Abstract
Fortunately, the majority of children conceived through assisted reproductive technologies (ARTs) appear healthy; however, metabolic abnormalities, including elevated glucose and increased and altered adipose tissue deposition, have been reported in adolescents. To parse out factors that may be responsible, we investigated the effects of two different ARTs—in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI)—as well as somatic cell nuclear transfer (SCNT) on glucose clearance, body weight, and body composition of young adult mice. Female and male mice generated through ART weighed more than control (naturally conceived [STOCK]) mice at birth. No differences in body weight were observed in males up to 8 wk of age. ART females took longer than control mice to clear a glucose bolus, with glucose clearance most impaired in SCNT females. IVF females secreted more insulin and had a higher insulin peak 15 min after glucose injection compared with all other groups. Male mice exhibited no differences in glucose clearance, but IVF males required more insulin to do so. SCNT females weighed more than IVF, ICSI, and STOCK females, and they had higher fat content than ICSI females and higher leptin levels than all other groups. These results show that glucose parameters are altered in young adult mice conceived through techniques associated with ART before onset of obesity and may be responsible for its development later in life. The present study suggests that more investigation regarding the long-term effects of manipulations associated with ART is warranted.
Keywords: assisted reproductive technology,; in vitro fertilization,; insulin
Impaired glucose tolerance and insulin resistance emerge in male and female mice generated by techniques associated with assisted reproductive technology and somatic cell nuclear transfer.
INTRODUCTION
Assisted reproductive technologies (ARTs) are frequently used in a clinical setting to help individuals who are experiencing difficulty conceiving through natural methods, and it is estimated that more than 1% of U.S. births, and up to 3% of European births, are a result of ARTs [1]. The majority of these children appear to be healthy, but reports that ARTs are associated with an increased incidence of imprinting disorders, congenital defects, and altered metabolism have appeared [2, 3].
Some children (age, 8–18 yr) conceived through in vitro fertilization (IVF) have higher systolic and diastolic blood pressure, elevated fasting glucose levels, and a trend toward increased total body adipose tissue compared with naturally conceived children of the same age [4, 5]. Little is known about possible long-term effects of these procedures on offspring, because these children are only now entering young adulthood. The relatively short time during which ARTs have been in use, coupled with the possibility that offspring may have disorders of various kinds, highlights the utility and importance of using laboratory animal models with short life spans, such as the mouse, to provide vital information about the long-term effects of ARTs.
Mammalian cloning by somatic cell nuclear transfer (SCNT) requires that numerous manipulations be made to the developing embryo; some of these manipulations are shared by ARTs. SCNT mice develop an obese phenotype [6, 7], and in those reports, control mice conceived in vivo but exposed to in vitro culture conditions and mechanical embryo handling before transfer to a surrogate dam also developed an obese phenotype that was midway between that of mice conceived naturally and undergoing no manipulations and that of SCNT mice [7]. This suggests that in vitro culture conditions or manipulation of embryos per se may lead to permanent alterations in growth as well as other phenotypes not yet described [8]. Consistent with this, it has been reported that the combination of superovulation, placement in culture media during development to the blastocyst stage, and embryo transfer can affect behavior as well as cardiovascular and metabolic measures in rodents [9, 10].
Because in vitro manipulations appear to be associated with some of the phenotypic changes involved in SCNT, the purpose of the present study was to determine the effect of the in vitro manipulations used in two of the most common variations of ART in humans, IVF and intracytoplasmic sperm injection (ICSI), on body weight and glucose regulation of young adult male and female mice in comparison with offspring generated through SCNT or natural matings.
MATERIALS AND METHODS
Animals
Animal groups included B6C3F1 mice that were derived from natural in vivo fertilization (STOCK), IVF, ICSI, or cloning through SCNT. For STOCK, IVF, and ICSI groups, C57BL/6 females and C3H/He males were used to produce B6C3F1 mice. For SCNT, B6C3F1, and B6D2F1 mice (C57BL/6 × DBA/2 hybrids) were used to collect nuclear donor cells and oocytes, respectively. All pups were cross-fostered at birth to lactating CD-1 mothers in litters of five. All mice were produced at the University of Hawaii. The majority of mice were tested at the University of Cincinnati, but two small cohorts (containing each treatment group) were tested at the University of Hawaii. Tests were conducted under the same conditions and by the same personnel who performed the procedures at the University of Cincinnati. All animal protocols were approved by the Institutional Animal Care and Use Committees of both the University of Hawaii and the University of Cincinnati.
Controls
Female mice underwent unilateral tubal ligation before mating with a male. Because IVF, ICSI, and SCNT mice are born in smaller-than-normal litters, unilateral tubal ligation was used to reduce the number of oocytes produced and fertilized and to reduce the possible effects of litter size on body weight and metabolic parameters. Litter sizes ranged from two to eight pups. Ten male and eight female STOCK mice from five different litters were used in the present study.
Experimental Groups
Collection of oocytes.
For IVF, ICSI, and SCNT, oocytes were obtained from 10- to 12-wk-old females after superovulation by consecutive injections of equine chorionic gonadotropin (5 IU) and human chorionic gonadotropin (5 IU) administered 48 h apart. At 13–15 h after injection with human chorionic gonadotropin, cumulus-oocyte complexes were collected. For ICSI and SCNT, the cumulus-oocyte complexes were treated with 0.1% hyaluronidase to remove the cumulus cells.
In vitro fertilization.
Sperm collected from the cauda epididymis were incubated in TYH medium [11] for 1–2 h at 37°C under 5% CO2 to induce sperm capacitation. After incubation, a small amount of sperm suspension was added to the TYH medium containing the cumulus-oocyte complexes and cultured for 5–6 h for IVF. After IVF, fertilized oocytes with two pronuclei were transferred to CZB medium and cultured for 24 h to obtain embryos at the 2-cell stage. From one to five embryos were transferred to the oviducts of pseudopregnant surrogate dams, and from one to four pups were delivered per surrogate. Twelve male and 10 female IVF animals from 12 litters were used in our experiments.
Intracytoplasmic sperm injection.
These animals were produced according to the method of Kimura and Yanagimachi [12] with minor modifications [13]. Oocytes were prepared as described above. Sperm collected from the cauda epididymis were incubated in CZB medium. Twenty minutes later, a drop of sperm suspension was mixed with 12% (w/v) polyvinylpyrrolidone (PVP) in Hepes-CZB. The head of each sperm was separated from the tail by applying pulses to the head-tail junction by means of a Piezo-driven pipette (PrimTech). Only the sperm head was injected into each oocyte. Sperm-injected oocytes were cultured in CZB medium for 24 h to obtain embryos at the 2-cell stage. From four to five embryos were transferred to the oviducts of surrogate dams, and from one to three pups were delivered per surrogate. A total of 16 surrogates were used for ICSI. Twelve male and 12 female ICSI mice were used in the present study.
Somatic cell nuclear transfer.
SCNT mice were generated using the Honolulu method [14] with minor modifications [15]. Cumulus cells collected from B6C3F1 females were mixed with 12% PVP in Hepes-CZB. The oocytes were enucleated in HEPES-CZB containing 5 μg/ml of cytochalasin B with a glass pipette. After enucleation, each cumulus cell nucleus was injected into an oocyte cytoplasm, and reconstructed oocytes were kept in CZB medium for 2 h before activation. For activation, reconstructed oocytes were incubated with 10 mM SrCl2 and cytochalasin B in Ca2+-free CZB medium for 6 h. During the 6-h activation period and the 4-h culture in CZB medium that followed (total, 10 h), 50 nM trichostatin A (TSA) was added in both media as described previously [15]. The reconstructed oocytes were then transferred to new CZB medium and cultured without TSA for additional 14–18 h to obtain cloned embryos at the 2-cell stage. Because of the low success rate of SCNT, from 27 to 32 embryos were transferred to the oviducts of each surrogate, and from one to four pups were delivered per surrogate. Four surrogates were used for SCNT. Nine female SCNT mice were used in the present study. Immature Sertoli cells have been successfully used to generate male clones, whereas use of adult Sertoli cells has lower efficiency [16]. The greater efficiency using immature Sertoli cells likely results from their small size and lack of differentiation when they are harvested from neonates within days of birth [16, 17]. We felt that using clones generated through the use of immature Sertoli cells would not be appropriate for comparison with female clones produced using cumulus cells harvested from adult females. Thus, for the present study, cumulus cells were used as somatic cell donors, and only female SCNT mice were generated.
Embryo transfer.
IVF, ICSI, and SCNT embryos at the 2-cell stage were transferred into oviducts of pseudopregnant surrogate mothers (CD-1) that had been mated with vasectomized males of the same strain during the previous night. Surrogate mothers and natural mothers (STOCK group) were euthanized at 19.5 days postcoitus to collect live-term fetuses by cesarean section. Litter sizes of surrogate dams ranged from one to four, whereas litter sizes for naturally mated dams ranged from 2 to 10. All pups were cross-fostered at birth to lactating CD-1 foster dams in litters of five and were weaned at 21 days of age. Weaned mice were housed individually, with a 12L:12D photoperiod, and were provided with water and standard, low-fat, pelleted laboratory chow (Harlan Teklad LM485) ad libitum, except when noted otherwise.
Body Weight and Composition
Mice were weighed at birth and at least once per week thereafter, approximately 2 h before onset of the dark cycle. Body composition of mice was determined following death using whole-body nuclear magnetic resonance imaging (EchoMRI; Echo Medical Systems) at approximately 8 wk of age, when body weight had recovered from the glucose tolerance test (see below). This technique allowed determination of the percentage of lean and adipose tissue for each carcass.
Glucose Tolerance Test
Mice were challenged with an intraperitoneal glucose tolerance test (IPGTT) at approximately 8 wk of age. For the procedure, mice were transferred to a clean cage and fasted overnight for 16 h with ad libitum access to water. Whole-blood glucose levels were determined using a handheld glucometer (Freestyle; Abbott Laboratories), and 20-μl blood samples were collected from the tip of the tail using heparinized capillary tubes for plasma insulin determination. Following baseline glucose measurements and blood collection, mice were injected intraperitoneally with glucose (20% solution, 1.5 mg/g). Blood samples and blood glucose readings were then taken 15, 30, 45, 60, and 120 min postinjection. Food was returned immediately following the last time point.
Hormone Assays
Blood was centrifuged at 3800 rpm for 20 min at 4°C, and the plasma was collected and stored at −80°C until analysis for insulin using an enzyme-linked immunosorbent assay (Crystal Chem). At death, blood was collected for determination of plasma leptin. The blood was processed similarly to that for insulin, and plasma leptin levels were determined using a commercially available leptin radioimmunoassay kit (Millipore).
Data Analysis
One-way ANOVA and two-way repeated-measures ANOVA were used to determine significant treatment effects. When main effects were found, Holm-Sidak post hoc tests were used to make comparisons with the control group. All analyses were made using SigmaStat (version 3.1; Systat). Values greater than 2 SD from the mean were considered to be outliers. These criteria were established before analysis and resulted in the removal of two STOCK females, one STOCK male, one IVF female, and one ICSI female from analyses.
RESULTS
Body Weight and Body Composition
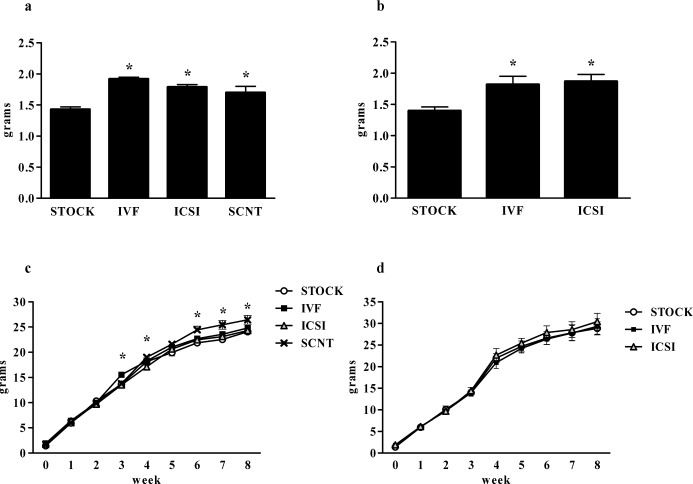
Both female and male mice generated through each of the ART techniques (IVF and ICSI) and females generated through SCNT weighed significantly more at birth than same-sex controls generated through natural matings (P < 0.001 for both females and males) (Fig. 1, a and b). This phenomenon was transient for both sexes; by 1 wk of age, no significant differences in body weight were seen among groups for either sex. At 3 wk of age, IVF females weighed significantly more than STOCK, ICSI, and SCNT females, although this effect was absent by 4 wk of age. At 4 wk of age, SCNT females weighed significantly more than IVF females. By 6 wk of age, body weights of SCNT females were greater than those of STOCK, IVF, and ICSI females, and this difference persisted through the duration of the present study (P < 0.01) (Fig. 1c). In males, no difference was observed in weekly body weights (Fig. 1d).
FIG. 1.
Body weights of female and male offspring produced through natural mating, ARTs, and SCNT. Results are reported as birth weights of female (a) or male (b) offspring as well as weekly body weights of female (c) and male (d) offspring through the duration of the 8-wk study. Data are presented as the mean; error bars represent the SEM. Asterisks denote significant differences versus the control (STOCK) condition (P < 0.05).
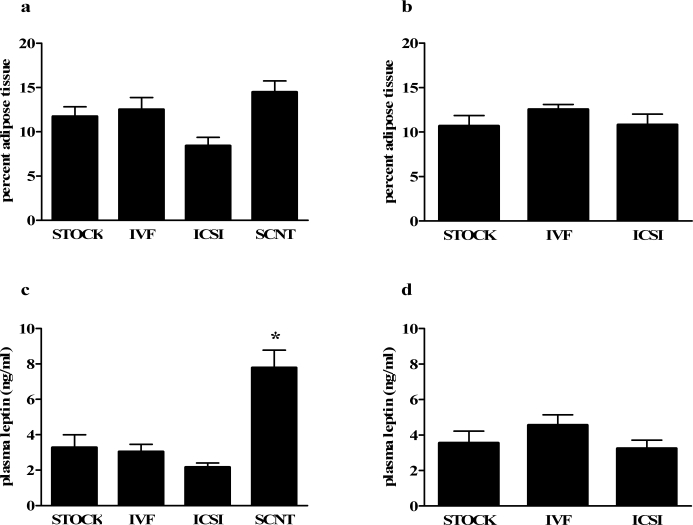
At 8 wk of age, female SCNT mice had a greater percentage of adipose tissue than ICSI females (P < 0.05) (Fig. 2a), but no differences were seen between ART-generated mice and STOCK females. No differences in percentage adipose tissue were evident in 8-wk-old males (Fig. 2b). No differences in percentage lean tissue were observed among female or male mice (data not shown).
FIG. 2.
Body composition and plasma leptin levels of offspring produced through natural mating, ARTs, and SCNT. Results are reported as percentage adipose tissue of female (a) and male (b) offspring and plasma leptin levels of female (c) and male (d) mice. Data are presented as the mean; error bars represent the SEM. Asterisks denote significant differences versus the STOCK group (P < 0.05).
Plasma Leptin
The SCNT females had significantly higher plasma leptin levels than STOCK, IVF, and ICSI groups at 8 wk of age (P < 0.05) (Fig. 2c). Method of conception had no effect on male plasma leptin values (Fig. 2d).
Glucose Tolerance Test
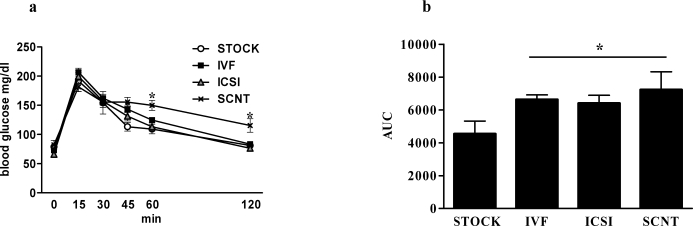
Females.
Females had similar basal and peak glucose levels; however, glucose levels of SCNT and IVF females were elevated 45 min postinjection relative to levels in STOCK animals (P < 0.05). At 60 min, SCNT glucose levels were higher than ICSI and STOCK glucose levels, and at 120 min, glucose levels of SCNT females were higher than those of all other treatments (P < 0.01) (Fig. 3a). Glucose area under the curve (AUC) was greater for SCNT, IVF, and ICSI females than for STOCK females (P < 0.05) (Fig. 3b).
FIG. 3.
Blood glucose response to IPGTT in female offspring generated through natural mating, ARTs, and SCNT. Results are reported as blood glucose level before (0 min) and following glucose administration (a) and as the glucose AUC (b). Data are presented as the mean; error bar represent the SEM. Asterisks denote significant differences versus STOCK females (P < 0.05).
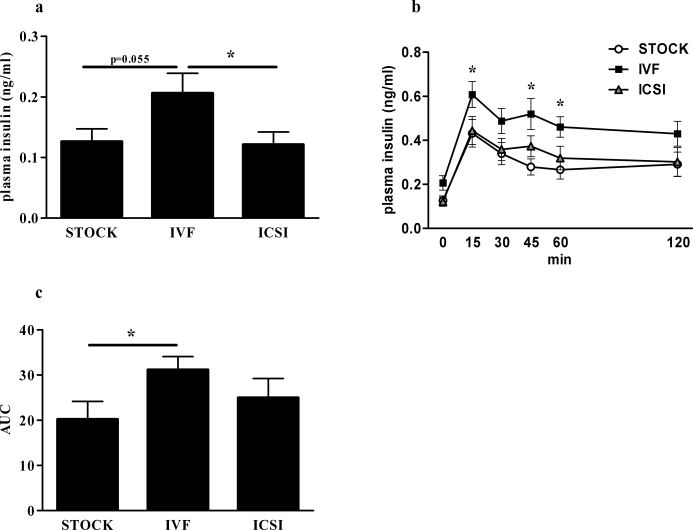
The IVF and SCNT females had significantly elevated fasting plasma insulin in comparison with STOCK females (P < 0.05) (Fig. 4a). Following intraperitoneal glucose injection, female IVF mice exhibited significantly higher insulin peaks at 15 min compared with STOCK females (P < 0.05) (Fig. 4b). Insulin AUC did not differ among females (Fig. 4c).
FIG. 4.
Basal plasma insulin and insulin response to IPGTT in female mice. Results are reported as basal plasma insulin levels (a), plasma insulin response to glucose administration (b), and plasma insulin AUC (c). Data are presented as the mean; error bars represent the SEM. Asterisks denote significant differences versus the STOCK group (P < 0.05).
Males.
Baseline glucose levels were not different among any of the male groups, nor were any differences found at any time point following glucose injection nor glucose AUC (data not shown). However, male IVF mice had higher fasting plasma insulin than male ICSI mice (P < 0.05) and a trend toward higher fasting insulin in comparison with STOCK males (P = 0.055) (Fig. 5a). Insulin levels of IVF males were significantly higher than those of ICSI males at 15 min postinjection and were higher than those of STOCK males at 15, 45, and 60 min postinjection (P < 0.05) (Fig. 5b). IVF males secreted more insulin than STOCK males in response to the glucose bolus, as evidenced by a greater AUC (Fig. 5c).
FIG. 5.
Basal plasma insulin and insulin response to IPGTT in male mice. Results are reported as basal plasma insulin levels (a), plasma insulin response to glucose administration (b), and plasma insulin AUC (c). Data are presented as the mean; error bar represent the SEM. Asterisks denote significant differences versus the STOCK group (P < 0.05).
DISCUSSION
In the present study, both female and male mice conceived by IVF or ICSI weighed more than STOCK controls at birth. The effects of IVF, ICSI, and SCNT on birth weight of offspring are consistent with previous findings from our laboratory and others in which SCNT or in vitro culture and manipulation resulted in fetal overgrowth in utero [7, 18, 19]. This is in contrast to some studies of human children conceived using ART, because those children had a lower birth weight than age-matched, naturally conceived children [20]. However, one must keep in mind that the model of ART in the present study used embryos from healthy, fertile, young adult females and males. In a clinical setting, those who seek ART are generally older and have underlying conditions that affect fertility. Studies have indicated that the practice of transferring multiple embryos to women who are attempting to conceive may be partially responsible for the low birth weights observed, because single embryo transfer is not associated with lower birth weights of IVF-conceived children [20].
Because embryo transfer is associated with a lowered success rate in producing full-term pups, dams that were used for natural conception underwent a unilateral tubal ligation to produce similar litter sizes. Tubal ligation did reduce the litter size but did not completely normalize them to that of ART and SCNT litters, so we cannot discount the possibility that the reduced number of full-term pups in the ART and SCNT groups affected birth weight. However, we have previously demonstrated that litter size is not solely responsible for effects on birth weight [7]. Additionally, all litters were cross-fostered after delivery into groups of five pups to control for postnatal effects of litter size.
The increased body weight at birth was transient in all groups; weights were no longer different by 1 wk of age. As we expected, SCNT females began to gain more weight compared to the other groups at 6 wk of age, and this difference persisted for the duration of the 8-wk study. No differences were found in body weight among IVF, ICSI, and STOCK groups throughout this study, suggesting that procedures associated with these techniques, such as exposure to in vitro culture, IVF, and mechanical handling and injection, may not responsible for the greater body weight observed in SCNT mice at this early age.
The SCNT females had significantly higher plasma leptin levels at 8 wk of age compared with all other groups. This finding is interesting, in that circulating leptin levels are positively correlated with adiposity, and SCNT females had amounts of adipose tissue similar to those of STOCK and IVF females. However, subcutaneous adipose tissue secretes more leptin than visceral adipose [21], suggesting that the pattern of adipose tissue accrual may be different in SCNT mice. Recent reports suggest that children conceived using IVF have atypical body composition, and that these differences may be depot-specific [5]. This difference observed in SCNT mice may result from a similar effect, and it may be too early to see it in the IVF and ICSI mice that do not differ in weight from the STOCK females at that time point. Future studies will assess patterns of adipose deposition in offspring exposed to in vitro manipulations.
Female mice generated by IVF, ICSI, and SCNT had similar fasting glucose and 15-min peak glucose levels in response to a glucose challenge. However, IVF and SCNT females took longer to clear the glucose load compared with STOCK controls. Furthermore, IVF and SCNT females had higher fasting insulin levels than STOCK animals, and IVF females had a significantly higher insulin peak at 15 min than STOCK females, suggesting that IVF and SCNT females are less glucose tolerant. Recently, it has been reported that children conceived using IVF have elevated fasting glucose levels and that factors such as impaired fertility, parental parameters, and body size could not account for these differences [4]. The children in those studies ranged from 8 to 18 yr of age and did not have elevated insulin levels, but it should be pointed out that the mice used in the present study would compare to an age older than 8–18 yr, and that comparable offspring may, indeed, develop symptoms of insulin resistance in the future.
At 8 wk of age, method of conception did not affect body composition, leptin levels, or the ability to clear a glucose bolus in male mice. However, males generated by IVF had higher fasting insulin, higher glucose-stimulated insulin levels, and a greater overall glucose AUC, suggesting that like their female counterparts, more insulin is necessary for IVF males to clear a glucose load in comparison with STOCK and ICSI mice.
The present study suggests that techniques associated with SCNT and ARTs, especially IVF, alter glucose tolerance of 8-wk-old male and female mice. Although both sexes of ART-generated mice exhibited alterations in the ability to clear a glucose load, the changes manifested in different ways. That is, IVF males released more insulin to maintain fasting and stimulated glucose levels similar to those of ICSI and STOCK males. However, the elevations of insulin in SCNT and IVF females were not sufficient to maintain glucose levels comparable to those of STOCK females, suggesting a sex-specific effect of in vitro exposure to media, mechanical manipulation, or both on glucose homeostasis in offspring.
Consistent with the data presented here, sex-dependent differences have been reported elsewhere, including hypertrophy of the liver and lungs in females conceived through ICSI using either fresh or frozen sperm [22]. Furthermore, behavioral differences seen in mice conceived using techniques associated with ART may be sexually dimorphic. Female mice generated through ICSI engaged in less exploration and locomotion and exhibited impaired performance on memory tasks, whereas no differences were seen in male mice generated by ICSI [22]. Additionally, sex steroids are known to affect adipose deposition and glucose clearance, with estrogen enhancing insulin sensitivity and testosterone having the opposite effect [23]. This may account for the higher insulin levels observed in males. In these studies, all females had lower peak glucose levels in comparison with their male counterparts. These reports, in concert with the present findings, imply that there may be multiple sexually dimorphic effects of in vitro manipulation on energy homeostasis and other parameters that require more comprehensive examination [24].
For the present study, male clones were not generated, because cloning using adult male Sertoli cells or fibroblasts has much lower success rates than cloning using adult cumulus cells. Although immature Sertoli cells have been successfully used as donor cells for SCNT, this is when those cells are small and undifferentiated. For the present purposes, we did not feel that an adequate comparison could be made between female clones generated from adult cumulus cells and male clones produced using immature and undifferentiated Sertoli cells [16]. However, in light of the sex differences noted in the ART groups, a male SCNT group should be investigated as well.
The mechanism by which ARTs, SCNT, and related techniques alter phenotype is not known. Aspects of exposure to in vitro culture media or mechanical manipulation and microinjection may play a role. Additionally, genetic reprogramming of somatic cell nuclei likely plays a significant role, because SCNT mice have alterations in some measures of energy homeostasis that are in excess of those exhibited by IVF and ICSI mice. It may be that these alterations occur secondary to epigenetic modifications, because the obese phenotype of mice cloned through SCNT is not passed on to offspring when naturally mated [7]. Fauque et al. [25] have reported that the combination of superovulation, IVF, and placement in a culture medium alters early embryo development and DNA methylation within the H19 imprinting control region and H19 proximal promoter. They also noted wide individual variability in the methylation status of genes in manipulated blastocysts and embryos compared with naturally conceived and gestated embryos that appeared to be dependent on the culture media used, providing a possible explanation for the finding that whereas the majority of children conceived by ARTs appear to be healthy, they also appear to have a greater predisposition to health problems compared with children conceived through natural methods. TSA, a potent histone deacetylase (HDAC) inhibitor, was added to the culture during some steps of SCNT. TSA improves the success rate of SCNT and is thought to do so by preventing hypermethylation of DNA [15], yet female offspring generated by SCNT weighed more than IVF or ICSI mice. This suggests that the state of methylation is not solely responsible for the phenotype observed.
Interestingly, male and female mice generated by IVF appear to have more aberrant glucose clearance than their ICSI counterparts. This is unexpected, because we hypothesized that the greater mechanical manipulation associated with ICSI would result in more significant changes. Spermatazoa penetrate the oocyte without assistance in IVF, but in ICSI, the tail is removed from the spermatozoa and the head is microsurgically injected into the oocyte, bypassing several steps that occur in natural fertilization and introducing foreign material into the oocyte that would not normally occur [26]. Furthermore, oocytes used in ICSI, as well as in SCNT, require additional treatment to remove cumulus cells. For these reasons, we expected that the phenotype of ICSI offspring would be more severe than that of the IVF group.
The in vitro culture environment and duration, however, can expose gametes and embryos to stress and damage by reactive oxygen species. The length of time in culture media correlates with the amount of damage that occurs [27], and the amount of time embryos are exposed to culture media and the composition of that culture media differ depending on the technique used. IVF requires less mechanical manipulation than ICSI, but it also requires 1–2 h for capacitation of sperm, 5–6 h for incubation of the sperm with the cumulus-oocyte complexes to facilitate fertilization, and a 24-h incubation period to obtain 2-cell embryos for transfer. ICSI requires 20 min of incubation before injection, followed by the 24-h incubation period to obtain embryos. Thus, in the present study, the IVF procedure may have exposed the gametes to more oxidative damage than those procedures used in ICSI. SCNT also requires a longer exposure to culture than in ICSI. These possibilities remain to be tested.
Based on previous studies, the obese phenotype of SCNT mice develops around 8 wk of age, and around 9–10 wk in mice that were naturally conceived but underwent in vitro manipulation [7]. The IPGTT likely was performed around the time of dynamic metabolic changes of IVF and ICSI mice, and it may be that differences would have emerged in the ICSI mice had they been tested at a later time point. Future studies will assess the time course of phenotypic changes in offspring undergoing different in vitro manipulations.
The placenta plays a critical role in regulating passage of nutrients, growth factors, and hormones to the developing fetus, and it may also play a role in the metabolic differences seen in offspring. This has been observed in models of intrauterine growth restriction and prenatal stress, in which changes in maternal nutrition and/or endocrine function affect placental transfer of nutrients, oxygen, hormones, and other factors to the fetus. This, in turn, can alter the metabolism of the offspring [28–30].
We previously demonstrated that cloned mice generated through SCNT weigh more than naturally bred mice [7, 31]. In addition, females that underwent in vitro manipulation and transfer were also heavier than naturally bred mice, although significantly less so than SCNT mice. These changes became evident between 8 and 10 wk of age. Our current research adds to these findings by investigating the effects of in vitro manipulations that occur in IVF and ICSI, which are responsible for an estimated 1–3% of human births in developed nations [1]. Similar to recent reports by Ceelen et al. [4], we also found a link between ART and alterations in glucose homeostasis. Although the mice used in the present study were young adults and none was obese in comparison with STOCK animals, differences in glucose tolerance and insulin sensitivity were already evident. This is important, because it suggests that insulin resistance may have already developed before onset of obesity, providing valuable insight regarding how altered glucose metabolism and obesity may arise in offspring exposed to in vitro manipulations [7, 22, 24].
These data suggest long-term effects of procedures associated with ARTs on glucose homeostasis in rodents that are manifest before any changes in body weight or composition. However, one must use caution when extrapolating findings in mice to the human condition.
Footnotes
Supported by National Institute of Child Health and Human Development ( HD055030), National Institute of Diabetes and Digestive and Kidney Diseases (068273), and University of Maryland Nutrition Obesity Research Center (P30 DK072488).
REFERENCES
- Vela G, Luna M, Sandler B, Copperman AB.Advances and controversies in assisted reproductive technology. Mt Sinai J Med 2009; 76: 506–520. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA.Growth and development of children born after in vitro fertilization. Fertil Steril 2008; 90: 1662–1673. [DOI] [PubMed] [Google Scholar]
- Allen C, Reardon W.Assisted reproduction technology and defects of genomic imprinting. BJOG 2005; 112: 1589–1594. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA.Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab 2008; 93: 1682–1688. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Roos JC, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA.Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab 2007; 92: 3417–3423. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Wakayama T, Yamazaki Y, Akutsu H, Woods SC, Kondo S, Yanagimachi R, Sakai RR.Phenotype of cloned mice: development, behavior, and physiology. Exp Biol Med (Maywood) 2003; 228: 1193–1200. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Wakayama T, Akutsu H, Yamazaki Y, Lachey JL, Wortman MD, Seeley RJ, D'Alessio DA, Woods SC, Yanagimachi R, Sakai RR.Cloned mice have an obese phenotype not transmitted to their offspring. Nat Med 2002; 8: 262–267. [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I.Large offspring syndrome in cattle and sheep. Rev Reprod 1998; 3: 155–163. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM.Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A 2004; 101: 1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins AJ, Ursell E, Panton R, Papenbrock T, Hollis L, Cunningham C, Wilkins A, Perry VH, Sheth B, Kwong WY, Eckert JJ, Wild AE, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol Reprod 2008; 78: 299–306. [DOI] [PubMed] [Google Scholar]
- Toyoda Y, Yokoyama M, Hoshi T.Studies on fertilization of mouse eggs in vitro. I. In vitro fertilization of eggs by fresh epididymal sperm. J Anim Reprod 1971; 16: 147–151. [Google Scholar]
- Kimura Y, Yanagimachi R.Development of normal mice from oocytes injected with secondary spermatocyte nuclei. Biol Reprod 1995; 53: 855–862. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Wakayama T, Yanagimachi R.Contribution of cumulus cells and serum to the maturation of oocyte cytoplasm as revealed by intracytoplasmic sperm injection (ICSI). Zygote 2001; 9: 277–282. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R.Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 1998; 394: 369–374. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mizutani E, Ohta H, Hikichi T, Thuan NV, Wakayama S, Bui HT, Wakayama T.Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. Biochem Biophys Res Commun 2006; 340: 183–189. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ogonuki N, Mochida K, Yamamoto Y, Takano K, Kohda T, Ishino F, Ogura A.Effects of donor cell type and genotype on the efficiency of mouse somatic cell cloning. Biol Reprod 2003; 69: 1394–1400. [DOI] [PubMed] [Google Scholar]
- Inoue K, Noda S, Ogonuki N, Miki H, Inoue S, Katayama K, Mekada K, Miyoshi H, Ogura A.Differential developmental ability of embryos cloned from tissue-specific stem cells. Stem Cells 2007; 25: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Young LE, Wilmut I, McEvoy TG.In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod 2000; 15(suppl 5):68–86. [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Sinclair KD, Young LE, Wilmut I, Robinson JJ.Large offspring syndrome and other consequences of ruminant embryo culture in vitro: relevance to blastocyst culture in human ART. Hum Fertil (Camb) 2000; 3: 238–246. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Prein J, Smit JJ, Vermeiden JP, Spreeuwenberg M, van Leeuwen FE, Delemarre-van de Waal HA.Growth during infancy and early childhood in relation to blood pressure and body fat measures at age 8–18 years of IVF children and spontaneously conceived controls born to subfertile parents. Hum Reprod 2009; 24: 2788–2795. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF.Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res 2002; 34: 616–621. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira PN, Perez-Crespo M, Sanchez-Martin M, Ramirez MA, Pericuesta E, Bilbao A, Bermejo-Alvarez P, de Dios Hourcade J, de Fonseca FR, Gutierrez-Adan A.Long-term effects of mouse intracytoplasmic sperm injection with DNA-fragmented sperm on health and behavior of adult offspring. Biol Reprod 2008; 78: 761–772. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W.Gender differences in insulin resistance, body composition, and energy balance. Gend Med 2009; 6(suppl 1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez de Fonseca F, Pintado B, Gutierrez-Adan A.Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci U S A 2004; 101: 5880–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauque P, Jouannet P, Lesaffre C, Ripoche MA, Dandolo L, Vaiman D, Jammes H.Assisted reproductive technology affects developmental kinetics, H19 imprinting control region methylation and H19 gene expression in individual mouse embryos. BMC Dev Biol 2007; 7: 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R.Intracytoplasmic injection of spermatozoa and spermatogenic cells: its biology and applications in humans and animals. Reprod Biomed Online 2005; 10: 247–288. [DOI] [PubMed] [Google Scholar]
- Enkhmaa D, Kasai T, Hoshi K.Long-time exposure of mouse embryos to the sperm produces high levels of reactive oxygen species in culture medium and relates to poor embryo development. Reprod Domest Anim 2009; 44: 634–637. [DOI] [PubMed] [Google Scholar]
- Bauer MK, Harding JE, Bassett NS, Breier BH, Oliver MH, Gallaher BH, Evans PC, Woodall SM, Gluckman PD.Fetal growth and placental function. Mol Cell Endocrinol 1998; 140: 115–120. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, O'Connor TG, Glover V.Prenatal stress and neurodevelopment of the child: focus on the HPA axis and role of the placenta. Dev Neurosci 2009; 31: 285–292. [DOI] [PubMed] [Google Scholar]
- Owens JA.Endocrine and substrate control of fetal growth: placental and maternal influences and insulin-like growth factors. Reprod Fertil Dev 1991; 3: 501–517. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Wakayama T, Blanchard RJ, Blanchard DC, Yanagimachi R.Postnatal growth and behavioral development of mice cloned from adult cumulus cells. Biol Reprod 2000; 63: 328–334. [DOI] [PubMed] [Google Scholar]