There has been long-standing interest in the relationship between cigarette smoking and colorectal cancer. Epidemiological studies that were conducted 40 years ago failed to detect any association with smoking, and essentially, all causative factors were assumed to be found in the diet, which fit the wide range of incidence in gastrointestinal cancers found worldwide (1). Even the most current epidemiological meta-analyses have reported modest relative risks (RRs; range = 1.18–1.20) for incident colorectal cancers among cigarette smokers, with an absolute risk increase of 10.8 cases per 105 person-years (2,3). Considering the enormous health hazards caused by cigarette smoking to the heart and lungs, some people might consider the role of cigarette smoking in colorectal cancer to be relatively trivial.
Between 1990 and 2000, a greater understanding of the pathogenesis of colorectal cancer began to unfold. Colorectal cancer actually consists of several different diseases that evolve through distinct genetic pathways but were initially indistinguishable to the pathologist. This problem gave rise to the hypothesis that these forms of cancer may also have different etiologies (Figure 1). The first identifiable subset of colorectal cancer was a group of approximately 15% with a unique mutational signature called microsatellite instability (MSI) (8). Between 20% and 25% of MSI tumors are linked to the hereditary form of colorectal cancer—Lynch syndrome (9)—but the rest occur sporadically and are not familial. MSI tumors have a large number of point mutations and insertion or deletion mutations at simple repeat sequences called microsatellites. Another 30% of colorectal cancers were found to have an epigenetic signature called the CpG island methylator phenotype (CIMP). In these CIMP tumors, the predominant problem is the abnormal silencing of genes by the methylation of promoters embedded in CpG islands (10). Overlap occurs between these two groups because 75%–80% of MSI tumors occur in the context of methylation-induced silencing of the MLH1 gene, which then causes MSI. Because of these complexities, it has taken some time to recognize the patterns involved, but the ability to recognize specific subsets of colorectal cancers has provided an opportunity to deconvolute the origins of these tumors.
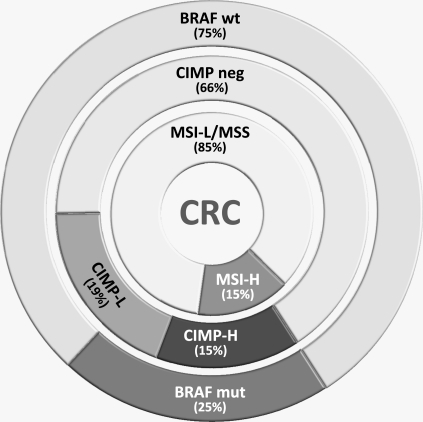
Figure 1.
Molecular characterization of colorectal cancer (CRC). CRCs can be characterized by the presence or absence of microsatellite instability (MSI), the CpG island methylator phenotype (CIMP), or BRAF mutation status. Whereas BRAF mutation status is a simple categorical variable (wild type [wt] or mutant [mut]), measurements of MSI and CIMP are quantitative variables and have been characterized as “high” (MSI-H and CIMP-H, respectively) and “low” (MSI-L and CIMP-L, respectively). The analysis can vary depending on the methods used to determine MSI and CIMP. This figure is adapted from a conceptual characterization by Jass (4) with distribution data from unselected CRC patients (5,6). The work reported by Limsui et al. (7) used a cohort of middle-aged women. Variations in the distribution of tumors in these categories can be caused by technical aspects in measuring MSI or CIMP or by differences in the biology of tumors that might be present in the cohort of patients selected for study. Also, the variables are not independent because CIMP is responsible for most (but not all) of the MSI tumors and BRAF mutations are highly associated with CIMP. Cigarette smoking is best associated with those CRCs with CIMP-H, MSI-H, and BRAF mutations.
It has been previously reported (11) that cigarette smoking is related to the group of colorectal cancers with MSI. Also, a link between cigarette smoking and colorectal cancers with CIMP has been noted (12,13). Molecular epidemiologists also recognized the link between tobacco smoking and BRAF mutations in colorectal cancer, which are intimately linked with CIMP and MSI (12). The Multiethnic Cohort Study reported (14) a relationship between smoking in the context of meat intake and a NAT2 “rapid” genotype, and the Colon Cancer Family Registry collaboration has reported (11) an association between MSI in rectal cancers and cigarette smoking. More recently, the Molecular Epidemiology of Colorectal Cancer Study in Israel has found that men who smoked were more likely to develop colorectal cancers with BRAF mutations (15). By focusing on specific subsets, the epidemiology and presumed causation of colorectal cancer have begun to be clarified.
The article by Limsui et al. (7) in this issue of Journal used the Iowa Women's Health Study database to report associations between cigarette smoking and molecularly defined subsets of colorectal cancer. First, women who had ever been a smoker had an increased risk for overall colorectal cancer incidence (RR = 1.20), similar to previous reports (11–13). But, individuals who had ever been smokers had substantially higher risks for colorectal cancers with MSI (RR = 1.66), CIMP (RR = 1.46), and BRAF mutations (RR = 1.57), compared with never-smokers. These risks were higher yet for current smokers (for MSI tumors, RR = 1.99; for CIMP tumors, RR = 1.88; and for BRAF-mutated tumors, RR = 1.92). No relationships were identified between cigarette smoking variables and non-MSI, non-CIMP, and/or BRAF mutation–negative colorectal cancers. Smokers were more likely to have proximal colorectal cancers, as expected from colorectal cancers with these molecular characteristics. The recognition of the unique nature of these colorectal cancer subsets was required to make these associations.
There were some unique aspects of the study by Limsui et al. (7) that might limit or modify the generalizability of these observations. First, this was a study of women from Iowa between the ages of 55 and 69 years. This characteristic is perhaps a greater strength than a weakness because few other studies have focused upon this population. Smoking was only modestly associated with the overall incidence of colorectal cancer but was more strongly associated with MSI colorectal cancers. In this study, 26% of the colorectal cancers had MSI (specifically, MSI-high), which is higher than the percentages in other cohorts. Limsui et al. used a novel set of microsatellite targets to define MSI (ie, four mononucleotide repeats, five dinucleotide repeats, one complex marker, and ≥30% instability = MSI), which is nonstandard and a possible explanation for their findings. Alternatively, colorectal cancers with MSI are more commonly found in older women. Similarly, there are no standardized definitions for CIMP (and there are confusing designations for high and low levels of CIMP), and investigators might identify slightly different subsets by the use of different sets of CIMP markers. The figure indicates the distribution of colorectal cancers by molecular characterization, adapting a concept developed by Jass (4), with data from our previous studies (5,6). It is apparent that if one were to expand or contract the proportion of tumors in any of the categories, the epidemiological associations might change as well.
The most important messages to take from the study by Limsui et al. is that colorectal cancer is a complex collection of diseases and that causation might be different for each of the categories. Cigarette smoking is associated with a specific subset of these tumors—those that are proximal, have MSI, come from a background of CIMP, and have BRAF mutations. As satisfying as it might be to gain these insights, we still have much more to understand. We need to know how smoking interacts with other environmental factors, such as diet. Moreover, the methylation pathway is particularly interesting because epigenetic changes, unlike point mutations and chromosomal rearrangements, are potentially reversible. Our task now is to understand how to translate these finding into improved screening and prevention-based strategies.
Funding
National Cancer Institute (R01 CA72851 and CA129286); National Institutes of Health; Baylor Research Institute.
Footnotes
C. R. Boland and A. Goel are fully responsible for the concepts presented and all aspects of writing this manuscript. The authors have no conflicts of interest to disclose.
References
- 1.Wynder EL, Shigematsu T. Environmental factors of cancer of the colon and rectum. Cancer. 1967;20(9):1520–1561. doi: 10.1002/1097-0142(196709)20:9<1520::aid-cncr2820200920>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 3.Tsoi KK, Pau CY, Wu WK, Chan FK, Griffiths S, Sung JJ. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2009;7(6):682–688. doi: 10.1016/j.cgh.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology. 2007;50(1):113–130. doi: 10.1111/j.1365-2559.2006.02549.x. [DOI] [PubMed] [Google Scholar]
- 5.Goel A, Arnold CN, Niedzwiecki D, et al. Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 2003;63(7):1608–1614. [PubMed] [Google Scholar]
- 6.Goel A, Nagasaka T, Arnold CN, et al. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132(1):127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102(14):1012–1022. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 9.Peltomaki P, Lothe RA, Aaltonen LA, et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 1993;53(24):5853–5855. [PubMed] [Google Scholar]
- 10.Toyota M, Issa JP. CpG island methylator phenotypes in aging and cancer. Semin Cancer Biol. 1999;9(5):349–357. doi: 10.1006/scbi.1999.0135. [DOI] [PubMed] [Google Scholar]
- 11.Poynter JN, Haile RW, Siegmund KD, et al. Associations between smoking, alcohol consumption, and colorectal cancer, overall and by tumor microsatellite instability status. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2745–2750. doi: 10.1158/1055-9965.EPI-09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samowitz WS, Albertsen H, Sweeney C, et al. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98(23):1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 13.Slattery ML, Curtin K, Sweeney C, et al. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120(3):656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 14.Nothlings U, Yamamoto JF, Wilkens LR, et al. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18(7):2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rozek LS, Herron CM, Greenson JK, et al. Smoking, gender, and ethnicity predict somatic BRAF mutations in colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(3):838–843. doi: 10.1158/1055-9965.EPI-09-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]