Abstract
Cerebral ischemia–reperfusion (I/R) insults result in neuronal cell death, brain tissue loss, and severe neurological deficits. However, the underlying mechanism is still not fully understood. Heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 belongs to a family of RNA-binding proteins that plays a central role in pre-mRNA processing. Recent studies have revealed that hnRNP A2/B1 may be involved in the progress of I/R; therefore, the present study aimed to examine expression patterns of hnRNP A2/B1 to better understand posttranscriptional regulations in cerebral I/R insults. Focal cerebral I/R models were induced by right middle cerebral artery occlusion (MCAO) for 120 min followed by 3, 6, 12, 24, 48, and 72 hr of reperfusion in male Sprague-Dawley rats. We employed immunohistochemistry to examine expression of hnRNP A2/B1 in rat cerebral cortex (including cingulate cortex, striate cortex, temporal cortex, and piriform cortex) and hippocampus after I/R insults. Results showed that expression of hnRNP A2/B1 was significantly downregulated in cerebral cortex and hippocampus from 3 to 24 hr of reperfusion after MCAO for 120 min, but significantly upregulated at 48 hr of reperfusion. Unexpectedly, translocation of hnRNP A2/B1 from nucleus to cytoplasm and even to neurites was observed in cerebral cortex at 3 hr of reperfusion, reaching a peak at 24 hr of reperfusion, but not in hippocampus, indicating different posttranscriptional regulation patterns in different brain regions. Interestingly, translocation of hnRNP A2/B1 was only observed in cerebral cortex with MCAO but not in the opposite side, suggesting an I/R–specific expression pattern in the brain. Our data suggest that hnRNP A2/B1 participates in posttranscriptional regulation of neurons in cerebral cortex and hippocampus that suffered I/R insults, although posttranscriptional regulation is more extensive in neuronal cells of cerebral cortex than in hippocampus. (J Histochem Cytochem 58:695–705, 2010)
Keywords: ischemia–reperfusion insults, middle cerebral artery occlusion, heterogeneous nuclear ribonucleoprotein A2/B1, post-transcriptional regulation, nuclear-cytoplasmic-neuritic translocation
Stroke is the third most common cause of death after heart attack and cancer, and has profound negative social and economic effects (Pignataro et al. 2009). The brain is the organ most vulnerable to ischemic insults because of its high metabolic rate, relatively low oxygen stores, and insufficient reserves of high-energy carbohydrates (Macdonald and Stoodley 1998). Ischemic insults usually result in severe functional losses in the central nervous system. Cellular responses to ischemia and hypoxia are complex, and are characterized by alteration in the expression of a number of stress-related genes and corresponding proteins that are necessary to maintain brain homeostasis. Reperfusion plays an important role in the pathophysiology of cerebral ischemia. The principal processes are energy failure, loss of cell ion homeostasis, acidosis, increased intracellular calcium, excitotoxicity, and free radical–mediated toxicity. Reperfusion of ischemic tissue produces an influx of inflammatory cells and oxygen that can cause increases in oxygen-derived free radicals (Macdonald and Stoodley 1998). Free radicals and inflammatory mediators may contribute to the breakdown of the blood–brain barrier. The ischemic arterioles are maximally dilated and may impair autoregulation, so that reperfusion causes cerebral blood flow in excess of normal reactive hyperemia. These factors may contribute to water and osmolytes in the blood pouring into the ischemic brain and aggravating brain edema. Neurons may die by necrosis or apoptosis, and neuroprotective approaches are still lacking. Although humans possess protective mechanisms, few are effective against neuronal damage.
Recently, the role of posttranscriptional regulation has drawn increasing attention. A growing body of evidence suggests that some pathological incidents in ischemia–reperfusion (I/R) are associated with gene expression and posttranscriptional regulation. A number of DNA microarrays have been used in recent years to study brain ischemia. It has been suggested that a change of expression in 74 genes with brain metabolic status serves as a molecular definition of the penumbra (Soriano et al. 2000). A recent study using DNA microarrays revealed transcriptional activation of NADPH oxidase in brain-derived neurotrophic factor–treated cortical cell cultures (Kim et al. 2002). A similar study by our group (Xu et al. 2009) also showed transcriptional and splicing regulation in human umbilical vein endothelial cells under hypoxic stress conditions. These findings strongly suggest that transcriptional and posttranscriptional regulation of neurons are helpful for a better understanding of the mechanism of cerebral I/R insults. However, details of posttranscriptional regulation in brain receiving I/R insults remain unclear.
Heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 is a pre-mRNA–binding protein belonging to the hnRNP family that participates in various biological events, such as transcriptional regulation, telomere-length maintenance, pre-mRNA splicing, nucleo-cytoplasmic transport of mRNA, and mRNA localization, translation, and stability (Dreyfuss et al. 2002). The human hnRNP A2 gene is located at chromosome 7p15 and contains 12 exons. Inclusion of all 12 exons generates the B1 isoform, whereas removal of exon 2 (36 nucleotides) produces the A2 isoform. Recent studies have demonstrated that hnRNP A2/B1 is located in nucleoplasm of neurons of cerebral cortices, hippocampus, olfactory regions, caudate-putamen, and supraoptic nucleus of the hypothalamus (Mizukami et al. 2000). Parallel studies on the two isoforms showed that hnRNP A2/B1 may be involved in posttranscriptional regulation of neurons of Alzheimer's disease cerebral cortex and hippocampus (Ishikawa et al. 2004; Mizukami et al. 2005). Further studies on differentially expressed hnRNP proteins in neuronal cells may partly reveal mechanisms of cerebral I/R insults. To better understand the potential roles of hnRNP A2/B1 protein in neuronal posttranscriptional regulation, we studied whether neuronal cells after I/R insults displayed differences in expression and localization of hnRNP A2/B1 protein. We utilized an immunohistochemical approach to study rat cerebral cortex (including cingulate cortex, striate cortex, temporal cortex, and piriform cortex) and hippocampus after I/R insults, using a panel of monoclonal antibodies against hnRNP A2/B1 protein.
Materials and Methods
Surgical Procedure
Right middle cerebral artery occlusion (MCAO), inducing cerebral focal ischemia, or sham surgery was performed in male Sprague-Dawley (SD) rats weighing 260 to 280 g. Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985) was followed. All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee. Animals were allowed free access to food and water and housed in a climate-controlled environment (25C). The animals were randomly divided into seven groups: a sham surgery group without ischemia (n=5), and six groups with 120-min ischemia followed by reperfusion for different times (3, 6, 12, 24, 48, or 72 hr; n=6 in each group). Rats were subjected to 120-min right MCAO using the intraluminal filament technique. Briefly, rats were anesthetized with chloral hydrate (350 mg/kg, IP). The right common carotid artery was exposed at the level of the external carotid artery (ECA) and internal carotid artery (ICA) bifurcation. A 4-0 monofilament nylon suture was inserted into the ECA from the common carotid artery bifurcation and pushed into the ICA for 17–20 mm until a slight resistance was felt, to block the origin of the middle cerebral artery. One hundred twenty min after MCAO, the suture was slowly and carefully withdrawn. The sham-operated rats were not subjected to MCAO, except for exposure of the ECA and the ICA. Afterward, the skin incision was sutured, and the animals were maintained in an incubator at 37C until they regained consciousness. Animals were then returned to their cages and closely monitored, with body temperature kept at 37 ± 0.5C. An observer blinded to the identity of the groups assessed the neurological deficits at 3, 6, 12, and 24 hr after reperfusion (before euthanization) using the forelimb akinesia test (also called the postural tail-hang test), whereas the spontaneous rotational test was used as a criterion for evaluating the ischemic insults. Animals not showing behavioral deficits at the above time points after reperfusion were excluded from the study.
Preparation of Histological Samples
At 3, 6, 12, 24, 48, or 72 hr after recirculation, animals were re-anesthetized. Thoraxes were exposed, and blood was removed by transcardial perfusion with normal saline, followed by cold 4% PBS-buffered paraformaldehyde. The whole brains were removed and postfixed in 4% PBS-buffered paraformaldehyde for 48 hr. A standard block, at a level 1.0–1.8 mm posterior to the bregma, was obtained and embedded in paraffin. A series of consecutive sections (5 μm in thickness) was prepared from the paraffin-embedded tissues for conventional histological examination and immunocytochemistry according to our previous reports (Shang et al. 2006; Xu et al. 2009).
Infract Area Measurement With 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining
At 3, 6, 12, or 24 hr after recirculation, rats were killed by decapitation under anesthesia. The rat brain was then cooled in ice-cold saline for 20 min and cut into 2-mm-thick coronal sections in a cutting block. Then the brain slices were stained with 2% TTC at 37C for 30 min in a dark room. The infarct tissue was unstained, whereas the normal tissue was stained red. The posterior surface of each section was imaged via digital camera (Nikon E4100; Tokyo, Japan), and the areas were calculated with the image analysis system Image-Pro Plus (version 5.1; Media Cybernetics, Bethesda, MD).
Immunocytochemistry
After dewaxing and rehydration, sections were washed three times with 0.01 M PBS. Endogenous peroxidase activity was inactivated by incubation in 0.3% H2O2 in methanol for 15 min. Then microwaves were used for 20 min for antigen retrieval. After washing in PBS, the sections were incubated in 10% normal goat serum for 30 min at room temperature to prevent nonspecific immunoreactions. The sections were then incubated with an anti-hnRNP A2/B1 monoclonal antibody (1:1000; Sigma-Aldrich, St. Louis, MO) containing 0.3% Triton X-100 at 4C overnight. Sections were then washed in PBS and incubated with biotinylated goat anti-mouse IgG secondary antibody (1:500; Zymed Laboratories, South San Francisco, CA) for 1 hr at room temperature. Finally, sections were incubated with avidin-biotin-peroxidase reagents for 1 hr, and the resulting peroxidase activity was revealed by incubating the slides with a 0.5 mg/ml of 3,3′-diaminobenzidine solution and 0.03% H2O2 for 3–5 min. The sections were then washed in distilled water, dehydrated in a graded series of alcohols, cleared in xylenes, and finally coverslipped. Immunoreactions of the brain regions (cerebral cortex, including cingulate cortex, striate cortex, temporal cortex, piriform cortex, and hippocampus) receiving MCAO were then observed under a microscope (Olympus; Tokyo, Japan). For negative control of immunocytochemistry, the anti-hnRNP A2/B1 antibody was replaced with PBS.
Morphometric Analysis
Images of the stained sections (five sections per animal) were captured with a microscope using a 20× objective connected to a three-color (red/green/blue) CCD camera and a PC computer. Two representative brain regions receiving MCAO but not the opposite side, cerebral cortex (including the cingulate cortex, striate cortex, temporal cortex, and piriform cortex) and hippocampus were analyzed. The number of immunoreactive (IR) neurons per visual field (2.5 × 1.8 mm2) was calculated with the Image-Pro Plus software, using stereological methods. In detail, each image was captured with 1280 × 1024 pixel resolution. First, the image was optimized by clicking the Enhance/Equalize/Best Fit tool in the Image-Pro Plus software. Next, the menu Measure/Calibration/Intensity was used for optical density calibration of the image (black level = 0, and incident level = 255). IR neurons were manually circumscribed using the Area of Interest (AOI) tool in the software. The command Select Colors in the Count/Size dialog box was then applied to manually set the color threshold representing the positive immunostaining signals. To define the mean optical density (MOD) and total per area (TPA) of the IR neurons, the Select Measurements in the Measure menu in the Count/Size dialog box was used. Finally, the data were displayed in the Ranges Statistics window. The parameter MOD × TPA was regarded as density of hnRNP A2/B1 expression level in rat brain, where TPA equals area of positive signals/AOI.
Although hnRNP A2/B1 is a typical nuclear protein, in our studies, the hnRNP A2/B1–immunopositive signals were observed not only in nuclei but also in cytoplasm and even in neurites. Accordingly, a neuron with extensive expression of hnRNP A2/B1 in cytoplasm and neurites was considered and counted as nuclear-cytoplasmic-neuritic translocation. The number of nuclear-cytoplasmic-neuritic translocated neurons per visual field (2.5 × 1.8 mm2) was determined and quantified using the Image-Pro Plus software.
Quantification of Nuclear-cytoplasmic-neuritic Translocated Cells
Cells were counted using a light microscope at a magnification of ×200 coupled to a CCD camera that sent the image to a PC computer and displayed it on the monitor. The image was in turn forwarded to the Image-Pro Plus software. Neurons with hnRNP A2/B1 translocation from nucleus to cytoplasm and even to neurite were scored as positive only if the cell body image was well-defined. Endothelial cells, astrocytes, and glial cells were manually filtered according to their shape, size, and laminar organization. The number of neurons in the section could then be obtained from the calculation of accumulated averages for appropriate statistical quantities using the Image-Pro Plus software. To ensure that IR cells would not be counted twice and that the area in each section would be consistent, every fifth section of the entire cortex (including cingulate cortex, striate cortex, temporal cortex, and piriform cortex) was considered in the quantification with careful manual examination.
Data Analysis
All data were expressed as mean ± SEM. Statistical analysis of morphometric quantification of hnRNP A2/B1-IR axons and expression of hnRNP A2/B1 was performed by means of the one-way ANOVA test. Student's t-test was used to compare two means of different groups for significance at p<0.05 and p<0.01.
Results
Normal Expression of hnRNP A2/B1 in the Rat Brain
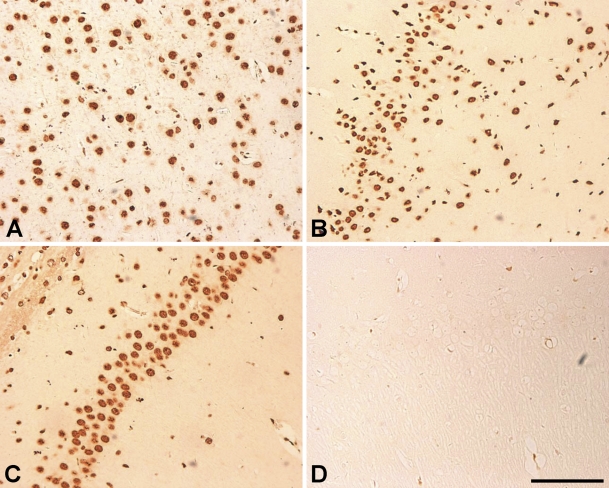
In sham-operated SD rat brain, hnRNP A2/B1-IR cells were abundantly present in cerebral cortex (including cingulate cortex, striate cortex, temporal cortex, and piriform cortex) and hippocampus, with higher density in the cerebral cortex (Figures 1A and 1B) than in the hippocampus (Figure 1C). In the cerebral cortex, the hnRNP A2/B1-IR cells were scattered evenly throughout layers II–VI. Strong staining signals were observed in the nucleoplasm of neurons, and weak immunoreactivity was observed in the cytoplasm and proximal dendrites of neurons. The negative control showed no typical IR signals (Figure 1D).
Figure 1.
Immunohistochemical localization of heterogeneous nuclear ribonucleoprotein (hnRNP) A2/B1 in cerebral cortex and hippocampus of the sham-operated group. There is a large amount of hnRNP A2/B1 expression in cerebral cortex (A,B) and a relatively high level restricted to the pyramidal layer of the hippocampus (C). The negative control shows no immunoreactive signals (D). Bar = 100 μm.
Morphometric Evaluation of Ischemic Neuronal Damage
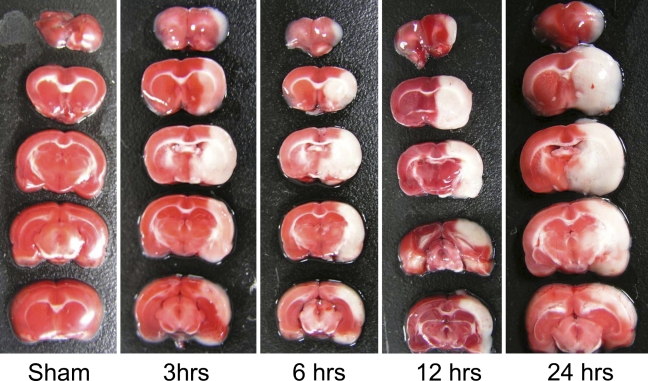
We used TTC staining to examine the morphological changes of rat brain receiving I/R treatment. Data showed that 120 min of ischemia followed by 3, 6, 12, and 24 hr of recirculation produced damages to the ipsilateral cortex, striatum, hypothalamus, amygdala, and hippocampus (Figure 2). The neuronal damage after MCAO occurs mainly in the hemisphere ipsilateral to the surgical region, not in the opposite regions. There was no significant difference in infarct volume among the groups that received 3-, 6-, 12-, and 24-hr recirculation after MCAO.
Figure 2.
Morphological changes in rat brain receiving ischemia–reperfusion (I/R) treatment stained with 2,3,5-triphenylterazolium chloride. One hundred twenty min of ischemia followed by 3, 6, 12, and 24 hr of recirculation produced damage to the ipsilateral cortex, striatum, hypothalamus, amygdala, and hippocampus.
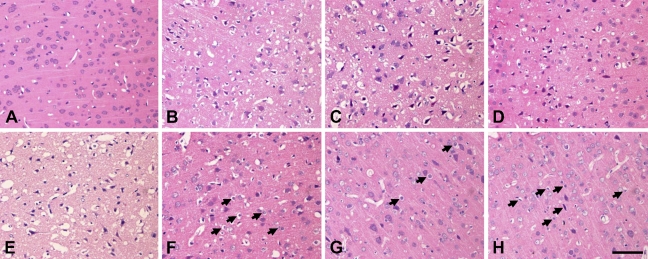
Distribution of neuron damage was evaluated by hematoxylin and eosin staining to reflect the degree of disorganization. Brains of sham-operated SD rats did not show any obvious ischemic cell changes in cerebral cortex (Figure 3A), whereas very slight cell edema occurred in the hippocampus (data not shown). In cerebral cortex, morphological changes of the neurons became evident in the temporal lobe after 3 hr of reperfusion (Figure 3B). At 6 hr and 12 hr after reperfusion, extended and severe morphological changes of neuronal cells were detected from the cingulate to the piriform cortex regions. Temporal and striate cortex showed extended ischemic cell necrosis, with only a few neurons with minor changes remaining (Figures 3C and 3D). At 24 hr after ischemia, delayed neuronal cell death extended to the neurons of the whole cortex, with few normal neurons remaining (Figure 3E). At 48–72 hr of reperfusion, major parts of the cortical layers from III to VI showed significant cell edema, but a few neurons showed irreversible cell necrosis (Figures 3F–3H, arrowheads). These cells are neurons, because they usually showed an irregular nucleus and the cell body was larger than that of the glial cells. In contrast to the cerebral cortex, neuronal damage was relatively minor in the hippocampus. Within the dorsal hippocampus, ischemic cell damage could be found in a major portion of subfields CA1 and CA2 and the border zone between CA3 and CA4. After 24 hr of reperfusion, neurons of the cerebral cortex were morphologically preserved with only slight edema, compared with the whole CA1–CA4 pyramidal stratum neurons (data not shown).
Figure 3.
Hematoxylin and eosin staining of the cerebral cortex at 3 to 72 hr of reperfusion after 120 min of right middle cerebral artery occlusion (MCAO), including the sham-operated group (A), and 3 hr (B), 6 hr (C), 12 hr (D), 24 hr (E), 48 hr (F), and 72 hr (G,H) of recirculation. The sham-operated group did not show any obvious ischemic cell changes (A); 3 hr of recirculation resulted in edema and necrosis (B); and 6 and 12 hr of recirculation showed extensive ischemic cell necrosis (C,D). Twenty-four hr of recirculation showed extensive damage and cell death, with few normal neurons remaining (E); 48 hr and 72 hr showed significant cell edema, and a few neurons showed irreversible cell necrosis (F–H, arrows). Bar = 100 μm.
Changes of hnRNP A2/B1 Protein Expression in Rat Brain
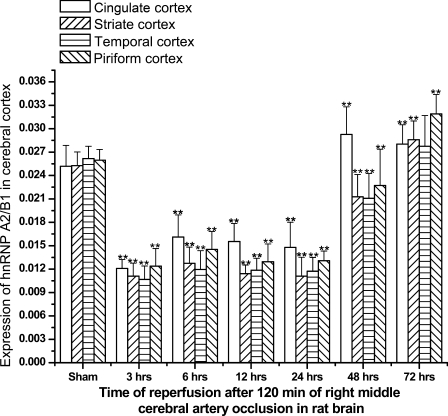
At 3, 6, 12, and 24 hr of reperfusion, the number of hnRNP A2/B1-IR neurons decreased notably and neurons were distributed diffusively throughout all layers of the cerebral cortex. However, the extent of decrease of hnRNP A2/B1-IR neurons was different at different reperfusion times in different regions of cerebral cortex (Figure 4). For example, at 24 hr of reperfusion, the immunoreactivity for hnRNP A2/B1 neurons decreased much less in the striate or temporal lobe cortex than in other subfields of the brain such as cingulate cortex or piriform cortex. At 48 hr after I/R, the number of hnRNP A2/B1-IR neurons and the immunoreactivity began to increase in cerebral cortex compared with the 24 hr postischemic group, even higher than that in sham control in cingulate cortex. At 72 hr after I/R, the number of hnRNP A2/B1-IR neurons and immunoreactivity in cerebral cortex dramatically increased much more than in the sham control (Figure 4). It could be seen that expression of hnRNP A2/B1-IR neurons began to diminish at 3 hr after reperfusion and continuously decreased between 6 hr and 24 hr after ischemia insults. Changes of hnRNP A2/B1-IR cells in the ischemic striate cortex and temporal lobe cortex were most dramatic, and decreased by 50–60% at 6–24 hr after recirculation, compared with sham-operated controls (p<0.01; Figure 4). The I/R resulted in more-progressive neuronal death of cortex cells, and the level of nucleoplasm immunoreactivity was significantly reduced. At 24 hr after I/R, most of the hnRNP A2/B1-IR cells were morphologically damaged, whereas the residual nerve cells showed intense IR signals to anti-hnRNP A2/B1 antibody.
Figure 4.
Diagram showing expression of hnRNP A2/B1 in cerebral cortex in different groups of Sprague-Dawley rats subjected to I/R brain insults. **p<0.01, compared with sham group.
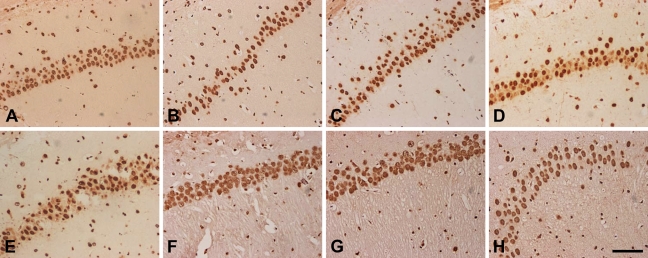
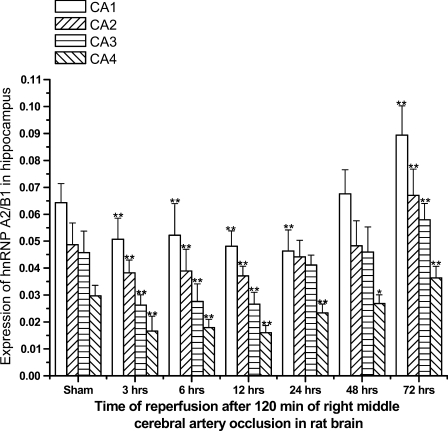
In hippocampus, hnRNP A2/B1-IR neurons were distributed mainly in the pyramidal cell layer. We observed an intense A2/B1 immunoreactivity in neuronal nucleoplasm from CA1 to CA4 subfields. In addition, nuclei of glial cells and ependymal cells were also immunolabeled with anti–hnRNP A2/B1 antibodies (data not shown), but much less intensely than neuronal nuclei. At 3, 6, 12, and 24 hr after I/R insults, immunoreactivity of hnRNP A2/B1 was intense from subfields CA1 to CA3, whereas neurons in CA4 were less intensely IR for hnRNP A2/B1. However, immunoreactivity of hnRNP A2/B1 was much more abundant than that in cerebral cortex. Compared to sham-operated animals, the number and immunoreactivity of hnRNP A2/B1-IR neurons decreased slightly at 3, 6, and 12 hr after reperfusion and increased dramatically between 24 and 72 hr after ischemia. Especially at 48 and 72 hr of reperfusion, we observed that the level of nucleoplasmic immunoreactivity was significantly upregulated, with much more intensity than that of the sham-operated group (Figures 5 and 6).
Figure 5.
Immunocytochemistry staining of hnRNP A2/B1 in subfield CA1 of the hippocampus at 3 hr to 72 hr of reperfusion after 120 min of right MCAO, including the sham-operated group (A), and 3 hr (B), 6 hr (C), 12 hr (D), 24 hr (E), 48 hr (F) and 72 hr (G,H) of recirculation. A large number of hnRNP A2/B1-immunoreactive (hnRNP A2/B1-IR) neurons were distributed mainly in the pyramidal cell layer (A). At 3, 6, 12, and 24 hr of recirculation, hnRNP A2/B1-IR expression was reduced after ischemia insults (B–E). At 48 and 72 hr of recirculation, hnRNP A2/B1-IR neurons were increased (F–H). Bar = 100 μm.
Figure 6.
Diagram showing expression of hnRNP A2/B1 in the hippocampus in brains of different groups of Sprague-Dawley rats subjected to I/R insults at 3, 6, 12, 24, 48, and 72 hr of recirculation. *p<0.05 and **p<0.01, compared with the sham group.
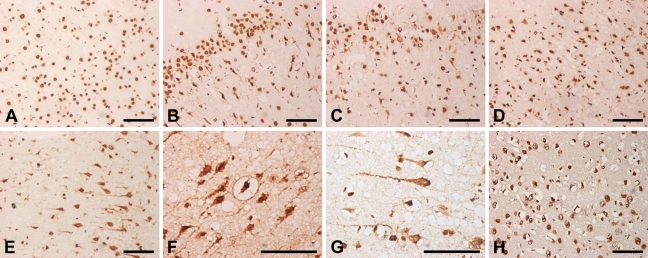
hnRNP A2/B1 Nuclear-cytoplasmic-neuritic Translocation in Cerebral Cortex of Rat Brain
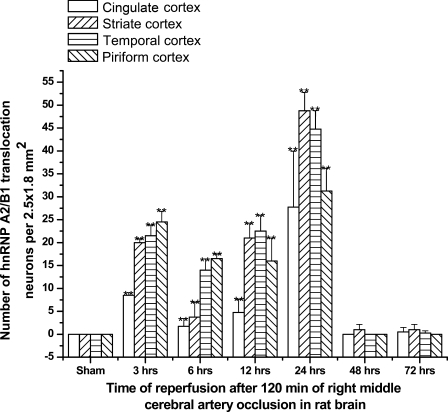
In the sham group, immunocytochemistry observations demonstrated nucleoplasmic localization of hnRNP A2/B1, whether in cerebral cortex or hippocampus (Figure 7A). After 3, 6, 12, or 24 hr of I/R in cerebral cortex, unexpectedly, we observed that hnRNP A2/B1 proteins were translocated from nucleus to cytoplasm, even to neuronal dendrite and growth cone of axons (Figures 7B–7D). At 3 hr of reperfusion, the number of hnRNP A2/B1 translocated neurons could be observed as sparsely distributed in cerebral cortex (Figure 7B), and continuously increased between 6 and 12 hr after ischemia (Figures 7C and 7D). At 24 hr after ischemia, the number of hnRNP A2/B1-IR translocated neurons reached the peak (Figures 7E–7G). At 48 hr after I/R, the number of hnRNP A2/B1-IR translocated neurons was reduced dramatically to the minimum level (Figure 7H). Interestingly, neuronal damage was relatively severe in striate cortex and temporal lobe, and the number of hnRNP A2/B1-IR translocated neurons was relatively more than that in the other cortex, as summarized in Figure 8. However, this observation was not made in hippocampus (data not shown), indicating a different expression pattern of hnRNP A2/B1 in the brain post–I/R insults.
Figure 7.
Immunocytochemical staining of hnRNP A2/B1 translocated neurons in cerebral cortex at 3 hr to 72 hr of reperfusion after 120 min of right MCAO, including the sham-operated group (A), and 3 hr (B), 6 hr (C), 12 hr (D), 24 hr (E–G), and 48 hr (H) of recirculation. At 3 hr of recirculation, hnRNP A2/B1 translocated neurons were sparsely distributed in the cerebral cortex (B). At 6 and 12 hr of recirculation, hnRNP A2/B1 translocated neurons were also observed (C,D). At 24 hr of recirculation, hnRNP A2/B1-IR translocated neurons reached a peak (E–G). At 48 hr of recirculation, hnRNP A2/B1-IR neurons were reduced dramatically to a minimum level (H). Bar = 100 μm.
Figure 8.
Diagram showing nuclear-cytoplasmic-neuritic translocation of hnRNP A2/B1 proteins in cerebral cortex in different groups subjected to I/R insults at 3, 6, 12, 24, 48, and 72 hr of recirculation. **p<0.01, compared with sham group.
Discussion
The life histories of mRNAs as they pass through the cell are largely governed by continually evolving sets of ribonucleoproteins (Lu and Schneider 2004). Here, we have shown for the first time that hnRNP A2/B1 expresses dynamic changes in the cerebral cortex and hippocampus of rat brain after I/R insults by immunocytochemistry. Furthermore, in the cerebral cortex of the ischemic side, but not the opposite region, we found that hnRNP A2/B1 protein translocated from nucleus to cytoplasm and even to growth cone of axons. In contrast, this phenomenon was not observed in the hippocampus.
There are some reports showing that the hippocampus is more vulnerable than the cerebral cortex. Blood is supplied to the hippocampus by many arteries, especially the anterior choroidal artery and the posterior cerebral artery; the cerebral cortex areas are mainly supplied by the middle cerebral artery. In our study, neurons of the cerebral cortex suffered more serious damage after I/R than those of the hippocampus, suggesting that different areas respond differently to I/R injuries. As a member of the hnRNP family, hnRNP A2/B1 seemed to play an important role in posttranscriptional regulation of cerebral cortex, and hippocampal neurons suffered from I/R insults. The altered expression level of hnRNP A2/B1 in hippocampus and cerebral cortex may therefore reveal that the regulatory mechanisms in different brain regions are quite different, providing new clues for development of therapeutic approaches to the treatment of stroke.
The ischemic penumbra has been seen as non-functional but still-viable brain tissue surrounding the irreversibly damaged ischemic core. Ischemia usually insults on translation inhibition during both ischemia and early reperfusion in both core and penumbra. Our results are consistent with previous reports that the expression level of the hnRNP A2/B1 protein notably decreases in cerebral cortex and hippocampus during 3, 6, 12, and 24 hr of reperfusion. These early decreases in hnRNP A2/B1 density are not due to edema. Actually, after the immunohistochemical staining of the hnRNP A2/B1 signals, it is easy to distinguish the edema signals of the neurons. The neurons with edema usually show irregular staining of the nucleus, and the hnRNP A2/B1 expression is mainly located in the nucleus during the early period of reperfusion after ischemia insults. In other words, although there is edema in the rat brain that has received I/R insults, the positive signals of hnRNP A2/B1 as a nuclear protein can be ascertained from the optical density analysis of the images. I/R also alters cellular regulatory systems that control neuronal protein synthesis. It is possible that activation of a genetic program may lead to expression of specific proteins required for cell survival. It has been documented that posttranscriptional regulatory mechanisms, specifically modulation of mRNA stability, can play a major role in gene expression. In many cases, it has been proven that mRNA turnover depends on mRNA binding proteins that specifically recognize adenosine uridine (AU)-rich elements or other sequence motifs in the 3′-untranslated region (3′-UTR), and either suppress or favor RNase degradation (Garayoa et al. 2003; Griffin et al. 2004). In accord with previous studies, hnRNP A2 has also been shown to bind to 3′-UTR AU-rich elements of glucose transporter-1 mRNA and to mediate its rapid decay under ischemic or hypoglycemic conditions (Garayoa et al. 2003; He et al. 2009). In this sense, we can speculate that the observed downregulation of hnRNP A2/B1 expression after hypoxic treatment would diminish its destabilizing mRNA function, and may even allow the binding of other RNA-stabilizing proteins to the 3′-UTR of the transcripts involved. The decrease of the destabilizing role of hnRNP A2/B1 mRNA due to the ischemic/hypoxic environment could be a key element contributing to the upregulated expression of ischemic/hypoxia–inducible genes with hnRNP A2/B1–binding elements in their transcripts (Kamma et al. 2001; Garayoa et al. 2003). On the other hand, it has been reported that hnRNP A2/B1 is related to the binding and elongation of single-stranded telomere repeats, protecting them from a nuclease and promoting telomerase activity (Ford et al. 2002). In addition, hnRNP A2 appears to participate in DNA repair (He et al. 2009). We observed that at 48 hr after I/R injury, expression of hnRNP A2/B1 dramatically increased, probably in association with the renovation of damaged neurons.
The most surprising result of our study is that hnRNP A2/B1 protein translocated from nucleus to cytoplasm, even to the growth cone of axons. This was observated in cerebral cortex but not in hippocampus. Because hnRNP A2 is a component of RNPs that travel along microtubules from the soma to distant dendrite sites, shuttling hnRNP proteins also have a function in the cytoplasm and are involved in the transport of mRNAs out of the nucleus. Other results suggest that hnRNP A2/B1 protein may have cytoplasmic functions in specific cells or tissues. At 3 hr post–I/R insults, relocalization of hnRNP A2/B1-IR from nucleus to cytoplasm has been observed in the ischemic side of the cerebral cortex, reaching a peak at 24 hr of reperfusion. Because some mRNAs and ribosomes are also present in neuronal dendrites, recent reports indicate that RNA binding protein is involved in pre-mRNA splicing of intact neuronal dendrites and may play a crucial role in synaptic plasticity by regulating RNA expression (Glanzer et al. 2005; Ule and Darnell 2006). In addition, it has been reported that some viral mRNAs and alternatively spliced mRNAs are probably exported to the cytosol as intron-retaining transcripts. It is thus possible that splicing proteins, such as the splicing factor tra2-β 1, could be distributed in dendrites with relative abundance (Daoud et al. 2002). Moreover, the hnRNP A/B family has been shown to influence splice site selection and exon skipping (Kamma et al. 1995). In accordance with these reports, hnRNP A2/B1 may regulate the structure and function of neuraxons during I/R insults, which may provide new clues on the role of endogenous neuroprotection in putative splicing regulation patterns in cerebral cortex and hippocampus in this animal model.
It is well known that hnRNP B1 is a splicing variant of hnRNP A2, with a mini-exon in the N-terminal encoding 12 amino acids. hnRNP B1 has two tandem repeats of RNA recognition motifs and a glycine-rich domain relevant to protein–protein interaction and RNA–protein interaction. Previous studies have suggested that hnRNP A2/B1 displayed a different response to Alzheimer's disease pathology (Ishikawa et al. 2004; Mizukami et al. 2005). Molecular evidence for the involvement of hnRNP A2/B1 in cerebral I/R injury will be required for a better understanding of their differential biological actions.
As for future studies to identify the cofactors of hnRNP A2/B1, its targets during axonogenesis and its mode of regulation should provide the necessary information to explain how hnRNP A2/B1 affects each target separately in cerebral I/R injury. In conclusion, the current work suggests that hnRNP A2/B1 may be associated with aberrant posttranscriptional regulation of cerebral I/R injury of the rat brain.
Acknowledgments
This work is supported by The General Program of the National Natural Science Foundation of China (grants 30973107, 30772293, and 30600365); the National Key Technologies R&D Program for New Drugs (grants 2009ZX09103-616, 2009ZX09503-002, and 2009ZX09301-002); the National Basic Research Project (973 program) (grant 2006CB504100); the General Program of the State Key Laboratory of Medical Neurobiology (grant 09-03); and the Major Program for Science and Technology Research of the Beijing Municipal Bureau (grant 7061004).
The authors thank Dr. Changhong Ren, Dr. Bing Hou, and Dr. Zhiwei Xu for assistance in preparing animal models.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Daoud R, Mies G, Smialowska A, Olah L, Hossmann KA, Stamm S (2002) Ischemia induces a translocation of the splicing factor tra2-beta 1 and changes alternative splicing patterns in the brain. J Neurosci 22:5889–5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfuss G, Kim VN, Kataoka N (2002) Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol 3:195–205 [DOI] [PubMed] [Google Scholar]
- Ford LP, Wright WE, Shay JW (2002) A model for heterogeneous nuclear ribonucleoproteins in telomere and telomerase regulation. Oncogene 21:580–583 [DOI] [PubMed] [Google Scholar]
- Garayoa M, Man YG, Martinez A, Cuttitta F, Mulshine JL (2003) Downregulation of hnRNP A2/B1 expression in tumor cells under prolonged hypoxia. Am J Respir Cell Mol Biol 28:80–85 [DOI] [PubMed] [Google Scholar]
- Glanzer J, Miyashiro KY, Sul JY, Barrett L, Belt B, Haydon P, Eberwine J (2005) RNA splicing capability of live neuronal dendrites. Proc Natl Acad Sci USA 102:16859–16864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ME, Hamilton BJ, Roy KM, Du M, Willson AM, Keenan BJ, Wang XW, et al. (2004) Post-transcriptional regulation of glucose transporter-1 by an AU-rich element in the 3′UTR and by hnRNP A2. Biochem Biophys Res Commun 318:977–982 [DOI] [PubMed] [Google Scholar]
- He Y, Rothnagel JA, Epis MR, Leedman PJ, Smith R (2009) Downstream targets of heterogeneous nuclear ribonucleoprotein A2 mediate cell proliferation. Mol Carcinog 48:167–179 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mizukami K, Iwakiri M, Kamma H, Ikonomovic MD, Dekosky ST, Asada T (2004) Immunohistochemical study of hnRNP B1 in the postmortem temporal cortices of patients with Alzheimer's disease. Neurosci Res 50:481–484 [DOI] [PubMed] [Google Scholar]
- Kamma H, Fujimoto M, Fujiwara M, Matsui M, Horiguchi H, Hamasaki M, Satoh H (2001) Interaction of hnRNP A2/B1 isoforms with telomeric ssDNA and the in vitro function. Biochem Biophys Res Commun 280:625–630 [DOI] [PubMed] [Google Scholar]
- Kamma H, Portman DS, Dreyfuss G (1995) Cell type-specific expression of hnRNP proteins. Exp Cell Res 221:187–196 [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, Gwag BJ (2002) Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J Cell Biol 159:821–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JY, Schneider RJ (2004) Tissue distribution of AU-rich mRNA-binding proteins involved in regulation of mRNA decay. J Biol Chem 279:12974–12979 [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Stoodley M (1998) Pathophysiology of cerebral ischemia. Neurol Med Chir (Tokyo) 38:1–11 [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ishikawa M, Iwakiri M, Ikonomovic MD, Dekosky ST, Kamma H, Asada T (2005) Immunohistochemical study of the hnRNP A2 and B1 in the hippocampal formations of brains with Alzheimer's disease. Neurosci Lett 386:111–115 [DOI] [PubMed] [Google Scholar]
- Mizukami K, Kamma H, Ishikawa M, Dreyfuss G (2000) Immunohistochemical study of the hnRNP A2 and B1 in the rat forebrain. Neuroreport 11:3099–3102 [DOI] [PubMed] [Google Scholar]
- Pignataro G, Scorziello A, Di Renzo G, Annunziato L (2009) Post-ischemic brain damage: effect of ischemic preconditioning and postconditioning and identification of potential candidates for stroke therapy. FEBS J 276:46–57 [DOI] [PubMed] [Google Scholar]
- Shang A, Zhou D, Wang L, Gao Y, Fan M, Wang X, Zhou R, et al. (2006) Increased neuroglobin levels in the cerebral cortex and serum after ischemia-reperfusion insults. Brain Res 1078:219–226 [DOI] [PubMed] [Google Scholar]
- Soriano MA, Tessier M, Certa U, Gill R (2000) Parallel gene expression monitoring using oligonucleotide probe arrays of multiple transcripts with an animal model of focal ischemia. J Cereb Blood Flow Metab 20:1045–1055 [DOI] [PubMed] [Google Scholar]
- Ule J, Darnell RB (2006) RNA binding proteins and the regulation of neuronal synaptic plasticity. Curr Opin Neurobiol 16:102–110 [DOI] [PubMed] [Google Scholar]
- Xu Z, Hou B, Zhang Y, Gao Y, Wu Y, Zhao S, Zhang C (2009) Antidepressive behaviors induced by enriched environment might be modulated by glucocorticoid levels. Eur Neuropsychopharmacol 19:868–875 [DOI] [PubMed] [Google Scholar]