Synopsis
Neurologists frequently evaluate patients complaining of vision loss, especially when the patient has been examined by an ophthalmologist who has found no ocular disease. A significant proportion of patients presenting to the neurologist with visual complaints will have non-organic or functional visual loss. While there are examination techniques which can aid in the detection and diagnosis of functional visual loss, the frequency with which functional visual loss occurs concomitantly with organic disease warrants substantial caution on the part of the clinician. Furthermore, purely functional visual loss is never a diagnosis of exclusion, and must be supported by positive findings on examination that demonstrate normal visual function. The relationship of true psychological disease and functional visual loss is unclear and most patients respond well to simple reassurance.
Keywords: functional visual loss, nonorganic visual loss
Introduction
Neurologists are frequently called upon to evaluate patients complaining of vision loss. Similar to other areas of neurology, a significant proportion of these patients will have non-organic disease [1–3]. Nonorganic visual loss is frequently called functional visual loss [4–6]. The ability to differentiate between organic and functional visual loss has important clinical and medicolegal implications [7, 8].
Neuro-Ophthalmology and Nonorganic Dysfunction
One of the most common interactions between the neurologist and the ophthalmologist occurs over patients with nonorganic visual dysfunction. It is estimated that such cases constitute up to 5% of a general ophthalmologist’s practice, frequently resulting in neurologic consultation after no ocular cause is identified [9, 10]. Using objective measurements, one can identify non-physiologic responses and can sometimes prove unequivocally the organic integrity of the visual system [9, 11–15].
Functional visual loss is never a diagnosis of exclusion; positive findings are required to make the diagnosis. Occasionally, these positive findings may not be related to the patient’s primary complaint. Furthermore, it is not enough to demonstrate that the patient’s responses are non-physiologic. This is a helpful adjunct and acts as confirmatory evidence in the diagnosis of a nonorganic disorder. However, organic and nonorganic disease can and do coexist. In reviews of two neuro-ophthalmologists’ experiences, 53% of patients with evidence of functional visual loss had coexistent organic disease [14, 16]. A recent series of patients with idiopathic intracranial hypertension found 6% had concurrent functional visual loss, complicating the decision to proceed with surgical intervention [17]. A purely nonorganic disorder can only be diagnosed if the maneuvers used during examination prove normal function of the system being tested.
Patients with evidence of nonorganic visual dysfunction are typically described as “functional” in an attempt to avoid having to distinguish between those cases which are intentionally feigned and those which are thought to be outside the realm of the patient’s consciousness. There are three major categories of functional disorders: (1) somatoform disorders (also commonly referred to as “hysteria”), (2) factitious disorders, and (3) malingering [18]. In general, malingering implies purposeful feigning or exaggeration of symptoms usually for clear secondary gain, while somatoform disorders are thought to occur outside the patient’s conscious awareness [9, 11, 13–15]. Factitious disorders are characterized by intentionally produced symptoms for the purpose of assuming the sick role [18]. Differentiating among these various diagnostic categories remains difficult and within the psychiatrist’s realm; demonstrating the non-physiologic nature of the complaint is in the neurologist’s and neuro-ophthalmologist’s realm.
Neurologists and neuro-ophthalmologists are particularly adept at demonstrating either the organic or nonorganic nature of a symptom or sign because they evaluate an organ system that respects certain anatomical rules that are not intuitively understood by the patient. In addition, the visual system, more than other parts of the sensory system, is closely observable and measurable. Armed with knowledge of neuroanatomy and neurophysiology, a working understanding of basic ophthalmologic tools, and a little sleight of hand, one can demonstrate integrity of the visual system.
Like any evaluation of visual loss, one’s approach begins by first differentiating monocular from binocular visual loss. This allows the physician to appropriately localize potential organic lesions and to strategize the history and physical. Next, the physician should further refine the examination by whether the visual loss is primarily central (visual acuity loss) versus peripheral (visual field loss) so that techniques tailored to each case can assist the clinician in his or her determination of whether the responses are non-physiologic.
Monocular Visual Acuity Loss
Loss of visual acuity is a common presenting complaint of the functional patient. When it assumes a monocular pattern, the ideal testing situation is established. All of these tests are designed to take advantage of the fact that during binocular vision it is difficult to separate out what each eye sees. If a patient is feigning monocular visual loss, he or she may try to close one eye or the other during the examination. The success of these tests depends on a skilled examiner and each test can be described to the patient in a manner that obscures the true intent of the test for the purposes of detecting functional visual loss. One of the best tests available to the neurologist is a test of stereopsis which requires good vision and good binocular fusion. The widely available, and relatively inexpensive, Titmus or Randot Stereo Tests (Stereo Optical Co., Inc., Chicago, IL) are examples. Presenting stereopsis as a test of “how the eyes work together” is usually successful. The degree of stereopsis on a set of standardized tests has been correlated with the minimum visual acuity required in each eye (Table 1) [19].
Table 1.
Relationship of Stereopsis to Visual Acuity
| Stereopsis (arc second) | Visual acuity |
|---|---|
| 40 | 20/20 |
| 43 | 20/25 |
| 52 | 20/30 |
| 61 | 20/40 |
| 78 | 20/50 |
| 94 | 20/70 |
| 124 | 20/100 |
| 160 | 20/200 |
Data from Levy NS, Glick EB. Stereoscopic perception and Snellen visual acuity. Am J Ophthalmol. Oct 1974;78(4):722–724.
Colored lenses will allow only similarly colored light to pass through. If a patient wears glasses with one green and one red lens while viewing an eye chart with alternate green and red letters, the function and acuity of each eye can be individually assessed. This can be presented to a patient as a “color test.” Similarly, the patient can be given polarized glasses with different axes in each lens and asked to read a polarized eye chart with some letters perceptible only to one eye or the other.
Another set of tests works on the principle of “fogging” [9, 11, 13–15]. While the patient views the eye chart with both eyes open, the “good” eye is subtly fogged so that any useful binocular vision must be a result of “bad” eye function. Using the phoropter, an instrument of refraction, convex lenses of high strength are placed over the good eye so that binocular vision of 20/20 would prove 20/20 vision in the “bad eye.” Unfortunately, this particular test relies on a piece of specialized equipment that is usually unavailable to the neurologist. All of these tests have the advantage of not only proving that the presumed bad eye has vision, but they also document the amount of vision present, which is critical to demonstrating normal function, thereby proving purely functional loss.
Other less quantitative tests may be necessary to prove better vision than that claimed by the patient. These maneuvers are similar to those performed on the patient with severe binocular visual loss (see below), except that the good eye in the monocular cases must be occluded during testing, with the following exceptions. First, in the patient claiming profound monocular visual loss, the absence of a relative afferent pupillary defect (Marcus Gunn or swinging flashlight sign) makes functional visual loss, refractive error, or media opacity more likely. Second, one could also place a four diopter prism over the better-seeing eye of a patient complaining of substantial monocular visual loss. If the patient reports seeing two images when viewing an object that they previously claimed to be unable to see, one should suspect at least a degree of functional visual loss, as a patient with organic disease will only see one image [20]. There are other variations of this test utilizing a single prism that require minimal slight-of-hand, but presumably have a reduced chance of being foiled by the astute malingerer [21]. Finally, binocular visual fields can be performed to see if the patient has sufficient vision in the affected eye to prohibit plotting of the physiologic blind spot of the normal eye. If the bad eye is truly nonseeing, the physiologic blind spot of the good eye should be plottable.
Binocular Visual Acuity Loss
More difficult are those cases of binocular visual loss. Here is where the level of expectation as communicated by the examiner becomes extremely important. The patient is asked to read the eye chart from the bottom upwards, obscuring the other lines, and beginning with the smallest available, usually 20/10. The examiner allows for significant time on each line, repeatedly encouraging the patient to make the best guesses he or she can, and explaining that he or she should be able to see the chart. Frequently, by the time the patient reaches the 20/20 or 20/25 lines, good vision is established; this may be particularly useful in the highly suggestible patient, rather than the obvious malingerer. Similarly, lenses that when combined are the equivalent of plain glass can be placed over the patient’s refraction while the examiner suggests that these will magnify the letters on the chart. Another feature of functional patients is that they will frequently claim the same visual acuity when the distance from the eye chart is halved. The physiology of vision is such that if a patient sees the 20/100 line at 20 feet, they should be able to see the 20/50 line at 10 feet. This form of visual acuity testing has been shown to be highly specific and sensitive for functional visual loss [22].
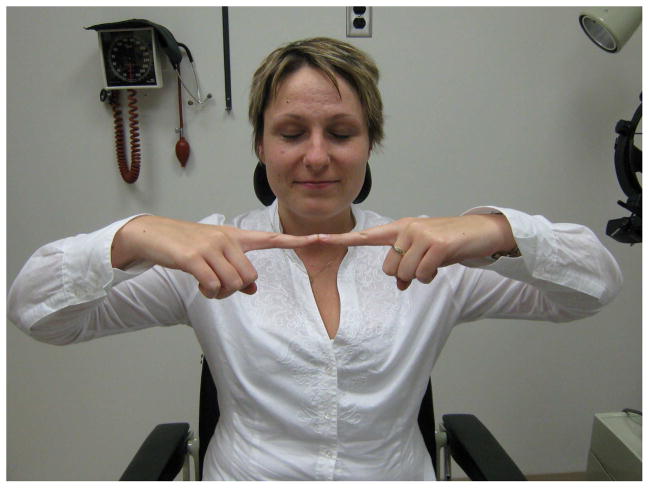
Less quantitative maneuvers that at least establish the presence of vision may be necessary in those patients with professed severe bilateral visual loss. In the mirror test, a large mirror is rotated back and forth in the vertical axis in front of the patient [9, 11, 13]. It is very difficult for a seeing patient to avoid following this moving image. Similarly, the optokinetic drum or tape will elicit appropriate fast and slow phases of nystagmus in eyes that have at least 20/200 vision [11, 13]. Under the guise of coordination tests, the “blind” person can be asked to “wiggle your fingers,” “open and close your fists,” then “do this” (while the examiner quickly performs another simple movement of the hands) [6]. The seeing patient may mimic the new movement before realizing the slip. Simply observing the patient may be informative. The “blind” patient that easily maneuvers around physical obstacles in the examining room, or that flinches when an object or bright light is suddenly presented, likely has at least a component of functional visual loss. Additional findings confirming nonphysiologic tendencies include a failure to direct the eyes to look at their own hand and an inability to touch the two index fingertips together when so instructed (Figure 1); these tests of proprioception are easily passed by a truly blind person. Spasm of the near triad (purposeful convergence with associated pupillary miosis) may also be witnessed. It has been observed that patients with functional visual loss frequently wear sunglasses (46%) in the clinic, and yet have no ocular findings to provide an organic reason for this [9, 23].
Figure 1.
(A) A person who is truly blind can touch the tips of the fingers properly. (B) A person with functional visual loss is often unable to touch the tips of the fingers properly. From Biousse V, Newman NJ. Neuro-ophthalmology Illustrated. New York: Thieme 2009; p. 504 with permission.
Visual Field Loss
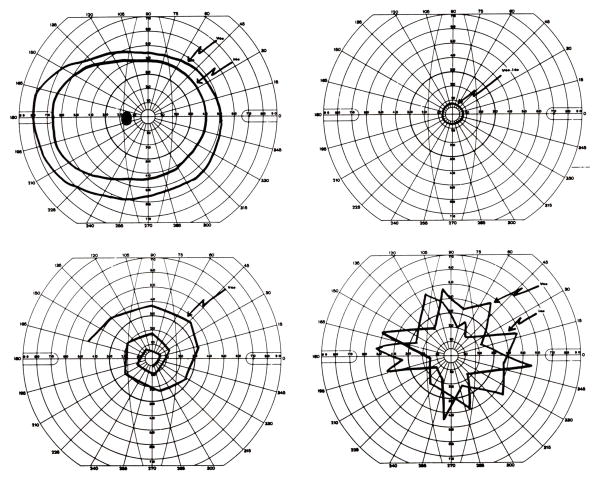
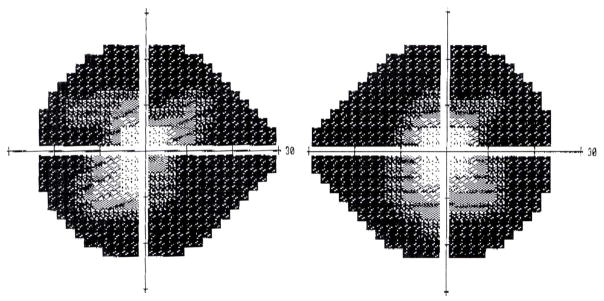
Functional visual loss may assume the form of visual field loss. The most common visual field complaint is that of concentric loss of peripheral vision, like “tunnel vision.” The field may be constricted to 5–10° centrally, yet the patient has no difficulty maneuvering around objects in the periphery. The classic configuration on tangent screen testing is that of circular constriction that does not expand appropriately when the distance between the patient and the tangent screen is increased [9, 11–15] (Figure 2). This “tunnel field can also be confirmed with confrontation testing at different distances. Visual fields performed kinetically on the Goldmann perimeter may show a similar constriction with nonphysiologic overlap of isopters (i.e., the patient claims to see the smaller, less bright object at the same place as the larger, brighter test object). Alternatively, the functional patient may plot out a continuous spiral or a jagged inconsistent star pattern (Figure 3). It is not unusual for the functional patient with complaints other than visual field loss to have visual fields plotted in these patterns. In those cases, the visual field abnormalities can act as additional evidence of functional visual loss. Automated static perimetry is generally not helpful in the assessment of suspected functional patients. Poor testing parameters and inconsistent responses do not differentiate between the patient with nonphysiologic visual dysfunction and the organic patient unable to adequately perform this test. Some have suggested that square or cloverleaf appearances are seen more frequently in cases of functional visual loss [9], but this cannot be relied upon as diagnostic of functional visual loss (Figure 4).
Figure 2.
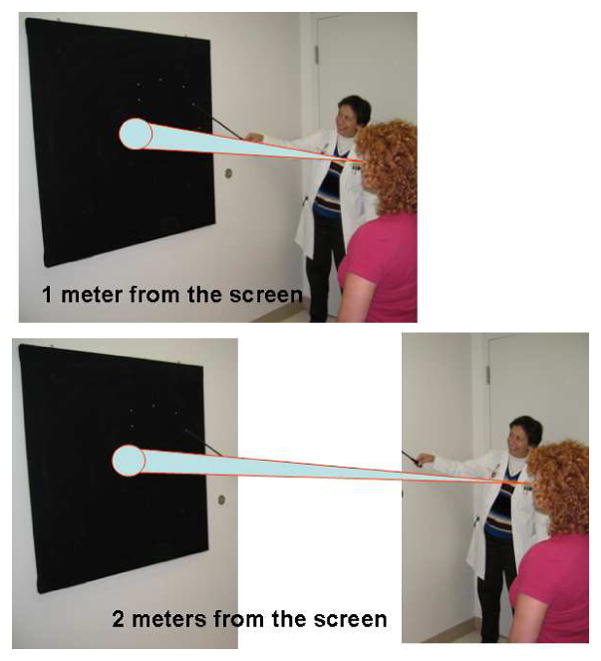
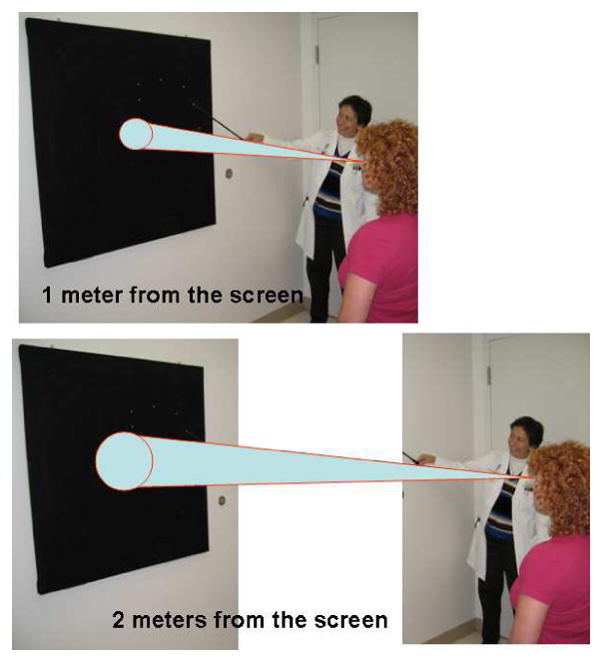
(A) Nonorganic constriction of visual field on tangent screen test. The size of the visual field does not increase when the patient is moved farther away from the screen. (B) Organic constriction of visual field on tangent screen test. The size of the visual field increases when the patient is moved farther away from the screen. From Biousse V, Newman NJ. Neuro-ophthalmology Illustrated. New York: Thieme 2009; p. 507 with permission.
Figure 3.
Goldmann perimeter visual fields. (A) Normal visual field (B) Constricted visual field of a functional patient. Note that the constriction is to the same degree for two different sized stimuli. (C) Spiral visual field of a functional patient. (D) Star-pattern visual field with inconsistent responses in a functional patient. From Newman NJ. Gen Hosp Psych 1993;15:105, with permission.
Figure 4.
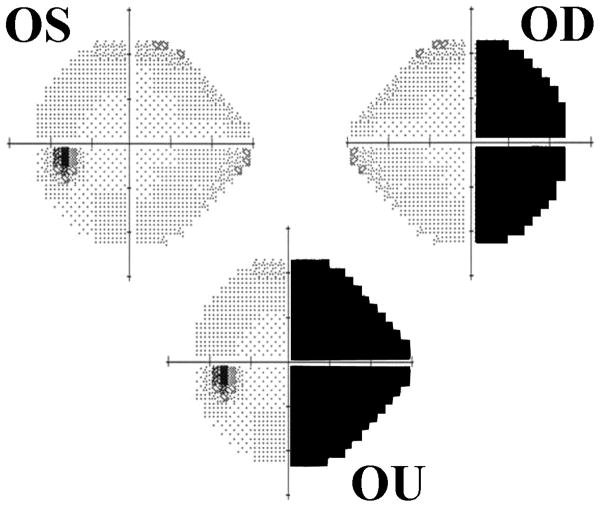
Nonphysiologic static (Humphrey) perimetry. Note the box-like appearance of the right eye’s visual field and the cloverleaf-like pattern of loss in the left eye’s visual field in a patient claiming severe bilateral visual loss. Goldmann perimetry confirmed nonphysiologic visual field loss.
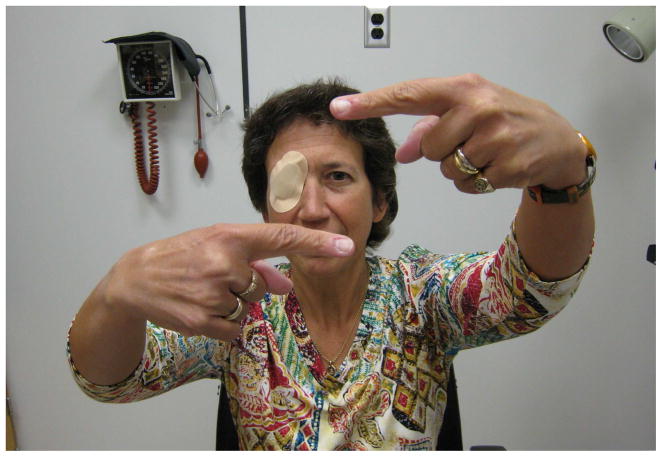
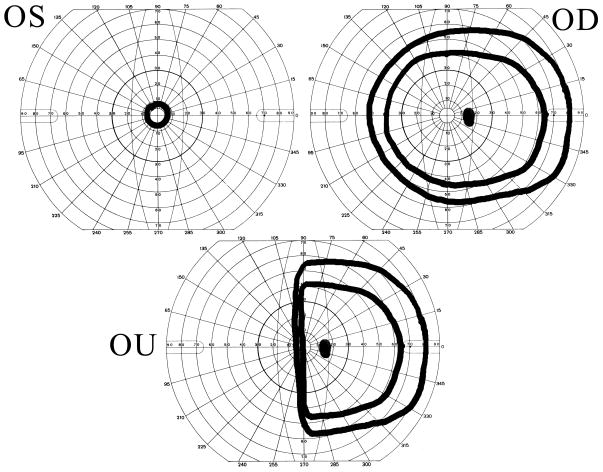
Other patterns of visual field loss are less common. Monocular hemianopias can often be proven functional by the patient’s performance on binocular visual field testing. In the patient with organic disease, the good eye visual field will compensate for most of the missing bad eye field. With the functional patient, the hemianopia may persist binocularly [12, 13, 24] (Figure 5). Similarly, in the patient with professed severe monocular visual loss, a binocular visual field (with both eyes open) may reveal a nonphysiologic constriction or absence of the visual field on the side of the monocular loss, a region clearly seen on prior testing of the good eye alone (Figure 6). Patients with true bitemporal hemianopias will not be able to see objects beyond the point of fixation because they will lie entirely within the missing temporal fields of vision [12, 13, 25]. Functional patients with bitemporal hemianopia are rarely medically sophisticated enough to demonstrate this. Central scotomas or arcuate defects are unlikely manifestations of functional visual loss and should prompt a careful search for true organic pathology [16].
Figure 5.
Monocular and binocular visual field in a functional patient with monocular hemianopia of the right eye. In the binocular field we see that the hemianopia persists, whereas in organic disease the normal nasal visual field of the right eye would compensate for the deficit in the temporal field of the left eye.
Figure 6.
Goldmann visual fields in a patient claiming severe monocular visual loss in the left eye. The right eye is normal and the left eye shows severe tubular constriction. In the binocular visual field, there is non-physiologic constriction to the left side, a region clearly seen by the patient previously when testing the right eye alone.
Ancillary Testing
A thorough neuro-ophthalmic evaluation, complete with appropriate special maneuvers specifically directed toward revealing nonphysiologic responses, will usually be sufficient to not only establish the presence of nonorganic visual dysfunction, but to prove normal function of the system. In those cases in which completely normal function cannot be demonstrated, but a nonorganic etiology is suspected, ancillary tests are occasionally useful. Visual evoked potentials of normal and symmetric amplitude and latency in a patient with profound monocular visual loss are confirmatory evidence of a functional etiology [11]. Although not as widely available, multifocal visual evoked potentials may demonstrate normal electrophysiological responses in the region of purported visual loss [26]. However, an abnormal visual evoked response is less helpful. Voluntary alteration or obliteration of evoked potentials is not uncommon and may be inapparent even to a trained observer [25, 27]. A similar situation exists with pattern and multifocal electroretinograms: a normal and symmetric response argues against the presence of severe organic disease and an abnormal test is inconclusive [28, 29, 30]. The flash electroretinogram measures the function of predominantly the outer retinal layers of cells involved in vision. It should be abnormal in a patient with diffuse retinal dysfunction but will be normal in a patient with more distal organic disease such as optic neuropathy, chiasmal neuropathy, or retrochiasmal visual dysfunction. Neuroimaging may help rule out obvious compressive or vascular lesions, but negative studies do not establish the diagnosis as functional. Indeed, Moster et al. found lesions on nuclear medicine imaging studies (SPECT and PET) while evaluating two patients with suspected functional visual loss [31]. A functional disturbance must never be a diagnosis of exclusion.
Functional Visual Loss: A Marker of Psychiatric Disease?
Evidence of a functional visual disturbance may be seen in a range of patients from the “deliberate malingerer” to the “indifferent hysteric” to the “suggestible innocent” [15]. Patients vary in their degree of awareness, fraud, and suggestibility. Few, if any, of the maneuvers outlined above distinguish among these underlying differences in motive and etiology. As a result, the prognosis, management, and therapy of these patients will differ markedly.
As a group, patients with functional visual disturbances may not have as high an incidence of true psychiatric disease as has been previously assumed [10, 14, 15, 32, 33]. Krill and Newell [34] claimed “no uniform psychological factors” in their 59 patients with functional visual loss. Similarly, Van Balen and Slijper [35] found no difference on psychological testing between 43 children with functional visual loss and age-matched controls. Older studies report the incidence of “psychiatric disease” in patients presenting with functional visual loss as ranging from 70% to 100% [10, 36–39]. However, many of these patients were given diagnoses not accepted under the current classification of psychiatric disorders including headache, mixed psychoneurosis, tension state, fatigue, vertigo, cardiovascular spasm, convulsion, hysteria, psychosis, low intellect, overly dependent, compulsive, anxious, and overly independent [10]. A recent series of 140 patients reported by Lim et al. found 39% of adults and 18% of children had an underlying history of psychiatric disease, and that a significant proportion of both adults and children reported concomitant psychosocial events (36%). These events were more likely to be trauma in adults and social in children [40]. In another series of 71 consecutive children with functional visual loss, 27% had previously diagnosed psychiatric disturbances, 31% had significant home or school stress, and 23% wanted glasses. Fourteen percent of the patients had no identifiable cause [41]. However, both of these studies were limited by retrospective, nonsystematic screening for psychiatric disease.
Review of the literature also led Kathol et al. to conclude that psychiatric disease is far from uniformly present, and that many patients with functional visual loss do not appear to have psychiatric syndromes or personality disorders [10, 32, 33]. They reexamined 42 of their own patients with functional visual loss an average of 4 years after the initial diagnosis: 22 were diagnosed with a DSM-III psychiatric diagnosis and/or personality disorder at some time during the course of their observation; 23 had persistent findings of nonphysiologic visual disturbances at follow-up, but only eight of these 23 patients had a diagnosed psychiatric disorder and few were either socially or economically impaired. One criticism of this study is that patients were included as examples of functional visual loss even if the only abnormalities were incidentally discovered, non-physiologically constricted, tubular, or spiral visual fields. It is not surprising that patients such as these would not be assigned a DSM-III psychiatric diagnosis. The authors concluded that some patients with functional visual loss are merely suggestible. This is not a new theory, as Babinski postulated in 1909 that isolated “hysterical” symptoms were a product of the patient’s suggestibility [42].
The reemergence of the idea of suggestibility has led several authors to emphasize the role of simple reassurance in the treatment of many patients with functional visual disturbances [13, 15, 32, 33, 40]. In some cases, patients may be convinced that their symptoms are not signs of serious illness, are commonly seen by the examiner, and will likely improve with time. This form of therapy can begin in the clinician’s office and may be sufficient to preclude further psychiatric management. It is generally unhelpful to directly confront patients who are malingering; indeed, the explanation to the patient on why the findings are nonphysiologic may merely educate them on how to better evade detection in the future.
Differences in underlying motive and etiology may help explain the generally poor response of functional visual loss to a wide range of treatments, including psychotherapy [10, 15, 32, 33, 40]. Kathol et al. [10] retrospectively reviewed the response of patients with nonorganic visual dysfunction to a variety of therapies including hypnosis, behavior modification, psychotherapy, and nonspecific treatments such as “special” glasses, eye drops, placebos, surgery, prayer, sham lumbar puncture, and electroconvulsive therapy. No treatment was more beneficial than another and there was no significant difference in the outcome of those receiving psychiatric treatment and those not. Eleven percent of the patients in Lim et al.’s series were referred for counseling and the remainder only needed reassurance [40]. However, patients with functional visual loss may have a wide variety of underlying psychiatric and nonpsychiatric diagnoses. Obviously, prospective randomized studies that attempt to separate patients by underlying diagnoses are necessary before conclusions can be drawn regarding the success of specific therapies.
Organic Dysfunction Mimicking Functional Visual Loss
Some organic disorders can present with visual loss combined with symptoms and signs of psychiatric disease that may lead the clinician to falsely presume that the visual loss is functional in nature [40, 43–46]. Early recognition of these disorders may aid in timely evaluation and institution of appropriate therapy. In several of these disorders, the neuro-ophthalmologic examination may provide evidence of organic pathology and suggest the location and nature of the underlying problem. This will allow for subsequent directed evaluation and management.
Neoplasms involving the central nervous system, especially in the pituitary-hypothalamic region and frontal lobes, may cause visual loss associated with psychiatric features. Up to 50% of patients with intracranial mass lesions will develop psychiatric symptoms and occasionally these will be the presenting manifestation [46]. Patients with pituitary tumors, especially those that secrete adrenocorticotropic hormone (ACTH) or that cause panhypopituitarism, may present with depression [47, 48]. Similarly, mass lesions involving the hypothalamic region, including cranipharyngioma and neurosarcoid, may result in personality changes and frank psychiatric symptoms [49–52].
Examination of the visual system may show evidence of compression of the optic nerves or chiasm on visual field testing and ophthalmoscopic examination. Central scotomas, arcuate defects, and subtle bitemporal defects are likely to be manifestations of organic disease. Unrecognized tumors of the frontal lobes may result in papilledema and visual loss associated with apathy, depression, and personality changes. Without adequate funduscopic examination, the visual complaints might be attributed to functional visual loss [46]. A paraneoplastic photoreceptor degeneration can result in rapid visual loss initially misdiagnosed as nonorganic in etiology [53–55]. Ophthalmoscopic examination combined with electroretinography will ultimately result in correct diagnosis.
Strokes may occasionally mimic functional visual loss. For example, top-of-the-basilar infarction can present with visual disturbances and behavioral alterations [56]. Careful examination may reveal abnormalities of vertical gaze and visual field defects suggestive of occipital lobe ischemia. The posterior form of Alzheimer’s disease can also present with visual complaints and psychiatric/cognitive disturbances that are not infrequently thought to represent functional complaints. Signs can include prominent visual agnosia, visual-spatial difficulties, and even frank visual field defects [57, 58]. Multiple sclerosis is another disorder where visual complaints are often combined with unusual patient affects that may at first appear to be non-organic disease. The neuro-ophthalmologic history and examination can be useful in revealing clinical evidence of demyelination involving the afferent and efferent visual systems.
The clinician must also be cautious while evaluating patients with known psychiatric disease to not quickly dismiss complaints of visual loss as functional. Some of the psychiatric patient’s visual complaints may be a direct result of psychiatric medications [59, 60]. The classic example is the pigmentary retinopathy caused by thioridazine (Mellaril) [61, 62], but this is seen much less frequently since the largest manufacturers of the drug discontinued production in 2005. Early complaints include difficulties with night vision and a brownish tinge to vision. Chlorpromazine (Thorazine) remains on the market and can also cause pigmentary retinopathy, but usually only at high doses [63]. Retinal screening examinations may reveal abnormalities prior to symptoms. Phenothiazines can also cause opacities of the cornea or lens. Ocular surface disease is a frequent cause of decreased vision and can result from antidopaminergic-induced pseudoparkinsonism resulting in decreased blink, and consequent corneal surface symptoms. Anitcholinergics can also contribute to ocular surface disease through decreased tear production. Other anticholinergic effects that affect vision are decreased accommodative ability and aggravation of narrow-angle glaucoma.
Conclusions
The neurologist frequently interacts with the ophthalmologist regarding patients with suspected functional visual loss. Simple examination techniques combined with the judicious use of ancillary testing can confirm the non-physiologic nature of the visual complaints and often prove normal visual function. A neurologist who has a patient with visual loss should always seek consultation with an ophthalmologist to ensure there is no underlying ophthalmic explanation. Similarly, if hysteria or malingering is suspected in a patient without overt visual system involvement, ophthalmologic examination may reveal confirmatory signs of non-organic dysfunction or instead discover findings supporting an organic, neurologic cause. Communication between the neurologist and ophthalmologist regarding the specific questions to be addressed can help to focus the examination and allow the ophthalmologist to provide aid in the diagnosis of functional visual loss with some of their specialized tools. If uncertainty remains, consultation with a neuro-ophthalmologist is likely appropriate, because many of these tests are beyond the scope of those usually performed by a general ophthalmologist and require significant patience and time on the part of the examiner.
Acknowledgments
Support:
This work was supported in part by a departmental grant (Department of Ophthalmology) from Research to Prevent Blindness, Inc, New York, New York, by core grant P30-EY06360 (Department of Ophthalmology) from the National Institutes of Health/National Eye Institute, and PHS Grants KL2-RR025009 (Dr. Bruce) from the Clinical and Translational Science Award Program, National Institutes of Health/National Center for Research Resources. Dr. Bruce was a recipient of the American Academy of Neurology Practice Research Fellowship. Dr. Newman is a recipient of a Research to Prevent Blindness Lew R. Wasserman Merit Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berlin RM, Ronthal M, Bixler EO, et al. Psychiatric symptomatology in an outpatient neurology clinic. J Clin Psychiatry. 1983 Jun;44(6):204–206. [PubMed] [Google Scholar]
- 2.Kirk C, Saunders M. Primary psychiatric illness in a neurological out-patient department in North East England. An assessment of symptomatology. Acta Psychiatr Scand. 1977 Oct;56(4):294–302. doi: 10.1111/j.1600-0447.1977.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirk CA, Saunders M. Psychiatric illness in a neurological out-patient department in North East England. Use of the General Health Questionnaire in the prospective study of neurological out-patients. Acta Psychiatr Scand. 1979 Nov;60(5):427–437. doi: 10.1111/j.1600-0447.1979.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 4.Biousse V, Newman NJ. Neuro-ophthalmology illustrated. New York: Thieme; 2009. Nonorganic neuro-ophthalmic symptoms and signs; pp. 501–510. [Google Scholar]
- 5.Miller NR. Neuro-ophthalmologic manifestations of nonorganic disease. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 6. Vol. 2. Philadelphia: Lippincott William & Wilkins; 2005. pp. 1315–1334. [Google Scholar]
- 6.Newman NJ. Neuro-ophthalmology and psychiatry. Gen Hosp Psychiatry. 1993 Mar;15(2):102–114. doi: 10.1016/0163-8343(93)90106-x. [DOI] [PubMed] [Google Scholar]
- 7.Wasfy IA, Wasfy E, Aly TA, et al. Ophthalmic medicolegal cases in Upper Egypt. Int Arch Med. 2009;2(1):1. doi: 10.1186/1755-7682-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mavrakanas NA, Schutz JS. Feigned visual loss misdiagnosed as occult traumatic optic neuropathy: diagnostic guidelines and medical-legal issues. Surv Ophthalmol. 2009 May–Jun;54(3):412–416. doi: 10.1016/j.survophthal.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Miller BW. A review of practical tests for ocular malingering and hysteria. Surv Ophthalmol. 1973 Jan–Feb;17(4):241–246. [PubMed] [Google Scholar]
- 10.Kathol RG, Cox TA, Corbett JJ, et al. Functional visual loss: I. A true psychiatric disorder? Psychol Med. 1983 May;13(2):307–314. doi: 10.1017/s0033291700050923. [DOI] [PubMed] [Google Scholar]
- 11.Kramer KK, La Piana FG, Appleton B. Ocular malingering and hysteria: diagnosis and management. Surv Ophthalmol. 1979 Sep–Oct;24(2):89–96. doi: 10.1016/0039-6257(79)90126-7. [DOI] [PubMed] [Google Scholar]
- 12.Keane JR. Neuro-ophthalmic signs and symptoms of hysteria. Neurology. 1982 Jul;32(7):757–762. doi: 10.1212/wnl.32.7.757. [DOI] [PubMed] [Google Scholar]
- 13.Smith CH, Beck RW, Mills RP. Functional disease in neuro-ophthalmology. Neurol Clin. 1983 Nov;1(4):955–971. [PubMed] [Google Scholar]
- 14.Keltner JL, May WN, Johnson CA, et al. The California syndrome. Functional visual complaints with potential economic impact. Ophthalmology. 1985 Mar;92(3):427–435. [PubMed] [Google Scholar]
- 15.Thompson HS. Functional visual loss. Am J Ophthalmol. 1985 Jul 15;100(1):209–213. doi: 10.1016/s0002-9394(14)75008-1. [DOI] [PubMed] [Google Scholar]
- 16.Scott JA, Egan RA. Prevalence of organic neuro-ophthalmologic disease in patients with functional visual loss. Am J Ophthalmol. 2003 May;135(5):670–675. doi: 10.1016/s0002-9394(02)02254-7. [DOI] [PubMed] [Google Scholar]
- 17.Ney JJ, Volpe NJ, Liu GT, et al. Functional visual loss in idiopathic intracranial hypertension. Ophthalmology. 2009 Sep;116(9):1808–1813. e1801. doi: 10.1016/j.ophtha.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 19.Levy NS, Glick EB. Stereoscopic perception and Snellen visual acuity. Am J Ophthalmol. 1974 Oct;78(4):722–724. doi: 10.1016/s0002-9394(14)76312-3. [DOI] [PubMed] [Google Scholar]
- 20.Golnik KC, Lee AG, Eggenberger ER. The monocular vertical prism dissociation test. Am J Ophthalmol. 2004 Jan;137(1):135–137. doi: 10.1016/s0002-9394(03)00865-1. [DOI] [PubMed] [Google Scholar]
- 21.Chen CS, Lee AW, Karagiannis A, et al. Practical clinical approaches to functional visual loss. J Clin Neurosci. 2007 Jan;14(1):1–7. doi: 10.1016/j.jocn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Zinkernagel SM, Mojon DS. Distance doubling visual acuity test: a reliable test for nonorganic visual loss. Graefes Arch Clin Exp Ophthalmol. 2009 Jun;247(6):855–858. doi: 10.1007/s00417-008-1019-9. [DOI] [PubMed] [Google Scholar]
- 23.Bengtzen R, Woodward M, Lynn MJ, et al. The “sunglasses sign” predicts nonorganic visual loss in neuro-ophthalmologic practice. Neurology. 2008 Jan 15;70(3):218–221. doi: 10.1212/01.wnl.0000287090.98555.56. [DOI] [PubMed] [Google Scholar]
- 24.Keane JR. Hysterical hemianopia. The ‘missing half’ field defect. Arch Ophthalmol. 1979 May;97(5):865–866. doi: 10.1001/archopht.1979.01020010423002. [DOI] [PubMed] [Google Scholar]
- 25.Mills RP, Glaser JS. Hysterical bitemporal hemianopia. Arch Ophthalmol. 1981 Nov;99(11):2053. doi: 10.1001/archopht.1981.03930020931022. [DOI] [PubMed] [Google Scholar]
- 26.Massicotte EC, Semela L, Hedges TR., 3rd Multifocal visual evoked potential in nonorganic visual field loss. Arch Ophthalmol. 2005 Mar;123(3):364–367. doi: 10.1001/archopht.123.3.364. [DOI] [PubMed] [Google Scholar]
- 27.Bumgartner J, Epstein CM. Voluntary alteration of visual evoked potentials. Ann Neurol. 1982 Nov;12(5):475–478. doi: 10.1002/ana.410120511. [DOI] [PubMed] [Google Scholar]
- 28.Reiss AB, Biousse V, Yin H, et al. Voluntary alteration of full-field electroretinogram. Am J Ophthalmol. 2005 Mar;139(3):571–572. doi: 10.1016/j.ajo.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 29.Rover J, Bach M. Pattern electroretinogram plus visual evoked potential: a decisive test in patients suspected of malingering. Doc Ophthalmol. 1987 Jun;66(3):245–251. doi: 10.1007/BF00145238. [DOI] [PubMed] [Google Scholar]
- 30.Vrabec TR, Affel EL, Gaughan JP, et al. Voluntary suppression of the multifocal electroretinogram. Ophthalmology. 2004 Jan;111(1):169–176. doi: 10.1016/j.ophtha.2003.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Moster ML, Galetta SL, Schatz NJ. Physiologic functional imaging in “functional” visual loss. Surv Ophthalmol. 1996 Mar–Apr;40(5):395–399. doi: 10.1016/s0039-6257(96)80068-3. [DOI] [PubMed] [Google Scholar]
- 32.Kathol RG, Cox TA, Corbett JJ, et al. Functional visual loss: II. Psychiatric aspects in 42 patients followed for 4 years. Psychol Med. 1983 May;13(2):315–324. doi: 10.1017/s0033291700050935. [DOI] [PubMed] [Google Scholar]
- 33.Kathol RG, Cox TA, Corbett JJ, et al. Functional visual loss. Follow-up of 42 cases. Arch Ophthalmol. 1983 May;101(5):729–735. doi: 10.1001/archopht.1983.01040010729005. [DOI] [PubMed] [Google Scholar]
- 34.Krill AE, Newell FW. The diagnosis of ocular conversion reaction involving visual function. Arch Ophthalmol. 1968 Mar;79(3):254–261. doi: 10.1001/archopht.1968.03850040256006. [DOI] [PubMed] [Google Scholar]
- 35.van Balen AT, Slijper FE. Psychogenic amblyopia in children. J Pediatr Ophthalmol Strabismus. 1978 May–Jun;15(3):164–167. doi: 10.3928/0191-3913-19780501-11. [DOI] [PubMed] [Google Scholar]
- 36.Linhart WO. Field findings in functional disease; report of 63 cases. Am J Ophthalmol. 1956 Jul;42(1):75–84. doi: 10.1016/0002-9394(56)90012-5. [DOI] [PubMed] [Google Scholar]
- 37.Friesen H, Mann WA. Follow-up study of hysterical amblyopia. Am J Ophthalmol. 1966 Dec;62(6):1106–1115. doi: 10.1016/0002-9394(66)92560-8. [DOI] [PubMed] [Google Scholar]
- 38.Rada RT, Meyer GG, Krill AE. Visual conversion reaction in children. I. Diagnosis. Psychosomatics. 1969 Jan–Feb;10(1):23–28. doi: 10.1016/S0033-3182(69)71787-X. [DOI] [PubMed] [Google Scholar]
- 39.Gross MP, Sloan SH. Patients with eye symptoms and no organic illness: an interdisciplinary study. Psychiatry Med. 1971 Oct;2(4):298–307. doi: 10.2190/7lg4-6tb4-w4q8-hcmq. [DOI] [PubMed] [Google Scholar]
- 40.Lim SA, Siatkowski RM, Farris BK. Functional visual loss in adults and children patient characteristics, management, and outcomes. Ophthalmology. 2005 Oct;112(10):1821–1828. doi: 10.1016/j.ophtha.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Taich A, Crowe S, Kosmorsky GS, et al. Prevalence of psychosocial disturbances in children with nonorganic visual loss. J AAPOS. 2004 Oct;8(5):457–461. doi: 10.1016/j.jaapos.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Babinski J. Demembrement de l’hysterie traditionnelle: pithiatisme. La Semaine Medicale. 1909;29:3–8. [Google Scholar]
- 43.Hall RCW. Psychiatric presentations of medical illness: somatopsychic disorders. New York: Springer: Scientific & Medical Books; 1980. [Google Scholar]
- 44.Hayes JR, Butler NE, Martin CR. Misunderstood somatopsychic concomitants of medical disorders. Psychosomatics. 1986 Feb;27(2):128–130. 133. doi: 10.1016/s0033-3182(86)72723-0. [DOI] [PubMed] [Google Scholar]
- 45.Cummings JL. Organic psychosis. Psychosomatics. 1988 Winter;29(1):16–26. doi: 10.1016/S0033-3182(88)72418-4. [DOI] [PubMed] [Google Scholar]
- 46.Binder RL. Neurologically silent brain tumors in psychiatric hospital admissions: three cases and a review. J Clin Psychiatry. 1983 Mar;44(3):94–97. [PubMed] [Google Scholar]
- 47.Cohen SI. Cushing’s syndrome: a psychiatric study of 29 patients. Br J Psychiatry. 1980 Feb;136:120–124. doi: 10.1192/bjp.136.2.120. [DOI] [PubMed] [Google Scholar]
- 48.Kelly WF, Checkley SA, Bender DA, et al. Cushing’s syndrome and depression--a prospective study of 26 patients. Br J Psychiatry. 1983 Jan;142:16–19. doi: 10.1192/bjp.142.1.16. [DOI] [PubMed] [Google Scholar]
- 49.Baskin DS, Wilson CB. Surgical management of craniopharyngiomas. A review of 74 cases. J Neurosurg. 1986 Jul;65(1):22–27. doi: 10.3171/jns.1986.65.1.0022. [DOI] [PubMed] [Google Scholar]
- 50.Delaney P. Neurologic manifestations in sarcoidosis: review of the literature, with a report of 23 cases. Ann Intern Med. 1977 Sep;87(3):336–345. doi: 10.7326/0003-4819-87-3-336. [DOI] [PubMed] [Google Scholar]
- 51.Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol. 1985 Sep;42(9):909–917. doi: 10.1001/archneur.1985.04060080095022. [DOI] [PubMed] [Google Scholar]
- 52.Heffernan A, Cullen M, Towers R, et al. Sarcoidosis of the hypothalamus. A case report with a review of the literature. Hormones. 1971;2(1):1–12. [PubMed] [Google Scholar]
- 53.Sawyer RA, Selhorst JB, Zimmerman LE, et al. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol. 1976 May;81(5):606–613. doi: 10.1016/0002-9394(76)90125-2. [DOI] [PubMed] [Google Scholar]
- 54.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987 Mar;105(3):372–375. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 55.Thirkill CE, FitzGerald P, Sergott RC, et al. Cancer-associated retinopathy (CAR syndrome) with antibodies reacting with retinal, optic-nerve, and cancer cells. N Engl J Med. 1989 Dec 7;321(23):1589–1594. doi: 10.1056/NEJM198912073212307. [DOI] [PubMed] [Google Scholar]
- 56.Caplan LR. “Top of the basilar” syndrome. Neurology. 1980 Jan;30(1):72–79. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- 57.Katz B, Rimmer S. Ophthalmologic manifestations of Alzheimer’s disease. Surv Ophthalmol. 1989 Jul–Aug;34(1):31–43. doi: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 58.Mendez MF, Mendez MA, Martin R, et al. Complex visual disturbances in Alzheimer’s disease. Neurology. 1990 Mar;40(3 Pt 1):439–443. doi: 10.1212/wnl.40.3_part_1.439. [DOI] [PubMed] [Google Scholar]
- 59.Fraunfelder FT, Meyer SM. Ocular toxicology. In: Duane TD, editor. Clinical Ophthalmology. Vol. 5. Philadelphia: J.B. Lippincott; 1988. [Google Scholar]
- 60.Grant WM. Toxicology of the eye. Springfield, IL: Charles C. Thomas; 1986. [Google Scholar]
- 61.May RH, Selymes P, Weekley RD, et al. Thioridazine therapy: results and complications. J Nerv Ment Dis. 1960;130:230. [Google Scholar]
- 62.Meredith TA, Aaberg TM, Willerson WD. Progressive chorioretinopathy after receiving thioridazine. Arch Ophthalmol. 1978 Jul;96(7):1172–1176. doi: 10.1001/archopht.1978.03910060006002. [DOI] [PubMed] [Google Scholar]
- 63.Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127–141. doi: 10.2165/00002018-200831020-00003. [DOI] [PubMed] [Google Scholar]