Abstract
The aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor, mediates many biological processes, including fetal development. In this study, we examined AhR protein expression in human placentas from normal (N) and severe preeclamptic (sPE) pregnancies, as well as human fetal tissues from the second trimester of pregnancy, using immunohistochemistry and/or Western blot analysis. In the placentas, the AhR immunoreactivity was present primarily in syncytiotrophoblasts. The AhR staining was also seen in endothelium of large blood vessels in villi and endothelium of umbilical cord arteries and veins. No difference in AhR protein levels was found between N and sPE placentas. In fetal tissues, the AhR immunoreactivity was localized in lung, kidney, esophagus, pancreas, liver, testicle, thymus gland, retina, and choroid, mainly in epithelial cells, whereas it was absent in heart, brain, sclera, and thoracic aorta. These findings suggest that the AhR plays a critical role in syncytiotrophoblasts of human placentas and epithelium of many fetal organs. These data also imply that human placentas and those fetal organs with high AhR expression (e.g., lung, kidney, liver, pancreas, and thymus gland) during fetal development are highly susceptible to environmental toxicants such as dioxin. (J Histochem Cytochem 58:679–685, 2010)
Keywords: AhR, placenta, fetus, pregnancy
It is now clear that the aryl hydrocarbon receptor (AhR) can act on many types of cells and mediate a series of biological processes, including cell division, apoptosis, cell differentiation (Puga et al. 2009), actions of estrogen and androgen (Safe and McDougal 2002), immune system homeostasis (Stevens et al. 2009), and reproduction (Okey 2007; Hernández-Ochoa et al. 2009), indicating critical roles for the AhR in the regulation of multiple biological processes.
The AhR has long been studied as the receptor for environmental toxicants/carcinogens such as dioxin. Upon binding to these toxicants, the AhR can adversely affect developing embryos and fetuses, as well as male and female reproductive systems (Okey 2007; Hernández-Ochoa et al. 2009), in which the fetus appears to be more sensitive to those environmental toxicants than the adult (Peterson et al. 1993; Hernández-Ochoa et al. 2009). Specifically, prenatal exposure of many species (e.g., mice, rats, rabbits, monkeys, and possibly humans) to dioxin could cause fetotoxicity, leading to decreased litter size and increased prenatal mortality (Peterson et al. 1993; Hernández-Ochoa et al. 2009). Moreover, such an exposure can induce delayed puberty, decreased sperm count, and reduced fertility (Hernández-Ochoa et al. 2009). Interestingly, knockout of the AhR gene in mice also leads to similar adverse effects. For example, AhR-knockout female mice have difficulties in establishing implantation and maintaining pregnancy and have decreased litter size and increased neonatal death (Fernandez-Salguero et al. 1997; Abbott et al. 1999; Benedict et al. 2000; Baba et al. 2005), indicating important roles for the AhR in pregnancy, fetal growth, and neonatal survival.
Preeclampsia is a life-threatening complication of pregnancy, accounting for almost 15% of pregnancy-associated deaths, and one of the major causes of iatrogenic prematurity among newborns (Page 2002). The pathogenesis of preeclampsia is thought to act at three levels: defective placentation, placental ischemia, and endothelial cell dysfunction.
Expression of AhR mRNA has been identified in various human tissues, among which, placenta, lung, and liver appear to express the highest levels of AhR mRNA, with low levels of expression found in brain, kidney, and skeletal muscle (Manchester et al. 1987; Dolwick et al. 1993a; Shmueli et al. 2003). Expression of AhR protein and mRNA has also been reported in mouse and rabbit placentas (Tscheudschilsuren et al. 1999; Kitajima et al. 2004) and in a variety of mouse fetal tissues, e.g., liver, lung, and kidney in gestational days 12–16 (Abbott et al. 1995). However, information on AhR protein expression and cellular distribution in human placentas from N and preeclamptic (PE) pregnancies, as well as in the developing fetus, is lacking. Herein, we examined AhR protein expression in human placentas and fetal tissues using immunohistochemistry and/or Western blot analyses.
Materials and Methods
Placenta Collection and Fetal Tissue Microarray
Placentas were obtained immediately after delivery from women with normal-term pregnancies (N; n=9; eight were from vaginal delivery and one from cesarean section delivery), and from women who had developed sPE pregnancies (n=10; all were from cesarean section delivery) as described (Chung et al. 2004). Preeclampsia was defined according to the guidelines of the National Institutes of Health (National High Blood Pressure Education Program 2000). Preeclampsia was considered severe if one or more of the following criteria were present: maternal blood pressure higher than or equal to 160/110 mm Hg on two separate readings; proteinuria more than 2+ by dipstick or more than 2 g/24 hr; visual disturbances; pulmonary edema; epigastric or right upper quadrant pain; impaired liver function; thrombocytopenia; or fetal growth restriction. None of the study subjects had signs of infection. Smokers were excluded. Patient ages were similar (23 ± 1.2 years) between N and sPE pregnancies. Gestation ages for N pregnancies (39 ± 0.2 weeks) were significantly (p<0.05) higher than those for sPE pregnancies (33 ± 1.2 weeks). Fetal weights for N pregnancies (3433 ± 96.2 g) were higher (p<0.05) than in sPE pregnancies (1811 ± 309.1 g). Each of these parameters was analyzed by using the Student's t-test. Collection of placentas was approved by the Institutional Review Boards, Meriter Hospital and University of Wisconsin, Madison, and followed the recommended guidelines for using human subjects. Placental villi from beneath the chorionic and basal plates were quickly dissected (∼10 g each), snap-frozen, and stored in liquid nitrogen for Western blot analysis. Additional placental tissues from N and sPE pregnancies and umbilical cords from normal-term human placentas (n=10) were fixed overnight at 4C in 4% paraformaldehyde in 10 mM PBS and embedded in paraffin for immunohistochemistry.
Two human fetal tissue microarrays (catalog number BE01015) were purchased from Biomax (Rockville, MD). This tissue microarray contained 12 types of fetal tissues: lung (n=5), kidney (n=5), esophagus (n=4), pancreas (n=5), liver (n=5), testicle (n=5), thymus gland (n=5), retina, choroid, and sclera (n=2), brain (n=5), heart (n=5), thoracic aorta (n=1 subject), eyeball (n=1, which was missing on the tissue microarray purchased). According to the manufacturer's information, these tissue samples were obtained following induced abortion, and the fetuses had an average 4.8 ± 0.1–month gestation (ranging from 4 to 7 months).
Immunohistochemistry
Immunolocalization of the AhR was visualized by indirect detection via the avidin:biotinylated-peroxidase complex method (Vector Laboratories; Burlingame, CA) as previously described (Chung et al. 2004). The tissue sections were cut at 5 μm thick. For each sample, two adjacent tissue sections were mounted on one positively charged glass microscope slide (Fisherbrand Superfrost Plus; Fisher Scientific, Pittsburgh, PA). For antigen retrieval, the tissue sections and tissue microarrays were deparaffinized, dehydrated, and boiled in a 10 mM citrate buffer solution (pH 6.0) in a microwave for 10 min. Endogenous peroxidase activity was quenched by immersing the tissue array in 3% H2O2 in methanol for 10 min. After blocking the nonspecific binding with 1% horse serum albumin for 20 min, one tissue section per slide and one tissue microarray were probed with a rabbit AhR antibody (4 μg/ml and 2 μg/ml for the placental tissues and the human fetal tissue microarray, respectively; Biomol International, Plymouth Meeting, PA) for 1 hr. This antibody has been used to detect human AhR using Western blot analysis and immunohistochemistry (Juan et al. 2006). Another tissue section on the same slide and another tissue microarray were probed with preimmune rabbit IgG (Vector Laboratories) at the same concentration as the primary antibody and served as negative controls. The tissue sections and tissue microarrays were washed in PBS containing 0.3% Triton-X100, and then incubated with a biotinylated horse anti-rabbit antibody (Vector Laboratories) for 30 min. The specific immunoreactivity was visualized by 3-amino-9-ethylcarbazole (Vector Laboratories). Images were recorded under a Nikon microscope equipped with a Spot Insight QE CCD camera. To semi-quantitatively compare the AhR staining intensity in fetal tissues with the sample size per tissue ≥4, images were taken under a low-power (4x) objective, which covered the whole area of each tissue core, at exactly the same exposure time and light intensity. The images were uniformly converted to 8-bit gray-scale images. For each type of tissue with n ≥ 4, the optical density (OD) value was measured by the Image-J imaging analysis software (National Institutes of Health; Bethesda, MD). The OD value for each tissue core was calculated as: OD(AhR antibody staining) − OD(preimmune rabbit IgG). The tissue sections and tissue microarrays were then counterstained lightly with Harris Modified Hematoxylin (Fisher Scientific), and the images were recorded again for qualitative analysis.
Western Blot Analysis for AhR Protein in Placentas
Western blot analysis was carried out as described by Chung et al. (2004). Placental tissues were pulverized in liquid nitrogen using a mortar and pestle, followed by homogenization in buffer [50 mM HEPES, 0.1 M NaCl, 10 mM EDTA, 4 mM sodium pyrophosphate, 10 mM sodium fluoride, 2 mM sodium orthovanadate (pH 7.5), 1 mM phenyl methyl sulfonyl fluoride, 1% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml aprotinin] using a rotor-stator homogenizer (PawerGen700; Fisher Scientific, Hampton, NH), and further lysed by sonication. After centrifugation, protein concentrations of the supernatant were determined with BSA (fraction V; Sigma, St. Louis, MO) as standards.
The protein samples (100 μg) were separated on 10% SDS-PAGE gels, and electrically transferred to polyvinylidine difluoride membranes. The membranes were first probed with the AhR antibody (1:2000; Biomol International) followed by reprobing with either glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:4000; Research Diagnostics, Flanders, NJ) or β-actin (1:5000; Ambion, Austin, TX) antibody as a loading control. Human umbilical vein endothelial cells (HUVEC) isolated from normal-term placentas were used as a positive control for AhR (Juan et al. 2006). Proteins were visualized using enhanced chemiluminescence reagents (Amersham Biosciences; Piscataway, NJ), followed by exposure to chemiluminescence films. The immunoreactive signals were analyzed by a densitometer.
Statistics Procedures
Data for the AhR protein levels were analyzed using the Student's t-test. Data for the AhR staining intensity in the fetal tissue microarrays were analyzed using one-way ANOVA followed by pair-wise comparisons (Dunn's method) (SigmaStat; Jandel Co., San Rafael, CA); p≤0.05 was considered significant.
Results
Placentas
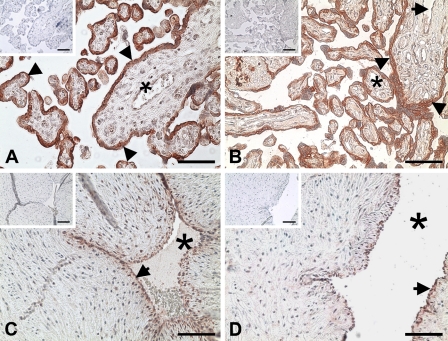
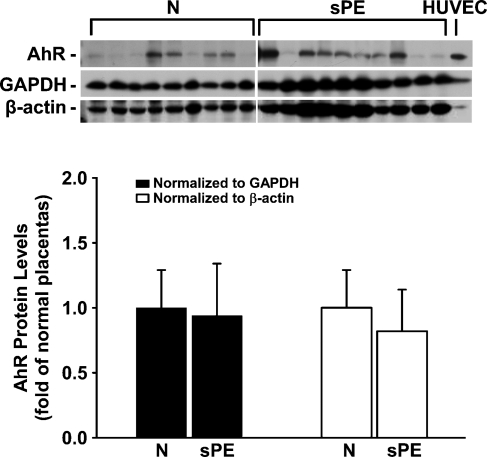
Positive reddish staining for the AhR was observed primarily in syncytiotrophoblasts and endothelial cells in some large blood vessels, as well as in endothelial cells of umbilical cord veins and arteries (Figure 1). No staining was observed in the IgG control (see the small inset in each panel in Figure 1). In Western blot analysis, the AhR antibody detected a single band at ∼95 kDa in HUVEC and in human placental tissues (Figure 2), corresponding to the human AhR molecular mass reported (Juan et al. 2006). The AhR protein levels after normalization with GAPDH or β-actin were similar between N and sPE placentas (Figure 2).
Figure 1.
Immunolocalization of the aryl hydrocarbon receptor (AhR) in human placentas from women with normal (N) and severe preeclamptic (sPE) pregnancies and umbilical cord arteries and veins. The adjacent tissue sections were incubated with either a rabbit AhR antibody or preimmune rabbit IgG (insets). Reddish color indicates positive staining for the AhR. Representative images for N placentas (A), sPE placentas (B), umbilical cord arteries (C), and veins (D) are shown. Arrowheads, syncytiotrophoblasts; arrows, endothelial cells; asterisks, lumen of blood vessels. Bar = 100 μm.
Figure 2.
Western blot analysis for AhR protein in human placentas from women with N and sPE pregnancies. Human placentas were obtained from N (n=9) and sPE (n=10) pregnancies. Representative Western blots are shown for AhR, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and β-actin. One band was detected at 95 kDa for the AhR, corresponding to its positive control human umbilical vein endothelial cells (HUVEC). Data normalized to GAPDH or β-actin are expressed as fold of N placentas.
Fetal Tissues
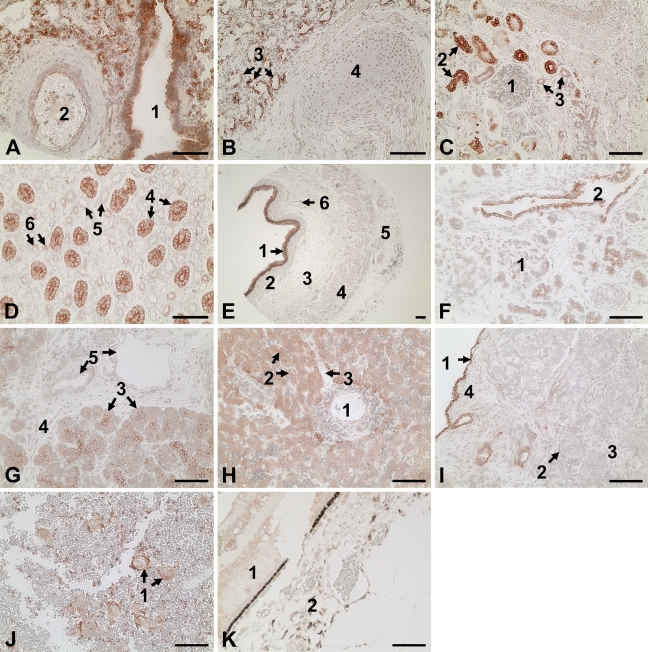
The AhR immunoreactivity was present in lung, kidney, liver, esophagus, pancreas, testicle, thymus gland, and retina, in which AhR staining was primarily localized in epithelial cells (Figure 3). In contrast, no AhR staining was observed in brain, heart, thoracic aorta, choroid, and sclera (not shown). Immunoreactivity was not seen in the tissue microarrays probed with the preimmune rabbit IgG (not shown).
Figure 3.
Immunolocalization of the AhR in human fetal tissues. Reddish color indicates positive staining for the AhR. A representative image for each tissue is shown. (A,B) Lung: (1) bronchiole, (2) blood vessel, (3) alveoli, and (4) cartilage. (C,D) Kidney: (1) maturing glomerulus with Bowman's capsule, (2) proximal convoluted tubules, (3) distal convoluted tubules, (4) collecting ducts, (5) thick segments of Henle's loop, and (6) thin segments of Henle's loop. (E) Esophagus: (1) multilayered epithelium, (2) lamina propria, (3) circular muscle, (4) longitudinal muscle, (5) adventitious tissue, and (6) blood vessel. (F,G) Pancreas: (1) islets of Langerhans, (2) interlobular duct, (3) exocrine acinous cells, (4) interlobular connective tissue, and (5) blood vessels. (H) Liver: (1) central vein, (2) hepatic cords, and (3) sinusoidal endothelium. (I) Testicle: (1) germinal epithelium, (2) seminiferous tubules, (3) intertubular tissue, and (4) tunica albuginea. (J) Thymus gland: (1) Hassall's corpuscles. (K) Retina: (1) retina, (2) choroid. Bar = 100 μm.
In lung, intensive AhR staining was localized in the epithelial cells lining the lumen of bronchus, bronchiole, and alveoli (Figures 3A and 3B). Weak AhR staining was observed in the endothelial and smooth-muscle cells of large blood vessels. No AhR staining was seen in the connecting tissue or in the cartilage.
In kidney, the strongest AhR staining was observed in the epithelial cells forming the collecting ducts (Figures 3C and 3D). Moderate AhR staining was found in epithelial cells lining the thin segment loop of Henle, but no staining was observed in the thick segment loop of Henle. AhR staining was intense in the proximal convoluted tubule, but was absent in the maturing glomeruli and Bowman's capsules.
In esophagus, AhR immunoreactivity was located in the multilayered epithelial cells (Figure 3E). No AhR staining was found in circular and longitudinal muscle layers.
In pancreas, intensive AhR staining was found in the exocrine acinous cells and epithelial cells lining the interlobular ducts and intercalated ducts (Figures 3F and 3G). Scattered AhR staining was observed in the endocrine islets of Langerhans. No staining was observed in interlobular connective tissue.
In liver, AhR staining was exclusively present in the epithelial hepatocytes but not in any other cells, including vascular endothelial cells (Figure 3H).
In testis, AhR staining was seen in a single layer of germinal epithelial cells, whereas no staining was present in seminiferous tubules and intertubular tissues (Figure 3I).
In thymus gland, AhR staining was restricted to the swirled epithelial structures known as Hassall's corpuscles in the medulla of thymus gland, whereas AhR staining was absent in densely packed cortex (Figure 3J).
In retina, weak AhR staining was observed in retina and in some fibroblast-like cells in choroid (Figure 3K).
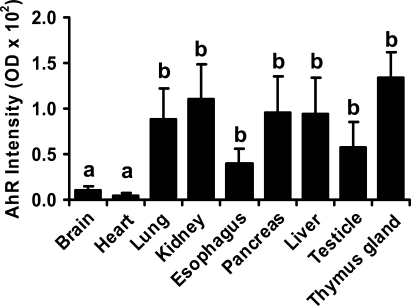
The semi-quantification analysis revealed that the AhR immunoreactivity in lung, kidney, pancreas, esophagus, liver, testicle, and thymus gland was higher (p≤0.05) than that in heart and brain (Figure 4), which was consistent with our qualitative results (Figure 3).
Figure 4.
Semiquantitative analysis of the AhR staining in human fetal tissues. Data are expressed as means ± SEM optical density value. Means with different letters differ (p<0.05).
Discussion
In this study, we have demonstrated for the first time that AhR protein is expressed in human placentas, primarily in syncytiotrophoblasts and endothelial cells, as well as in endothelial cells of umbilical cord veins and arteries from normal-term human placentas. We have also shown that AhR is expressed in the majority of human fetal tissues (e.g., lung, kidney, liver, esophagus, pancreas, testicle, thymus gland, and retina) printed on the tissue microarray, mainly located in epithelial cells. Thus, together with the evidence resulting from the AhR knockout studies (Fernandez-Salguero et al. 1997; Abbott et al. 1999; Benedict et al. 2000; Baba et al. 2005), these data suggest that AhR plays a critical role in syncytiotrophoblasts in human placentas and epithelial cells of those human fetal tissues during fetal development. These data also imply that these tissues may be highly susceptible to environmental toxicants such as dioxin and 3-methylcholanthrene, which are known to have negative impacts on fetal growth and development (Peterson et al.1993; Fernandez-Salguero et al. 1997; Abbott et al. 1999; Benedict et al. 2000).
The current findings that AhR protein levels were similar between human N and sPE placentas suggest that AhR might not be a key factor in sPE placentas. However, such a similarity might also be attributed to the shorter gestation age in sPE as compared with that in N pregnancies observed in the current study since the developmental differences in AhR expression have been recognized (Abbott et al. 1995). Moreover, we also cannot exclude the possibility that AhR protein expression in N and sPE placentas may differ from that in mild PE placentas, given that the pathogenesis of mild PE and sPE may not be completely the same (Gregg 2004). This is supported in a recent study by Baker et al. (2009), who reported that midgestation dyslipidemia was coupled only with mild PE, but not with sPE pregnancies.
Protein expression of the AhR has been reported in the placenta of mouse and rabbit (Tscheudschilsuren et al. 1999; Kitajima et al. 2004). In the current study, our observation of the intensive AhR staining and relatively high expression of AhR protein in human placentas is consistent with previous reports demonstrating the high density of dioxin binding sites (Manchester et al. 1987) and high AhR mRNA levels (Dolwick et al.1993a) in human placentas. These data are also in agreement with findings in mouse placentas at days 9 and 10 of gestation (Kitajima et al. 2004). Our data, however, are not in agreement with a report on rabbit placentas from early pregnancy in which AhR protein was undetectable in syncytiotrophoblasts, although a low level of AhR mRNA was identified (Tscheudschilsuren et al. 1999). It is unclear what causes these different expression patterns in AhR protein in placentas; however, these discrepancies might be due to different species and/or different stages of pregnancy.
Expression of AhR mRNA has been identified in many human tissues (Okey 2007). However, the tissue and cellular distribution of AhR protein in the human fetus is unknown. Our findings of intensive AhR staining in lung, kidney, and liver in the human fetus imply relatively high AhR expression in these fetal tissues. In adult humans, AhR mRNA is highly expressed in lung, pancreas, liver, and heart, with lower levels of expression in brain and kidney (Dolwick et al. 1993b). Similarly, in the current study, we also found that in human fetuses, the AhR protein level was relatively high in lung, pancreas, and liver, and was almost undetectable in brain. In contrast, a relatively high AhR level in fetal kidney and a nearly undetectable AhR level in fetal heart were observed in the current study. Although these discrepancies could be due to different expression between AhR mRNA and protein, an alternative explanation is that there exists a developmental expression in AhR in each individual human tissue. This is supported by the observation made by Abbott et al. (1995), who reported that in the mouse embryo, AhR protein and mRNA were expressed predominantly in heart and brain at gestational days 10 and 11 and were later dramatically decreased.
An important question arises regarding the physiological functions of AhR in the placenta and fetus. It is known that AhR-null female mice have difficulty in maintaining pregnancy, as well as decreased litter size, presumably partially owing to inability to support embryo/fetal development in uteri (Abbott et al. 1999). Moreover, knockout of AhR in mice also causes impaired vascular development and decreased growth of neonatal organs such as liver (Fernandez-Salguero et al. 1997; Abbott et al. 1999; Benedict et al. 2000; Baba et al. 2005; Lahvis et al. 2005). Conversely, constitutively active AhR in mice leads to a higher frequency of tumors, enlarged liver and kidney, and increased thymic atrophy (Andersson et al. 2002; Brunnberg et al. 2006). All of these studies indicate important roles for AhR in the regulation of cell growth and differentiation. Thus, together with the finding of the presence of many AhR ligands [e.g., indigo, indirubin, and 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE)] in animal and human tissues (presumably endogenously produced) (Okey 2007; Nguyen and Bradfield 2008), we propose that upon binding to endogenous AhR ligands yet to be identified in the human placenta and/or fetus, the AhR can mediate growth and differentiation of placental trophoblasts and fetal epithelial cells. In addition, given that the AhR was detected in endothelial cells of large blood vessels within the placental villi and umbilical cord, it is also possible that the AhR may also participate in regulation of placental vascular functions such as vascular remodeling, vasoconstriction, and vasodilatation.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL-64703 and HD-38843 (to JZ), and by a Department of Ob/Gyn Research and Development grant, University of Wisconsin-Madison (to JZ).
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Abbott BD, Birnbaum LS, Perdew GH (1995) Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn 204:133–143 [DOI] [PubMed] [Google Scholar]
- Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JJ (1999) Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol 155:62–70 [DOI] [PubMed] [Google Scholar]
- Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, et al. (2002) A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci USA 99:9990–9995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y (2005) Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol 25:10040–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AM, Klein RL, Moss KL, Haeri S, Boggess K (2009) Maternal serum dyslipidemia occurs early in pregnancy in women with mild but not severe preeclampsia. Am J Obstet Gynecol 201:293.e1–4 [DOI] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA (2000) Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci 56:382–388 [DOI] [PubMed] [Google Scholar]
- Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A (2006) The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure: effects in vital organs. Toxicology 224:191–201 [DOI] [PubMed] [Google Scholar]
- Chung JY, Song Y, Wang Y, Magness RR, Zheng J (2004) Differential expression of VEGF, EG-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab 89:2484–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolwick KM, Schmidt JV, Carver LA, Swanson HI, Bradfield CA (1993a) Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol 44:911–917 [PubMed] [Google Scholar]
- Dolwick KM, Swanson HI, Bradfield CA (1993b) In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci USA 90:8566–8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ (1997) Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 34:605–614 [DOI] [PubMed] [Google Scholar]
- Gregg AR (2004) Hypertension in pregnancy. Obstet Gynecol Clin North Am 31:223–241 [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Karman BN, Flaws JA (2009) The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol 77:547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan SH, Lee JL, Ho PY, Lee YH, Lee WS (2006) Antiproliferative and antiangiogenic effects of 3-methylcholanthrene, an aryl-hydrocarbon receptor agonist, in human umbilical vascular endothelial cells. Eur J Pharmacol 530:1–8 [DOI] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Fujishita A, Masuzaki H, Koji T, Ishimaru T (2004) Expression of the arylhydrocarbon receptor in the peri-implantation period of the mouse uterus and the impact of dioxin on mouse implantation. Arch Histol Cytol 67:465–474 [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW, Glover E, Pitot HC, McElwee MK, Bradfield CA (2005) The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol Pharmacol 67:714–720 [DOI] [PubMed] [Google Scholar]
- Manchester DK, Gordon SK, Golas CL, Roberts EA, Okey AB (1987) Ah receptor in human placenta: stabilization by molybdate and characterization of binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 3-methylcholanthrene, and benzo[a]pyrene. Cancer Res 47:4861–4868 [PubMed] [Google Scholar]
- National High Blood Pressure Education Program (2000) Working group report on high blood pressure in pregnancy. NHLBI (NIH) Publication No. 00-3029
- Nguyen LP, Bradfield CA (2008) The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21:102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okey AB (2007) An aryl hydrocarbon receptor odyssey to the shores of toxicology: the Deichmann Lecture, International Congress of Toxicology-XI. Toxicol Sci 98:5–38 [DOI] [PubMed] [Google Scholar]
- Page NM (2002) The endocrinology of pre-eclampsia. Clin Endocrinol (Oxf) 57:413–423 [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL (1993) Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol 23:283–335 [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL (2009) The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol 77:713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, McDougal A (2002) Mechanism of action and development of selective aryl hydrocarbon receptor modulators for treatment of hormone-dependent cancers. Int J Oncol 20:1123–1128 [PubMed] [Google Scholar]
- Shmueli O, Horn-Saban S, Chalifa-Caspi V, Shmoish M, Ophir R, Benjamin-Rodrig H, Safran M, et al. (2003) GeneNote: whole genome expression profiles in normal human tissues. C R Biol 326:1067–1072 [DOI] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, Bradfield CA (2009) The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscheudschilsuren G, Hombach-Klonisch S, Küchenhoff A, Fischer B, Klonisch T (1999) Expression of the arylhydrocarbon receptor and the arylhydrocarbon receptor nuclear translocator during early gestation in the rabbit uterus. Toxicol Appl Pharmacol 160:231–237 [DOI] [PubMed] [Google Scholar]