Abstract
Nestin is an intermediate filament protein that is known as a neural stem/progenitor cell marker. It is expressed in undifferentiated central nervous system (CNS) cells during development, but also in normal adult CNS and in CNS tumor cells. Additionally, nestin is expressed in endothelial cells (ECs) of CNS tumor tissues and of adult tissues that replenish by angiogenesis. However, the regulation of nestin expression in vascular endothelium has not been analyzed in detail. This study showed that nestin expression was observed in proliferating endothelial progenitor cells (EPCs), but not in mature ECs. In adherent cultured cells derived from bone marrow cells, EPCs that highly expressed nestin also expressed the endothelial marker CD31 and the proliferation marker Ki67. ECs cultured without growth factors showed attenuated nestin immunoreactivity as they matured. Transgenic mice that carried the enhanced green fluorescent protein under the control of the CNS-specific second intronic enhancer of the nestin gene showed no reporter gene expression in EPCs. This indicated that the mechanisms of nestin gene expression were different in EPCs and CNS cells. Immunohistochemistry showed nestin expression in neovascular cells from two distinct murine models. Our results demonstrate that nestin can be used as a marker protein for neovascularization. (J Histochem Cytochem 58:721–730, 2010)
Keywords: nestin, endothelial cell, endothelial progenitor cell, bone marrow cell, angiogenesis, neovascularization
Nestin was originally described as a neural stem/progenitor cell marker that appeared during development of the central nervous system (CNS), and it was defined as a class VI intermediate filament protein (Lendahl et al. 1990). Nestin expression is downregulated when CNS stem/progenitor cells differentiate into neurons or glial cells (Frederiksen and McKay 1988; Dahlstrand et al. 1995). The expression of the nestin protein is also observed in adult CNS stem/progenitor cells, both in vitro and in vivo (Reynolds and Weiss 1992; Morshead et al. 1994). In addition to embryonic and adult CNS stem/progenitor cells, nestin is expressed in CNS tumor cells, and the degree of nestin expression correlates with the malignancy of the CNS tumor (Tohyama et al. 1992). Thus, nestin is generally recognized as a marker protein of undifferentiated CNS cells at the stage that precedes exit from the cell cycle and commitment of the mature progeny to a specific lineage. Complexly, endothelial cells (ECs) in CNS tumor tissue were also positive for nestin (Dahlstrand et al. 1992), irrespective of its expression in tumor cells (Sugawara et al. 2002). Nestin expression in vascular ECs was also reported in a variety of adult human non-CNS tissues, including the pancreas (Klein et al. 2003), the corpus luteum in the ovary, and the full-term placenta (Mokry et al. 2004). Nestin expression in the ECs of adult tissues that replenish by angiogenesis and in the endothelium of vascular neoplasms (Shimizu et al. 2006) and cancers (Aihara et al. 2004; Teranishi et al. 2007) suggests that nestin is also a marker for angiogenesis. However, the regulation of nestin expression in the vascular endothelium has not been analyzed in detail, particularly with respect to neovascularization.
Bone marrow–derived endothelial progenitor cells (EPCs) are responsible for neovascularization in postnatal life (Asahara et al. 1999; Takahashi et al. 1999). A large number of reports on EPCs have been accumulated as a potential tool in regenerative medicine (Schachinger et al. 2006a,b; Erbs et al. 2007; Martin-Rendon et al. 2008) or a therapeutic target in oncology (Gao et al. 2008). Although a unique EPC marker has not been identified, EPCs, which most investigators obtain after the in vitro culture, are characterized as cells with high proliferative potential that display typical endothelial characteristics [e.g., expression of endothelial markers and uptake of acetylated low-density lipoprotein (Ac-LDL)] and differentiate into ECs in vitro (Timmermans et al. 2009). In the present study, we studied adherent cultured cells derived from bone marrow cells (Lin et al. 2000) to establish culture conditions that would sequentially induce the growth of both proliferative EPCs and committed mature ECs. We demonstrated that nestin expression was observed only in proliferating EPCs, not in mature ECs. Furthermore, vascular nestin expression was not activated by the CNS-specific enhancer of the nestin gene. This indicated that the nestin expressed in EPCs was cytochemically similar to that expressed in CNS stem/progenitor cells, but the mechanisms of nestin gene expression were different in EPCs and cells of the CNS. Finally, we used two distinct murine models to clarify nestin expression in neovascularization immunohistochemically.
Materials and Methods
Animals
Two strains of adult 8- to 10-week-old mice (20–25 g) were utilized: wild-type C57BL/6J mice, purchased from SLC (Shizuoka, Japan), and E/nestin:EGFP–transgenic mice on the C57BL/6J genetic background (Kawaguchi et al. 2001; Johansson et al. 2002) to study neural-specific nestin gene expression. All animal-related procedures were approved by the Laboratory Animal Care and Use Committee of Keio University and were conducted in accordance with the guidelines of the National Institutes of Health.
EC Culture
Primary Culture
Femurs and tibias were dissected free of attached muscles, crushed with a pestle, and suspended in αMEM (#11900, Gibco Invitrogen; Carlsbad, CA) supplemented with 10% FBS and 1% penicillin G (10,000 U/ml)-streptomycin sulfate (10,000 μg/ml) (PS). Then cell suspensions were filtered through a 70-μm filter (Cell Strainer #352350, Falcon; Bedford, MA). EPCs were cultured from mononuclear cells (MNCs) under the culture conditions previously reported, with some modifications (Dimmeler et al. 2001; Strehlow et al. 2003). Briefly, MNCs were isolated from bone marrow cells by Ficoll density-gradient centrifugation (Ficoll-Paque Plus, 1.077 g/ml, GE Healthcare; Uppsala, Sweden). Cells (1 × 106 cell/ml) were plated on fibronectin-coated 6-well plates (#140675, Nunc; Roskilde, Denmark) or fibronectin-coated 8-well chamber slides (0.75-cm2 per well, #5732-008, Iwaki; Chiba, Japan) in endothelial basal medium supplemented with 5% FBS, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), recombinant analog of insulin-like growth factor-1 (R3-IGF-1), epidermal growth factor (EGF), hydrocortisone, ascorbic acid, and gentamicin/amphotericin-B (EGM-2-MV Bullet Kit CC-3202, Lonza; Walkersville, MD). The medium was changed after 24 hr to remove non-adherent cells, and medium was renewed every week. At 21 days in vitro (DIV), cells were fixed and processed for indirect immunofluorescence.
For mature EC cultures, cells were lifted by incubation with 0.25% trypsin and 1 mM EDTA at 21 DIV and replated on fibronectin-coated 8-well chamber slides (Iwaki) at a density of 2 × 105 cell/ml. To allow endothelial maturation, cells were cultivated in endothelial maturation medium, which consisted of endothelial basal medium with the supplements mentioned above, but without growth factors (bFGF, R3-IGF-1, and EGF). After 14 DIV, cells were fixed for immunocytochemistry.
Cell Line Culture
Cells of a mouse brain endothelioma cell line (bEnd.3 cells CRL-2299, American Type Culture Collection; Manassas, VA) were cultured according to the manufacturer's instructions. Briefly, cells were maintained in DMEM (#12699; Gibco Invitrogen) supplemented with 10% FBS and 1% PS. The medium was renewed every 3 to 4 days. At the first passage, 2.5 × 105 cells were seeded on 8-well chamber slides (Iwaki). After 4 DIV, cells were processed for immunocytochemistry.
Primary Neural Stem/progenitor Cell Culture
Neurospheres were generated from adult forebrain as described previously (Kawaguchi et al. 2001; Shimazaki et al. 2001; Murayama et al. 2002). Briefly, the striata from E/nestin:EGFP–transgenic mice were dissected, collected into PBS containing 0.6% glucose, and then treated with the trypsin solution. After 15 min incubation at 37C, the tissue pieces were triturated and added to a trypsin inhibitor solution. Dissociated cells (5000 cells/ml) were seeded in the neurosphere culture medium composed of DMEM-F12 (1:1), glucose (0.6%), glutamine (2 mM), sodium bicarbonate (13.4 mM), HEPES (5 mM), insulin (25 μg/ml), transferrin (100 μg/ml), progesterone (20 nM), sodium selenate (30 nM), and purescine (60 μM), supplemented with recombinant human EGF (20 ng/ml) and recombinant human bFGF (20 ng/ml). Cells were cultured for 7 DIV and formed floating cell clusters of neural stem/progenitor cells (neurospheres). Neurospheres were collected and placed with media onto poly-O/fibronectin–coated 8-well chamber slides (Iwaki) 1 hr before fixation for immunocytochemistry.
Indirect Immunofluorescence
Cells were cultured on 8-well chamber slides (Iwaki), and freshly isolated MNCs were attached by centrifugation (500 rpm for 2 min) to glass slides (Cytoslide-coated #5991056, Thermoshandon; Kalamazoo, MI). Then they were fixed in 4% paraformaldehyde (PFA) in PBS for 15 min at room temperature. Mouse forebrain and a complex of the angiogenesis assay tubes retrieved from mice were cut into sections (14-μm thickness) with a cryostat (FINETEC CM3050S, Leica; Wetzlar, Germany). Samples were incubated in 0.3% Triton X-100 in PBS, then at 4C overnight with the following antibodies and dilutions in 10% normal goat or donkey serum: mouse monoclonal anti-nestin (1:300, clone Rat-401, Developmental Studies Hybridoma Bank of Iowa University; Iowa City, Iowa, or 556309, BD Pharmingen; San Jose, CA), rabbit polyclonal antibody NCL-Ki67p (1:100, NE128EW, Novocastra; Newcastle upon Tyne, UK), rabbit polyclonal anti–von Willebrand factor (vWF, 1:10, sc-14014, Santa Cruz Biotechnology; Santa Cruz, CA), rat anti-mouse CD31 (1:10, 550274, BD Biosciences; San Jose, CA). Slides for Ki67 were pretreated in a microwave oven in REAL target retrieval solution (S2031, Dako; Glostrup, Denmark) according to the high-temperature antigen unmasking technique. After washing with PBS, cells or sections were incubated with anti-mouse IgG conjugated to Alexa 488 (Molecular Probes Invitrogen; Carlsbad, CA), Alexa 555 for nestin, or anti-rabbit IgG conjugated to Alexa 488 for Ki67. Signals for vWF and CD31 were amplified with anti-rabbit or anti-rat horseradish peroxidase (Jackson ImmunoResearch; West Grove, PA) and tyramide signal amplification kits (Perkin Elmer; Waltham, MA). Nuclei were stained with Hoechst 33258 (Sigma; Taufkirchen, Germany). Controls with omission of the primary antibody were processed in parallel for all histochemistry. Cells and sections were observed with an AxiCom fluorescent microscope with an MRm digital camera or LSM510 META (Zeiss; Jena, Germany). Florescent images were prepared from data files with AxioVision 4 software or Zeiss Image Browser Ver. 3.5 software, and with Adobe Photoshop software (San Jose, CA). Linear adjustments in brightness or contrast were applied to the entire image when necessary. All immunofluorescence assays were performed in more than two independent experiments to confirm the immunoreactivity.
The number of immunopositive cells was counted in five, non-overlapping visual fields at a magnification of ×200. The percentages of immunopositive cells were calculated per 100 live cells, visualized with Hoechst staining.
Flow Cytometry
MNCs were suspended in Hanks balanced salt solution supplemented with 2% FBS, 10 mM HEPES (HBSS+) at 2–8 × 106 cell/ml. Propidium iodide (PI, 2 μg/ml) was added for dead cell discrimination. The cell suspensions were filtered through a cell strainer–capped tube (#358235, Falcon) and then analyzed with a FACS Vantage (Becton Dickinson; Franklin Lakes, NJ). PI fluorescence was measured first, and a live cell gate was defined that excluded the cells positive for PI. Crude green fluorescent protein (GFP) expression of MNCs was measured, and GFP-positive cells were gated by comparison with a negative control (wild-type mouse bone marrow MNCs). The proportions of GFP-positive cells among all live cells were obtained by analyzing the cumulative data from ∼5 × 106 live MNCs isolated from each animal.
In Vivo Angiogenesis Assay
Angiogenesis assay tubes (Directed In Vivo Angiogenesis Assay #3450-048-SK, Trevigen Inc.; Gaithersburg, MD) were prepared and implanted into the dorsal flank of adult mice according to the manufacturer's instructions. Mice were placed under general anesthesia with an inhalant anesthetic (1.5% isoflurane with 0.35 l/min N2O and 0.15 l/min O2). After surgery, mice were housed for 7 days and sacrificed. The angiogenesis assay tubes were removed from the animals. A substrate and vessel complex within the tube was retrieved, fixed in 4% PFA at 4C overnight, and then cryoprotected in 30% sucrose in PBS at 4C overnight. Then the samples were embedded in optimum cutting temperature compound (Tissue Tek 4583, Sakura Finetec U.S.A.; Torrance, CA) and frozen with liquid nitrogen for immunohistochemistry.
Cold Injury of the Mouse Brain
Adult mice were anesthetized as described above and placed on a stereotactic frame (SR-5, Narishige; Tokyo, Japan). Rectal (core) temperature was measured and maintained at around 37C with a heating pad. Cold injury was induced in the mouse forebrain according to a modification of a previously reported method (Maeda et al. 1997; Flentjar et al. 2002; Sewell et al. 2004) to produce a more severe injury that would promote angiogenesis at the lesion. The scalp was incised at the midline and the skull was exposed. The right parietal bone was carefully drilled with an electric drill and a hole was made slightly larger than 2 mm in diameter. A 2-mm-diameter metal probe, cooled in liquid nitrogen, was placed through the drilled hole and applied to the dura mater for 30 sec. The incision was sutured, and mice were allowed to recover from anesthesia on a heating pad. Mice were housed for 14 days before sacrifice. Mice were sacrificed under deep anesthesia with a transcardial perfusion of PBS, followed by 4% PFA. The forebrain was removed and processed for immunohistochemistry as described above.
Results
Nestin Is Expressed in Newly Generated Vascular ECs In Vitro
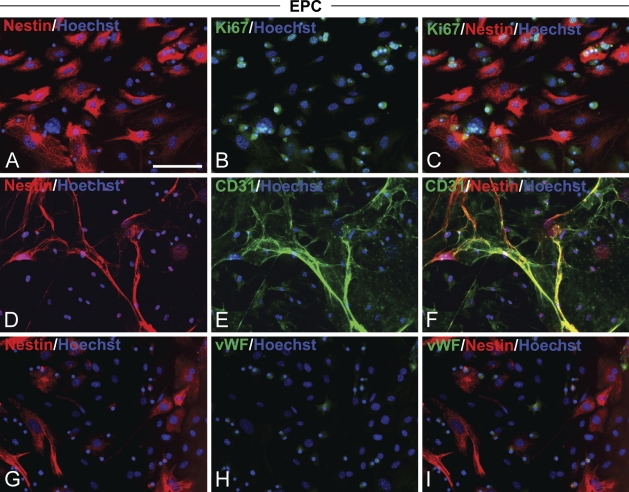
To test whether the nestin protein was expressed in vascular ECs, ECs were derived from adult murine bone marrow cells and cultured under adherent culture conditions in endothelial proliferation medium. Native nestin expression was observed in 11.5 ± 0.04% (mean ± SD, n=2) of MNCs freshly isolated from adult bone marrow cells. Primary cells were cultured at subconfluence for a period of 21 DIV. The cultured cells thoroughly expressed nestin (Figures 1A, 1D, and 1G), and most were positive for the proliferation marker Ki67 (Figure 1B). The EC lineage of cultured cells was confirmed by staining with antibodies against two marker proteins expressed in both EPCs and mature ECs, CD31 (Kanayasu-Toyoda et al. 2003) (Figure 1E) and vascular endothelium cadherin (VE-cadherin) (Peichev et al. 2000) (data not shown), or by detecting the uptake of 1,1′dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate Ac-LDL (DiI–Ac-LDL) (Voyta et al. 1984) (data not shown). Only a small number of cells expressed the mature EC marker vWF (Kuwana et al. 2004) (Figure 1H). This immunocytochemical phenotype indicated that the culture could generate proliferating immature ECs, namely EPCs, from adult bone marrow MNCs. The Ki67+ CD31+ EPCs were also positive for nestin (Figures 1C and 1F). Thus, nestin immunoreactivity in EPCs coincided with the expression of the proliferation marker and immature endothelial marker proteins.
Figure 1.
Nestin expression in endothelial progenitor cells (EPCs). Mouse bone marrow mononuclear cells (MNCs) were cultured in endothelial growth medium supplemented with growth factors. After 21 days in vitro (DIV), cells were fixed and stained to detect nestin (A,D,G, red). (B) A proliferation marker, Ki67 (green). Endothelial markers CD31 (E, green) and von Willebrand Factor (vWF) (H, green) of the same images shown in A,D,G. (C,F,I) Merged images. Double-positive cells expressed nestin and Ki67, or nestin and CD31. (A–I) Nuclei were stained with Hoechst (blue). Bar = 100 μm.
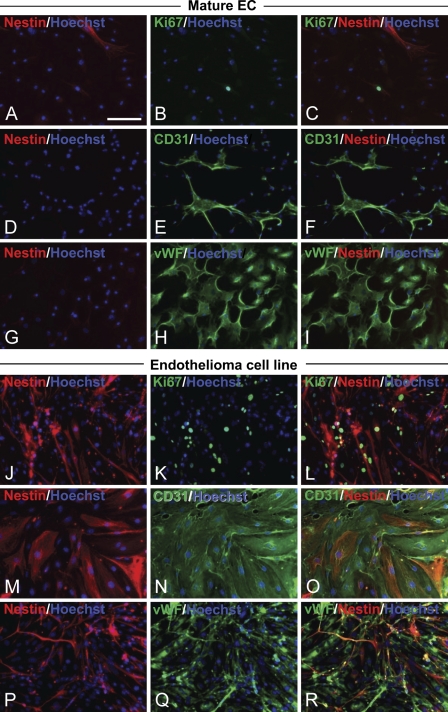
The EPCs were then passaged and cultured without growth factors for 14 DIV to facilitate differentiation into mature ECs. In contrast to the EPCs, the mature ECs rarely expressed nestin (Figures 2A, 2D, and 2G), and only an occasional cell was Ki67 immunoreactive (Figure 2B). The mature ECs were totally positive for both CD31 and vWF (Figures 2E and 2H). Nestin immunoreactivity was not detected in the CD31+ cells or the vWF+ cells (Figures 2F and 2I). Therefore, cultured ECs derived from the bone marrow showed attenuated nestin immunoreactivity as they matured.
Figure 2.
Downregulation of nestin expression in mature endothelial cells (ECs) and nestin immunoreactivity in neoplastic ECs. (A–I) Immunostaining of mature ECs. EPCs were cultured another 14 DIV without growth factors [epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor-1 (IGF-1)] to allow endothelial maturation. (A,D,G) Nestin (red), (B) Ki67 (green), (E) CD31 (green), (H) vWF (green), and (C,F,I) merged images. vWF-positive, CD31-positive, and Ki67 were occasionally positive (0 or 1 cell/high-power field) in mature ECs that did not express nestin. (J–R) Immunostaining of the EC line derived from mouse brain endothelioma. (J,M,P) Nestin (red), (K) Ki67 (green), (N) CD31 (green), (Q) vWF (green), and (L,O,R) merged images. Endothelioma cells positive for Ki67 and endothelial markers also expressed nestin. (A–R) Nuclei were stained with Hoechst (blue). Bar = 100 μm.
We next tested nestin expression in a mouse brain endothelioma cell line (Figures 2J, 2M, and 2P). The cells of the EC line highly expressed Ki67 (Figure 2K), CD31 (Figure 2N), VE-cadherin (data not shown), and vWF (Figure 2Q). Nestin immunoreactivity was clearly detected in the Ki67+ CD31+ vWF+ proliferating neoplastic ECs (Figures 2L, 2O, and 2R). Nestin expression in EPCs and cells of the endothelioma cell line and its downregulation in mature ECs were confirmed by Western blot and RT-PCR (data not shown).
Vascular Nestin Is Expressed Without the Neural-specific Nestin Gene Enhancer
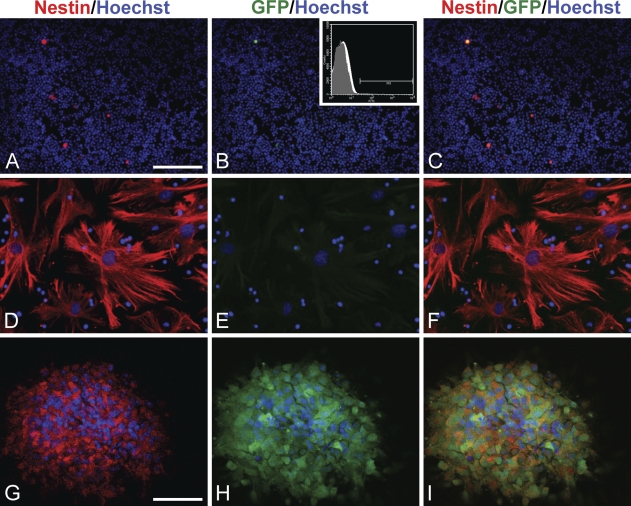
Because nestin is commonly used as a marker protein for CNS stem/progenitor cells, we examined whether the neural-specific nestin gene was expressed in EPCs. To this end, we utilized transgenic mice carrying enhanced green fluorescent protein (EGFP) under the control of the neural-specific second intronic enhancer of the nestin gene (E/nestin:EGFP) (Kawaguchi et al. 2001; Johansson et al. 2002). Bone marrow cells and forebrain striatum cells were obtained from adult transgenic mice and were selectively cultured for EPCs and neural stem/progenitor cells, respectively. More than 10% of the MNCs isolated from transgenic mice showed immunoreactivity for native nestin (13.2%, Figure 3A). This was consistent with the expression levels observed in MNCs from wild-type mice. Unexpectedly, a very small number of MNCs also expressed GFP (Figure 3B). This indicated that the neural-specific nestin gene enhancer was activated under these conditions. We confirmed GFP expression in transgenic mice MNCs by flow cytometry analysis. We found that 0.04 ± 0.03% (mean ± SD, n=5) of transgenic mice MNCs expressed GFP (Figure 3B, inset). Immunocytochemistry indicated that GFP-positive MNCs indeed expressed the nestin protein (Figure 3C). Consistent with findings from EPCs cultured from the MNCs of wild-type mice, EPCs derived from transgenic mice MNCs expressed the nestin protein, but GFP was not detected in nestin+ EPCs (Figures 3D–3F). As a control, we also tested neurospheres composed of neural stem/progenitor cells. The neurospheres were immunoreactive for nestin protein and expressed GFP (Figures 3G–3I). Thus, the vascular nestin protein expressed in EPCs was not activated by the neural-specific enhancer of the nestin gene.
Figure 3.
Expression of nestin protein and neural-specific nestin gene enhancer activity in EPCs and neural stem/progenitor cells. (A–C) Microscopic images of freshly isolated MNCs obtained from adult transgenic mice carrying enhanced green fluorescent protein (EGFP) under the control of the neural-specific nestin enhancer (E/nestin) (E/nestin:EGFP). (A) Immunoreactivity for nestin protein (red) was observed in some native MNCs. (B) Expression of GFP (green) was observed in very few MNCs. (B, inset) GFP-positive MNCs could be detected by flow cytometry. (C) A merged image. (D–F) Microscopic images of EPCs. MNCs from adult E/nestin:EGFP–transgenic mice were cultured for 21 DIV, and then stained. (D) Nestin (red), (E) GFP (green), and (F) a merged image. Note that nestin-positive EPCs are GFP negative. (G–I) Confocal images of neural stem/progenitor cells cultured from adult E/nestin:EGFP–transgenic mouse forebrain. (G) Nestin (red), (H) GFP (green). (I) Merged image; coexpression of GFP and nestin (yellow) indicated that the neural-specific nestin enhancer was active in nestin-positive stem/progenitor cells of neural lineage. Bars: A–F = 100 μm; G–I = 40 μm.
Nestin Is Expressed in Newly Generated Vascular ECs In Vivo
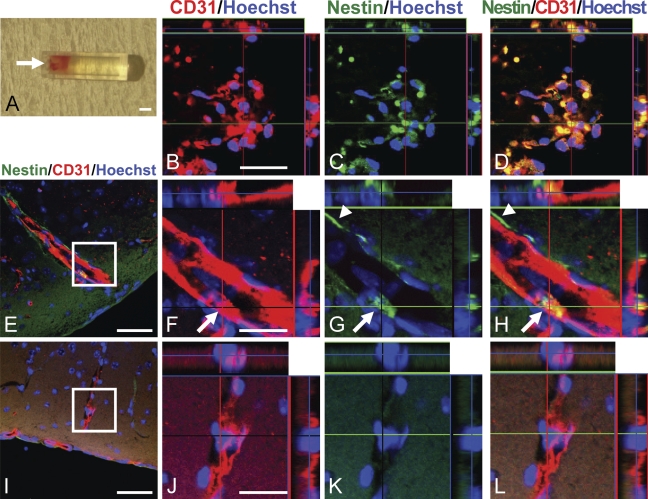
To examine whether the vascular nestin protein could be used as a marker for neovascularization in vivo, we captured neoangiogenic tissues in a silicone tube that was implanted into mice. The tube was filled with a substrate that contained growth factors designed to promote angiogenesis into the tube from the surrounding connective tissues. Thus, neovascular tissue grew through the open end of the tube and developed on the substrate (Figure 4A, arrow). Seven days after implantation, the tubes were retrieved and substrate complexes were removed and processed for immunostaining. Confocal microscopy revealed that all CD31+ ECs were also positive for nestin (Figures 4B–4D). This indicated that the newly generated ECs that developed in the implanted tube expressed nestin.
Figure 4.
Expression of nestin protein in neovascular cells in vivo. (A) In vivo neovascular branches grew for 7 days into an implanted silicone tube designed for a neoangiogenesis assay. Arrow indicates the open end of the tube where vascular ECs entered the tube. (B–D) Confocal images of neovascularization. (B) CD31 (red), (C) nestin (green), and (D) a merged image. Nestin was expressed in every CD31-positive EC in the tube. (E–H) Confocal images of neovascularization at the injury site of the mouse forebrain 14 days after cold injury. (E) CD31-positive (red) vasculature increased at the injury site. (F–H) The area denoted by a rectangle in E is shown at higher magnification to show staining for CD31 (F, red), nestin (G, green), and a merged image (H). An EC within the robust vasculature in the injured brain was double-positive for CD31 and nestin (arrows). A patchy perivascular lining of nestin-positive cells was also observed on the parenchymal border of CD31-positive ECs (arrowheads). (I–L) Images of the contralateral, uninjured side of the forebrain from the same mouse. (I) Fine, scattered CD31-positive (red) vasculature was observed in the uninjured mouse brain. (J–L) The area denoted by a rectangle in I is shown at higher magnification to show staining for CD31 (J, red), nestin (K, green), and coexpression in a merged image (L). Note that ECs in the normal brain did not express nestin. Bars: A = 1 mm; B–D,F–H,J–L = 20 μm; E,I = 50 μm.
Next, we tested vascular nestin expression in another in vivo model of neovascularization, cold injury of the mouse brain. Robust CD31+ vasculature was observed in the injured cortex (Figure 4E). A CD31/nestin double-positive EC phenotype was observed in the vasculature (Figures 4F–4H, arrow). We also encountered nestin+ CD31− perivascular cells on the parenchymal border of the CD31+ ECs (Figures 4G and 4H, arrowheads). On the other hand, no nestin positivity could be found in CD31+ mature ECs in the normal cortex on the contralateral side of the same brain (Figures 4I–4L). Therefore, two distinct in vivo models of neovascularization demonstrated that nestin was expressed in newly generated ECs.
Discussion
This study demonstrated that newly generated, proliferative ECs expressed vascular nestin in vitro and in vivo. Endothelial nestin expression was marked in undifferentiated EPCs at the stage that preceded exit from the cell cycle. Nestin expression was downregulated when EPCs matured into ECs in vitro. Likewise, in the CNS, nestin was expressed in neural stem/progenitor cells and was downregulated upon differentiation (Frederiksen and McKay 1988; Dahlstrand et al. 1995). Some (∼10%) bone marrow MNCs were found to express nestin. When MNCs were cultured and induced toward an endothelial lineage, EPCs positive for the proliferation marker Ki67 and the endothelial marker CD31 showed upregulated nestin expression. Upon maturation, vWF-positive ECs showed downregulated nestin expression. The upregulation of nestin in tissue stem/progenitor cells beyond the germ layers was also reported in muscle (Sejersen and Lendahl 1993; Zimmerman et al. 1994; Kachinsky et al. 1995), testis (Frojdman et al. 1997), and teeth (Terling et al. 1995). Thus, we can speculate that nestin may be a marker for various tissue stem/progenitor cells. However, nestin expression in tissue stem/progenitor cells should be distinguished from that in proliferating ECs, particularly when detected with in vivo histochemistry. For instance, in a study of the adult pancreas, it was reported that nestin-positive cells resided in pancreatic ducts. This suggested a potential role as endocrine progenitors (Zulewski et al. 2001). However, a more recent study demonstrated that all nestin-positive cells in the pancreas coexpressed vascular endothelial markers (Klein et al. 2003). In CNS stem/progenitor cells, nestin formed intermediate filament bundles with other intermediate filaments, including vimentin (Eliasson et al. 1999). Nestin is thought to play a role in distributing vimentin from copolymerized intermediate filaments to daughter cells during progenitor cell division (Chou et al. 2003). Further investigations will be required to elucidate whether nestin filaments in EPCs possess biological functions similar to those in CNS stem/progenitor cells.
The nestin gene consists of four exons and three introns (Zimmerman et al. 1994). Neural-specific nestin expression is driven by the second intronic enhancer of the nestin gene (Zimmerman et al. 1994), which is highly conserved evolutionarily (Lothian and Lendahl 1997). In this study, we used E/nestin:EGFP–transgenic mice that carried EGFP under the control of the second intronic enhancer of the nestin gene, and we showed reporter gene expression in CNS stem/progenitor cells (Kawaguchi et al. 2001; Johansson et al. 2002). However, we found that nestin-positive EPCs derived from E/nestin:EGFP mice were thoroughly negative for EGFP. This indicated that vascular nestin expression was not driven by the neural-specific nestin gene enhancer. These results suggested that the transcriptional mechanisms that regulated proliferative EPCs were different from those that functioned in CNS stem/progenitor cells. Alternatively, the first intron of the nestin gene has been shown to consistently direct reporter gene expression in developing muscle of transgenic mice (Zimmerman et al. 1994). This first intron may also be an endothelial-specific element of the nestin gene that can induce nestin expression in EPCs. This hypothesis is supported by findings from Aihara et al. (2004), who used a tube formation assay with human umbilical vein ECs and reported that a tyrosine kinase domain–deleted VEGF receptor effectively abolished tube formation under the control of the first intron. Unexpectedly, our results in transgenic mice showed that a small number of adult bone marrow MNCs (0.04% of total MNCs) expressed native nestin concomitant with the expression of EGFP, which was driven by the neural-specific second intronic enhancer. However, ∼10% of adult MNCs expressed nestin, but not EGFP. Further studies are necessary to determine whether this E/nestin:EGFP–positive subtype derived from bone marrow cells has multipotency and whether it might originate from the neural crest (Nagoshi et al. 2008).
We used angiogenesis assay tubes to study in vivo angiogenesis. These silicone cylinders were filled with angiogenic modulating factors designed to allow vascular ECs to grow into the tube after implantation into mouse subcutaneous tissue (Guedez et al. 2003). This assay system eliminated the problem of cellular contamination with tissue progenitor cells. In vivo neovascularization generated in the tube after 7 days clearly demonstrated nestin expression in CD31-positive ECs. This was consistent with the nestin expression observed in cells cultured in endothelial proliferation conditions in vitro. Furthermore, the neovascularization induced in murine brain tissues by cold injury showed that CD31-positive ECs expressed nestin. On the other hand, nestin expression was not observed in ECs from the uninjured side of the brain cortex, where neovascularization is not thought to occur in normal adults. Taken together, our results showed that nestin was expressed in proliferating ECs and may be useful as a marker protein for neovascularization. Importantly, vascular nestin expression should be verified by coexpression of an endothelial marker protein such as CD31, because, in situ, tissue stem/progenitor cells may also express nestin. The observations in this study are consistent with previous in vivo reports concerning nestin expression in angiogenesis (Aihara et al. 2004; Shimizu et al. 2006; Teranishi et al. 2007). Moreover, our in vitro results clearly showed that vascular nestin expression was downregulated at the transition from a proliferating EPC to a mature, postmitotic EC.
Acknowledgments
This study was supported by grants-in-aid for scientific research from the Japanese Ministry of Education, Culture, Science, Sports, and Technology (KAKENHI #19591702) (to JN), and the Keio Gijuku Academic Development Funds (to JN).
The authors thank Sadafumi Suzuki for technical assistance.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Aihara M, Sugawara K, Torii S, Hosaka M, Kurihara H, Saito N, Takeuchi T (2004) Angiogenic endothelium-specific nestin expression is enhanced by the first intron of the nestin gene. Lab Invest 84:1581–1592 [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228 [DOI] [PubMed] [Google Scholar]
- Chou YH, Khuon S, Herrmann H, Goldman RD (2003) Nestin promotes the phosphorylation-dependent disassembly of vimentin intermediate filaments during mitosis. Mol Biol Cell 14:1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrand J, Collins VP, Lendahl U (1992) Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res 52:5334–5341 [PubMed] [Google Scholar]
- Dahlstrand J, Lardelli M, Lendahl U (1995) Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 84:109–129 [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, et al. (2001) HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest 108:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson C, Sahlgren C, Berthold CH, Stakeberg J, Celis JE, Betsholtz C, Eriksson JE, et al. (1999) Intermediate filament protein partnership in astrocytes. J Biol Chem 274:23996–24006 [DOI] [PubMed] [Google Scholar]
- Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, et al. (2007) Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation 116:366–374 [DOI] [PubMed] [Google Scholar]
- Flentjar NJ, Crack PJ, Boyd R, Malin M, de Haan JB, Hertzog P, Kola I, et al. (2002) Mice lacking glutathione peroxidase-1 activity show increased TUNEL staining and an accelerated inflammatory response in brain following a cold-induced injury. Exp Neurol 177:9–20 [DOI] [PubMed] [Google Scholar]
- Frederiksen K, McKay RD (1988) Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci 8:1144–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE (1997) The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation 61:243–249 [DOI] [PubMed] [Google Scholar]
- Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V (2008) Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science 319:195–198 [DOI] [PubMed] [Google Scholar]
- Guedez L, Rivera AM, Salloum R, Miller ML, Diegmueller JJ, Bungay PM, Stetler-Stevenson WG (2003) Quantitative assessment of angiogenic responses by the directed in vivo angiogenesis assay. Am J Pathol 162:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Lothian C, Molin M, Okano H, Lendahl U (2002) Nestin enhancer requirements for expression in normal and injured adult CNS. J Neurosci Res 69:784–794 [DOI] [PubMed] [Google Scholar]
- Kachinsky AM, Dominov JA, Miller JB (1995) Intermediate filaments in cardiac myogenesis: nestin in the developing mouse heart. J Histochem Cytochem 43:843–847 [DOI] [PubMed] [Google Scholar]
- Kanayasu-Toyoda T, Yamaguchi T, Oshizawa T, Hayakawa T (2003) CD31 (PECAM-1)-bright cells derived from AC133-positive cells in human peripheral blood as endothelial-precursor cells. J Cell Physiol 195:119–129 [DOI] [PubMed] [Google Scholar]
- Kawaguchi A, Miyata T, Sawamoto K, Takashita N, Murayama A, Akamatsu W, Ogawa M, et al. (2001) Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol Cell Neurosci 17:259–273 [DOI] [PubMed] [Google Scholar]
- Klein T, Ling Z, Heimberg H, Madsen OD, Heller RS, Serup P (2003) Nestin is expressed in vascular endothelial cells in the adult human pancreas. J Histochem Cytochem 51:697–706 [DOI] [PubMed] [Google Scholar]
- Kuwana M, Okazaki Y, Yasuoka H, Kawakami Y, Ikeda Y (2004) Defective vasculogenesis in systemic sclerosis. Lancet 364:603–610 [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD (1990) CNS stem cells express a new class of intermediate filament protein. Cell 60:585–595 [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothian C, Lendahl U (1997) An evolutionarily conserved region in the second intron of the human nestin gene directs gene expression to CNS progenitor cells and to early neural crest cells. Eur J Neurosci 9:452–462 [DOI] [PubMed] [Google Scholar]
- Maeda M, Akai F, Yanagihara T (1997) Neuronal integrity and astrocytic reaction in cold injury: an immunohistochemical investigation. Acta Neuropathol 94:116–123 [DOI] [PubMed] [Google Scholar]
- Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM (2008) Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J 29:1807–1818 [DOI] [PubMed] [Google Scholar]
- Mokry J, Cizkova D, Filip S, Ehrmann J, Osterreicher J, Kolar Z, English D (2004) Nestin expression by newly formed human blood vessels. Stem Cells Dev 13:658–664 [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, et al. (1994) Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13:1071–1082 [DOI] [PubMed] [Google Scholar]
- Murayama A, Matsuzaki Y, Kawaguchi A, Shimazaki T, Okano H (2002) Flow cytometric analysis of neural stem cells in the developing and adult mouse brain. J Neurosci Res 69:837–847 [DOI] [PubMed] [Google Scholar]
- Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, et al. (2008) Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2:392–403 [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, et al. (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95:952–958 [PubMed] [Google Scholar]
- Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, et al. (2006a) Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J 27:2775–2783 [DOI] [PubMed] [Google Scholar]
- Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, et al. (2006b) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 355:1210–1221 [DOI] [PubMed] [Google Scholar]
- Sejersen T, Lendahl U (1993) Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci 106:1291–1300 [DOI] [PubMed] [Google Scholar]
- Sewell DL, Nacewicz B, Liu F, Macvilay S, Erdei A, Lambris JD, Sandor M, et al. (2004) Complement C3 and C5 play critical roles in traumatic brain cryoinjury: blocking effects on neutrophil extravasation by C5a receptor antagonist. J Neuroimmunol 155:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S (2001) The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci 21:7642–7653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Sugawara K, Tosaka M, Imai H, Hoya K, Takeuchi T, Sasaki T, et al. (2006) Nestin expression in vascular malformations: a novel marker for proliferative endothelium. Neurol Med Chir (Tokyo) 46:111–117 [DOI] [PubMed] [Google Scholar]
- Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, et al. (2003) Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 107:3059–3065 [DOI] [PubMed] [Google Scholar]
- Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T (2002) Nestin as a marker for proliferative endothelium in gliomas. Lab Invest 82:345–351 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, et al. (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438 [DOI] [PubMed] [Google Scholar]
- Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, et al. (2007) Identification of neovasculature using nestin in colorectal cancer. Int J Oncol 30:593–603 [PubMed] [Google Scholar]
- Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J (1995) Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol 39:947–956 [PubMed] [Google Scholar]
- Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J (2009) Endothelial progenitor cells: identity defined? J Cell Mol Med 13:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ (1992) Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest 66:303–313 [PubMed] [Google Scholar]
- Voyta JC, Via DP, Butterfield CE, Zetter BR (1984) Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99:2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman L, Parr B, Lendahl U, Cunningham M, McKay R, Gavin B, Mann J, et al. (1994) Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12:11–24 [DOI] [PubMed] [Google Scholar]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, et al. (2001) Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 50:521–533 [DOI] [PubMed] [Google Scholar]