Abstract
Inhibins and activins are important regulators of the female reproductive system. A novel inhibin subunit, named βC, has been identified and demonstrated to be expressed in several human tissues. We demonstrate here that inhibin βC is expressed in human placenta. Expression of the inhibin βC subunit was demonstrated at the protein level by means of immunohistochemical evaluation and at the transcriptional level by an inhibin βC-specific RT-PCR analysis. Expression of inhibin βC was detected in the human chorionic carcinoma cell lines JEG and BeWo. Although the precise role of this novel inhibin subunit in human placenta development and homeostasis is unclear, analogies with other inhibin subunits and the strong expression of βC in normal human trophoblast cells and chorionic carcinoma cells suggest that βC may be involved in autocrine/paracrine signaling pathways, angiogenesis, decidualization, and tissue remodeling under normal and malignant conditions. Additionally, JEG and BeWo express βC and, therefore, can be used as a cell culture model for further functional analysis of this subunit in the human placenta. (J Histochem Cytochem 58:751–757, 2010)
Keywords: placenta, immunohistochemistry, immunofluorescence, RT-PCR, inhibin βC, chorionic carcinoma cell lines
Inhibin/activin proteins are disulphide-linked heterodimeric or homodimeric proteins, which, together with transforming growth factor (TGF) β1–3, bone morphogenetic proteins, Müllerian inhibiting substance, and several other proteins, belong to the TGF-β superfamily (Vale et al. 1988; Chang et al. 2002). Activins are homodimers or heterodimers composed of two different β subunits (βA and βB), each encoded by a single gene. These β subunits either form activins by dimerizing with a second β subunit or, alternatively, form inhibins by dimerizing with the α subunit. Thus, depending on the subunit combination, there are two forms of inhibin [inhibin A (α-βA) and inhibin B (α-βB)] and three isoforms of activin [activin A (βA–βA), activin B (βB–βB), and activin AB (βA–βB)] (Vale et al. 1988). Moreover, two additional β subunits, βC (Hötten et al. 1995) and βE (Fang et al. 1996), have been identified in humans.
Primarily isolated from the gonads, inhibins and activins are synthesized in several other gynecological organs, including normal and pathological human endometrium (Mylonas et al. 2004,2009; Worbs et al. 2007) and normal and pathological placenta (Petraglia et al. 1991; Mylonas et al. 2006a,b,2007). During pregnancy, the well-characterized inhibin α, βA, and βB subunits are also expressed in the placental decidua, the syncytiotrophoblast, and the trophoblast (Petraglia et al. 1991; McCluggage et al. 1998; Debieve et al. 2000), suggesting different roles for these subunits, such as paracrine modulators of reproductive function (Welt et al. 2002). Interestingly, higher inhibin levels in human serum have been described in preeclampsia (Muttukrishna et al. 1997) and Down syndrome (Aitken et al. 1996), demonstrating a substantial function during pregnancy. Additionally, inhibin α, βA, βB, and βC subunits, follistatin, β-glycan, and the activin receptor were detected in placental tissue from both uncomplicated term pregnancies and term pregnancies with preeclampsia (Casagrandi et al. 2003), consistent with elevated levels of inhibin A and activin A in maternal serum (Muttukrishna et al. 1997).
The novel inhibin βC subunit is predominantly expressed in rat and human livers (Esquela et al. 1997; Zhang et al. 1997; Vejda et al. 2003). A βC transcript was also demonstrated in human ovary, placenta, and testis (Loveland et al. 1996). The precise role of the βC subunit has not yet been elucidated. Mice deficient in the activin βC gene appeared normal and viable (Lau et al. 2000). In an immortalized mouse hepatocyte cell line (AML12), an increase in the rate of DNA synthesis by activin βC could be demonstrated (Wada et al. 2005). Additionally, a possible autocrine growth modulator function in liver regeneration has been suggested (Gold et al. 2005). Interestingly, ectopic expression of this subunit induced apoptosis in hepatoma cells (Chabicovsky et al. 2003; Vejda et al. 2003). Whether, and to what extent, the βC subunit can also act as a mitotic agent in human placental tissue is still unclear.
Only limited data on the histological expression of the inhibin βC subunit in normal human placenta exist. Moreover, the synthesis of this subunit in chorionic carcinoma cell lines BeWo and JEG is also quite unclear. These chorionic carcinoma cell lines are available now for over 30 years and are often used as in vitro model to evaluate normal and pathological placental endocrine function (Sullivan 2004). Therefore, the aim of this study was to evaluate the synthesis of the inhibin βC subunit in normal human placental tissue and in chorionic carcinoma cell lines BeWo and JEG.
Materials and Methods
Tissue Samples
Placental tissues were obtained from six placentas of women giving birth at the First Department of Obstetrics and Gynecology of the Ludwig–Maximilians-University Munich, Munich, Germany. Whether gestation can influence inhibin/activin production in the placenta is still controversial and, therefore, normal placental tissue samples were acquired. Tissue specimens of normal pregnancies were obtained in the course of an elective cesarean section for breech presentation, during the 38th week of gestation, to avoid any influencing factors due to the physiological “stress” of normal delivery (Mylonas et al. 2006a).
Immunohistochemistry
IHC was performed by a combination of pressure cooker heating and the standard streptavidin–biotin–peroxidase complex using the goat-IgG VECTASTAIN Elite ABC Kit (Vector Laboratories; Burlingame, CA) as previously described (Kimmich et al. in press). Briefly, paraffin-fixed tissue sections were dewaxed using xylol for 15 min and dehydrated twice in 100% ethanol. Endogenous peroxidase activity was quenched by immersion in 3% hydrogen peroxide (Merck; Darmstadt, Germany) in methanol for 20 min. After washing, the slides were subjected to antigen retrieval for 5 min in a pressure cooker using sodium citrate buffer (pH 6.0), containing 0.1 M citric acid and 0.1 M sodium citrate in distilled water. After cooling to room temperature, sections were washed twice in PBS. Nonspecific binding was blocked by incubating the sections with Ultra V Block (Lab Vision; Fremont, CA) for 45 min at room temperature. Sections were then incubated at 4C overnight with the inhibin βC polyclonal goat antibody (R&D Systems; Wiesbaden, Germany) at a dilution of 1:50 in Ultra V Block. After washing with PBS, sections were incubated with biotinylated anti-goat antibody (Vector Laboratories) for 30 min at room temperature. After incubation with the avidin–biotin–peroxidase complex (diluted in 10 ml PBS; Vector Laboratories) for 30 min and repeated washing with PBS, the sections were visualized using the ABC substrate buffer (VECTASTAIN Elite ABC Kit) and the chromagen 3,3′-diaminobenzidine (Dako; Glostrup, Denmark) at a concentration of 1 mg/ml for 2 min. Sections were counterstained with Mayer's acidic hematoxylin and dehydrated in an ascending series of ethanol (50–98%). After xylol treatment, sections were mounted. Negative controls were performed by replacing the primary antibody with normal goat IgG as an isotype control at the same dilution used for the primary antibody. Positive cells showed a brownish color, and negative controls, as well as unstained cells, showed a blue color. Sections were examined using a Leitz photomicroscope (Wetzlar, Germany). Digital images were obtained with a digital camera system (JVC; Yokohama, Japan) and were saved on computer (Diskus Software; Hilgers, Königswinter, Germany).
Cells and Cell Culture
The chorionic carcinoma cells BeWo and JEG were kindly provided by Dr. B. Ugele (First Department of Obstetrics and Gynecology, Ludwig–Maximilians-University Munich, Munich, Germany). Cells were cultured in Quantum 263 medium (PAA; Pasching, Austria) supplemented with antibiotics at 37C in a humidified atmosphere with 5% CO2 as previously described (Brüning et al. 2009; Kimmich et al. in press).
Immunofluorescence Analysis
Cells grown on glass cover slips were fixed with acetone for 10 min at room temperature and washed twice with PBS. Nonspecific binding was blocked by incubating the sections with Ultra V Block for 15 min at room temperature. Thereafter, the slides were incubated with the inhibin βC antibody (1:50 in dilution medium; Dako) overnight at 4C, followed by incubation with a Cy3-conjugated donkey–anti-goat antibody (1:500; Dianova, Hamburg, Germany) as previously described (Kimmich et al. in press). The slides were embedded in mounting buffer containing 4,6-diamino-2-phenylindole for staining the nuclei. Then, the slides were embedded with VECTASHIELD Mounting Medium (AXXORA; Lörrach, Germany) and examined with an Axiophot photomicroscope (Zeiss; Jena, Germany). Digital images were obtained with a digital camera system (AxioCam; Zeiss) and saved using the AxioVision software (Version 4.7; Zeiss).
RT-PCR Analysis
RNA was extracted from cells using the NucleoSpin RNA II kit (MACHEREY-NAGEL; Düren, Germany) as previously described (Kimmich et al. in press). Reverse transcription was performed with M-MLV reverse transcriptase and oligo(dT) (Promega; Mannheim, Germany) as recommended by the supplier. PCR was performed in an Eppendorf Mastercycler with GoTaq (Promega). Primer sequences were (5′–3′) GCAGCCCGGGTGAGAGTTGG (forward primer) and ACTGCACCCACAGGCCTC (reverse primer), which amplify a 393-bp product of the inhibin βC cDNA, as previously described (Kimmich et al. in press). PCR cycling was performed after a 5-min initiation at 94C with 36 cycles of 1 min at 94C, 1 min at 57C, and 2 min at 72C, followed by a 5-min extension at 72C. For cDNA quality control, actin primers (Stratagene; Amsterdam, The Netherlands), which amplify a 661-bp product, were used. As a further control, cDNA was omitted (water control) to identify any PCR contamination. PCR products were separated on a 1.5% agarose gel with pBR328 ladder markers (Roth; Karlsruhe, Germany). Gels contained the SYBR Safe dye (1:10,000 dilution; Invitrogen, Karlsruhe, Germany), and after a complete run, they were transferred on a UV-permeable tray to a BioRad Image Analyzer (Munich, Germany). The image file was exported as a TIFF file and imported into PowerPoint to crop and label the figure.
Results
Immunolabeling of Inhibin βC
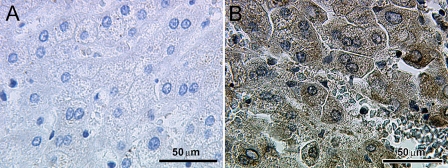
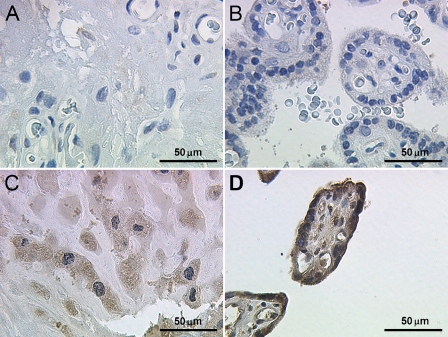
To test the specificity of the βC subunit antibody, evaluation of the immunohistochemical staining was performed using appropriate positive controls, including normal human liver specimens. The inhibin βC antibody (Figures 1A and 1B) stained hepatocytes, confirming previous results (Hötten et al. 1995; Schmitt et al. 1996; Zhang et al. 1997; Vejda et al. 2003). In normal placental tissue, inhibin βC was primarily expressed in the cytoplasm of extravillous trophoblast cells, whereas immunostaining in syncytiotrophoblast cells was moderate but weaker than trophoblast cells (Figures 2A–2D).
Figure 1.
Immunohistochemical staining reaction of inhibin βC in normal human liver tissue. Normal human liver tissue was used as a positive control for inhibin βC immunolabeling. Negative controls were performed by replacing the primary antibody with normal goat IgG as an isotype control. (A) No staining was detected in the negative control. (B) The inhibin βC subunit was detected in hepatocytes of normal human liver tissue, but not in erythrocytes.
Figure 2.
Immunohistochemical staining reaction of inhibin βC in normal human placenta tissue. No staining was detected in the negative controls for trophoblast cells (A) or syncytiotrophoblast cells (B). (C) Invasive trophoblast cells were strongly stained for inhibin βC. (D) Additionally, syncytiotrophoblast cells also showed cytoplasmic staining for the novel inhibin βC subunit.
Expression Analysis of Inhibin βC in Human Chorionic Carcinoma Cell Lines by Immunofluorescence
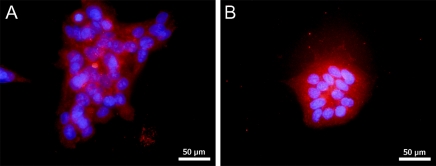
Chorionic carcinoma cells are malignant cell lines derived from invasive trophoblast cells. We, therefore, tested the expression of inhibin βC in the human trophoblast cancer cell lines BeWo and JEG. Both cell lines expressed the novel βC subunit at the protein level, as demonstrated by positive immunohistochemical staining in the cytoplasm (Figures 3A and 3B).
Figure 3.
Localization of inhibin βC in BeWo and JEG. The carcinoma cell lines were analyzed by immunofluorescence for expression of inhibin βC. Positive cytoplasmic staining was detected in BeWo (A) and JEG (B) cells.
RT-PCR Analysis
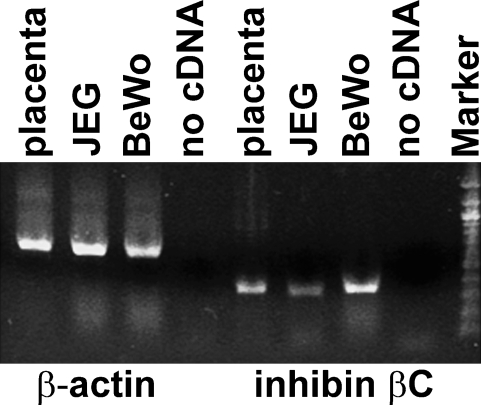
To analyze the inhibin βC subunit expression at the transcriptional level, RNA was extracted from samples of human placental tissue, transcribed into cDNA, and analyzed by PCR using specific primers. Figure 4 demonstrates that the mRNA of the inhibin βC subunit (393-bp PCR product) is expressed in normal placental cells. Further, expression of this subunit in the chorionic carcinoma cell lines BeWo and JEG was confirmed at the transcriptional level by RT-PCR analysis.
Figure 4.
Inhibin βC expression in normal placental tissue and chorionic carcinoma cell lines. Normal placental tissue and BeWo and JEG cells were analyzed by RT-PCR for the expression of the inhibin βC subunit. PCR products from inhibin βC mRNA were detected in each sample. Interestingly, the inhibin βC mRNA level was less intense in JEG cells compared with BeWo cells and placental tissue. Furthermore, the water control to demonstrate any PCR contamination was negative.
Discussion
Inhibins and activins were initially characterized as endocrine and paracrine hormonal regulators of the hypothalamic–pituitary–gonadal axis. In addition, the human placenta expresses inhibin/activin mRNA (Petraglia et al. 1991), and inhibin/activin subunits are thought to be the primary source of maternal circulating inhibin and activin (Florio et al. 2001). Although the precise physiological roles of placental inhibins and activins are still unclear, possible functions could include decidualization (Jones et al. 2006), trophoblast differentiation (Caniggia et al. 1997), immunomodulatory functions (Keelan et al. 2000), steroidogenesis (Ni et al. 2000; Ferreira et al. 2008), and apoptosis (Chen et al. 2002). Moreover, these proteins are intriguing as possible prognostic serological markers, because inhibin subunits and the entire inhibin or activin proteins have been associated with placenta-associated diseases such as preeclampsia and HELLP syndrome (Muttukrishna et al. 1997; Craig et al. 2000; Seufert et al. 2004; Mylonas et al. 2006a; Grill et al. 2009).
The inhibin/activin βC subunit is predominantly expressed in hepatocytes (Hötten et al. 1995; Schmitt et al. 1996; Zhang et al. 1997; Vejda et al. 2003). However, this subunit has been detected in cells from several other organs, including normal or malignant prostate and ovarian, testicular, endometrial, and pituitary tissues (Loveland et al. 1996; Mellor et al. 2000; Gold et al. 2004,2009; Kimmich et al. in press). The inhibin βC gene was detected in placental tissue from uncomplicated term pregnancies and pregnancies with preeclampsia (Casagrandi et al. 2003). We have demonstrated the presence of the inhibin βC subunit at the transcriptional level and at the protein level in the normal human placenta. Additionally, chorionic carcinoma cell lines also express this novel subunit. These cell lines have become valuable in vitro tools for studying placental physiology, invasion, and differentiation (King et al. 2000; Al-Nasiry et al. 2007; Serrano et al. 2007). Moreover, knowledge of the expression patterns of the β subunits has become tremendously important, because activin signaling may be a promising molecular therapeutic target for a variety of diseases, including metabolic diseases and malignant tumors (Tsuchida et al. 2009). Because the precise function of this novel subunit in human reproduction is still unclear, these cell lines can be used for further analysis of normal and pathological placental functions.
The precise role of the βC subunit has not yet been elucidated. Mice deficient in the activin βC gene, the activin βE gene, or both appeared normal and viable (Lau et al. 2000), leading to the hypothesis that this subunit has a minor role in human reproduction (Gold et al. 2009). Therefore, it is not surprising that the research focus has concentrated on liver function. In an immortalized mouse hepatocyte cell line (AML12) and in primary rat hepatocytes, activin βC increases the rate of DNA synthesis (Wada et al. 2005). Moreover, the βC subunit was identified as an autocrine growth modulator in liver regeneration, leading to mitosis in a subset of hepatocytes (Gold et al. 2005). However, ectopic expression of this subunit induced apoptosis in hepatoma cells (Chabicovsky et al. 2003; Vejda et al. 2003). Whether, and to what extent, the βC subunit can also act as a mitotic agent in human placental tissue is still unclear.
One major issue remains unresolved, the question of putative inhibin or activin synthesis and the physiological function of these proteins in vivo. The formation of homodimeric activin C (βC–βC), heterodimeric activins AC (βA–βC), BC (βB–βC), and CE (βC–βE), and inhibin C (α–β) has been demonstrated by ectopic expression of the respective subunits in different cell models (Mellor et al. 2000; Ushiro et al. 2006). Interestingly, activin C (βC–C) does not activate activin A (βA–βA)-responsive promoters, suggesting that the βC subunit regulates the levels of bioactive activin A (βA–βA) through the formation of signaling-incompetent activin AC heterodimers (Mellor et al. 2003; Butler et al. 2005; Gold et al. 2009). Therefore, the βC subunit might function as an antagonist of activin function and thereby regulate activin signaling (Mellor et al. 2000,2003). However, data that confirm secretion of the intact inhibins or activins are still lacking because of the lack of appropriate immunoassays.
In conclusion, inhibin βC mRNA and protein were detected in normal placental tissue by immunohistochemical and molecular assays. Additionally, chorionic carcinoma cell lines, which are often used to evaluate normal placental function, also express this novel subunit. However, the functional role of inhibin in the normal and pathological human placenta is still quite unclear, and therefore, inhibin subunits may have pleiotropic but distinct roles during pregnancy and gestational diseases.
Acknowledgments
This study was partially supported by the FöFoLe Program of the Ludwig–Maximilians-University Munich (297/03), the Friedrich–Baur-Institute of the Ludwig–Maximilians-University Munich, and the Weigland Stipendium Program of the Ludwig–Maximilians-University Munich (to IM). A.B. and I.M. are supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG BR 3641/3-1).
We thank Mrs. C. Kuhn, Mrs. S. Kunze, Mrs. S. Schulze, and Mrs. I. Wiest for their excellent work with the placental samples. Additionally, we thank Prof. Jeschke and Prof. Friese for their help in conducting this study, and Mr. P. Fuchs for his help with the images.
This article is distributed under the terms of a License to Publish Agreement (http://www.jhc.org/misc/ltopub.shtml). JHC deposits all of its published articles into the U.S. National Institutes of Health (http://www.nih.gov/) and PubMed Central (http://www.pubmedcentral.nih.gov/) repositories for public release twelve months after publication.
References
- Aitken DA, Wallace EM, Crossley JA, Swanston IA, van Pareren Y, van Maarle M, Groome NP, et al. (1996) Dimeric inhibin A as a marker for Down's syndrome in early pregnancy. N Engl J Med 334:1231–1236 [DOI] [PubMed] [Google Scholar]
- Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R (2007) The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod 22:1304–1309 [DOI] [PubMed] [Google Scholar]
- Brüning A, Makovitzky J, Gingelmaier A, Friese K, Mylonas I (2009) The metastasis-associated genes MTA1 and MTA3 are abundantly expressed in human placenta and chorionic carcinoma cells. Histochem Cell Biol 132:33–38 [DOI] [PubMed] [Google Scholar]
- Butler CM, Gold EJ, Risbridger GP (2005) Should activin betaC be more than a fading snapshot in the activin/TGFbeta family album? Cytokine Growth Factor Rev 16:377–385 [DOI] [PubMed] [Google Scholar]
- Caniggia I, Lye SJ, Cross JC (1997) Activin is a local regulator of human cytotrophoblast cell differentiation. Endocrinology 138:3976–3986 [DOI] [PubMed] [Google Scholar]
- Casagrandi D, Bearfield C, Geary J, Redman CW, Muttukrishna S (2003) Inhibin, activin, follistatin, activin receptors and beta-glycan gene expression in the placental tissue of patients with pre-eclampsia. Mol Hum Reprod 9:199–203 [DOI] [PubMed] [Google Scholar]
- Chabicovsky M, Herkner K, Rossmanith W (2003) Overexpression of activin beta(C) or activin beta(E) in the mouse liver inhibits regenerative deoxyribonucleic acid synthesis of hepatic cells. Endocrinology 144:3497–3504 [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev 23:787–823 [DOI] [PubMed] [Google Scholar]
- Chen YG, Lui HM, Lin SL, Lee JM, Ying SY (2002) Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 227:75–87 [DOI] [PubMed] [Google Scholar]
- Craig K, Pinette MG, Blackstone J, Chard R, Cartin A (2000) Highly abnormal maternal inhibin and beta-human chorionic gonadotropin levels along with severe HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome at 17 weeks' gestation with triploidy. Am J Obstet Gynecol 182:737–739 [DOI] [PubMed] [Google Scholar]
- Debieve F, Pampfer S, Thomas K (2000) Inhibin and activin production and subunit expression in human placental cells cultured in vitro. Mol Hum Reprod 6:743–749 [DOI] [PubMed] [Google Scholar]
- Esquela AF, Zimmers TA, Koniaris LG, Sitzmann JV, Lee SJ (1997) Transient down-regulation of inhibin-betaC expression following partial hepatectomy. Biochem Biophys Res Commun 235:553–556 [DOI] [PubMed] [Google Scholar]
- Fang J, Yin W, Smiley E, Wang SQ, Bonadio J (1996) Molecular cloning of the mouse activin beta E subunit gene. Biochem Biophys Res Commun 228:669–674 [DOI] [PubMed] [Google Scholar]
- Ferreira MC, Cavallo IK, Florio P, Petraglia F, Reis FM (2008) Activin betaA subunit, follistatin and follistatin-like 3 are expressed in the endometrium of ovariectomized rats and regulated by estrogen replacement. J Mol Histol 39:535–541 [DOI] [PubMed] [Google Scholar]
- Florio P, Cobellis L, Luisi S, Ciarmela P, Severi FM, Bocchi C, Petraglia F (2001) Changes in inhibins and activin secretion in healthy and pathological pregnancies. Mol Cell Endocrinol 180:123–130 [DOI] [PubMed] [Google Scholar]
- Gold E, Jetly N, O'Bryan MK, Meachem S, Srinivasan D, Behuria S, Sanchez-Partida LG, et al. (2009) Activin C antagonizes activin A in vitro and overexpression leads to pathologies in vivo. Am J Pathol 174:184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EJ, O'Bryan MK, Mellor SL, Cranfield M, Risbridger GP, Groome NP, Fleming JS (2004) Cell-specific expression of beta C-activin in the rat reproductive tract, adrenal and liver. Mol Cell Endocrinol 222:61–69 [DOI] [PubMed] [Google Scholar]
- Gold EJ, Zhang X, Wheatley AM, Mellor SL, Cranfield M, Risbridger GP, Groome NP, et al. (2005) betaA- and betaC-activin, follistatin, activin receptor mRNA and betaC-activin peptide expression during rat liver regeneration. J Mol Endocrinol 34:505–515 [DOI] [PubMed] [Google Scholar]
- Grill S, Rusterholz C, Zanetti-Dallenbach R, Tercanli S, Holzgreve W, Hahn S, Lapaire O (2009) Potential markers of preeclampsia—a review. Reprod Biol Endocrinol 7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hötten G, Neidhardt H, Schneider C, Pohl J (1995) Cloning of a new member of the TGF-beta family: a putative new activin beta C chain. Biochem Biophys Res Commun 206:608–613 [DOI] [PubMed] [Google Scholar]
- Jones RL, Findlay JK, Salamonsen LA (2006) The role of activins during decidualisation of human endometrium. Aust N Z J Obstet Gynaecol 46:245–249 [DOI] [PubMed] [Google Scholar]
- Keelan JA, Zhou RL, Mitchell MD (2000) Activin A exerts both pro- and anti-inflammatory effects on human term gestational tissues. Placenta 21:38–43 [DOI] [PubMed] [Google Scholar]
- Kimmich T, Bruning A, Kaufl SD, Makovitzky J, Kuhn C, Jeschke U, Friese K, et al. (In Press) Inhibin/activin-betaC and -betaE subunits in the Ishikawa human endometrial adenocarcinoma cell line. Arch Gynecol Obstet. Published online December 11, 2009 (DOI: 10.1007/s00404-009-1310-y) [DOI] [PubMed]
- King A, Thomas L, Bischof P (2000) Cell culture models of trophoblast II: trophoblast cell lines—a workshop report. Placenta 21(suppl A):S113–119 [DOI] [PubMed] [Google Scholar]
- Lau AL, Kumar TR, Nishimori K, Bonadio J, Matzuk MM (2000) Activin betaC and betaE genes are not essential for mouse liver growth, differentiation, and regeneration. Mol Cell Biol 20:6127–6137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KL, McFarlane JR, de Kretser DM (1996) Expression of activin beta C subunit mRNA in reproductive tissues. J Mol Endocrinol 17:61–65 [DOI] [PubMed] [Google Scholar]
- McCluggage WG, Ashe P, McBride H, Maxwell P, Sloan JM (1998) Localization of the cellular expression of inhibin in trophoblastic tissue. Histopathology 32:252–256 [DOI] [PubMed] [Google Scholar]
- Mellor SL, Ball EM, O'Connor AE, Ethier JF, Cranfield M, Schmitt JF, Phillips DJ, et al. (2003) Activin betaC-subunit heterodimers provide a new mechanism of regulating activin levels in the prostate. Endocrinology 144:4410–4419 [DOI] [PubMed] [Google Scholar]
- Mellor SL, Cranfield M, Ries R, Pedersen J, Cancilla B, de Kretser D, Groome NP, et al. (2000) Localization of activin beta(A)-, beta(B)-, and beta(C)-subunits in humanprostate and evidence for formation of new activin heterodimers of beta(C)-subunit. J Clin Endocrinol Metab 85:4851–4858 [DOI] [PubMed] [Google Scholar]
- Muttukrishna S, Knight PG, Groome NP, Redman CW, Ledger WL (1997) Activin A and inhibin A as possible endocrine markers for pre-eclampsia. Lancet 349:1285–1288 [DOI] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Wiest I, Hoeing A, Vogl J, Shabani N, Kuhn C, et al. (2004) Inhibin/activin subunits alpha, beta-A and beta-B are differentially expressed in normal human endometrium throughout the menstrual cycle. Histochem Cell Biol 122:461–471 [DOI] [PubMed] [Google Scholar]
- Mylonas I, Schiessl B, Jeschke U, Vogl J, Makrigiannakis A, Kuhn C, Kunze S, et al. (2006a) Expression of inhibin/activin subunits alpha (-alpha), beta A (-beta (A)) and beta B (-beta (B)) in placental tissue of normal and intrauterine growth restricted (IUGR) pregnancies. J Mol Histol 37:43–52 [DOI] [PubMed] [Google Scholar]
- Mylonas I, Schiessl B, Jeschke U, Vogl J, Makrigiannakis A, Kuhn C, Schulze S, et al. (2006b) Expression of inhibin/activin subunits alpha (-alpha), betaA (-betaA), and betaB (-betaB) in placental tissue of normal, preeclamptic, and HELLP pregnancies. Endocr Pathol 17:19–33 [DOI] [PubMed] [Google Scholar]
- Mylonas I, Shabani N, Vogl J, Makovitzky J, Kunze S, Kuhn C, Schulze S, et al. (2007) Inhibin/activin subunits are immunohistochemically expressed in complete and partial hydatidiform moles. Anticancer Res 27:1995–2000 [PubMed] [Google Scholar]
- Mylonas I, Worbs S, Shabani N, Kuhn C, Kunze S, Schulze S, Dian D, et al. (2009) Inhibin-alpha subunit is an independent prognostic parameter in human endometrial carcinomas: analysis of inhibin/activin-alpha, -betaA and -betaB subunits in 302 cases. Eur J Cancer 45:1304–1314 [DOI] [PubMed] [Google Scholar]
- Ni X, Luo S, Minegishi T, Peng C (2000) Activin A in JEG-3 cells: potential role as an autocrine regulator of steroidogenesis in humans. Biol Reprod 62:1224–1230 [DOI] [PubMed] [Google Scholar]
- Petraglia F, Garuti GC, Calza L, Roberts V, Giardino L, Genazzani AR, Vale W, et al. (1991) Inhibin subunits in human placenta: localization and messenger ribonucleic acid levels during pregnancy. Am J Obstet Gynecol 165:750–758 [DOI] [PubMed] [Google Scholar]
- Schmitt J, Hotten G, Jenkins NA, Gilbert DJ, Copeland NG, Pohl J, Schrewe H (1996) Structure, chromosomal localization, and expression analysis of the mouse inhibin/activin beta C (Inhbc) gene. Genomics 32:358–366 [DOI] [PubMed] [Google Scholar]
- Serrano MA, Macias RI, Briz O, Monte MJ, Blazquez AG, Williamson C, Kubitz R, et al. (2007) Expression in human trophoblast and choriocarcinoma cell lines, BeWo, Jeg-3 and JAr of genes involved in the hepatobiliary-like excretory function of the placenta. Placenta 28:107–117 [DOI] [PubMed] [Google Scholar]
- Seufert R, Neubert S, Tanner B, Schaffrath M, Pollow K, Kolbl H (2004) Inhibins and activin A in hypertensive disorders of pregnancy and HELLP-syndrome. Zentralbl Gynakol 126:148–153 (in German) [DOI] [PubMed] [Google Scholar]
- Sullivan MH (2004) Endocrine cell lines from the placenta. Mol Cell Endocrinol 30:103–119 [DOI] [PubMed] [Google Scholar]
- Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K (2009) Activin signaling as an emerging target for therapeutic interventions. Cell Commun Signal 7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro Y, Hashimoto O, Seki M, Hachiya A, Shoji H, Hasegawa Y (2006) Analysis of the function of activin betaC subunit using recombinant protein. J Reprod Dev 52:487–495 [DOI] [PubMed] [Google Scholar]
- Vale W, Rivier C, Hsueh A, Campen C, Meunier H, Bicsak T, Vaughan J, et al. (1988) Chemical and biological characterization of the inhibin family of protein hormones. Recent Prog Horm Res 44:1–34 [DOI] [PubMed] [Google Scholar]
- Vejda S, Erlach N, Peter B, Drucker C, Rossmanith W, Pohl J, Schulte-Hermann R, et al. (2003) Expression of activins C and E induces apoptosis in human and rat hepatoma cells. Carcinogenesis 24:1801–1809 [DOI] [PubMed] [Google Scholar]
- Wada W, Medina JJ, Kuwano H, Kojima I (2005) Comparison of the function of the beta(C) and beta(E) subunits of activin in AML12 hepatocytes. Endocr J 52:169–175 [DOI] [PubMed] [Google Scholar]
- Welt C, Sidis Y, Keutmann H, Schneyer A (2002) Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227:724–752 [DOI] [PubMed] [Google Scholar]
- Worbs S, Shabani N, Mayr D, Gingelmaier A, Makrigiannakis A, Kuhn C, Jeschke U, et al. (2007) Expression of the inhibin/activin subunits (-alpha, -betaA and -betaB) in normal and carcinogenic endometrial tissue: possible immunohistochemical differentiation markers. Oncol Rep 17:97–104 [PubMed] [Google Scholar]
- Zhang YQ, Shibata H, Schrewe H, Kojima I (1997) Reciprocal expression of mRNA for inhibin betaC and betaA subunits in hepatocytes. Endocr J 44:759–764 [DOI] [PubMed] [Google Scholar]