Abstract
In ischemic acute kidney injury, renal blood flow is decreased. We have previously shown that reperfused, transplanted kidneys exhibited ischemic injury to vascular endothelium and that preservation of peritubular capillary endothelial integrity may be critical to recovery from ischemic injury. We hypothesized that bone marrow–derived (BMD) endothelial progenitor cells (EPCs) might play an important role in renal functional recovery after ischemia. We tested this hypothesis in recipients of cadaveric renal allografts before and for 2 weeks after transplantation. We found that the numbers of circulating CD34-positive EPCs and CD146-positive endothelial cells (ECs) decreased immediately after ischemia–reperfusion. In renal allograft tissues obtained 1 hr after reperfusion, CD34-positive cells were more frequently observed along the endothelial lining of peritubular capillaries compared with non-ischemic controls. Moreover, 0–17.5% of peritubular capillary ECs were of recipient origin. In contrast, only 0.1–0.7% of tubule cells were of recipient origin. Repeat graft biopsy samples obtained 35 and 73 days after transplant did not contain capillary ECs of recipient origin, whereas 1.4% and 12.1% of tubule cells, respectively, were of recipient origin. These findings suggest that BMD EPCs and ECs may contribute to endothelial repair immediately after ischemia–reperfusion. (J Histochem Cytochem 58:687–694, 2010)
Keywords: ischemic acute renal failure, endothelial cells, vasculature, vascular pathophysiology
Acute renal failure or acute kidney injury (AKI) is a common disorder associated with high morbidity and high mortality (Liano and Pascual 1998; Clermont et al. 2002; Uchino et al. 2005; Xue et al. 2006; Ali et al. 2007; Himmelfarb and Ikizler 2007). In most patients, AKI is caused by an ischemic insult, but the pathogenesis and the pathophysiology leading to sustained AKI, or recovery, are poorly understood. Renal blood flow is markedly decreased following the initial ischemic insult (Arendshorst et al. 1976; Conger et al. 1991; Alejandro et al. 1995; Conger and Weil 1995; Vinot et al. 1995; Conger 1997; Corrigan et al. 1999), but the mechanisms underlying decreased renal blood flow have not been completely elucidated in humans. We have shown that damage to the renal vasculature in humans occurs after an ischemic insult and that maintaining or regaining peritubular capillary endothelial integrity may be critical to recovery from postischemic AKI (Kwon et al. 2008). Putative angioblasts have been isolated from peripheral blood and have been shown to form capillary networks in culture (Asahara et al. 1997), and bone marrow–derived (BMD) cells have been shown to contribute to endothelial repair in an experimental model of glomerulonephritis (Rookmaaker et al. 2003). Moreover, BMD endothelial cells (ECs) have been identified in peritubular capillaries and have been shown to be significantly increased in postischemic mouse kidneys compared with controls (Duffield et al. 2005). We hypothesized that BMD circulating endothelial progenitor cells (EPCs) might play an important role in renal functional recovery after ischemia–reperfusion by participating in endothelial repair of the kidney. We tested this hypothesis in recipients of freshly transplanted kidneys from deceased donors, because this has been regarded as an optimal model for clarifying the pathophysiology of ischemic AKI in humans (Alejandro et al. 1995; Vinot et al. 1995; Kwon et al. 1998,1999,2008,2009; Corrigan et al. 1999).
Materials and Methods
Subjects
The study group consisted of 16 consecutive consenting cadaveric renal allograft recipients. Written informed consent was obtained from each recipient, and the study protocol was approved by the Pennsylvania State College of Medicine Institutional Review Board (IRB protocol no. 21215). All allograft recipients were treated with intraoperative alemtuzumab and 1 g of methylprednisolone, and maintenance immunosuppression with mycophenolate mofetil and tacrolimus. Immunohistochemical experiments have shown that alemtuzumab does not interact with or bind to ECs. All subjects were followed for at least 3 weeks after transplantation to monitor renal graft function and to detect confounding variables. No subject had an episode of acute rejection during the period.
Protocol
Immediately before and 2 hr, 3 days, and 14 days after transplant, a 5-ml aliquot of blood was collected from each subject to quantify circulating CD34-positive EPCs and CD146-positive ECs. Eight healthy donors of living donor transplants with creatinine clearance >80 ml/min in 24-hr urine and normal urinalysis served as the control group. Eight recipients (Subjects 1, 2, 5, 6, 10, 11, 12, and 16) also consented to an intraoperative needle biopsy of the allograft ∼1 hr after completion of vascular anastomosis and restoration of reperfusion of the transplanted kidney. Renal allograft tissues were analyzed for CD34-positive cells by immunohistochemistry, and for cells of recipient bone marrow origin by fluorescence in situ hybridization (FISH). Graft function was monitored daily by measuring serum creatinine concentration. Five recipients of cadaveric renal allografts showed depressed renal graft function throughout the first 2 post-transplantation weeks, as judged by serum creatinine levels >1.5 mg/dl on postoperative day 14, and were designated the “sustained-AKI” group. One of those patients (Subject 8) required dialysis treatment after transplant. The other 11 recipients showed prompt recovery of graft function, as judged by serum creatinine ≤1.5 mg/dl on postoperative day 14, and were designated the “recovery” group. Subjects 1 and 2 had repeat graft biopsies on postoperative days 73 and 35, respectively; these samples showed no evidence of acute rejection. For non-ischemic control tissue, we used renal biopsy specimens from two patients undergoing laparoscopic nephrectomy for renal cell cancer; these patients had normal kidney function (serum creatinine 1.0 and 1.2 mg/dl, respectively) and no systemic or nephrologic disease affecting the kidney. Control kidney tissues were obtained from a grossly normal part of the kidney prior to clamping of the renal artery during nephrectomy.
Quantitation of Circulating CD34-positive EPCs and CD146-positive ECs by Fluorescence-activated Cell Sorting
Sequential gating was performed to quantify CD34-positive and CD146-positive cells using the ISHAGE (International Society of Hematotherapy and Graft Engineering) protocol and FITC-conjugated antibody to CD34, phycoerythrin-conjugated antibody to CD146 (P1H12), and PerCP-Cy5.5–conjugated antibody to CD45, all from BD Biosciences (San Jose, CA).
Identifying CD34-positive Cells in Renal Allograft Tissues by Immunohistochemistry
Mouse monoclonal anti-human CD34 Class II antibody (clone QBEnd-10; DakoCytomation, Oxford, UK), diluted 1:200, was used to assess CD34-positive cells in renal allograft tissues. Texas Red–conjugated phalloidin, which stains filamentous actin, was purchased from Molecular Probes (Eugene, OR) and diluted 1:200 to identify vasculature in the kidney. Monoclonal mouse anti-human von Willebrand Factor (vWF) antibody was purchased from DAKO A/S (Glostrup, Denmark) and diluted 1:50 to examine the integrity of ECs. Our protocols for tissue preparation and immunofluorescence staining have been described previously (Kwon et al. 2008,2009). Image analysis for immunohistochemistry was performed by three-dimensional reconstruction of serial optical sections of kidney tissue using laser confocal fluorescence microscopy and volume-rendering software (Kwon et al. 2008,2009).
Identifying Cells of Recipient Bone Marrow Origin by FISH
To identify cells from recipient bone marrow in the kidney, FISH was performed for X and Y chromosomes in four male subjects who had received kidney grafts from female donors (F-to-M subjects). The DNA probe CEP Y (DYZ1) satellite III was purchased from Abbott Molecular, Inc. (Des Plaines, IL). The probe for human X α satellite, pBamX-7, was directly labeled by fluorophore Alexa-594 using the Invitrogen ARES labeling kit (Carlsbad, CA). Nuclei were stained with 4′,6-diamidino-2-phenylindole. Cells showing a Y chromosome were considered to be of recipient bone marrow origin. Two males who received kidney grafts from male donors (M-to-M) and one female who received a kidney graft from a female donor (F-to-F) were used as positive and negative controls for the Y chromosome, respectively. ECs were identified by immunohistochemistry using mouse monoclonal anti-human CD31 (PECAM-1; Fitzgerald Industries International, Inc., Concord, MA), diluted 1:100, prior to FISH.
Statistical Analysis
The characteristics of allograft recipients and donors were expressed as means ± 1 standard error. Differences in patient characteristics and in numbers of cells in the recovery and sustained-AKI groups at each time point were assessed using Student's unpaired t-test. Differences in the numbers of cells at different time points in each functional group and in the controls were assessed by one-way ANOVA with posthoc multiple comparisons. The quartile distributions of circulating CD34-positive EPCs and CD146-positive ECs at each time point in recovery and sustained-AKI groups were illustrated using box plots. All statistical analysis was performed using SPSS 11.5 software (SPSS, Inc.; Chicago, IL).
Results
Clinical Features
Clinical characteristics of the renal allografts and patient population are shown in Table 1. The study group consisted of 16 consecutive consenting recipients of cadaveric renal allografts, ranging in age from 24 years to 76 years. The donors ranged in age from 2 years to 59 years. Total ischemic times were between 7 hr 16 min and 24 hr 11 min. Five allograft recipients (Subjects 4, 5, 8, 10, and 16) had sustained AKI, defined as serum creatinine >1.5 mg/dl on postoperative day 14. The remaining 11 recipients exhibited rapid recovery of graft function. The gender distribution (female/male, 2/9 vs 2/3) and mean age (49.5 ± 4.4 years vs 54.4 ± 2.6 years) of patients in the recovery and sustained-AKI groups, respectively, were similar, as were the mean total ischemic times (744.4 ± 95.8 min vs 848.2 ± 109.3 min; p=0.52832) and mean donor ages (34.3 ± 4.7 years vs 45.4 ± 5.2 years). Subjects 1 and 16, who had surgical complications (bleeding from vascular anastomosis site resulting in hypotension in Subject 1 and perigraft hematoma in Subject 16 on the day of transplantation), were analyzed until the time of the event.
Table 1.
Characteristics of patient population
| Subject | Age (years) | Sex | Ethnicity | Cause of ESRD | Transplant | Donor age (years) | Donor gender | Total ischemic time (hr:min) |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | W | ADPKD | CADRT | 55 | F | 16:26 |
| 2 | 42 | M | W | PKD on HD | CADRT | 2 | F | 13:31 |
| 3 | 24 | M | AA | s/p Graft failure | CADRT | 49 | M | 8:28 |
| 4 | 58 | M | AA | DM II | CADRT | 34 | M | 10:10 |
| 5 | 53 | F | W | HTN | CADRT | 48 | F | 16:39 |
| 6 | 46 | M | W | DM I | CADRT; SPK | 18 | M | 8:21 |
| 7 | 46 | M | W | DM I | CADRT; SPK | 23 | M | 9:47 |
| 8 | 61 | F | W | s/p Graft failure | CADRT | 59 | M | 18:53 |
| 9 | 37 | F | AA | GN | CADRT | 32 | F | |
| 10 | 56 | M | W | DM II | CADRT | 56 | M | 8:26 |
| 11 | 64 | M | Indian | DM II | CADRT | 49 | F | 16:33 |
| 12 | 76 | M | W | Unknown | CADRT | 39 | F | 24:11 |
| 13 | 44 | F | W | DM I | CADRT; SPK | 37 | F | 10:50 |
| 14 | 40 | M | W | DM I | CADRT; SPK | 23 | F | 7:16 |
| 15 | 56 | M | W | DM I | CADRT; SPK | 50 | M | 8:41 |
| 16 | 44 | M | AA | HTN | CADRT | 30 | F | 16:33 |
ADPKD, autosomal dominant polycystic kidney disease; CADRT, cadaveric renal transplant; DM, diabetes mellitus; ESRD, end stage renal disease; HD, hemodialysis; HTN, hypertension; s/p, status post; SPK, simultaneous cadaveric pancreas and kidney transplant; W, white; AA, African American. Bold numbers indicate subjects who had intraoperative graft biopsy. Intraoperative biopsy tissue from Subject 2 could not be used, owing to inadequate sample size. Subjects 1 and 2 had a repeat graft biopsy on postoperative day 73 and 35, respectively.
Circulating CD34-positive EPCs and CD146-positive ECs
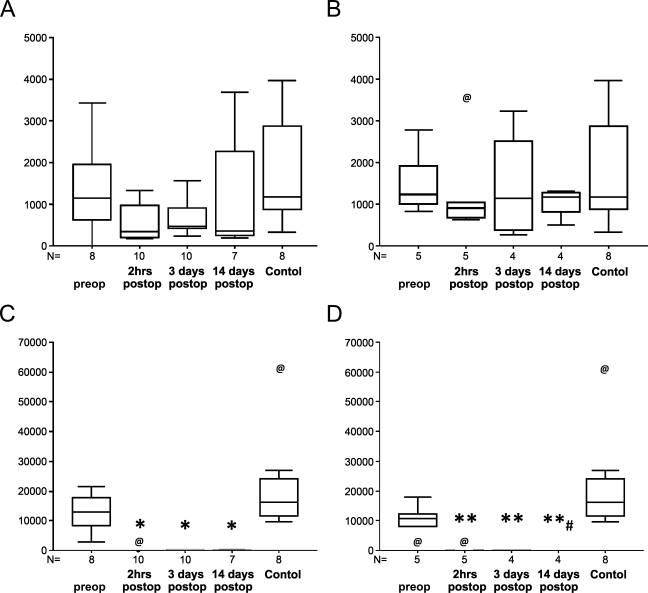
Before transplantation, the median numbers of CD34-positive EPCs in 5 ml of processed peripheral blood were similar in patients destined to have renal functional recovery (1140) and sustained AKI (1238), and in controls (1179) [not significant (NS); Figure 1 and Table 2]. All recipients in the recovery and sustained-AKI groups showed decreases in CD34-positive EPCs immediately after surgery, to a median of 347 and 904 cells/5 ml, respectively. On postoperative days 3 and 14, the median numbers of CD34-positive EPCs were 476 and 353 cells/5 ml, respectively, in the recovery group, and 1142 and 1179 cells/5 ml, respectively, in the sustained-AKI group. The median numbers of circulating CD146-positive ECs before transplantation were 12,945 and 10,770 cells/5 ml in the recovery and AKI groups vs 16,140 cells/5 ml in controls (NS). These cells almost completely disappeared, however, from the peripheral circulation immediately after transplant surgery and remained almost absent for the next 2 weeks (p<0.05 vs. healthy controls). In patients who recovered graft function, the difference between the preoperative value and the values on all postoperative days attained statistical significance (p<0.05). However, on postoperative day 14, the number of circulating CD146-positive ECs in the recovery group was significantly higher than in the sustained-AKI group (p<0.05).
Figure 1.
Box plots of circulating CD34-positive endothelial progenitor cells (A,B) and CD146-positive endothelial cells (ECs) (C,D) (per 5 ml of processed peripheral blood) in cadaveric renal allograft recipients showing prompt recovery of graft function (A,C), in those who sustained acute kidney injury (B,D) immediately before (preop) and 2 hr, 3 days, and 14 days after transplant (postop), and in healthy controls. N indicates the number of samples analyzed in each group. @ indicates an outlier; *p<0.05 vs control and preop; **p<0.05 vs control; #p<0.05 vs recovery on 14 days postop.
Table 2.
Circulating CD34-positive EPCs and CD146-positive ECs in recipients of cadaveric renal allografts (per 5 ml of processed peripheral blood)
| Recovery |
Sustained AKI |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Postoperative day |
Postoperative day |
||||||||
| Preop | 0 | 3 | 14 | Preop | 0 | 3 | 14 | Control | |
| n | 8 | 10 | 10 | 7 | 5 | 5 | 4 | 4 | 8 |
| CD34+EPC | |||||||||
| Mean | 1,358 | 545 | 684 | 1,323 | 1,553 | 1,349 | 1,446 | 1,042 | 1,770 |
| SEM | 358 | 128 | 136 | 506 | 321 | 486 | 591 | 161 | 432 |
| Median | 1,140 | 347 | 476 | 353 | 1,238 | 904 | 1,142 | 1,179 | 1,179 |
| CD146+EC | |||||||||
| Mean | 12,799 | 173* | 146* | 141* | 10,026 | 176** | 83** | 15**,# | 21,656 |
| SEM | 2,092 | 143 | 56 | 44 | 2,454 | 109 | 42 | 9 | 5,492 |
| Median | 12,945 | 19 | 56 | 165 | 10,770 | 45 | 75 | 8 | 16,140 |
*p<0.05 vs control and preop; **p<0.05 vs control; #p<0.05 vs recovery on 14 days postop; EPC, endothelial progenitor cell; EC, endothelial cell; AKI, acute kidney injury.
CD34-positive Cells in Renal Allograft Tissues
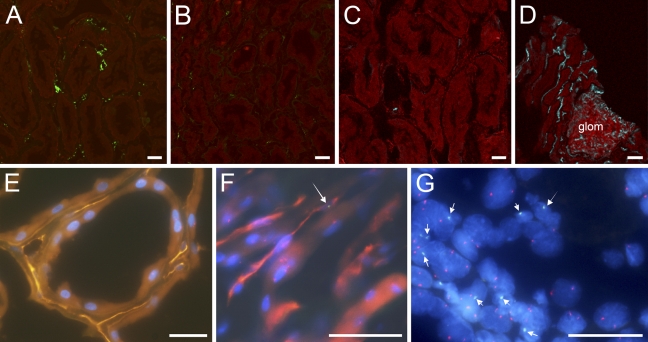
Graft biopsy tissues showed variable degrees of damage to the peritubular capillary endothelium, as assessed by diminished vWF staining (Figure 2B). Few CD34-positive cells were detected in non-ischemic control tissues (Figure 2C). In renal allograft tissues (Figure 2D), however, these cells were observed more frequently along the endothelial lining of peritubular capillaries than in controls.
Figure 2.
Fluorescence microscopy of human renal tissues showing ECs staining for von Willebrand Factor (vWF) in a non-ischemic control (A) and in a cadaveric renal allograft after ischemia–reperfusion (B), and CD34-positive ECs in a non-ischemic control (C) and in a cadaveric renal allograft after ischemia–reperfusion (D). Staining for vWF-positive and CD34-positive ECs is shown in green and blue, respectively, and staining for actin is shown in red. After ischemia–reperfusion, compared with respective controls (A,C), vWF-positive ECs decreased (B), whereas CD34-positive ECs increased (D). Images were obtained by three-dimensional reconstruction of serial images of kidney tissue sections. Immunohistochemistry was followed by fluorescence in situ hybridization to detect X and Y chromosomes in intraoperative renal graft biopsy tissues from two male subjects who had received kidney grafts from female donors: Subjects 11 (E) and 1 (F), and in a repeat graft biopsy sample from Subject 1 on postoperative day 73 (G). Arrows indicate Y chromosomes in green in an endothelial cell (F) and tubule cells (G), demonstrating cells of recipient bone marrow origin. Peritubular capillary ECs are identified by PECAM-1 in orange (E) or red (F). Red dots in nuclei indicate the X chromosome; glom denotes glomerulus. Bar = 20 μm.
Cells of Recipient Bone Marrow Origin in Renal Allograft Tissues
We identified cells of recipient bone marrow origin in renal allograft tissues after ischemia–reperfusion (Figures 2E–2G). Y chromosome–containing cells were deemed to be of recipient bone marrow origin in four male subjects who received kidney grafts from female donors. Two males who received kidney grafts from male donors and one female who received a kidney graft from a female donor were used as positive and negative controls, respectively, for the Y chromosome (Table 3). We found that in intraoperative renal allograft tissues obtained after reperfusion, 0–17.5% of peritubular capillary ECs and 0.1–0.7% of tubule cells were of recipient origin. In contrast, no peritubular capillary ECs of recipient origin were observed in graft biopsy samples obtained 35 days and 73 days after transplantation, whereas 1.4% and 12.1% of tubule cell nuclei, respectively, were of recipient origin (Table 3).
Table 3.
Cells of recipient bone marrow origin in cadaveric renal allografts
| Peritubular capillary ECs |
Tubule cells |
||||||
|---|---|---|---|---|---|---|---|
| X |
Y |
X |
Y |
||||
| Donor-to-recipient relation | Subject | Total nuclei (%) | Total nuclei (%) | Positive control (%) | Total nuclei (%) | Total nuclei (%) | Positive control (%) |
| Intraoperative biopsy after ischemia and reperfusion | |||||||
| F-to-M | |||||||
| 1 | 39.0 | 5.9 | 17.5 | 53.3 | 0 | 0.1 | |
| 12 | 43.1 | 1.9 | 5.7 | 47.2 | 0.4 | 0.7 | |
| 11 | 27.9 | 0 | 0 | 67.2 | 0.3 | 0.4 | |
| 16 | 63.0 | 2.8 | 8.3 | 91.7 | 0.1 | 0.2 | |
| 2.4 | 7.0 | 0.2 | 0.3 | ||||
| M-to-M; positive control for Y | |||||||
| 6 | 4.3 | 45.8 | 14.2 | 63.2 | |||
| 10 | 0 | 21.1 | 2.2 | 52.8 | |||
| 33.4 | 58.0 | ||||||
| F-to-F; negative control for Y | |||||||
| 5 | 0 | 0 | 22.8 | 0 | 0 | ||
| Postoperative repeat biopsy | |||||||
| F-to-M | |||||||
| POD 35 | 2 | 75.0 | 0 | 0 | 90.4 | 1.4 | 2.4 |
| POD 73 | 1 | 100.0 | 0 | 0 | 92.8 | 12.1 | 20.8 |
| 0 | 0 | 6.7 | 11.6 | ||||
Bold numbers indicate the median values in each group. POD, postoperative day; F-to-M, male subject who received a kidney graft from a female donor; M-to-M, male subject who received a kidney graft from a male donor; F-to-F, female subject who received a kidney graft from a female donor.
Discussion
Following the initial ischemic insult, renal blood flow was decreased by ∼30–70% in ischemic AKI (Arendshorst et al. 1976; Conger et al. 1991; Alejandro et al. 1995; Conger and Weil 1995; Vinot et al. 1995; Conger 1997; Corrigan et al. 1999). After accounting for transtubular backleak of the glomerular filtrate, we previously observed a 51% reduction in transglomerular filtration in cadaveric renal allograft recipients with sustained AKI compared with recipients showing prompt recovery (Kwon et al. 1998). Glomerular transcapillary hydraulic pressure difference (ΔP) depression has been reported to be the predominant cause of hypofiltration in this form of postischemic injury, and afferent vasoconstriction has been shown to be the proximate cause of ΔP depression (Alejandro et al. 1995). Employing the same model, we recently reported that maintaining the integrity of the peritubular capillary endothelium may be essential to recovery of renal function following postischemic AKI (Kwon et al. 2008). More recently, we also found that vascular endothelial damage incurred after ischemia–reperfusion can cause impaired vasodilatory ability because of decreased endothelial NO generation and may contribute to reduction in glomerular filtration (Kwon et al. 2009).
The endothelium is the largest organ in the body. The endothelium has been found to play roles in vasodilation as well as in controlling thrombotic tendencies and in initiating and maintaining inflammation (Segal et al. 2002). We have investigated the mechanism underlying the repair of damaged endothelium in individuals with ischemic AKI. In kidney tubules, tissue regeneration may result from proliferation of surviving dedifferentiated cells, from renal stem cells that reside inside the kidney and migrate to the site of regeneration, or from BMD cells that gain access to the injured structure and differentiate into mature cells (Poulsom et al. 2001; Gupta et al. 2002; Oliver et al. 2004; Krause and Cantley 2005). Results of animal model experiments on the contributory roles of BMD stem cells in the restoration of tubular epithelial cells during repair of the postischemic kidney (Duffield et al. 2005; Lin et al. 2005; Tögel et al. 2005,2007) have suggested that the repair of damaged tubular epithelial cells occurs independently of BMD stem cells, but that vasculotropic, paracrine actions of mesenchymal stem cells are important in recovery (Tögel et al. 2007). However, of the kidney cells in mice 28 days after ischemia–reperfusion injury, 8% are BMD cells and 81% of these are interstitial cells, which may include myofibroblasts and ECs (Lin et al. 2005). Implantation of BMD mononuclear cells into ischemic myocardium was shown to enhance angiogenesis and to improve cardiac function (Kimihata et al. 2001). Moreover, BMD cells have been reported to contribute to glomerular endothelial repair following thrombotic microangiopathy (Rookmaaker et al. 2002). Endothelial cell chimerism has been found in transplanted kidneys 6 months after transplantation and vascular rejection (Laagaaij et al. 2001). In addition, ECs have been reported to contribute to recovery from ischemic AKI in an experimental rat model (Brodsky et al. 2002). Regnerative cell therapy with EPCs may potentially offer a modality of targeted treatment to re-establish perfusion and restore function of the injured kidney (Becherucci et al. 2009). Our hypothesis is that after ischemia–reperfusion, BMD circulating EPCs and ECs are mobilized to the injured endothelium and contribute to endothelial repair in the kidney, leading to renal functional recovery. The present study was designed to test the hypothesis by utilizing cadaveric renal allografts as a model. ECs of recipient bone marrow origin were identified in the allograft tissues obtained ∼1 hr after restoration of reperfusion, and circulatory EPCs and ECs were serially quantified while the renal graft function was monitored after transplant.
Freshly transplanted cadaveric renal allografts were analyzed because they are known to be an optimal model for postischemic AKI in humans (Alejandro et al. 1995; Vinot et al. 1995; Kwon et al. 1998,1999,2008,2009; Corrigan et al. 1999). As previously reported (Asahara et al. 1997; Solovey et al. 1997), CD34 and CD146 (P1H12) cell surface antigens were employed to quantify circulating EPCs and ECs in recipients of a cadaveric renal allograft, respectively. Our findings suggest that BMD circulating EPCs and ECs had migrated to the injured endothelium of the kidney, inasmuch as the recipients of cadaveric renal allografts did not manifest signs suggesting simultaneous endothelial injury in other organ systems. The finding of ECs of recipient bone marrow origin in renal allograft tissues also supports this concept. The numbers of circulating CD34-positive EPCs among patients showing recovery and sustained AKI, compared with controls, did not reach statistical significance, possibly because of the small number of subjects studied. Nonetheless, the median values of CD34-positive EPCs on each postoperative day lead to a speculation that CD34-positive EPCs disappear from the circulation more precipitously after ischemia–reperfusion in patients with recovery of the graft function, compared with those destined to have sustained AKI. Circulating CD146-positive ECs decreased significantly in cadaveric renal allograft recipients compared with controls for at least 14 days after transplantation. Patients showing recovery of graft function had significantly lower numbers of CD146-positive ECs compared with preoperative numbers. In the current study, graft function was analyzed by monitoring daily serum creatinine concentration. Renal blood flow or ΔP may be more directly related to transglomerular filtration. The contributory role of BMD EPCs and ECs to the endothelial repair dictating renal blood flow, ΔP, and transglomerular filtration remains to be further studied. Taken together, our findings suggest that BMD EPCs and ECs may contribute to endothelial repair immediately after ischemia–reperfusion and that tubular repopulation with BMD cells occurs later.
Acknowledgments
We gratefully thank Denise Shenigo for helping in recruitment of some subjects. We also thank Dr. Lewis Harpster for assisting us in obtaining non-ischemic control tissue samples.
This article is a JHC article of the month. This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication with the exception of the JHC articles of the month which are immediately released for public access.
This study was supported by the Department of Medicine, Pennsylvania State College of Medicine. This project was also funded, in part, under a grant from the Pennsylvania Department of Health using tobacco settlement funds.
References
- Alejandro V, Scandling JD Jr, Sibley RK, Dafoe D, Alfrey E, Deen W, Myers BD (1995) Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J Clin Invest 95:820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, MacLeod A (2007) Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 18:1292–1298 [DOI] [PubMed] [Google Scholar]
- Arendshorst WJ, Finn WF, Gottschalk CW (1976) Micropuncture study of acute renal failure following temporary renal ischemia in the rat. Kidney Int Suppl 6(suppl):100–105 [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967 [DOI] [PubMed] [Google Scholar]
- Becherucci F, Mazzinghi B, Ronconi E, Peired A, Lazzeri E, Sagrinati C, Romagnani P, et al. (2009) The role of endothelial progenitor cells in acute kidney injury. Blood Purif 27:261–270 [DOI] [PubMed] [Google Scholar]
- Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS (2002) Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol 282:F1140–1149 [DOI] [PubMed] [Google Scholar]
- Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP (2002) Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 62:986–996 [DOI] [PubMed] [Google Scholar]
- Conger J (1997) Hemodynamic factors in acute renal failure. Adv Ren Replace Ther 4(suppl 1):25–37 [PubMed] [Google Scholar]
- Conger JD, Robinette JB, Hammond WS (1991) Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int 39:1087–1097 [DOI] [PubMed] [Google Scholar]
- Conger JD, Weil JV (1995) Abnormal vascular function following ischemia-reperfusion injury. J Investig Med 43:431–442 [PubMed] [Google Scholar]
- Corrigan G, Ramaswamy D, Kwon O, Sommer FG, Alfrey EJ, Dafoe DC, Olshen RA, et al. (1999) PAH extraction and estimation of plasma flow in human postischemic acute renal failure. Am J Physiol 277:F312–318 [DOI] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV (2005) Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115:1743–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME (2002) A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int 62:1285–1290 [DOI] [PubMed] [Google Scholar]
- Himmelfarb J, Ikizler TA (2007) Acute kidney injury: changing lexicography, definitions, and epidemiology. Kidney Int 71:971–976 [DOI] [PubMed] [Google Scholar]
- Kimihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, et al. (2001) Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 104:1046–1052 [DOI] [PubMed] [Google Scholar]
- Krause D, Cantley LG (2005) Bone marrow plasticity revisited: protection or differentiation in the kidney tubule? J Clin Invest 115:1705–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O, Corrigan G, Myers BD, Sibley R, Scandling JD, Dafoe D, Alfrey E, et al. (1999) Sodium reabsorption and distribution of Na+-K+-ATPase during postischemic injury to the renal allograft. Kidney Int 55:963–975 [DOI] [PubMed] [Google Scholar]
- Kwon O, Hong S, Ramesh G (2009) Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia/reperfusion. Am J Physiol Renal Physiol 296:F25–33 [DOI] [PubMed] [Google Scholar]
- Kwon O, Hong S, Sutton TA, Temm CJ (2008) Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol 295:F351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Alfrey E, Dafoe D, et al. (1998) Backleak, tight junctions and cell-cell adhesion in postischemic injury to the renal allograft. J Clin Invest 101:2054–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laagaaij EL, Cramer-Knijnenburg GF, van Kemenade FJ, van Es LA, Bruijn JA, van Krieken JH (2001) Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet 357:33–37 [DOI] [PubMed] [Google Scholar]
- Liano F, Pascual J (1998) Outcomes in acute renal failure. Semin Nephrol 18:541–550 [PubMed] [Google Scholar]
- Lin F, Moran A, Igarashi P (2005) Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115:1756–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q (2004) The renal papilla is a niche for adult kidney stem cells. J Clin Invest 114:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, et al. (2001) Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195:229–235 [DOI] [PubMed] [Google Scholar]
- Rookmaaker MB, Smits AM, Tolboom H, Wout KV, Martens AC, Goldschmeding R, Joles JA, et al. (2003) Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am J Pathol 163:553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rookmaaker MB, Tolboom H, Goldschmeding R, Zwaginga JJ, Rabelink TJ, Verhaar MC (2002) Bone-marrow-derived cells contribute to endothelial repair after thrombotic microangiopathy. Blood 99:1095. [DOI] [PubMed] [Google Scholar]
- Segal MS, Bihorac A, Koc M (2002) Circulating endothelial cells: tea leaves for renal disease. Am J Physiol 283:F11–19 [DOI] [PubMed] [Google Scholar]
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP (1997) Circulating activated endothelial cells in sickle cell anemia. N Engl J Med 337:1584–1590 [DOI] [PubMed] [Google Scholar]
- Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C (2005) Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 289:F31–42 [DOI] [PubMed] [Google Scholar]
- Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C (2007) Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292:F1626–1635 [DOI] [PubMed] [Google Scholar]
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294:813–818 [DOI] [PubMed] [Google Scholar]
- Vinot O, Bialek J, Canaan-Kuhl S, Scandling JD Jr, Dafoe D, Alfrey E, Myers BD (1995) Endogenous ANP in postischemic acute renal allograft failure. Am J Physiol 269:F125–133 [DOI] [PubMed] [Google Scholar]
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, et al. (2006) Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17:1135–1142 [DOI] [PubMed] [Google Scholar]