Abstract
This commentary provides an overview of the paper by Carletti et al. (this issue) that describes a role for microRNAs in granulosa cell survival.
The development and rupture of an ovulatory follicle is dependent upon the actions of the gonadotropins follicle-stimulating hormone and the midcycle surge of luteinizing hormone (LH), respectively. After rupture, the cellular remnants of the ovulatory follicle differentiate into steroid-secreting luteal cells. Continued survival of the granulosa cells following ovulation and their luteinization is critical for progesterone production and the ability to sustain pregnancy, and several studies [1–3] have documented a prosurvival effect from surge levels of LH on granulosa cells following its binding and signaling via LH/chorionic gonadotropin receptors (LHCGR).
The balance between induction of proapoptotic and antiapoptotic pathways in granulosa cells residing within an ovulatory follicle, however, is complex and not well understood. Factors downstream of LH signaling that determine whether granulosa cell survival or apoptosis is favored have been discovered and investigated individually. For example, progesterone has an antiapoptotic effect on luteinized granulosa cells in most species studied, thereby promoting their continued survival and allowing development of the corpus luteum [4–7]. Other examples of individual factors that play a role in determining granulosa cell survival in the ovulatory follicle include pituitary adenylate cyclase-activating polypeptide (PACAP), epidermal growth factor-like (EGF) ligands, hyaluronan and proteoglycan link protein 1 (HAPLN1), as well as phosphatase and tensin homolog (PTEN) [8–11]. Each of these components regulates distinct pathways that in turn ultimately regulate the induction of apoptosis. A report by Carletti et al. [12] in this issue of Biology of Reproduction provides insight regarding a novel mechanism whereby granulosa cell survival is maintained in the ovulatory follicle after LHCGR signaling through the synthesis and action of a specific microRNA (miRNA).
MicroRNA Synthesis and Function
The miRNAs are small (19–25 bp), noncoding RNAs that limit cellular protein expression [13]. In mammals, miRNAs are first synthesized as RNA transcripts between several tens and hundreds of kilobases long (referred to as primary miRNA [pri-miRNA]) by the DNA-dependent RNA polymerase-II (POLII) [14].
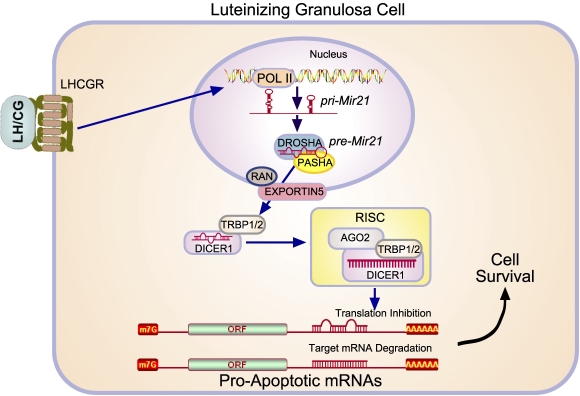
A pri-miRNA typically possesses multiple miRNAs and is cleaved in the nucleus into shorter (∼70-bp) precursor miRNAs. This reaction is performed by a protein complex consisting of the enzyme DROSHA and the double-stranded RNA-binding protein PASHA [15]. The precursor miRNA is exported from the nucleus by an EXPORTIN5/RAN complex and cleaved in the cytoplasm by DICER1 into a mature, 19- to 25-nucleotide miRNA duplex. The miRNA strand with lower base pairing stability (the guide strand) is loaded onto the RNA-induced silencing complex (RISC) composed of DICER1, TAR RNA-binding protein isoforms 1 or 2 (TRBP1/2), and the Argonaute protein AGO2 [13, 16]. The RISC complex is guided to its mRNA target by the miRNA strand, where it then prevents protein synthesis via two different mechanisms: inhibition of translation or RNase-mediated destruction of mRNA [17–19]. The former occurs when the imperfect homology between the miRNA and target mRNA interferes with translation, whereas the latter results from a perfect match between the miRNA/mRNA hybrid and the activation of RNase activity that leads to mRNA degradation (Fig. 1).
FIG. 1.
Signaling through the LHCGR induces the expression of the prosurvival microRNA Mir21 in luteinizing granulosa cells. Granulosa cells residing within the periovulatory follicle respond to LH to induce the transcription of primary (pri)Mir21, which is subsequently processed by DROSHA and PASHA. Following its export to from the nucleus by EXPORTIN5/RAN, DICER1 converts Mir21 into its mature form. Following its association with the components (e.g., DICER1, TAR-RNA binding protein [TRBP1/2], and the Argonaute protein AGO2) that form the RISC, Mir21 binds to target mRNAs and inhibits protein expression through their degradation or interference with translation. In the luteinizing granulosa cells of the ovulatory follicle, such targets include those that otherwise directly or indirectly promote apoptosis.
Recent studies have determined that mRNA degradation grossly affects protein production, whereas inhibition of translation leads to fine-scale adjustments in protein levels [20]. Moreover, as the inhibition of translation occurs with mismatches between the mRNA and the miRNA, numerous genes may be targets of a single miRNA. In fact, the levels of hundreds, and perhaps even thousands, of different proteins can be reduced through the action of a single miRNA [19, 20].
MicroRNA Action in the Ovulatory Follicle
Shortly after the discovery of miRNAs and the realization that they serve as master regulators of posttranscriptional events, a few studies were conducted to determine their presence, and in some cases targets, in the female reproductive tract [21–24]. A critical role for miRNAs in regulating female fertility, however, was firmly established through the generation of conditional Dicer1 null mutants, wherein its selective deletion in the ovary led to female reproductive effects, including inhibition of antral follicle development, induction of granulosa cell apoptosis, and reduced ovulatory efficiency [25–27].
Fiedler et al. [28] subsequently used a microarray approach to systematically identify differentially expressed miRNAs in granulosa cells before and after LHCGR activation. RNA was obtained from mouse granulosa cells before (0 h, nonluteinized cells) and 4 h after human chorionic gonadotropin (hCG) treatment, from which 212 unique miRNA species were identified. Of these, 13 miRNAs were differentially expressed between the 0 and 4 h post-hCG samples, with 10 being downregulated and 3 being upregulated. Two of the latter miRNAs, granulosa cell microRNA-132 (Mir132) and granulosa cell microRNA-212 (Mir212), were induced 17- and 34-fold, respectively, relative to basal levels (0 h, control) following hCG treatment. Both Mir132 and Mir212 are formed from the same pri-miRNA, which, as expected, is also induced in mouse granulosa cells through LHCGR signaling. Furthermore, the study demonstrated that inhibition of mouse granulosa cell Mir132 action using complementary locked nucleic acid (LNA) oligonucleotides increased C-terminal binding protein-1 (CTBP1) protein levels, which in turn may directly affect granulosa cell steroid production, because CTBP1 has been shown to regulate adrenal steroidogenesis by serving as a corepressor of steroidogenic factor-1 (SF1) activity [29].
The study by Fiedler et al. [28] led to the systematic evaluation of the miRNAs differentially expressed in luteinizing granulosa cells, and it serves as the foundation for the paper by Carletti et al. [12] in this issue of Biology of Reproduction. Carletti et al. observed that hCG treatment of equine chorionic gonadotropin (eCG)-primed immature mice led to a 30-fold maximum stimulation of pri-Mir21 in mural granulosa cells collected 4 h after hCG, whereas mature Mir21 mural granulosa cell levels increased to a 5.8-fold maximum 6 h after hCG. In contrast, regulation of mature Mir21 expression in nonluteinized mouse granulosa cells in vitro did not recapitulate what was observed in vivo, in that its abundance in cultured granulosa cells increased 5-fold upon plating, with no change following treatment of cells with hCG or cell-permeable 8-bromo-cAMP. In granulosa cells transfected with a Mir21-targeting LNA oligonucleotide (MIR21-LNA), the authors demonstrated that Mir21 could be depleted to approximately 4% of the levels present in cells treated with a scrambled or control LNA. Reduction of Mir21 levels to that extent resulted in a marked change in granulosa cell morphology that included their “rounding” and “lifting” off of the culture dish, both of which are hallmarks of apoptosis.
Molecular markers of apoptosis (positive annexin A5 staining determined by flow cytometry; cleaved caspase 3 determined by immunoblotting) increased hours after granulosa cells were transfected with the MIR21-LNA in a dose-dependent manner. The authors also noted that cleaved caspase 3 in granulosa cells decreased 6 h after administration of hCG in vivo, the time that coincides with increasing levels of Mir21. The in vivo effect of Mir21 on ovarian apoptosis was subsequently established through the intrabursal injection of a Mir21-targeting LNA possessing a nuclease-resistant phosphorothioate backbone before hCG administration. Apoptosis was significantly increased in Mir21-depleted ovaries compared to the contralateral ovary receiving only saline or scrambled LNA. Moreover, using this same in vivo anti-Mir21 LNA delivery approach, the number of cumulus-oocyte complexes recovered from the oviduct adjacent to the ovary treated with MIR21-LNA was significantly reduced compared to the ovary treated with scrambled LNA or the saline-treated control ovary. Thus, these findings demonstrate for the first time that a single miRNA (Mir21) plays a critical role in maintaining the survival of granulosa cells in the periovulatory follicle in response to LH (Fig. 1). This is also the first study to demonstrate a role for miRNAs, particularly an oncogenic miRNA such as Mir21, in the normal physiology of the ovary.
MicroRNAs and Ovarian Function: Future Directions
For studies that shed light on a novel or previously unappreciated biological process, more new questions surface than are answered. The study by Carletti et al. is no different in this respect, with the most prominent question being which mRNAs are posttranscriptionally regulated by Mir21 that protect luteinizing granulosa cells from apoptosis? Targets previously reported to be under the control of Mir21 were analyzed by the authors, but none was found to change substantially when granulosa cells were treated with the MIR21-LNA. A systematic genomic and proteomic determination of Mir21-regulated targets in granulosa cells from the ovulatory follicle would likely clarify this issue. In the study by Carletti et al., increased expression of Mir21 was also noted in the granulosa cells obtained following gonadotropin-mediated follicle development (i.e., after eCG injection), raising the question of whether this miRNA exhibits the same antiapoptotic activities during folliculogenesis. This question is particularly relevant in light of the recent published report demonstrating that conditional inactivation of DICER1 in follicular granulosa cells leads to increased primordial follicle numbers and accelerated early follicle recruitment [27]. Lastly, the mechanisms regulating ovarian Mir21 expression, or other miRNAs for that matter, are presently unknown and await further investigation.
Footnotes
Supported by NIH grants NCRR RR00163, NICHD HD42000, NICHD HD20869, and NICHD U54 HD055744.
REFERENCES
- Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA.Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci 2004; 82(E-suppl):E40–E52. [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS.Hormonal control of the cell cycle in ovarian cells: proliferation versus differentiation. Biol Reprod 1998; 59: 476–482. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Schwinof KM, Stouffer RL.Gonadotropin and steroid control of granulosa cell proliferation during the periovulatory interval in rhesus monkeys. Biol Reprod 2001; 65: 755–762. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markstrom E, Andersson M, Billig H.Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biol Reprod 2000; 63: 1457–1464. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Coukos G, Christofidou-Solomidou M, Montas S, Coutifaris C.Progesterone is an autocrine/paracrine regulator of human granulosa cell survival in vitro. Ann N Y Acad Sci 2000; 900: 16–25. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM.Progesterone receptor and the cell cycle modulate apoptosis in granulosa cells. Endocrinology 2004; 145: 5033–5043. [DOI] [PubMed] [Google Scholar]
- Peluso JJ.Multiplicity of progesterone's actions and receptors in the mammalian ovary. Biol Reprod 2006; 75: 2–8. [DOI] [PubMed] [Google Scholar]
- Ben-Ami I, Armon L, Freimann S, Strassburger D, Ron-El R, Amsterdam A.EGF-like growth factors as LH mediators in the human corpus luteum. Hum Reprod 2009; 24: 176–184. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Cahill N, Richards JS.Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 2008; 22: 2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Park HJ, Choi HS, Kwon HB, Arimura A, Lee BJ, Choi WS, Chun SY.Gonadotropin stimulation of pituitary adenylate cyclase-activating polypeptide (PACAP) messenger ribonucleic acid in the rat ovary and the role of PACAP as a follicle survival factor. Endocrinology 1999; 140: 818–826. [DOI] [PubMed] [Google Scholar]
- Liu J, Park ES, Curry TE, Jr, Jo M.Periovulatory expression of hyaluronan and proteoglycan link protein 1 (Hapln1) in the rat ovary: hormonal regulation and potential function. Mol Endocrinol 2010; 24: 1203–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti MZ, Fiedler SD, Christenson LK.MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod 2010; 83: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushati N, Cohen SM.MicroRNA functions. Annu Rev Cell Dev Biol 2007; 23: 175–205. [DOI] [PubMed] [Google Scholar]
- Cai X, Hagedorn CH, Cullen BR.Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004; 10: 1957–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN.The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425: 415–419. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R.Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 2005; 123: 631–640. [DOI] [PubMed] [Google Scholar]
- Hutvagner G, Zamore PD.A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002; 297: 2056–2060. [DOI] [PubMed] [Google Scholar]
- Martinez J, Tuschl T.RISC is a 5′-phosphomonoester-producing RNA endonuclease. Genes Dev 2004; 18: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N.Widespread changes in protein synthesis induced by microRNAs. Nature 2008; 455: 58–63. [DOI] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP.The impact of microRNAs on protein output. Nature 2008; 455: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro S, Song R, Park C, Zheng H, Sanders KM, Yan W.Cloning and expression profiling of small RNAs expressed in the mouse ovary. RNA 2007; 13: 2366–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK.MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A 2007; 104: 15144–15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, Ma XH, Ni H, Lei W, Yang ZM.MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem 2008; 283: 23473–23484. [DOI] [PubMed] [Google Scholar]
- Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW.Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod 2010; 82: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK.Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 2008; 149: 6207–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM.Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 2008; 22: 2336–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK.The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 2010; 315: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler SD, Carletti MZ, Hong X, Christenson LK.Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod 2008; 79: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammer EB, Sewer MB.Phosphorylation of CtBP1 by cAMP-dependent protein kinase modulates induction of CYP17 by stimulating partnering of CtBP1 and 2. J Biol Chem 2008; 283: 6925–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]