Abstract
Human and rat endometriotic lesions synthesize and secrete tissue inhibitor of metalloproteinase 1 (TIMP1). More TIMP1 localizes in the ovarian theca in an established rat model for endometriosis (Endo) when compared to surgical controls (Sham). We hypothesized that endometriotic TIMP1 secreted into peritoneal fluid (PF) negatively affects ovarian function and embryogenesis by altering the balance of matrix metalloproteinases (MMPs) and TIMPs. Three experiments were performed modulating TIMP1 in vitro and in vivo to investigate ovarian and embryonic anomalies. The first experiment demonstrated control embryos treated in vitro with endometriotic PF concentrations of TIMP1 developed abnormally. In the second experiment where TIMP1 was modulated in vivo, TIMP1-treated Sham rats had fewer zygotes, ovarian follicles, and corpora lutea (CLs) and poorer embryo quality and development, which is analogous to the findings in Endo rats. Importantly, Endo rats treated with a TIMP1 function-blocking antibody had zygote, follicle, and CL numbers and embryo quality similar to Sham rats. In addition, more TIMP1 inhibitory activity was found in ovaries from Endo and TIMP1-treated Sham rats than in ovaries from Sham or TIMP1 antibody-treated Endo rats. In experiment three, control rats (no surgery) treated with Endo PF had fewer follicles and CLs and increased TIMP1 localization in the ovarian theca whereas treatment with Endo PF stripped of TIMP1 or with Sham PF had no effect, providing further evidence that endometriotic TIMP1 sequesters in the ovary and inhibits MMPs necessary for ovulation. Collectively, these results showed that excessive TIMP1 was deleterious to ovulation and embryo development. Thus, novel TIMP1-modulating therapies may be developed to alleviate infertility in women with endometriosis.
Keywords: embryo,; endometriosis,; infertility,; ovary,; TIMP1
Modulating peritoneal levels of TIMP1 affects ovarian function and embryo development in rats with surgically induced endometriosis.
INTRODUCTION
Endometriosis is a gynecological disease that causes pain and infertility in women of reproductive age. Clear mechanisms causing the endometriosis-associated infertility have not been firmly established. Infertility in women with endometriosis may be associated with subtle, explicit or multifaceted abnormalities [1–8]. Anomalies have been identified in the ovary such as reduced rates of follicular growth, functional capacity of the preovulatory follicle, and early luteal function [1, 2, 4, 5]; in gametes and embryos, including reduced rates of fertilization and defects in embryo development [1, 6, 8–10]; and in endometrial function [7, 11].
Because of the ethical limitations of performing controlled studies of infertility in women with endometriosis, animal models provide a valuable tool to study risk factors, prevalence, and the pathogenesis and pathophysiologies of endometriosis [12, 13]. Rats with surgically induced endometriosis (Endo) display pathophysiologies similar to those of primates and humans with the disease, including pain and infertility [12–14]. An association between the presence of ectopic endometriotic implants and reduced fecundity in rats has been described [13, 15–17].
Expression of matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs) is reportedly involved in the pathogenesis and pathophysiologies of endometriosis occurring in women and in animal models [17–22]. The MMPs are a family of enzymes that degrade the extracellular matrix, including basement membrane components [23]. TIMPs inhibit MMPs to facilitate tightly controlled tissue remodeling and other biological functions. A 1:1 stoichiometric balance of these enzymes and their inhibitors is required for normal follicular development, ovulation, formation and regression of the corpora lutea (CL), embryo development, and embryo implantation [24–32].
The MMPs and TIMPs are synthesized and secreted by both eutopic and ectopic endometrium in both the human and the rat [19–21, 33, 34]. TIMP1 represents at least 10% to 15% of proteins secreted into the peritoneal cavity by both rat implants and human endometriotic lesions [33, 35]. Because products from endometriotic lesions in the peritoneal fluid (PF) bathe the ovary and enter the uterus through the oviducts, endometriotic TIMP1 may influence the entire reproductive system, including ovulation, oocyte quality, embryo development, and early spontaneous pregnancy loss [17].
Our long-term goal is to understand the affects of endometriotic lesion-secreted TIMP1 on reduced fecundity. In these studies, we used a well-established rat model of endometriosis to evaluate the affect of modulation of TIMP1 on ovarian function and preimplantation embryo development.
MATERIALS AND METHODS
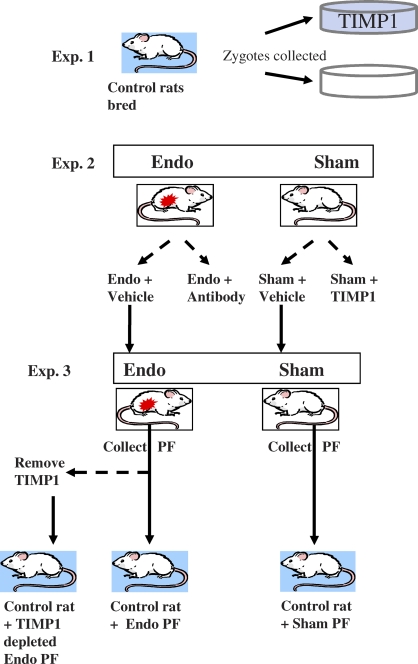
Three experiments were performed (Fig. 1). 1) TIMP1 was modulated in vitro to determine its effect on embryo development. 2) TIMP1 was modulated in vivo in Endo and Sham rats to determine the effect on embryo development and ovulation. 3) Control rats were treated with peritoneal fluid from Endo and Sham rats to determine the specificity of TIMP1 effects on ovulation.
FIG. 1.
In vitro and in vivo effects of modulating TIMP1 on preimplantation embryo development and ovulation. In experiment 1, zygotes were collected from control rats and cultured for 24 h in the presence and absence of TIMP1. In experiment 2, Endo rats were treated with a TIMP1 function-blocking antibody or vehicle, and Sham rats were treated with TIMP1 or vehicle. Zygotes were examined for embryo quality, and ovaries were evaluated for anomalies in ovulation. In experiment 3, PF (peritoneal fluid) was collected from Endo and Sham rats from experiment 2. TIMP1 was immunoprecipitated out of a fraction of Endo PF. These three PF pools were injected into control rats. Ovaries were evaluated for ovulatory dysfunction.
Animal Management
All the experiments were conducted with the approval of the University of Missouri Institutional Animal Care and Use Committee and in accordance with the National Research Council's “Guide for Care and Use of Laboratory Animals.” Mature female Sprague-Dawley rats (250 g; Harlan Laboratories, Inc., Madison, WI) exhibiting normal 4–5 d estrous cycles were housed in an environmentally controlled room with a 14L:10D cycle. Rat chow and water were available ad libitum. Rats were acclimated to the vivarium for 14 d prior to surgery.
Experiment 1: Modulation of TIMP1 During In Vitro Embryo Culture
To first determine if TIMP1 concentrations found in the PF of rats with endometriosis were detrimental to preimplantation embryo development, a pilot study was performed. Day 1 zygotes were collected from normal rats (no surgery) and cultured in vitro (Fig. 1).
To stimulate folliculogenesis and follicular maturation, immature Sprague-Dawley rats (n = 4, 50–60 g; Harlan Laboratories, Inc.) were injected with PG 600 (20 international units [IU]; Intervet, Millsboro, DE) and 2 days later injected with hCG (40 IU; EMD Biosciences, Gibbstown, NJ). The females were then cocaged with proven breeder males.
Females were euthanized the next afternoon in a CO2 chamber followed by a cervical dislocation. The ovaries and oviducts were excised and rinsed twice in HEPES-buffered Tyrode solution as described previously [36]. The oviducts were removed and the ampulla was nicked, allowing the release of the cumulus-oocyte complexes. Cumulus cells were removed using hyaluronidase in HEPES buffer (1 mg/ml; Sigma Aldrich, St. Louis, MO).
Embryos were cultured for 24 h to the two-cell stage in rat embryo culture media (protein-free minimal essential medium with amino acids, a kind gift of Dr. John Critser, University of Missouri) with (n = 16 embryos) or without (n = 6 embryos) recombinant rat TIMP1 (700 ng/ml; R&D Systems, Minneapolis, MN). This concentration of TIMP1 protein in the culture media is equivalent to PF TIMP1 concentrations in the endometrium of rats, which has been previously correlated with ovarian anomalies and subfertility [17].
After culture, removal of the zona pellucida, fixation and processing for immunolabeling was performed as described previously [17]. Two-cell embryos were evaluated for TIMP1 and nuclear pore complex protein localization. A mouse monoclonal antinuclear pore complex antibody MAb 414 (Abcam, Cambridge, MA) was used to evaluate nuclear envelope reassembly reflective of the blastomere/embryo quality. A rabbit polyclonal anti-TIMP1 antibody (Cell Applications, Inc., San Diego, CA) was used to detect TIMP1 in the embryos while nuclear localization was visualized by the DNA stain 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA). Species-appropriate, fluorescently conjugated secondary antibodies or biotin-conjugated antibodies followed by avidin-conjugated fluorescent labels were then used to detect primary antibodies. TIMP1 and nuclear pore complex localization were visualized by epifluorescence microscopy.
Photomicrographs were taken with a Nikon Eclipse 800 microscope (Nikon Instruments Inc., Melville, NY) equipped with Cool Snap camera (Roper Scientific, Tucson, AZ) and MetaMorph software (Universal Imaging Corp., Downington, PA), and color merged using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA). In addition to immunofluorescence, embryo quality was assessed by gross morphological analysis. Embryos were considered of good quality if they had two equal-sized blastomeres free of cytoplasmic fragmentation or granularity, normal blastomere adhesion, and normal appearing nuclei. Embryos were considered abnormal if any of these qualifications were not met.
Induction of Endometriosis
For experiments 2 and 3, endometriosis was surgically induced in rats (Endo) as previously described by Vernon and Wilson [13] and routinely performed in our laboratory [19, 21, 33, 34, 37, 38]. A unilateral ovariohysterectomy was performed, and sections of the removed uterus were autotransplanted into the arterial cascade of the small intestine. The remaining rats underwent a control surgery (Sham). Sham rats also had a unilateral ovariohysterectomy without autotransplantation of the uterine squares. After 4 wk of endometriotic lesion growth, reproductive cycles were synchronized but not superovulated with a LHRH agonist (40 μg/250 g rat; Sigma) to allow treatments to be administered on the same day. Reproductive cyclicity was monitored by evaluation of vaginal cytology. Only rats in estrus were used in this study.
Experiment 2: In Vivo Modulation of TIMP1
Prior studies from our laboratory [17] have demonstrated methylene blue dye injected into the peritoneal cavity can bathe the ovary, enter the oviducts, and traverse the reproductive tract. Therefore, experiments 2 and 3 used an intraperitoneal route of treatment delivery.
Sham rats (n = 5 per treatment) received intraperitoneal injections of recombinant rat TIMP1 (2.8 μg/kg; R&D Systems), GM6001 (N-[(2R)-2-(hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan methylamide), a synthetic broad-spectrum MMP inhibitor (3.9 mg/kg; Millipore; Billerica, MA), or PBS as a control. The recombinant TIMP1 dose was determined from concentrations of intraperitoneal TIMP1 previously reported in Endo rats [17]. The GM6001 dose was found to be efficacious at inhibiting many MMPs reported in an in vivo study of nude mice [39] and was then adjusted for rat body weight.
Endo rats (n = 5 per treatment) received intraperitoneal injections of a function-blocking TIMP1 antibody, (32 μg/kg rat; AbD Serotec; Raleigh, NC) or vehicle. The dose of TIMP1-blocking antibody originated from an in vivo study in male mice adjusted for rat body weight and was validated as the most efficacious dose to reduce TIMP1 in PF of control rats in our laboratory [40].
Treatments (intraperitoneal injections) started on the morning of the second proestrus after synchronization and were repeated every third day for a total of three injections. Rats were then caged with proven breeder males on the subsequent evening of proestrus. Mating was confirmed the next morning by the presence of a vaginal plug or the presence of sperm in a vaginal lavage, and this was considered as Day 1 of pregnancy. To determine differences in quality and development, preimplantation embryos were collected from each rat and fixed as described.
Evaluation of Embryo Quality Following In Vivo Modulation of TIMP1
Zygotes were evaluated by using the DNA stain DAPI combined with mouse monoclonal antitubulin antibody E7 (Developmental Studies Hybridoma Bank, Iowa City, IA) to evaluate chromosome alignment, spindle structure (mitotic blastomeres), and nuclear integrity as well as with a rabbit polyclonal antiproteasome antibody to detected the distribution and abundance of proteasomes, which is reflective of embryo quality. Epifluorescence microscopy combined with differential interference contrast (DIC) microscopy was used to analyze the zygotes. Proteasome intensity was quantified morphometrically using Image J (NIH, Bethesda, MD) by tracing each zygote and measuring pixel intensity of the proteasome channel after the background was subtracted.
Determination of the Effect of In Vivo Modulation of TIMP1 in Follicle and CL Numbers
Differences in the numbers of follicles and CLs between treatment groups were evaluated histologically and quantified morphometrically as described previously [15, 17]. These morphometric calculations were based on size so that no antral follicle, CL, or luteinized unruptured follicle (LUF) was counted more than once in each ovary [15]. All the morphological and morphometric analyses were performed by two investigators blinded to the study group. Differences in the numbers of follicles and in the number of CLs between treatments were analyzed by one-way ANOVA and Tukeys test for posthoc pairwise multiple comparisons.
Immunolocalization of TIMP1 in the Ovaries
Localization of TIMP1 protein in the ovaries was quantified to detect potential TIMP1 involvement in ovarian dysfunction in endometriosis. Immunohistochemistry was performed as previously described [17] except that the tissues were blocked in 1% hydrogen peroxide in methanol for 1 h. Slides were incubated with polyclonal rabbit anti-rat TIMP1 (1:100, 5 ng/ml, Cell Applications, Inc.) for 1 h and then with secondary antibody, an anti-rabbit IgG conjugated with Alexa Fluor 488 (1 μg /ml in PBS; Invitrogen) for 30 min. DAPI (Vector Laboratories, Burlingame, CA) was used to counterstain cell nuclei.
Immunofluorescent localization of TIMP1 in the ovarian follicular theca and CL was quantified morphometrically by area fraction as described previously [17]. All the data were reported as mean intensity per area. Both follicular and CL TIMP1 area fractions of treatment groups were normally distributed and compared using one-way ANOVA and Tukey posthoc pairwise multiple comparison procedures.
TIMP1 Protein Inhibitory Activity
To assess the inhibitory activity of TIMP1, reverse zymography was performed using a kit and protocol established by Dr. Dylan Edwards (University of East Anglia, Norwich, U.K.). To protect the integrity of the extracellular matrix degrading enzymes, protein extraction was performed using detergents instead of heat as described by Hu et al. [41]. Supernatants were then collected, and an aliquot was subjected to the DC Protein Assay (Bio-Rad Laboratories, Richmond, CA). Samples were either used immediately or stored at −80°C until use.
Protein samples (5 μg) were separated by SDS-PAGE using 12% acrylamide with gelatin (0.1% w/v) gels and gelatinases A and B (MMP2 and 9, respectively). A TIMP1 standard was included on each gel as a positive control. As the gel was destained, areas in the gel where TIMPs inhibited gelatinolytic activity were visualized as dark blue bands while the remainder of the gel lightened due to the enzyme's ability to break down the gelatin in the gel. This method was used to visualize free TIMPs but not MMP-TIMP complexes in the ovarian extracts.
Western blot analyses were performed to confirm the results of reverse zymography as previously described [42]. Total protein (5 μg) was separated by a 12% SDS gel and transferred to a nitrocellulose membrane. The membrane was incubated with polyclonal rabbit anti-rat TIMP1 (1:100, 5 ng/ml; Cell Applications, Inc.) overnight. A biotinylated secondary antibody, anti-rabbit IgG (50 μl/10 ml), was used before visualization of the bands by an ABC kit (Vector Laboratories) with diaminobenzidine as per the manufacturer's instructions.
Quantification of Ovarian Timp1 Gene Transcript Levels
Timp1 mRNA levels in the ovaries were quantified by performing quantitative real-time polymerase chain reaction (qRT-PCR). Total RNA was isolated from one-quarter of the ovary (∼25 mg) using the Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA) as per the manufacturer's instructions, including the optional on-column DNase digestion step with RNase-free DNase. The quantity and quality of the RNA were checked using a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA).
Ovarian RNA (500 ng) was reverse transcribed into cDNA using the First Strand cDNA Synthesis Kit for qRT-PCR (Roche, Basel, Switzerland) as per the manufacturer's instructions. The cDNA was diluted 10-fold prior to quantification by qRT-PCR.
Quantitative RT-PCR was performed using Taqman PCR Mix from Applied Biosystems (Foster City, CA) in an ABI 7500 Real-Time PCR System (Applied Biosystems); 18s gene expression was used as the endogenous control to normalize the target gene expression data. Primer/probe sets for Timp1 and 18s were purchased from Applied Biosystems. Normal rat estrus-stage uterus cDNA served as the arbitrary constant. Relative mRNA expression for Timp1 was calculated as described previously [43]. One-way ANOVA was used to detect statistically significant differences.
Ovarian Timp1 Transcript Localization
To localize the source of the Timp1 mRNA in the ovary, in situ hybridization was performed. All in situ hybridization was performed on paraffin-embedded tissues that were processed and sectioned specifically for the in situ hybridization. Slides were deparaffinized, rehydrated, and transferred to a pretreatment buffer of 10 mM Tris, 1 mM ethylenediaminetetraacetic acid, and 0.05% tween 20 (polysorbate 20, pH 7.6; Fisher Scientific, Pittsburgh, PA) at 95°C for 20 min to permeabilize the tissues for hybridization. Peroxidases were blocked before hybridization of the probe. Oligonucleotide probes were designed for a sense (AGC CCT TAT AAC CAG GTC CG) and antisense (CGG ACC TGG TTA TAA GGG CT) DNA probe with 100% identity to the exon spanning rat Timp1. Tissues were hybridized overnight at 37°C with a hybridization buffer containing 20% 20× SSC (3 M NaCl, 300 mM trisodium citrate, pH 7.0), 10 mg/ml bovine serum albumin, 20% (v/v) dextran sulfate (Fisher Scientific), 10 mg/ml sonicated salmon sperm (Invitrogen), and the oligonucleotide probe at 200 ng/ml. The remainder of the procedure was followed using the Vector Laboratories FISH protocol.
Experiment 3 In Vivo Treatments with PF
To determine the specificity of peritoneal endometriotic TIMP1 to the mechanisms of endometriosis-associated infertility, peritoneal washings from the vehicle-treated Endo and Sham rats were collected from the in vivo experiments (Fig. 1). Endo rat PF was pooled (n = 5) and divided. TIMP1 was immunoprecipitated from one-half with a mouse monoclonal anti-rat TIMP1(R&D Systems) attached to immobilized protein A-Trisacryl beads (Fisher Scientific) as per the manufacturer's instructions. Sham rat PF was also pooled (n = 5).
TIMP1 concentration in each pooled PF was tested by ELISA (RayBiotech, Inc., Norcross, GA) and for total protein as described above. About 10.8 mg of total protein/ml from each pooled peritoneal fluid group was snap frozen and lyophilized overnight. Lyophilized PF was reconstituted in sterile water for injection to normalize the amount of protein and amount of treatment injected into each treated rat.
Additional rats without surgery (n = 6 per treatment) were reproductively synchronized as before and injected with PF treatments after their second proestrus. Rats were euthanized the next morning (morning of estrus). Ovaries from these rats were collected and prepared as described before.
Differences in the numbers of follicles and CLs between treatment groups were evaluated histologically and quantified morphometrically. Localization of TIMP1 protein in the ovaries was detected and compared using one-way ANOVA and Tukey posthoc pairwise multiple comparison procedures. Also, gene transcript levels of Timp1 were determined in these ovaries to compare the specificity of endometriotic peritoneal TIMP1′s role in TIMP1 expression in the ovary; the levels were analyzed using a Kruskal-Wallis ANOVA on ranks.
Statistical Analyses
All the statistical tests described for each experiment were performed using the Sigma Stat package (Systat Software, Inc., Point Richmond, CA). A P value of < 0.05 was considered significant. The data were normally distributed and were reported as the mean ± the standard deviation (SD) except where noted.
RESULTS
Experiment 1: In Vitro Modulation of TIMP1 Alters Embryo Development
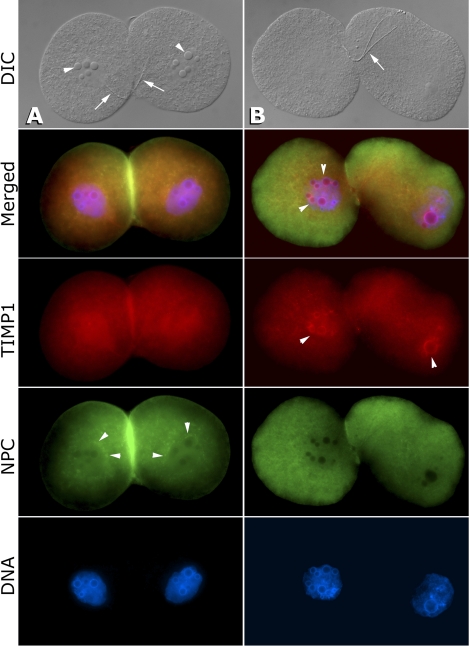
After 24 h of culture, all the two-cell embryos cultured from rats without surgical induction of endometriosis appeared morphologically normal. The TIMP1 protein (visualized in red, Fig. 2) was evenly dispersed throughout the blastomere cytoplasm with some accumulation around the nucleolus precursor bodies inside the nuclei. Nuclear pore complex-1 localization (visualized in green) around the nuclei indicated a normal reformation of a functional nuclear envelope. All the TIMP1-treated preimplantation embryos were morphologically abnormal, displaying elongated blastomeres with poor adhesion and granulated cytoplasm. Supernumerary nucleolus precursor bodies were observed in some blastomeres as well as nuclear accumulation of the TIMP1 signal, consistent with nuclear translocation of TIMP1-EGFP observed in human breast carcinoma cells [44]. Nuclear pore complexes were also less concentrated around the nuclei in TIMP1 two-cell embryos versus controls, indicating incomplete nuclear envelope assembly during cell division.
FIG. 2.
TIMP1 affects embryo development in vitro. A) Representative two-cell embryo cultured in control media for 24 h is morphologically normal with equal-sized blastomeres, equal-sized and -numbered nucleolus precursor bodies (arrowheads), and normal blastomere adhesion as visualized by DIC (differential interference contrast) microscopy. Remnants of the sperm flagellum (arrows), spanning both blastomeres, is still visible at this stage. Red indicates TIMP1 protein localization that is equally dispersed throughout the cytoplasm and nucleus. Green indicates NPC (nuclear pore complexes) that are concentrated around the nucleus (arrowheads), indicating good reformation of the nuclear envelope. Blastomere adhesion and the presence of NPC stacks in the cortical cytoplasm, probably in the form of annulate lamellae, increasing the bright green labeling area where adhering blastocysts contact each other. B) Representative two-cell embryo cultured with TIMP1 for 24 h is abnormal with elongated blastomeres, shrunken cytoplasm, and poor blastomere adhesion as well as abnormally small, supernumerary nucleoli (arrowheads in merged panel). Sperm flagellum, while split, is still prominent (arrow; DIC), suggesting a delayed postfertilization processing of sperm accessory structures. TIMP1 labeling is concentrated inside the nucleus and around the nucleoli (arrowheads in TIMP1 panel), possibly as a result of nuclear translocation of the endocytosed, extrinsic TIMP1 protein. NPC proteins are diffused across the cytoplasm, indicating dysfunctional nuclear envelope reassembly following embryo cleavage. Loose blastomere adhesion causes the lack of NPC signal between the blastomeres. Original magnification ×600.
Experiment 2: In Vivo Modulation of TIMP1 Affects Zygote Quality
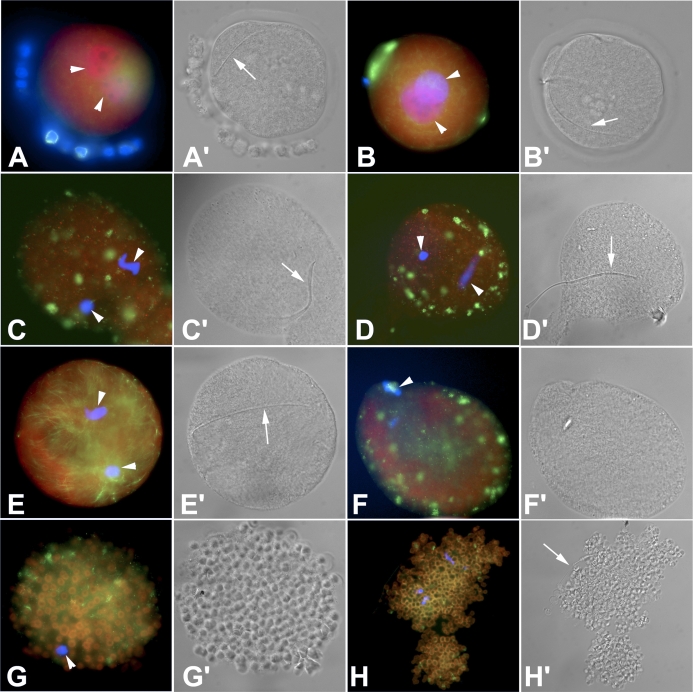
The morphology of zygotes collected from rats treated in vivo varied greatly (Fig. 3 and Table 1). Zygotes from Sham rats appeared normal with a round shape that was free of cytoplasmic fragmentation or granularity and that had a distinct microtubule network. There were fewer zygotes from Endo rats than from Sham rats; the zygotes from Endo rats had aberrantly clustered microtubules and an overabundance of proteasome staining, suggesting a cell under stress or even apoptosis [45]. Treatment of Sham rats using rTIMP1 resulted in fewer zygotes and in anomalies that were similar to those found in Endo rats, including aberrant microtubule network (multiple small cytoplasmic asters of microtubules) and an overabundance of proteasomes. When a TIMP1 function-blocking antibody was given to Endo rats, there was a significant restoration of normal microtubule distribution and a decrease in the intensity of proteasomal staining; the intensity was similar to the levels found in Sham rats. GM6001-treated rats yielded zygotes that had severe abnormalities, including proteasome accumulation and abnormal microtubule distribution; the zygotes were only recognizable as embryos due to the presence of a sperm tail (Fig. 3).
FIG. 3.
Modulation of TIMP1 affects embryo development in vivo. The red color indicates proteasome labeling, which is expected to be concentrated in the pronuclei of normal zygotes and increased/aggregated in the cytoplasm of stressed cells. The green color indicates microtubule localization. Under control conditions, a pair of apposed male and female pronuclei, a landmark of normal fertilization and zygotic development, develop from the chromosomes of the oocyte metaphase-II plate and the fertilizing sperm nucleus, respectively. A, B) Zygotes collected from the vehicle-treated Sham rats. The microtubule network appeared normal (green). Apposed pronuclei are visible (arrowheads). In A, the blue color is DNA staining of the corona radiate cell nuclei. A′, B′) DIC images from the Sham zygotes. The arrows indicate the sperm tail. C, D) Zygotes collected from the vehicle-treated Endo rats. Pronuclei (arrowheads, blue) are abnormally small and failed to reach apposition. Proteasomes are aggregated throughout the cytoplasm, indicative of a cell under stress. Microtubule network is disarranged (green), indicative of the underdevelopment of these zygote. C′, D′) DIC images from the Endo zygotes. Arrows indicate sperm tail, which is not completely incorporated in the ooplasm in a zygote shown in D′. E) Zygote collected from an Endo rat treated with a TIMP1 function-blocking antibody. While the pronuclei (arrowheads, blue) are small and not yet apposed, an extensive, well-developed microtubule network can be seen (green). Proteasome staining is distributed diffusely in the cytoplasm, indicating a reduction of stress compared to nontreated rats. E′) DIC image of TIMP1 function blocking antibody-treated Endo rat. Arrow indicates the sperm tail. F) Although this female Sham rat treated with TIMP1 had spermatozoa present in the vaginal lavage, the oocytes failed to fertilize. Similar to the zygotes from the Endo rats, there is an increase in cytoplasmic proteasome labeling as well as clustering of microtubules in cytoplasmic foci/asters. Only a single set of (oocyte) chromosomes is present (arrowhead). F′) DIC image of TIMP1-treated Sham rat indicates abnormally shrunken cytoplasm; the sperm tail is not present. G) Unfertilized oocyte collected from a Sham rat treated with GM6001. This oocyte has a severely abnormal phenotype with proteasome-containing ring structures indicative of the process of cell stress or death. Arrowhead points to a hypercondensed chromatin, indicating an immature/defective oocyte. G′) DIC image of GM6001-treated Sham rat oocyte clearly showing the unique proteasome-containing ring structures induced by this treatment. H) Zygotecollected from a Sham rat treated with GM6001. The severely abnormal phenotype is repeated in this disintegrating zygote with proteasome-containing ring structures and corrupted plasma membrane. H′) DIC image of the same zygote, showing the familiar ring structures. Arrow indicates remnants of a sperm tail. Original magnification ×600.
TABLE 1.
Affect of in vivo modulation of TIMP1 on zygote quality.

In Vivo Modulation of TIMP1 Changes Follicle and CL Numbers
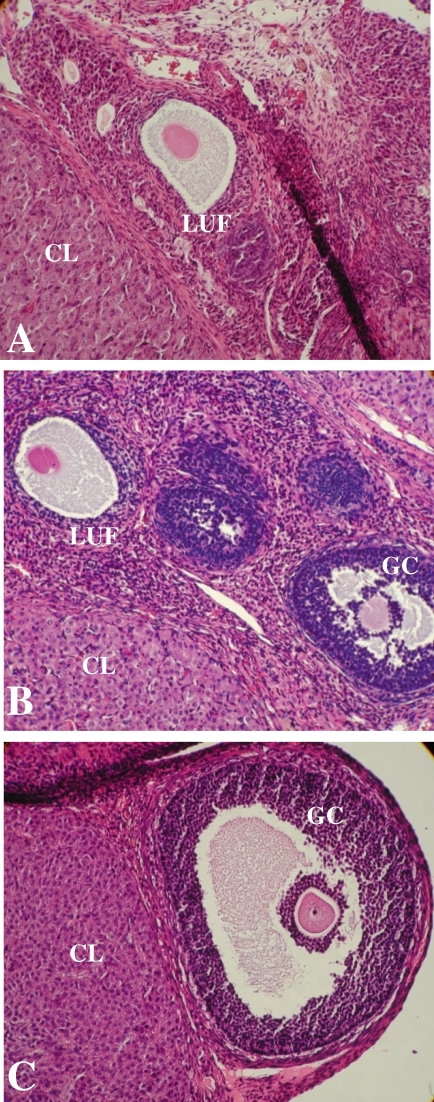
Significantly fewer antral follicles and CLs were present in Endo rat ovaries than in Sham rat ovaries (Table 2). TIMP1-treated Sham rats had fewer follicles and CLs than Sham rats, and the numbers were similar to those seen in Endo rats treated with vehicle. Endo rats treated with the TIMP1 function-blocking antibody had significantly more follicles and CLs than Endo rats or TIMP1-treated Sham rats, and the numbers were similar to those seen in Sham rats treated with vehicle. GM6001-treated rats had the same number of follicles and CLs as Sham rats and as TIMP1 antibody-treated Endo rats. In addition, LUFs were found in 80% of Endo rats (mean = 1 LUF/ovary) and 100% of TIMP1-treated Sham rats (mean = 1.6 LUF/ovary), but not in Sham rats (Fig. 4).
TABLE 2.
Modulation of TIMP1 in Sham and Endo rats alters ovarian function.
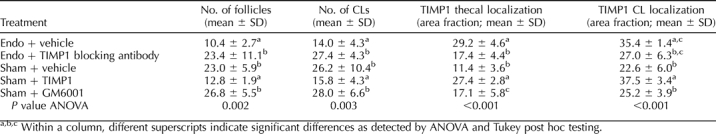
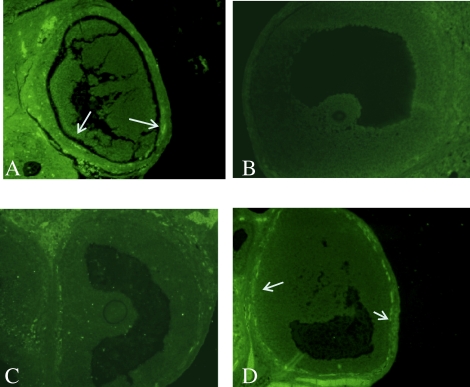
FIG. 4.
Modulation of TIMP1 affects ovulation. Luteinized unruptured follicle syndrome was found in both Endo rats (A) and Sham rats treated with TIMP1 (B) but not in Sham rats (C). This is indicative of ovulatory dysfunction associated with excessive TIMP1. Corpora lutea (CL), follicles with granulosa cells (GC), and luteinized unruptured follicles (LUF) are noted. Original magnification ×200.
In Vivo Modulation of TIMP1 Affects Ovarian TIMP1 Protein Localization and Inhibitory Activity
In agreement with our previous report [17], more TIMP1 protein was localized in ovarian thecal cells of Endo rats compared to Sham rats (Fig. 5 and Table 2). In Sham rats treated with rTIMP1, the TIMP1 localized around ovarian thecal cells at levels equal to those found in Endo rats. In contrast, TIMP1 antibody-treated Endo rats accumulated TIMP1 in the ovarian thecal cells at levels equal to Sham rats.
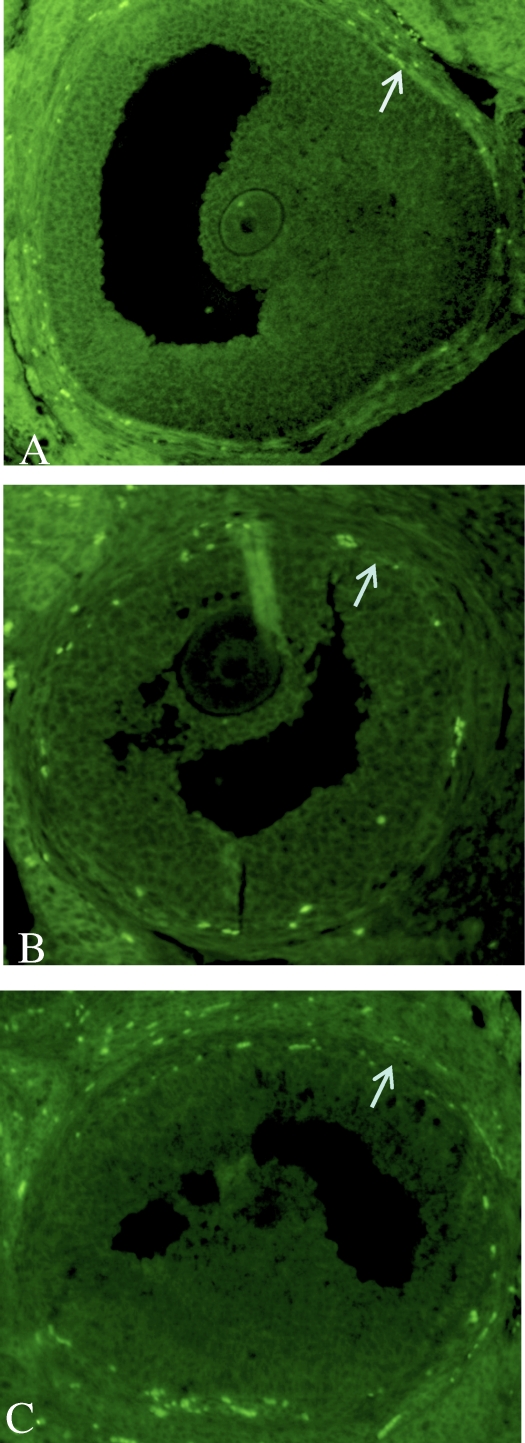
FIG. 5.
Differential TIMP1 localization in the ovary after in vivo treatments. More TIMP1 protein was localized in the thecal cells of antral follicles in Endo rats (A) than in Sham rats (B). C) Treatment of Endo rats with a TIMP1 function-blocking antibody reduced TIMP1 localization to levels equal to Sham. D) Sham rats treated with TIMP1 had an increase in TIMP1 localization in the ovarian theca, providing further evidence that TIMP1 from the peritoneal cavity can sequester into the ovary to influence its function. The arrows indicate increased thecal localization of TIMP1 in A and D. Original magnification ×200.
To determine the activity of the TIMP1 present in the ovary, reverse zymography was performed on ovarian extracts. More TIMP1 activity was found in Endo rats and TIMP1-treated Sham rats compared to any other treatment group (Fig. 6). Western blot analysis for TIMP1 confirmed the identity of the TIMP1 band on the reverse zymograms.
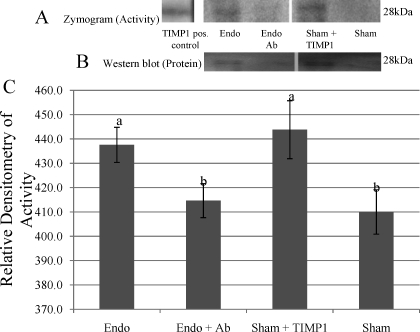
FIG. 6.
Modulation of TIMP1 affects ovarian TIMP1 inhibitory activity. A) A representative reverse zymogram. B) A representative Western blot analysis. C) Relative densitometry for TIMP1 inhibitory activity from reverse zymograms shows Endo rats and TIMP1-treated Sham rats had more TIMP1 inhibitory activity than Sham rats or TIMP antibody-treated Endo rats. Different lowercase letters indicate statistical significance (one-way ANOVA, P < 0.001). Error bars indicate ± SD.
Ovarian Timp1 mRNA Levels Do Not Change After In Vivo Treatments
Levels of Timp1 mRNA were quantified using qRT-PCR. Ovarian Timp1 mRNA levels were not different in any of the treatment groups (mean ± SD; Endo: 1.7 ± 0.2; Endo + TIMP1 antibody: 1.6 ± 0.4; Sham: 1.1 ± 0.3; Sham + TIMP1: 1.4 ± 0.3; P = 0.085). Because of the large variation of whole ovarian homogenate mRNA levels, in situ hybridization was performed to analyze the cell-specific location of Timp1. Localization of Timp1 mRNA to the theca of antral follicles as detected by in situ hybridization and evaluated by morphometric analysis showed no difference in Timp1 mRNA levels (mean ± SD; Endo: 17.9 ± 5.3; Endo + TIMP1 antibody: 23.5 ± 11.0; Sham: 20.8 ± 4.2; Sham + TIMP1: 21.8 ± 4.2; P = 0.480).
Experiment 3: Effects of PF Treatments on Normal Rats
The TIMP1 protein concentration in pooled PF collected from experiment 2 Endo rats was 2121.4 pg/mg of protein. TIMP1 was successfully immunoprecipitated from a portion of the Endo rat PR, where low amounts of TIMP1 (2.7 pg/mg protein) remained. The TIMP1 concentration in pooled PF from Sham rats was 1457.2 pg/mg protein.
The protein concentration of TIMP1 in the peritoneal fluid of experiment 3 control rats varied after the various PF treatments. The TIMP1 concentration in normal rats treated with Endo PF was significantly higher than control rats treated with Sham PF (Table 3). Peritoneal fluid from control rats treated with TIMP1-depleted Endo PF was statistically similar to the control rats treated with Sham PF.
TABLE 3.
Modulation of TIMP1 concentration in normal rats alters ovarian function.
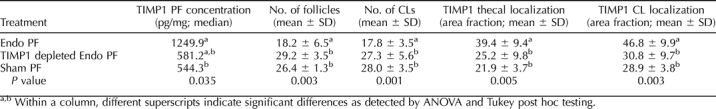
Significantly fewer antral follicles and fewer CLs were present in ovaries of control rats treated with Endo PF than in ovaries from control rats treated with either TIMP1-depleted Endo PF or Sham PF (Table 3) where the numbers are similar. Using immunohistochemistry and quantification of the fluorescence, rats treated with Endo PF had more TIMP1 localized in ovarian thecal cells compared to rats treated with either TIMP1-depleted Endo PF or Sham PF (Fig. 7 and Table 3).
FIG. 7.
Peritoneal fluid treatments affect TIMP1 protein localization in the ovary. Control rats treated with Endo PF (A) had more TIMP1 protein localization in thecal cells of antral follicles than control rats treated with TIMP1-depleted Endo PF (B) or Sham PF (C). Arrows point to the thecal layer. This provides further evidence that TIMP1 from the peritoneal cavity can be sequestered into the ovary, causing an imbalance between the MMPs and TIMPs. Original magnification ×200.
Gene expression of ovarian Timp1 from control rats treated with PF was quantified using qRT-PCR. There was no difference in Timp1 ovarian mRNA transcript levels among any of the treatment groups (data not shown, P = 0.7).
DISCUSSION
The results of our studies provide strong evidence that excessive levels of TIMP1 protein, such as those secreted by endometriotic lesions into the peritoneal cavity, negatively affects ovarian function and preimplantation embryo development. First, in a pilot study, in vitro culture of normal rat embryos with TIMP1 for only 24 h caused disruption of preimplantation embryo development similar to those found in Endo rats. This observation suggests that extrinsic, secreted TIMP1 can be taken up by oocytes or embryos, and even translocated from the cytoplasm to the nucleus. While there is not yet a clearly defined role for TIMP1 in nuclear function, a study demonstrated unambiguously the uptake of CHO-expressed/secrereted, chimeric TIMP1-EGFP protein by cocultured human breast carcinoma cells in vitro [44]. This supports our hypothesis that endometriotic lesion-secreted TIMP1 can translocate to ovary and/or oviduct and cause poor preimplantation embryo quality, developmental arrest, and the subsequent embryo loss found in endometriosis. We acknowledge the possibility that a nonspecific protein effect may exist. However, this data provided the first evidence suggesting that TIMP1 alters embryo development as well as justification for performing TIMP1-modulating treatments in vivo.
In vivo TIMP1-modulating treatments confirmed earlier reports in this model: Endo rats had fewer follicles and CLs, increased LUFs, and poorer embryo quality compared to Sham rats [15, 17]. Adding endometriotic levels of TIMP1 to the peritoneal cavity of Sham rats resulted in a recapitulation of the aberrant in vivo phenomena, providing more evidence that TIMP1 contributes to the reproductive dysfunction found in endometriosis. Most importantly, Endo rats treated with a TIMP1 function-blocking antibody had more follicles and CLs and normal embryo development compared to Endo rats; in fact, the numbers are equal to those seen in Sham rats. These data provides insights into the potential development of a clinically relevant anti-TIMP1 therapy to restore ovarian function and embryo quality and development.
Treating Sham rats with the synthetic broad-spectrum MMP inhibitor GM6001 demonstrated that blocking all the MMPs gives rise to a severely abnormal phenotype that is not seen in endometriosis. Thus, TIMP1 may be specifically responsible for the reproductive anomalies studied found in our research.
More TIMP1 protein was found to be localized in ovarian thecal cells in Endo rats and TIMP1-treated Sham rats compared to Sham rats, GM6001-treated Sham rats, or Endo rats treated with a TIMP1-blocking antibody. Intriguingly, Endo rats treated with a TIMP1 function-blocking antibody had reduced levels of TIMP1 protein localization in the ovarian theca that were equal to those seen in Sham rats. Consistently, TIMP1 activity in ovarian extracts measured by reverse zymography showed more TIMP1 inhibitory activity in Endo rats and TIMP1-treated Sham rats compared to the other three treatment groups.
However, ovarian Timp1 mRNA levels measured by qRT-PCR did not differ among Endo rats, Endo rats receiving TIMP1 antibody, Sham rats, or Sham rats treated with TIMP1. Due to the relatively small sample size (n = 5 per group) and the large variation within the groups, these negative findings should be interpreted cautiously. Yet, the results of the in situ hybridization of Timp1 confirmed the lack of differences among the groups. The mRNA data, along with the data indicating more TIMP1 protein localizing to the ovarian theca, are consistent with the hypothesis that excessive endometriotic TIMP1 sequesters into the ovary and affects ovarian function as opposed to an increase in TIMP1 production by the ovaries of Endo rats.
To determine whether TIMP1 and not another endometriotic factor present in PF is responsible for the results seen in this study, normal rats were given injections of PF from Endo rats that contained or lacked TIMP1 or from Sham rats. As expected, rats treated with neat Endo PF had more TIMP1 in their peritoneal fluid than those receiving either Sham or TIMP1-depleted Endo PF. The Endo PF-treated rats had fewer follicles and CLs as well as an increase in TIMP1 localization in their ovarian thecal cells than the other two treatment groups, adding further evidence to TIMP1′s role in ovarian dysfunction in endometriosis.
In summary, these results demonstrate that TIMP1 may be part of the mechanism(s) causing endometriosis-associated infertility. The anomalous phenotype of endometriosis can be recapitulated in Sham rats by adding exogenous TIMP1 at endometriotic levels in the ovary, oocyte, and embryo. More importantly, the endometriotic phenotype can be negated in Endo rats by treating with a TIMP1 function-blocking antibody restoring ovarian follicle and CL production and rescuing the preimplantation embryo. Ongoing studies in our laboratory are being conducted to determine whether the mechanism of action of TIMP1 is via MMP inhibition or whether other TIMP1 MMP-independent activities lead to the ovarian and embryonic abnormalities. Novel therapies modulating TIMP1 may be developed to restore fertility in women with endometriosis.
Acknowledgments
We would like to thank the laboratory staff in the Department of Obstetrics, Gynecology & Women's Health Division of Perinatal Research, in particular Henda Nabli, Randy Zimmer, Katherine Pelch, and Takeshi Nagamatsu, as well as Dr. John Critser and his laboratory staff, for their support.
Footnotes
Supported in part by the University of Missouri System Research Board and NIH HD57445-01 (K.S.T.), and the University of Missouri Food for the Twenty-First Century Program and NIH T32 RR-07004 (P.S.). Presented in part at the Forty-First Annual Meeting of the Society for the Study of Reproduction, May 27–30, 2008, Kona, Hawaii.
REFERENCES
- Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C.The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update 2002; 8: 95–103. [DOI] [PubMed] [Google Scholar]
- Garrido N, Pellicer A, Remohi J, Simon C.Uterine and ovarian function in endometriosis. Semin Reprod Med 2003; 21: 183–192. [DOI] [PubMed] [Google Scholar]
- Cahill DJ, Hull MG.Pituitary-ovarian dysfunction and endometriosis. Hum Reprod Update 2000; 6: 56–66. [DOI] [PubMed] [Google Scholar]
- Doody MC, Gibbons WE, Buttram VC., JrLinear regression analysis of ultrasound follicular growth series: evidence for an abnormality of follicular growth in endometriosis patients. Fertil Steril 1988; 49: 47–51. [DOI] [PubMed] [Google Scholar]
- Tummon IS, Maclin VM, Radwanska E, Binor Z, Dmowski WP.Occult ovulatory dysfunction in women with minimal endometriosis or unexplained infertility. Fertil Steril 1988; 50: 716–720. [PubMed] [Google Scholar]
- Hull MG, Williams JA, Ray B, McLaughlin EA, Akande VA, Ford WC.The contribution of subtle oocyte or sperm dysfunction affecting fertilization in endometriosis-associated or unexplained infertility: a controlled comparison with tubal infertility and use of donor spermatozoa. Hum Reprod 1998; 13: 1825–1830. [DOI] [PubMed] [Google Scholar]
- Groll M.Endometriosis and spontaneous abortion. Fertil Steril 1984; 41: 933–935. [DOI] [PubMed] [Google Scholar]
- Tanbo T, Omland A, Dale PO, Abyholm T.In vitro fertilization/embryo transfer in unexplained infertility and minimal peritoneal endometriosis. Acta Obstet Gynecol Scand 1995; 74: 539–543. [DOI] [PubMed] [Google Scholar]
- Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: a possible cause of infertility. Fertil Steril published online 5 Nov 2009; PMID: 19896655. [DOI] [PubMed]
- Bergendal A, Naffah S, Nagy C, Bergqvist A, Sjoblom P, Hillensjo T.Outcome of IVF in patients with endometriosis in comparison with tubal-factor infertility. J Assist Reprod Genet 1998; 15: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittaway DE, Vernon C, Fayez JA.Spontaneous abortions in women with endometriosis. Fertil Steril 1988; 50: 711–715. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL.Using rats as a research model for the study of endometriosis. Ann N Y Acad Sci 2002; 955: 318–327;.discussion, 340–342 and 396–406. [DOI] [PubMed] [Google Scholar]
- Vernon MW, Wilson EA.Studies on the surgical induction of endometriosis in the rat. Fertil Steril 1985; 44: 684–694. [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E.Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett 2001; 306: 185–188. [DOI] [PubMed] [Google Scholar]
- Moon CE, Bertero MC, Curry TE, London SN, Muse KN, Sharpe KL, Vernon MW.The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am J Obstet Gynecol 1993; 169: 676–682. [DOI] [PubMed] [Google Scholar]
- Pal AK, Biswas S, Goswami SK, Kabir SN.Effect of pelvic endometrial implants on overall reproductive functions of female rats. Biol Reprod 1999; 60: 954–958. [DOI] [PubMed] [Google Scholar]
- Stilley JA, Woods-Marshall R, Sutovsky M, Sutovsky P, Sharpe-Timms KL.Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 1. Biol Reprod 2009; 80: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HW, Wen Y, Chun SH, Nezhat C, Woo BH, Lake Polan M.Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-3 mRNA expression in ectopic and eutopic endometrium in women with endometriosis: a rationale for endometriotic invasiveness. Fertil Steril 2001; 75: 152–159. [DOI] [PubMed] [Google Scholar]
- Cox KE, Piva M, Sharpe-Timms KL.Differential regulation of matrix metalloproteinase-3 gene expression in endometriotic lesions compared with endometrium. Biol Reprod 2001; 65: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Zhou HE, Nothnick WB.The relevancy of the matrix metalloproteinase system to the pathophysiology of endometriosis. Front Biosci 2005; 10: 569–575. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Penney LL, Zimmer RL, Wright JA, Zhang Y, Surewicz K.Partial purification and amino acid sequence analysis of endometriosis protein-II (ENDO-II) reveals homology with tissue inhibitor of metalloproteinases-1 (TIMP-1). J Clin Endocrinol Metab 1995; 80: 3784–3787. [DOI] [PubMed] [Google Scholar]
- Osteen KG, Yeaman GR, Bruner-Tran KL.Matrix metalloproteinases and endometriosis. Semin Reprod Med 2003; 21: 155–164. [DOI] [PubMed] [Google Scholar]
- Matrisian LM.Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990; 6: 121–125. [DOI] [PubMed] [Google Scholar]
- Hulboy DL, Rudolph LA, Matrisian LM.Matrix metalloproteinases as mediators of reproductive function. Mol Hum Reprod 1997; 3: 27–45. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Osteen KG.Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol Reprod 2001; 64: 1285–1296. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Adler RR, Rappolee DA, Pedersen RA, Werb Z.Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev 1989; 3: 848–859. [DOI] [PubMed] [Google Scholar]
- Bagavandoss P.Differential distribution of gelatinases and tissue inhibitor of metalloproteinase-1 in the rat ovary. J Endocrinol 1998; 158: 221–228. [DOI] [PubMed] [Google Scholar]
- Wang H, Wen Y, Mooney S, Li H, Behr B, Polan ML.Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase expression in human preimplantation embryos. Fertil Steril 2003; 80Suppl 2:736–742. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Mann JS, Huang MH, Keeble SC.Gelatinase and proteoglycanase activity during the periovulatory period in the rat. Biol Reprod 1992; 46: 256–264. [DOI] [PubMed] [Google Scholar]
- Bany BM, Schultz GA.Tissue inhibitor of matrix metalloproteinase-3 expression in the mouse uterus during implantation and artificially induced decidualization. Mol Reprod Dev 2001; 59: 159–167. [DOI] [PubMed] [Google Scholar]
- Nothnick WB, Soloway P, Curry TE., JrAssessment of the role of tissue inhibitor of metalloproteinase-1 (TIMP-1) during the periovulatory period in female mice lacking a functional TIMP-1 gene. Biol Reprod 1997; 56: 1181–1188. [DOI] [PubMed] [Google Scholar]
- Nothnick WB.Disruption of the tissue inhibitor of metalloproteinase-1 gene results in altered reproductive cyclicity and uterine morphology in reproductive-age female mice. Biol Reprod 2000; 63: 905–912. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Vernon MW.Polypeptides synthesized and released by rat ectopic uterine implants differ from those of the uterus in culture. Biol Reprod 1993; 48: 1334–1340. [DOI] [PubMed] [Google Scholar]
- Sharpe-Timms KL, Zimmer RL, Jolliff WJ, Wright JA, Nothnick WB, Curry TE.Gonadotropin-releasing hormone agonist (GnRH-a) therapy alters activity of plasminogen activators, matrix metalloproteinases, and their inhibitors in rat models for adhesion formation and endometriosis: potential GnRH-a-regulated mechanisms reducing adhesion formation. Fertil Steril 1998; 69: 916–923. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Zimmer RL, Griffin WT, Penney LL.Polypeptides synthesized and released by human endometriosis differ from those of the uterine endometrium in cell and tissue explant culture. Fertil Steril 1993; 60: 839–851. [DOI] [PubMed] [Google Scholar]
- Gunasena KT, Villines PM, Critser ES, Critser JK.Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod 1997; 12: 101–106. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Lyon BP, Muse KN, Vernon MW.Follicular atresia and infertility in rats treated with a gonadotropin-releasing hormone antagonist. Endocrinology 1990; 127: 25–31. [DOI] [PubMed] [Google Scholar]
- Sharpe KL, Bertero MC, Muse KN, Vernon MW.Spontaneous and steroid-induced recurrence of endometriosis after suppression by a gonadotropin-releasing hormone antagonist in the rat. Am J Obstet Gynecol 1991; 164: 187–194. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Mathiesen B, Kindem K, Galappathi K.Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 2006; 66: 6699–6707. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Koike T, Sadoun E, Sage EH, Puolakkainen P.Inhibition of TIMP1 enhances angiogenesis in vivo and cell migration in vitro. Microvasc Res 2003; 65: 9–17. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhang X, Nothnick WB, Spencer TE.Matrix metalloproteinases and their tissue inhibitors in the developing neonatal mouse uterus. Biol Reprod 2004; 71: 1598–1604. [DOI] [PubMed] [Google Scholar]
- Piva M, Horowitz GM, Sharpe-Timms KL.Interleukin-6 differentially stimulates haptoglobin production by peritoneal and endometriotic cells in vitro: a model for endometrial-peritoneal interaction in endometriosis. J Clin Endocrinol Metab 2001; 86: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW.A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res 2001; 29: e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter LM, Garfield SH, Thorgeirsson UP.Tissue inhibitor of metalloproteinases-1 (TIMP-1) binds to the cell surface and translocates to the nucleus of human MCF-7 breast carcinoma cells. Biochem Biophys Res Commun 1999; 257: 494–499. [DOI] [PubMed] [Google Scholar]
- Blickwedehl J, McEvoy S, Wong I, Kousis P, Clements J, Elliott R, Cresswell P, Liang P, Bangia N.Proteasomes and proteasome activator 200 kDa (PA200) accumulate on chromatin in response to ionizing radiation. Radiat Res 2009; 167: 663–674. [DOI] [PubMed] [Google Scholar]