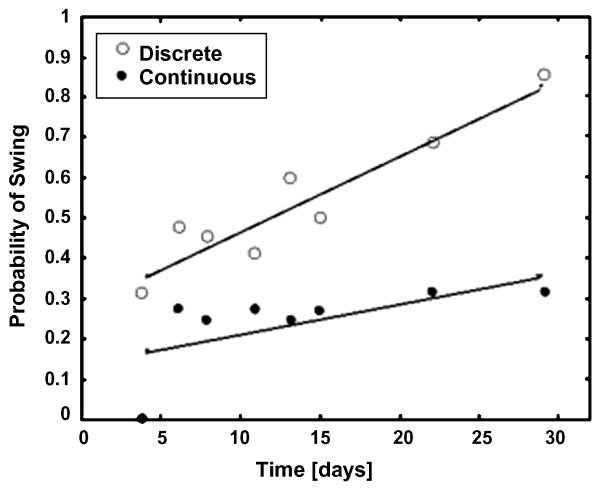
An advantage of using robotic devices is the ability to control the type of sensory information that the spinal cord receives by manipulating the kinetics and kinematics properties of the hindlimbs. An example of controlling the limb kinematics to facilitate stepping is shown in Figure 3 where the backward movement of the hindlimbs placed in a step-sliding device is manipulated to initiate the swing phase of a step. The initiation of the swing phase is one of the more difficult phases of the step cycle to normalize after a spinal cord injury. The example shows the details of how the mechanical manipulation of the limb has a significant impact on the probability of successfully initiating a swing phase (38). The probability of a successful swing phase significantly improves when the manipulation is imposed only under specified conditions rather than continuously. This observation is consistent with the concept that the sensory system tends to become less responsive to an identical stereotypical type of stimulation as opposed to a more unpredictable state. This adaptive feature of the sensory system and its functional significance is addressed in more detail below when describing robotic assistance in an “assist as needed” mode.
Fig. 3.
The probability of swing initiation (number of swings divided by number of pulls) was significantly reduced for the continuous slide algorithm (R2=0.36, p=0.11) when compared to the discrete pull algorithm (R2=0.94, p<0.0001) during 4 weeks of measurement following spinal cord contusion in the slide (S) group of rats (n=24, p<0.0001). (From [38])
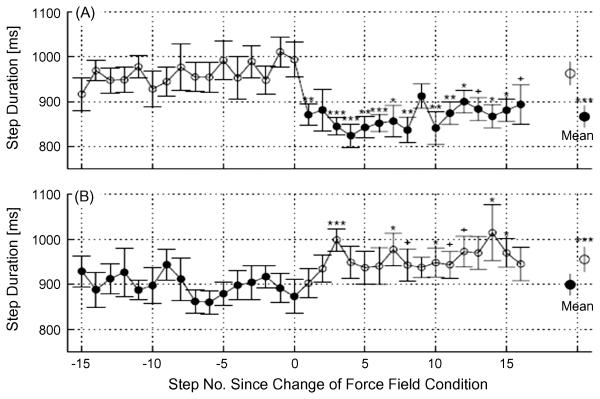
After a complete mid- thoracic spinal cord transection, robotic devices can be used to control the amount of loading that is sensed by the paw and assess the response of the spinal circuitry to repetitive applications of a given loading level during the stance phase of a step (43). The kinematics of the stepping is clearly a function of the level of the load placed on the paw. The duration of the step cycle decreases when the level of loading is increased and increases when the level of loading is reduced (Fig. 4). Many types of experiments in which the sensory information representing the kinetics and kinematics of the hindlimb motion are manipulated have shown that the neural networks controlling locomotion can readily provide a strategy that results in enabling the system to continue effective stepping (19, 30, 44). Robotic devices have been extremely useful not only in manipulating this sensory input but also in assessing the resulting changes in the kinematics and kinetics of the stepping.
Fig. 4.
Change in step duration with application (A) or removal (B) of the force field. Data are time aligned such that step one corresponds to the first step after application or removal of the field (filled symbols indicate the force field is on). Step durations of the indicated steps were first averaged for each rat across all exposures, and then averaged across both limbs for all rats (n=16), bars indicate SEM. Symbols indicate the significance of the difference between the mean of the steps prior to application or removal of the force field and each step after application or removal of the force field (***P<0.001, **P<0.01, *P<0.05, +P<0.1, paired t-test). Step duration decreased on the first step after the field was applied, but increased over the course of several steps when the force field was removed. Large circles and bars indicate the mean+/−SD of the step durations before and after the change in force field condition (***P<0.001, paired t-test). (From [43])
4. Step training increases the efficacy of selected neuromotor pathways
4.1. Effects of training mice and rats using a robotic device
Unlike the adult spinal cat the ability of adult mice and rats to recover some stepping ability after a complete mid-thoracic spinal cord transection with only step training as an intervention is minimal. Even the use of robotic devices is relatively ineffective in mice and rats that have received a complete lesion as an adult without some additional intervention that can increase the level of excitability of the spinal circuitry that normally controls stepping. One effective way to achieve this higher level of excitability is to administer a serotonergic agonist, such as quipazine (21). When quipazine was given 15 minutes prior to each robotically controlled training session of adult spinal mice, a significant improvement in bipedal stepping occurred over a period of weeks. This improvement in stepping was manifested as a more consistent trajectory of the ankle when the robotic device was in a passive mode (Fig. 5). An important feature of this type of step training was that the robotic device controlled the kinematics of the stepping in a relatively fixed pattern for a few minutes and then was returned to a passive mode whereby only the spinal circuits could generate the stepping. This alternating mode of assistance and non-assistance was used in our first attempts of using robotics to train spinal mice to avoid the possible phenomenon of “learned disuse”. In other words, would the spinal circuitry gradually become non-responsive to a fixed stereotypically imposed kinematics pattern?
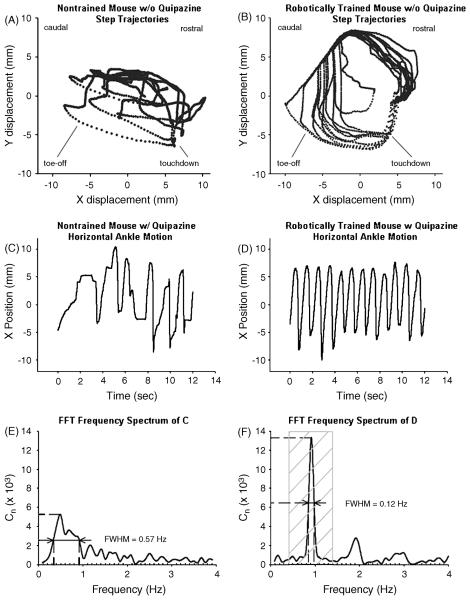
Fig. 5.
Step trajectories. Step trajectories of robotically trained mice showed consistent and rhythmic patterns. Trajectories recorded during the best 12 s periods of treadmill stepping of a representative nontrained mouse (A) and a representative robotically trained mouse (B) are shown. Ankle velocity is implicitly represented by the spacing of the data points. The rostral and caudal orientations of the mouse are noted, as well as the general regions of touchdown and toe-off. Fast Fourier transform analysis. FFT analysis provides information about step rhythm. Twelve seconds of horizontal ankle motion during stepping are shown for a nontrained mouse given quipazine that stepped arrhythmically (C) and for a trained mouse given quipazine that stepped rhythmically (D). The corresponding frequency spectrum and the FWHM of the peak at the primary stepping frequency for C and D are shown in E and F, respectively. Lower values of FWHM correspond to more rhythmic stepping. For successful spinal stepping on a treadmill at 3 cm/s, the peak corresponding to the primary stepping frequency lies between 0.4 and 1.4 Hz (F, crosshatched region). Cn represents the number of incidences during a test session that the mouse stepped at a particular frequency. (From [21])
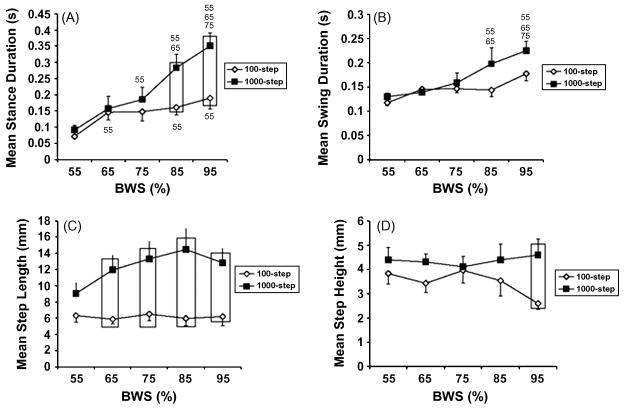
To begin to understand the criticalness of the amount of training needed to realize a sustainable improved level of stepping, rats that received a complete mid-thoracic lesion as neonates were trained to step beginning 4 weeks post-weaning (6). One group of rats was trained daily for 100 steps while another group was trained to perform 1000 steps per day. Stance duration, swing duration, and step length were higher in rats receiving 1000 versus 100 steps per day. Step height was also higher but only at the highest level of body weight support. (Fig. 6). These results emphasize the need for further refining the dose-response curve for the amount and frequency of training versus the amount of improvement in stepping that can be expected. The results to date suggest that these types of questions can be addressed in a highly quantitative manner by systematically performing experiments using a robotic device such as the one described above.
Fig. 6.
Training amount determines the responsiveness to hindlimb weight bearing. Mean stance duration (A), mean swing duration (B), mean step length (C), and mean step height (D) are shown across different levels of weight bearing for stepping tests at 8 cm • sec_1 performed after 4 weeks of training. A “55,” “65,” and “75” indicate that the data are significantly different from 55%, 65%, and 75% body weight support (BWS), respectively. For the 1000–step group in (A): the 55% BWS data are significantly different from the data at 75%, 85%, and 95% BWS; the 65% BWS data are significantly different from the data at 85% and 95% BWS; the 75% BWS data are significantly different from the data at 95% BWS. For the 100–step group in (A), the 55% BWS data are significantly different from the 65%, 85%, and 95% BWS data. For the 1000-step group in (B): the 55% and 65% BWS data are significantly different from the 85% and 95% BWS data, and the 75% BWS data are significantly different from the 95% BWS data. Boxes, significant difference between the 1000-step and 100-step groups. Values are mean ± standard error of the mean (SEM) calculated from 13 rats in each group. BWS is body weight support. (From [6])
4.2. Improved coordination of motor pools with motor training
To step successfully there must be a critical level of coordination of motor pools within and between the hindlimbs of the spinal animals when stepping bipedally. We learned that a very important component of the kinematics of stepping in complete spinal cats was the level of interlimb coordination that was gained with step training (11, 36). We examined detailed kinetics and kinematics features of stepping in a single hindlimb in trained and non-trained cats. When selecting the most successful stepping in either trained or non-trained cats the differences were not consistently dramatic and did not identify the cause of step failure. When we examined the coordination between the two hindlimbs relative to when there was a failure to continue successful stepping, however, we learned that the most common cause of failure was the gradually increasing levels of synchrony between the two limbs. This increased synchrony resulted in a higher likelihood of no weight bearing of either hindlimb at one phase of the step, i.e., when both limbs were beginning the swing phase at the same time. Although this type of coordination occurs normally when stepping at a rapid rate (galloping) in uninjured and injured animals, the ability to gallop is much more limited in the experimental setup for training spinal animals. For example, galloping involves modulation of the movement of the whole spine as well as the forelimbs, but in our controlled experimental setup the forelimbs are fixed on a platform and there is little possibility for modulating the curvature of the spine. The important point is that the level coordination among motor pools within and between limbs must reach a critical level of preciseness to generate consistent and successful stepping. The behavioral mechanisms that underlie the improvement in stepping ability with training are likely to be largely due to the improved coordination of motor pools.
4.3. Fewer neurons are activated during stepping in trained compared to non-trained spinal animals
It appears that after a complete spinal cord transection the sensorimotor circuits within the lumbosacral spinal cord become functionally refined as a result of motor training. This is consistent with the improved coordination of motor pools between the two hindlimbs with training as discussed above. In addition there is evidence for improved coordination among motor pools within the same limb (8). These results indicate the temporal pattern of activation of the neurons that are involved in locomotion, but do not directly address the number of neurons involved. Recent experiments using the cell activity marker c-fos are consistent with there being a marked reduction in the number of neurons that are activated within the lumbosacral segments during a bout of stepping in complete spinal rats that have been trained to step compared to those that have not been trained (31). The implications of these results are important in terms of the reorganization and control of the spinal circuitries by motor training and the determination of the mechanisms underlying this observation will be a significant accomplishment. It is also possible that the sensorimotor pathways are more sporadic and divergent in the non-trained compared to the trained state.
One interpretation for the reduced number of neurons that are activated throughout the gray matter of all of the lumbosacral segments in trained compared to non-trained rats is that after a spinal cord injury there is a proliferation of functional synapses resulting in less specificity in the movement, i.e., as occurs with spasticity. It is possible that these synapses may be present anatomically but not functionally in the uninjured state, perhaps analogous to axonal sprouting in the muscle following denervation. If this is true, then the training would essentially be serving to refine the network involved in locomotion by eliminating or “pruning” the more non-functional synaptic contacts. It is a common phenomenon for individuals after a severe, but incomplete, spinal cord injury to lose the ability to selectively activate specific muscle groups (37). An attempt to move one joint can commonly result in movement of the entire limb or even both limbs.
5. Robots working in an “assist-as-needed” mode: rationale and evidence for its advantages
Extending the concept noted above whereby a mechanical device moves the hindlimbs in a monotonous step-like pattern in an attempt to relearn how to step or even improve the quality of stepping, there appears to be significant limitations of this approach. There is reasonably clear evidence that imposing such a motion on the limbs of individuals with a complete spinal cord injury will result in a rather rapid progression from modest activation of the muscles to nearly no activation after 2 to 3 minutes of repetitive “stepping” (13). This seems to reflect a phenomenon similar to that which is often referred to as “learned disuse”. In this case, the point is that the sensory system becomes less responsive after identical and repetitive patterns of activation. This phenomenon seems to be analogous to the common adaptive response of the sensory system exposed to non-varying stimuli, e.g., becoming unaware of wearing a hat.
To avoid this type of phenomenon a computer control system for a robotic device has been developed for mice, rats, and humans that performs in a manner approximating that of a skilled therapist. A skilled therapist assists the stepping of an individual with a spinal cord injury in a body weight support system when and to the degree that assistance is needed to maintain a relatively effective stepping pattern. The therapist provides less assistance as the individual improves in stepping ability, until the eventual goal of no assistance is reached. Robotic systems that are controlled in an “assist as needed” basis have the potential to become more effective than a skilled therapist. These systems should be able to provide immediate visual feedback to the subject, indicating the relative amount of work being performed by the robot compared to the subject that is relearning to step.
The performance of a robotic device designed for training human subjects, called the PAM and POGO, seems to have most of the desired features (1, 2, 20). This device has “back drivable” capability so that the movement of the force arms can be controlled in an “assist as needed” mode. Another significant advantage of this device is that it accommodates the normal movement of the hip so that it is synchronized with the movement of the remainder of the lower limb thereby permitting stepping to occur as a more highly integrated system, i.e., more components of the normal highly integrated control of stepping involving head-to-toe components (see next section for further discussion of this concept). Preliminary evidence suggests that the PAM and POGO system can interact with complete spinal cord injured individuals in a manner similar to that of a highly skilled therapist. For example, the EMG patterns generated by the individual when the limbs are being “semi-controlled” by the robot or by the therapist are very similar. The device also can quantify the amount of work and power generated by the device relative to that generated by the individual. Feedback regarding knowledge of the amount of work performed by the subject can be observed online as well as stored for comparisons within and across training sessions. This feature provides a source of motivation for the individual as well as providing a quantitative assessment of the rate of progress that can be used by the therapists and clinicians.
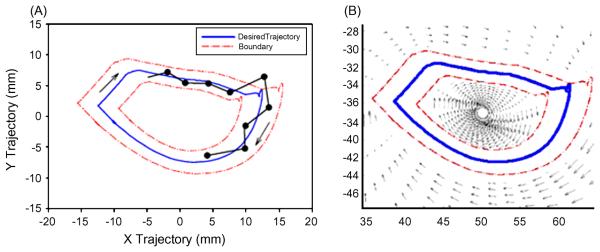
The underlying concept upon which this “assist as needed” approach is based is the inherent variation in the performance of every motor task, even for a movement as automatic as stepping (4). This variation indicates that the neural control system for locomotion is based on some fundamental probabilistic processes within the network of neurons that interprets sensory information associated with stepping and makes decisions based on these sensory patterns. Therefore, it seems logical that imposing a non-varying kinematics pattern on the limbs results in a high probability that there will be continuous interruption of each incremental sequence of kinematics events that the nervous system is designed to generate. The logic, therefore, is that continuous interruptions will occur and interfere with the normal progression of activation that the network has “intended” to execute. Consequently, it is less likely that these circuits which are continuously interrupted will learn the appropriate sequence of synaptic events that they are designed to carry out. A conceptual schematic of the point-to-point determination that results from a fixed compared to a trajectory guided “softly” with a window approach, i.e., in an “assist-as-needed” mode, that is generated with a continuous force field that “pushes” the ankle to move toward a desired trajectory is shown in Figure 7.
Fig. 7.
(A) Schematic of a semi-active fixed-trajectory paradigm for step training, where the desired limb trajectory (blue) is bounded by both inner and outer boundaries (red). The actual trajectory (black) that the neural circuits might induce is allowed to vary within the boundary. However, once the trajectory falls outside of the boundary, the robot will actively bring it back within the boundaries. The black line with periodic dots illustrates the potential positions that the intrinsic neural control might choose to generate for any given bin time. The probability that the neural control would move the limb to the exact position defined by the blue line, representing a fixed trajectory, is highly unlikely. As a result, theoretically, the neural control system is continuously disrupted by the fixed trajectory paradigm. This fixed trajectory, therefore, does not allow the neural control circuitry to respond to any of its intrinsic activation patterns, but rather forces the intrinsic circuitry to continuously respond to external perturbations. This strategy for control would seem to unnecessarily disrupt the spinal circuitry and in the process minimize or even preclude the intrinsic circuitry from interpreting relevant proprioceptive information required to generate a solution (i.e., make choices) and, thus, presumably prevent the circuitry from meaningful learning phenomena. (B) Soft robotic control schematics on how the semi-active control paradigm for step training is implemented. A moving window (red) bounds the desired trajectory (blue) of the mouse limb during stepping. Within the window, the robotic arm allows the mouse to vary its movement. However, when the neural control desired trajectory falls outside of the window, the robot will experience a convergent velocity field, that actively returns the mouse's limbs back within the window. This type of soft control is thought to approximate the “assist as needed” approach used by experienced therapists. (From [4])
Evidence of the validity of this concept was initially provided in an experiment in which complete spinal mice were trained to step with a robotic device that was programmed to train in one of three modes (5). In one mode a fixed trajectory was imposed on the hindlimbs of spinal mice during each training session (15 min/day, 5 days/week) over a period of 6 weeks. A second group of spinal mice were trained with a robot functioning in an “assist as needed” mode, but each limb was controlled independently. A third group of spinal mice were trained in the “assist as needed” mode, but the variation in the relationship between the two limbs was limited so that the stepping occurred in an alternating pattern, i.e., avoiding the incidence of hopping type of movements when the two limbs would become more synchronized in their stepping kinematics. The results were relatively clear in that those mice that were trained in an “assist as needed” mode with coordination between the limbs learned to step more consistently than either of the other two groups.
A further application of these results needs to be examined given the implications of the importance of variation of stepping kinematics as a feature of optimizing relearning to step. Specifically, theoretically there will be an optimal window for the amount of variation allowed, i.e., it can be too small and therefore too consistent in its trajectory or it can be too large to not have any corrective impact. It seems likely that the size of this “window” of variation will be a function of the severity of the injury and the state of recovery. For example, the size of the window in the initial stages of training most likely would be relatively large, but as the subject begins to step more successfully the window size could be reduced until ideally the variation would match that which occurs routinely in the uninjured state. It also seems likely that the magnitude of this variation will vary from individual to individual, even in the uninjured subject. Given that it is presently unclear how to determine the most effective window size, this issue needs careful examination with the likelihood that some progress can be made by incorporating basic principles of learning and models of learning.
6. Significance of the fidelity of the guidance provided by a robot
As a general principle as sources of the loss of control are diminished there are fewer options, less redundancy and less synergism among the components that make up the locomotor system. Therefore, with an increase in the severity of an injury utilization of those components that can become functional is less and less. Strikingly, there is not a direct proportionality in the loss of these individual sources of control and the loss of function. For example, there can be a marked loss of continuity above and below a lesion while a remarkable degree of normal locomotor control is maintained. The fact remains, however, that whatever sources remain functional, it becomes increasingly important that these sources function at their maximum potential.
How do these issues relate to the significance of the fidelity of the guidance that can be provided by a robotic device? Robotic devices can be designed to control individual muscles of individual joints or to exert more of a systemic control of the lower limbs (1, 29, 32). Robotic devices are being developed that engage as many components of the locomotor apparatus as possible to capitalize on their synergistic potential in attempting to facilitate recovery of locomotion (41). The device that is most widely distributed among the clinics has been an impressive first-generation effort (32), but given the experience to date it has obvious and severe limitations. For example, it essentially eliminates, or at least minimizes, the involvement of the hip in spite of the fact that even slight, but timely, shifts in the hip joints can greatly facilitate the swing phase of the step cycle. In addition, the normal pattern of loading of both limbs greatly depends on the appropriate dynamics of the hip, just with respect to the momentum that it can impart on the lower limb without any direct involvement of hip flexors or extensors. As a result of these limitations, critical components are precluded from contributing to the step cycle.
Efforts have been made to make newer robotic devices engage a more normal hip kinematics pattern and, therefore, to have the potential for facilitating more rapid and complete recovery of stepping for a larger number of individuals with spinal cord injuries (1, 2, 20). If these more intricate features of locomotion can be accommodated in addition to the “assist as needed” mode of control, the level of success of robotically mediated locomotor training will become significant. Again, the general principle is to optimize the use of every source of control remaining including mimicking as closely as possible the sensory input normally associated with locomotion. A significant level of function can be recovered by maximally utilizing those functional components that remain viable.
Acknowledgements
This work was supported by NIH NS16333, NIH HD44830, NIH NS42951, and the Christopher and Dana Reeve Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests : The authors declare that they have no competing financial interests.
References
- 1.Aoyagi D, Ichinose WE, Harkema SJ, Reinkensmeyer DJ, Bobrow JE. A robot and control algorithm that can synchronously assist in naturalistic motion during bodyweight-supported gait training following neurologic injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2007;15:387–400. doi: 10.1109/TNSRE.2007.903922. [DOI] [PubMed] [Google Scholar]
- 2.Aoyagi D, Ichinose WE, Harkema SJ, Reinkensmeyer DJ, Bobrow JE. An assistive robotic device that can synchronize to the pelvic motion during human gait training. Conf. Proc. IEEE Int. Conf. Rehab. Robotics. 2005:265–268. [Google Scholar]
- 3.Barbeau H, Fung J, Leroux A, Ladouceur M. A review of the adaptability and recovery of locomotion after spinal cord injury. Prog. Brain Res. 2002;137:9–25. doi: 10.1016/s0079-6123(02)37004-3. [DOI] [PubMed] [Google Scholar]
- 4.Cai LL, Courtine G, Fong AJ, Burdick JW, Roy RR, Edgerton VR. Plasticity of functional connectivity in the adult spinal cord. Phil. Trans. Roy. Soc. Lond. B: Biol. Sci. 2006;361:1635–1646. doi: 10.1098/rstb.2006.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J. Neurosci. 2006;26:10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, de Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrama. 2007;24:1000–1012. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- 7.Courtine G, Song B, Roy RR, Zhong H, Herrmann JM, Ao Y, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping mediated by indirect propriospinal relay connections after severe spinal cord injury. Nature Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Guzman CP, Roy RR, Hodgson JA, Edgerton VR. Coordination of motor pools controlling the ankle musculature in adult spinal cats during treadmill walking. Brain Res. 1991;555:202–214. doi: 10.1016/0006-8993(91)90343-t. [DOI] [PubMed] [Google Scholar]
- 9.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J. Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- 10.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery following spinalization in adult cats. J. Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- 11.de Leon RD, London NJS, Roy RR, Edgerton VR. Failure analysis of stepping in adult spinal cats. In: Binder MD, editor. Peripheral and Spinal Mechanisms in the Neural Control of Movement: Prog. Brain Res. Vol. 123. Elsevier Science B.V.; Netherlands: 1999. pp. 341–348. [DOI] [PubMed] [Google Scholar]
- 12.Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl. Physiol. 2004;96:1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- 13.Dietz V, Müller R. Degradation of neuronal function following a spinal cord injury: mechanisms and countermeasures. Brain. 2004;127:2221–2231. doi: 10.1093/brain/awh255. [DOI] [PubMed] [Google Scholar]
- 14.Ditunno JF, Jr., Barbeau H, Dobkin BH, Elashoff R, Harkema S, Marino RJ, Hauck WW, Apple D, Basso DM, Behrman A, Deforge D, Fugate L, Saulino M, Scott M, Chung J. Spinal cord injury locomotor trial group. Validity of the walking scale for spinal cord injury and other domains of function in a multicenter clinical trial. Neurorehabil Neural Repair. 2007;21:539–550. doi: 10.1177/1545968307301880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M, Scott M. Spinal cord injury locomotor trial group. Weight-supported treadmill vs. over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493. doi: 10.1212/01.wnl.0000202600.72018.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgerton VR, Courtine G, Gerasimenko Y, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne N, Burdick JW, Roy RR. Training locomotor networks. Brain Res. Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgerton VR, de Guzman CP, Gregor RJ, Roy RR, Hodgson JA, Lovely RG. Trainability of the spinal cord to generate hindlimb stepping patterns in adult spinalized cats. In: Shimamura M, Grillner S, Edgerton VR, editors. Neurobiological Basis of Human Locomotion. Japan Scientific Societies Press; Tokyo: 1991. pp. 411–423. [Google Scholar]
- 18.Edgerton VR, de Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Topical Review: Retraining the injured spinal cord. J. Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal circuitry after injury. Ann. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 20.Emken JL, Harkema SJ, Beres-Jones JA, Ferreira CK, Reinkensmeyer DJ. Feasibility of manual teach-and-replay and continuous impedance shaping for robotic locomotor training following spinal cord injury. IEEE Trans. Biomed. Eng. 2008;55:322–334. doi: 10.1109/TBME.2007.910683. [DOI] [PubMed] [Google Scholar]
- 21.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J. Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- 23.Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog. Brain Res. 2006;157:231–260. doi: 10.1016/s0079-6123(06)57016-5. [DOI] [PubMed] [Google Scholar]
- 24.Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: Comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp. Neurol. 2008;209:417–425. doi: 10.1016/j.expneurol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giszter SF. Spinal cord injury: present and future therapeutic devices and prostheses. Neurotherapeutics. 2008;5:147–162. doi: 10.1016/j.nurt.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav. Cogn. Neurosci. Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- 27.Grillner S, Brooks VB. Hand book of Physiology Section 1. American Physiological Society; Waverly, Maryland: 1981. The nervous system II. Motor control; pp. 1179–1236. [Google Scholar]
- 28.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J. Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 29.Hesse S, Uhlenbrock D, Werner C, Bardeleben A. A mechanized gait trainer for restoring gait in nonambulatory subjects. Arch. Phys. Med. Rehabil. 2000;81:1158–1161. doi: 10.1053/apmr.2000.6280. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task? Med. Sci. Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- 31.Ichiyama RM, Courtine G, Gerasimenko Yu P., Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J. Neurosci. doi: 10.1523/JNEUROSCI.1881-08.2008. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jezernik S, Colombo G, Keller T, Frueh H, Morari M. Robotic orthosis lokomat: A rehabilitation and research tool. Neuromodulation. 2003;6:108–115. doi: 10.1046/j.1525-1403.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- 33.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- 34.Lavrov I, Courtine G, Dy CJ, van den Brand R, Fong AJ, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR. Facilitation of stepping with epidural stimulation in spinal rats: Role of sensory input. J. Neurosci. doi: 10.1523/JNEUROSCI.1069-08.2008. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavrov IA, Gerasimenko YP, Courtine G, Ichiyama RM, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- 36.Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- 37.Maegele M, Müller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J. Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- 38.Nessler JA, Minakata K, Sharp K, Reinkensmeyer DJ. Robot-assisted hindlimb extension increases the probability of swing initiation during treadmill walking by spinal cord contused rats. J. Neurosci. Methods. 2007;159:66–77. doi: 10.1016/j.jneumeth.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 39.Nessler JA, Reinkensmeyer DJ, Sharp K, Kwak E, Minakata K, de Leon RD. Robotic assessment of locomotor recovery in spinal contused rats. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2004;4:2687–2690. doi: 10.1109/IEMBS.2004.1403771. [DOI] [PubMed] [Google Scholar]
- 40.Nessler JA, Timoszyk W, Merlo M, Emken J, Minakata K, Roy RR, de Leon RD, Edgerton VR, Reinkensmeyer DJ. Robotic device for studying hindlimb stepping in rodents after spinal cord injury. IEEE Trans. Neural Syst. Rehab. Eng. 2005;13:497–506. doi: 10.1109/TNSRE.2005.858432. [DOI] [PubMed] [Google Scholar]
- 41.Reinkensmeyer D, Aoyagi D, Emken J, Galvez J, Ichinose W, Kerdanyan G, Maneekobkunwong S, Minakata K, Nessler J, Weber R, Roy RR, de Leon R, Bobrow J, Harkema S, Edgerton V. Tools for understanding and optimizing robotic gait training. J. Rehabil. Res. Dev. 2006;43:657–670. doi: 10.1682/jrrd.2005.04.0073. [DOI] [PubMed] [Google Scholar]
- 42.Timoszyk W, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal transection. Brain Res. 2005:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 43.Timoszyk WK, de Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J. Neurophysiol. 2002;88:3108–3117. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- 44.Udoekwere UI, Ramakrishnan A, Mbi L, Giszter SF. Robot application of elastic fields to the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006;1:3684–3687. doi: 10.1109/IEMBS.2006.259633. [DOI] [PMC free article] [PubMed] [Google Scholar]