Abstract
Autogeny, the ability of a mosquito to mature an initial batch of eggs without bloodfeeding, is an alternative reproductive strategy with important implications for vector-borne disease transmission. Regulation of the major yolk protein (vitellogenin; Vg) genes during bloodmeal-induced oogenesis is well studied, but little is known about regulation of vitellogenesis in autogenous mosquitoes. We characterized the expression of four vitellogenin genes (Vg1a, Vg1b, Vg2a and Vg2b) in an autogenous strain of the West Nile Virus vector, Culex tarsalis. All vitellogenin genes were expressed during autogenous reproduction and following a bloodmeal, though the intensity and duration of expression varied between genes. Quantitative PCR analysis of vitellogenin transcription during autogeny revealed a similar temporal pattern to known vitellogenin expression profiles in anautogenous Aedes aegypti. Vitellogenin transcript, primarily produced from the Vg1b gene, was also detected in the larval and pupal stages of development, but no detectable vitellogenin protein was produced during this time period.
Introduction
The direct link between female mosquitoes’ requirement for vertebrate blood and individual fitness places enormous selective pressures on the efficiency and success of bloodfeeding behavior. Anautogenous female mosquitoes must take a bloodmeal at least once per gonotrophic cycle in order to supply nutrients for egg development. Unfortunately, this comes at the consequence of pathogen transmission and the spread of vector-borne diseases. For these reasons, bloodmeal-regulated egg development has dominated research in mosquito reproduction. However, some populations of medically important mosquitoes such as Culex tarsalis (Chao, 1958), Culex pipiens (Roubaud, 1929, Spielman, 1957), and Aedes albopictus (Bat-Miriam and Craig, 1966, Mori et al., 2008) are capable of developing their first batch of eggs autogenously, or in the absence of a bloodmeal. Even though most of these mosquitoes subsequently bloodfeed, autogeny is still of great interest because it can effectively delay the first bloodmeal (Nelson and Milby, 1982). This delay may reduce the probability of the mosquito becoming infected with and transmitting a pathogen, since fewer individuals survive the extrinsic incubation period (Reisen et al., 1983, Reeves, 1990). Expression of autogeny is also proposed to lead to maintenance or increases in vector population size during times of low host abundance, and may maintain vertically transmitted pathogens (Reeves, 1990, Eberle and Reisen, 1986).
Culex tarsalis is an extremely important and efficient vector of arboviruses (such as Western Equine Encephalitis Virus, Saint Louis Encephalitis Virus and most recently, West Nile Virus) in the Western United States (Reeves, 1990, Goddard et al., 2002, Turell et al., 2003, Reisen et al., 2004, Venkatesan et al. 2007). Because of this, traits that may impact vectorial capacity, such as autogeny, have been studied in natural and laboratory populations (Reeves, 1990, Eberle and Reisen, 1986). Unlike some mosquito vectors, where autogeny is found at fairly low levels (Mori et al., 2008), autogeny rates as high as 95% have been described in field Cx. tarsalis populations (Spadoni et al., 1974). The proportion of autogenous females varies geographically (Hardy and Reeves, 1973) and seasonally (Spadoni et al., 1974), likely due to genetic and environmental factors (Kardos, 1959, Hardwood and Halfhill, 1964, Reisen et al., 1984, Eberle and Reisen, 1986, Brust, 1991). While influenced by environmental conditions, autogeny in Cx. tarsalis has been found to be a dominant, multigenic trait that can be selected for in the laboratory (Eberle and Reisen, 1986).
Egg production during autogeny or anautogeny requires the synthesis of yolk proteins to supply the developing oocyte. Mosquito vitellogenins are the main yolk protein precursors, encoded by genes of approximately 6.5 kb that are found in multiple copies in the mosquito genome (Gemmill et al., 1986, Romans et al., 1995, Isoe and Hagedorn, 2007), likely as a result of ancestral gene duplication events (Chen et al., in review). Molecular regulation of one of the three vitellogenin genes (VgA1) in anautogenous Aedes aegypti has been thoroughly characterized (Kokoza et al., 2001, Raikhel et al., 2002, Attardo et al., 2005). Cx. tarsalis has four vitellogenin genes; two pairs which are more divergent (Vg1a/b and Vg2a/b, sharing ~64% nucleotide identity) and within the pairs, duplicates which share higher nucleotide identity (Vg1a and Vg1b, 98%; Vg2a and Vg2b, 97%) (Chen et al. in review). All four of the Cx. tarsalis genes share the highly conserved mosquito vitellogenin gene structure (Chen et al., in review; Isoe and Hagedorn, 2007; Sappington and Raikhel, 1998; Romans, 1995).
Despite an abundance of literature on the regulation of anautogeny in Ae. aegypti, our understanding of the molecular events regulating autogeny have been largely unexplored. To begin investigating this, we used an autogenous strain of Cx. tarsalis to characterize the expression of the vitellogenin gene family during autogeny, following blood feeding, and during immature mosquito development.
Results
Selection for a primarily autogenous line of Culex tarsalis, KNWR-au
A primarily autogenous line of Culex tarsalis (KNWR-au) was selected from a parent KNWR colony that exhibited low levels of autogeny. Autogeny was selected for >15 generations by propagating the colony in the absence of a blood source. Following 5 days post emergence, ~90% of KNWR-au females develop mature ovaries. Autogeny is limited to the first gonotrophic cycle. After depositing the autogenous egg raft, KNWR-au females are not able to initiate ovarian development until a bloodmeal is taken (and are therefore effectively anautogenous at this point).
Expression profiles of vitellogenin genes Vg1a,b and Vg2a,b are similar during blood meal regulated vitellogenesis in KNWR-au females
Despite extensive work with vitellogenin genes and their promoters in anautogenous mosquitoes, the focus of study has been primarily on VgA1 in Ae. aegypti (Kokoza et al., 2001, Nirmala et al., 2006, Li et al., 2008). We aimed to address whether all genes in the family were expressed similarly following blood feeding in KNWR-au, where four vitellogenin genes have been identified (Chen et al., in review). RNA was extracted from adult females before blood feeding and 3, 6, 12 and every 12 hours out to 96 hours post bloodmeal. While the presence and abundance of detectable transcript was variable between the genes before the first 12 hours, Vg1a,b and Vg2a,b were consistently expressed between 12 and 48 hours post blood meal and declined thereafter (Figure 1a).
Figure 1.
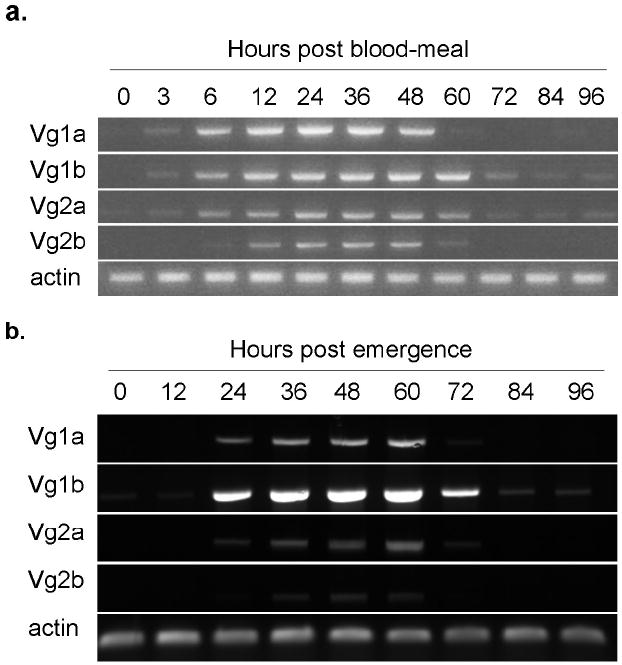
a. Expression of the four vitellogenin genes following a blood meal in KNWR-au. Females of the KNWR-au strain were allowed to emerge and deposit their autogenous egg raft before blood feeding. RNA for cDNA synthesis was extracted from groups of 10 females collected pre-blood meal (PBM) and at indicated time points following blood feeding out to 96 hours. RT-PCR was performed using primers specific to each of the four Culex tarsalis vitellogenin genes (Vg1a, Vg1b, Vg2a, Vg2b) and actin to control for RNA quality.
b. Expression of the four vitellogenin genes during autogenous ovarian development in KNWR-au. RNA for cDNA synthesis was extracted from groups of 10 females collected at indicated time points (0h and every 12 hours out to 96 hours) following eclosion from the pupal stage. RT-PCR was performed using primers specific to each of the four Culex tarsalis vitellogenin genes (Vg1a, Vg1b, Vg2a, Vg2b) and actin to control for RNA quality.
Expression profiles of Vg1a,b and Vg2a,b are similar during autogenous vitellogenesis in KNWR-au females
Autogenous females are able to synthesize vitellogenin using reserves carried over from larval development. Since egg maturation occurs within three to four days of adult emergence in KNWR-au, we examined the expression of all four vitellogenin genes from 12 hours to 96 hours post emergence. Similar to anautogenous development, Vg1a,b and Vg2a,b are expressed during this time period. All four transcripts are detectable between 24 to 60 hours following emergence with more variable expression among them before and after those time points (Figure 1b). Unlike in anautogenous Aedes aegypti, where expression of the vitellogenin genes is initiated within 3-12 hours following a blood meal, in autogenous Cx. tarsalis vitellogenin expression is detectable approximately 12-24 hours post-emergence. Vg1b expression appears qualitatively stronger than the other 3 Vg genes and low levels of detectable transcript were observed in newly emerged females.
In order to quantitatively characterize the expression of the two divergent Vg gene variants, primers were designed to amplify either Vg1 (both a and b) or Vg2 (both a and b); and expression quantified using quantitative rt-PCR. Larval and pupal stages were investigated to establish a baseline level of gene expression (Figure 2a), followed by autogenous expression in adults collected every 12 hours out to 96 hours post emergence (Figure 2b). All transcript levels were normalized to actin expression. Surprisingly, there was detectable expression of the Vg genes during larval and pupal development (Figure 2a). Vg1 had significantly higher expression levels which peaked in the early pupal stages, while Vg2 expression was lower and peaked in fourth instar larvae (Figure 2a). In the adult stages, profiles of Vg1 and Vg2 were similar; both genes peaked approximately 24 hours after first transcript was detected (48 hours after emergence) and declined ~48 hours after the first transcript (72 hours after emergence) (Figure 2b).
Figure 2.
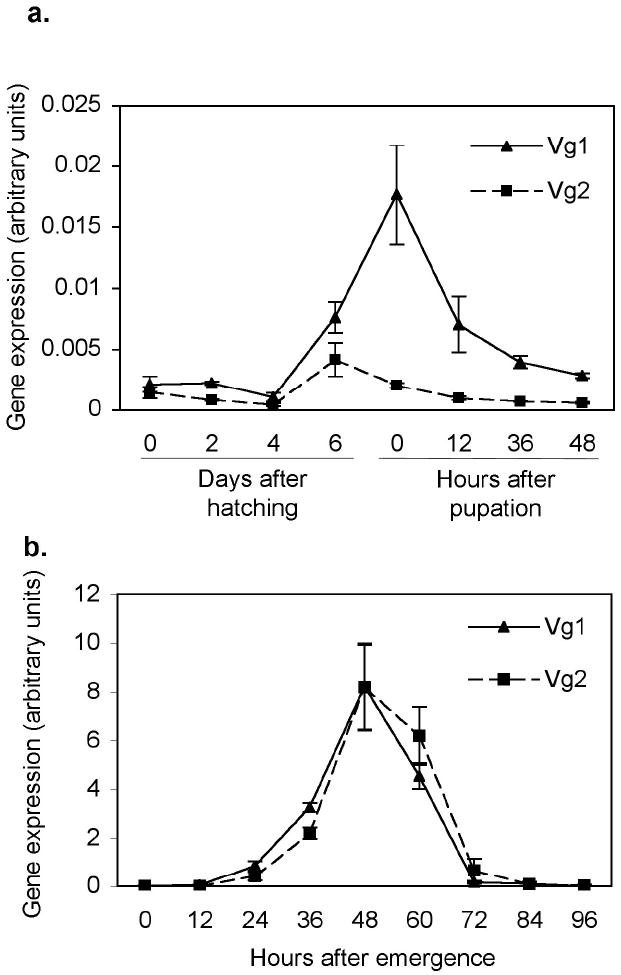
Quantitative rt-PCR for the expression of two groups of Culex tarsalis vitellogenin genes (Vg1a and b, Vg2a and b) during (a) immature development and (b) autogeny in KNWR-au. RNA for cDNA synthesis was extracted from ~200 newly hatched/first day, ~50-100 second day, 20 fourth day and 10 sixth day after hatching larvae, 10 females during each pupal stage, 10 females following emergence at indicated time points. QRT-PCR was performed with primers specific to either Vg1a and b or Vg2a and b. Data were normalized to actin expression. Each point represents the average of three replicates and the bars are the standard error of the mean. Note the difference in scale between a and b.
Vg1b, and low levels of Vg1a, Vg2a are expressed in larval and pupal development
Quantitative rt-PCR of Vg1 and Vg2 suggested developmental expression of one or both of the Vg1 genes and weaker expression of the Vg 2 gene(s) (Figure 2a). Therefore, expression of all four vitellogenin genes was further investigated in larvae (1, 2, 4, and 6 days after hatching) and female pupae (0, 12, 24 and 36 hours after pupation) (Figure 3a). Interestingly, Vg1b was abundantly and consistently expressed, while Vg1a and Vg2a were barely detectable in one replicate (and were not detected in the other two replicates) and Vg2b expression was never detected. Vg1b expression was detected at all larval and pupal stages tested. This suggests that the real time detection by Vg1 primers was likely due to amplification of Vg1b transcript.
Figure 3.
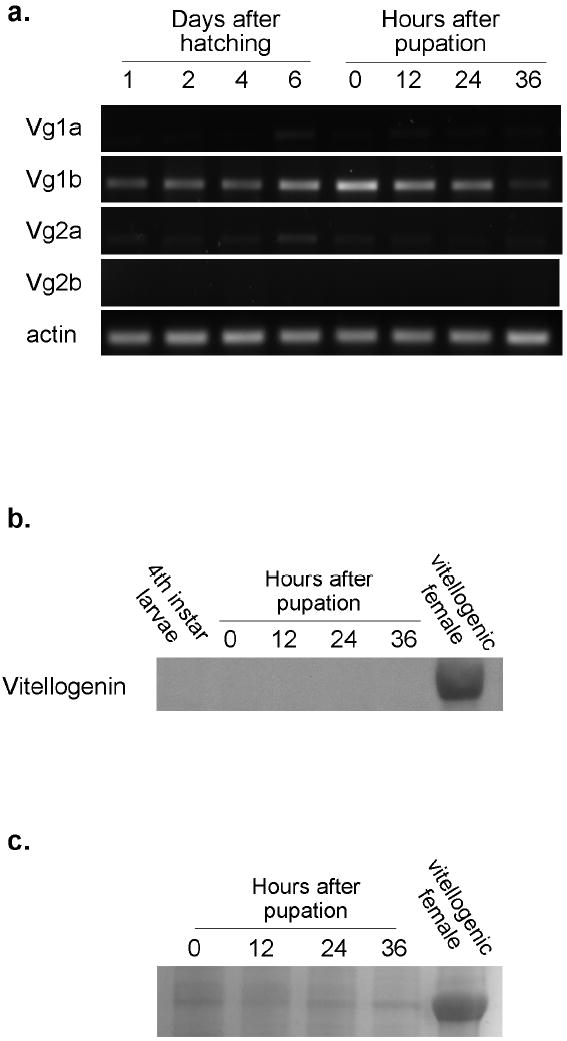
a. Expression of the four vitellogenin genes during larval and pupal development in KNWR-au. RNA for cDNA synthesis was extracted from groups of 10 females collected at indicated time points (0h and every 12 hours out to 96 hours) following eclosion from the pupal stage. RT-PCR was performed using primers specific to each of the four Cx. tarsalis vitellogenin genes (Vg1a, Vg1b, Vg2a, Vg2b) and actin to control for RNA quality. The experiment was repeated three times. b. Western blot analysis of vitellogenin production during larval and pupal development in KNWR-au. Protein was extracted from groups of 10 individuals as 4th instar larvae, 0, 12, 24, 36 hour pupae and 5 vitellogenic adult Cx. tarsalis females. Vitellogenin was detected using an antibody to the 68kD small subunit of Ae. aegypti vitellogenin. c. Coomassie blue stained gel of separated Cx. tarsalis pupal proteins and protein from vitellogenic adult Cx. tarsalis females.
No detectable vitellogenin protein is produced during immature development
In order to test if developmental expression of Vg1b lead to the production of protein, a western blot was performed with an antibody recognizing the 68 kD small subunit of Aedes vitellogenin. Protein was collected from fourth instar larvae and pupae at 0, 12, 24 and 36 hours after pupation. Protein from vitellogenic, bloodfed adult KNWR-au females was used as a positive control. There was no detectable protein in late-stage larvae or any of the pupal time points (Figure 3b). A Coomassie blue stained gel of the pupal time points revealed bands of similar molecular mass as adult vitellogenin (Figure 3c). The identity of these bands was not determined in the current study, but based on lack of detectable antibody signal and previous work in autogenous Aedes atropalpus (Zakharkin, 2001; Wheeler and Buck, 1996) we speculate that they represent hexamerin proteins (see discussion).
Discussion
Expression of multiple vitellogenin genes during reproduction is consistent with the hypothesis of a functional conservation of duplicated genes for increased dosage (Ohno, 1970, Clemments, 2000, Sugino and Innan, 2006, Tufail and Takeda, 2008). During previtellogenic development the fat body cells of the mosquito become polyploid, increasing the copy number of genes that will contribute to vitellogenin synthesis (Raikhel and Lea, 1983, Raikhel, 1987, Clemments, 2000). Multiple active copies of vitellogenin genes, subsequently multiplied by fat body cell ploidy, likely contribute to the rapid and abundant production of transcript for yolk protein. However, it is worth noting the variation in expression between gene copies. Vg2b is expressed at qualitatively lower levels and during fewer time points than the other three vitellogenin genes, while Vg1b consistently appears at higher levels and during more time points, especially during the autogenous period. Divergence in expression levels is a common consequence of gene duplications, and likely results from differences in promoter strength between genes (Li et al., 2005).
During autogenous development, the two groups of divergent genes (Vg1 vs Vg2) produce very similar amounts of transcript, and have nearly identical profiles, despite the differences seen in expression between the “a” and “b” copies of each gene (Figure 1b). This expression profile is similar to VgA1 following a blood meal in Aedes (Raikhel et al., 2002), except that autogenous transcription is initiated approximately 12-24 after emergence compared to the 3-6 hours following a blood meal. This is appropriate given what is known about the hormonal regulation in autogeny. Females undergo an initial period of development (under the control of Juvenile Hormone) during which the fat body gains competence to respond to the hormonal cues leading to vitellogenesis. In anautogenous mosquitoes this development halts at the previtellogenic arrest, while autogenous females continue through this stage of arrest (Klowden, 1997). While some autogenous mosquitoes, such as Toxorhynchites rutilus (Watts and Smith, 1978) and Wyeomya smithii (Smith and Brust, 1971) emerge with ovaries developed at or beyond the previtellogenic gate, Cx. tarsalis KNWR-au females emerge with their ovaries at Christopher’s stage I (Figure 4) and reach previtellogenesis at approximately 12-24 hours (data not shown). Vitellogenin gene expression ~12 - 24 hours post emergence suggests that KNWR-au females attain competence and receive esteroidogenic signals within this time frame.
Figure 4.
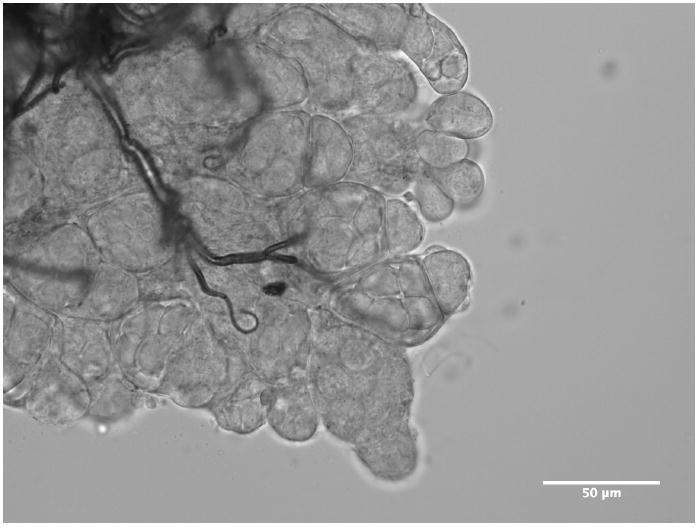
Micrograph of ovary from a newly-emerged (< 12 hours) Cx. tarsalis KNWR-au female.
In quantifying vitellogenin expression, we observed transcript expression prior to adult emergence (Figure 2a). Following further investigation, we found one of the Cx. tarsalis vitellogenin genes, Vg1b, to be consistently expressed during larval and pupal development (Figure 3a). Since vitellogenin promoters are frequently used to drive transgenes in mosquitoes (Nirmala et al., 2006, Chen et al., 2007, Li et al., 2008), this developmental expression is of practical importance. The physiological significance of Vg1b developmental expression remains to be determined. Western blots using the Ae. aegypti anti-vitellogenin 68kD subunit antibody did not reveal detectable protein (Figure 3b). This could be due to absence of translated product, suggesting that the Vg1b promoter is leaky, producing a functionally silent transcript. Alternatively, there may be no detectable signal because of the affinity of the antibody for Cx. tarsalis vitellogenin. A Coomassie blue stain of separated pupal proteins revealed protein of similar molecular mass as the small subunit of vitellogenin (~68 kD) (Figure 3c). Mosquito larval storage proteins, or hexamerins, are approximately the same molecular mass as vitellogenin (62.5, 66 and 72.5 kD in Aedes atropalpus) and are present through the pupal stages of autogenous females (Zakharkin, 2001; Wheeler and Buck, 1996). While hexamerins have not been examined in Cx. tarsalis, it is likely that the bands of similar mass are storage proteins and not vitellogenin. Hexamerins accumulate in the larval stages and serve as an amino acid reserve for metamorphosis and contribute autogenous vitellogenesis in Ae. atropalpus (Zakharkin, 2001; Wheeler and Buck, 1996). A further separated, Coomassie-stained, gel appeared to detect three bands in the early pupal stages of Cx. tarsalis (data not shown), as seen for storage protein subunits. While the inability of Aedes antibody to detect developmental Cx. tarsalis vitellogenin may be due to lower affinity, a dilution series of protein from a vitellogenic Cx. tarsalis female suggested the antibody could detect bands of similar Coomassie blue-stained intensity as the bands detected in Figure 3c (data not shown). Even if there are low levels of protein produced, it seems unlikely that it would contribute to autogenous yolk deposition, since females of this line do not emerge with vitellogenic ovaries (Figure 4) Therefore, any Vg1b product, if produced, may have an alternate function. This has been observed in the honey bee, Apis mellifera, in which vitellogenins are expressed and function in non-reproductive castes as antioxidants and to coordinate social behavior (Trenczek and Engels, 1986, Seehuus et al., 2006, Nelson et al., 2007, Corona et al., 2007). However, alternate functions of vitellogenins in mosquitoes have not been investigated.
In the analysis of promoter regions of the four vitellogenin genes of Cx. tarsalis Chen, et al (in review) identified a new gene 805bp upstream from Vg1b, called T37L for it’s homology to the human TRIM37 gene. This finding was unique to Vg1b; the head-to-head orientation and the short distance between the two genes suggest that the intergenic region may act as a bidirectional promoter. Similar to Vg1b, Cx. tarsalis T37L is transcriptionally expressed in all developmental stages and following emergence in autogenous females (Chen et al., in review). While the function of T37L in mosquitoes is not known, transcription of Vg1b during development could also be a consequence of shared regulatory elements with T37L, with little impact or function in autogenous egg development. Interestingly, the T37L gene and its association with one vitellogenin copy is highly conserved across mosquito genera (Chen et al., in review). Expression of Vg1b orthologues and the function of T37L warrant further investigation in Cx. tarsalis and other mosquito species.
Experimental Procedures
Insects
The Culex tarsalis KNWR colony was established in 2001 from wild caught mosquitoes in Kern County, California by W. Reisen (UC Davis). The colony began as a mixture of autogenous and anautogenous individuals from which autogeny was selected for >15 generations by propagating the colony without access to a blood source (referred to as KNWR-au). Mosquitoes were maintained at 27 C, 90% relative humidity on a 16:8 light-dark cycle. Larvae were panned at a density of 200 larvae/pan in 2.5 liters of distilled water and were fed a mixture (1:2:2) of ground fish food, bovine liver powder and rabbit pellets. Following emergence adults were provided 10% sucrose solution through a cotton wick. For experiments involving bloodfeeding, adult females who were already given the opportunity to lay an autogenous egg batch were fed on chicken blood (Rockland Immunochemicals) through a paraffin lined, warmed membrane feeder.
RNA extraction, RT-PCR and quantitative RT-PCR for gene expression
Total RNA was isolated from whole mosquitoes after being stored in RNAlater (Ambion) or processed directly in TriReagent (Ambion) using a motorized pestle according the manufacturer’s instructions. RNA was isolated from groups of ~200 newly hatched/first day, ~50-100 second day, 20 fourth day and 10 sixth day-old larvae. Sex was not determined in larvae, therefore groups were composed of males and females, while pupae and adults were sexed. RNA was isolated from 10 individual females per replicate at the pupal and adult stages. Isolated RNA was treated with DNAase (Ambion) to eliminate residual DNA contamination. cDNA was generated using 1μg of Dnase-treated RNA for reverse transcription using the Superscript RT for PCR kit (Invitrogen) in 20μl volumes with Oligo(dT)20 primers. No-RT controls were run for each sample. GenBank accession numbers for Vg sequences are GU017909-GU017912. For rt-PCR 2μl of cDNA was used as a template for amplification with following primers (5’-3’): Vg1SF: CAA-GGA-GGA-GGT-GTT-CTA-CG, Vg11SPR: ATG-CCT-TTG-TAA-ACA-GTT-CC, Vg12SPR: CCA-AAT-TCA-TTG-CTT-TCC-GA; Vg2SPF: CAA-CAA-GCA-GGA-GGA-AGA-TG, Vg21SPR: TCC-GTT-ACA-ACC-ATC-TAG-AG, Vg22SPR: GGC-AGT-TGT-ATC-TTC-CAA-GG. Each of the two main vitellogenin genes share a common forward primer (Vg1SPF and Vg2SPF) while four specific vitellogenin genes are amplified with a unique reverse primer (Vg11SPR, Vg12SPR, Vg21SPR, Vg22SPR). Primers were designed to be specific to all four vitellogenin genes from the sequences described (Chen et al. in review). Control actin primers were: actin1F: ATG-TTT-GAG-ACC-TTC-AAC-TCG-C, actin1R: TAA-CCT-TCR-TAG-ATT-GGG-ACG (GenBank accession number GU390398). PCR conditions for Vg primers were 94 °C for 3 min, followed by 30 cycles of: 94 °C for 30sec, 52 °C for 30sec, 72 °C for 1min, followed by a final extension of 72 °C for 5min. PCR conditions for actin primers were identical except that the annealing temperature was raised to 53 °C.
Quantitative rt-PCR was performed using an ABI 7300 Real-time PCR system (Applied Biosystems) in 96-well optical reaction plates (Applied Biosystems). Vg gene expression was normalized to actin expression. 5’-3’ primer sequences were: Vg1F: ACC-TTC-ACC-GGT-GTG-TAC-AAG-GTT, Vg1R: TTC-CGG-GAC-AAC-GTT-CAC-ATC-GTA; Vg2F: CTT-GTT-CGG-TGC-CAA-TCT-G, Vg2R: ACT-GGA-TTT-TGT-ACT-TGG-GC; actinF: GAC-TAC-CTG-ATG-AAG-ATC-CTG-AC, actinR: GCA-CAG-CTT-TTC-CTT-GAT-GTC-GC. Reactions were run in duplicate in 20μl volumes with 2μl of cDNA template using SYBR green chemistry (Qiagen). Each time point represents three independent replicates. PCR conditions were an initial incubation at 50 °C for 2min, followed by 95 °C incubation for 15 min, followed by 40 cycles of 95 °C for 15 sec, 55 °C for 30 sec and 72 °C for 30 sec.
Protein extraction and Western Blot
Samples were flash-frozen in liquid nitrogen and stored at −80C until processing. First, the utility of the Aedes anti-vitellogenin antibody for detecting Culex tarsalis vitellogenin was tested. More concentrated antibody was required to detect the 68kD band from vitellogenic Cx. tarsalis (1:500) than Ae. aegypti (1:10,000), suggesting a lower affinity for Cx. tarsalis vitellogenin. In order to assay developmental protein production 10 individuals (mixed sex larvae, female pupae) during development and 5 females for the vitellogenic female control were collected. Protein was extracted in cracking buffer and 1X protease inhibitor (Roche) as described (Attardo et al., 2003). Protein concentration was determined using Bradford reagent (Sigma) and 30 μg of protein loaded onto a 10% Tris gel and separated. For experiments involving total protein staining the gel was fixed in 50% ethanol, 10% acetic acid in water, stained in 0.1% Coomassie blue G250, 20% methanol, 10% acetic acid in water, washed and destained in 50% methanol 10% acetic acid in water. For Western blots, protein was transferred to a PVDF membrane (Millipore), and Ponceau-stained to ensure transfer and approximate equal loading. The membrane was blocked in 5% milk in Tris-buffered saline and 0.1% Tween-20 (TBS-T) for 1 hour, incubated overnight in 1:500 dilution of Aedes antibody that recognizes the 68kD subunit of vitellogenin in TBS-T, followed by a 1:5000 dilution of anti-mouse HRP secondary (Amersham) and developed in ECL substrate (Amersham). The western blot was repeated three times from independent samples.
Acknowledgments
We thank Dr. William Reisen for the original KNWR colony and Dr. Alex Raikhel for his kind gift of the monoclonal Aedes Vg antibody. Funding for this research was provided by NIH/NIAID grant R01AI067371 to JLR. KNPJ was partially supported by training grant T32AI007417.
References
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Attardo GM, Higgs S, Klingler KA, Vanlandingham DL, Raikhel AS. RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2003;100:13374–13379. doi: 10.1073/pnas.2235649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bat-Miriam M, Craig GB. Mutants in Aedes albopictus (Diptera: Culicidae) Mosquito News. 1966;26:13–22. [Google Scholar]
- Brust RA. Environmental regulation of autogeny in Culex tarsalis (Diptera: Culicidae) from Manitoba, Canada. J Med Ent. 1991;28:847–853. doi: 10.1093/jmedent/28.6.847. [DOI] [PubMed] [Google Scholar]
- Chao J. An autogenous strain of Culex tarsalis Coq. Mosquito News. 1958;18:134–136. [Google Scholar]
- Chen S, Armistead JS, Provost-Javier KN, Rasgon JL. Duplication, concerted evolution and purifying selection drive the evolution of mosquito vitellogenin genes. doi: 10.1186/1471-2148-10-142. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XG, Marinotti O, Whitman L, Jasinskiene N, James AA, Romans P. The Anopheles gambiae vitellogenin gene (VGT2) promoter directs persistent accumulation of a reporter gene product in transgenic Anopheles stephensi following multiple bloodmeals. Am J Trop Med Hyg. 2007;76:1118–1124. [PubMed] [Google Scholar]
- Clemments AN. The Biology of Mosquitoes: Volume 1 Development, Nutrition and Reproduction. New York, New York: CABI Publishing; 2000. [Google Scholar]
- Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci USA. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle MW, Reisen WK. Studies on autogeny in Culex tarsalis: 1. Selection and genetic experiments. J Am Mosq Cont Assoc. 1986;2:38–43. [PubMed] [Google Scholar]
- Gemmill RM, Hamblin M, Glaser RL, Racioppi LJV, Marx JL, White BN, Calvo JM, Wolfner IMF, Hagedorn HH. Isolation of mosquito vitellogenin genes and induction of expression by 20-hydroxyecdysone. Insect Biochem. 1986;16:761–774. [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerging Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood RF, Halfhill E. The Effect of Photoperiod on Fat Body and Ovarian Development of Culex tarsalis (Diptera: Culicidae) Ann Entomol Soc Amer. 1964;57:596–600. [Google Scholar]
- Hardy JL, Reeves WC. Emerging concepts of factors that limit the competence of Culex tarsalis to vector encephalitis viruses. Proc Mosq Vec Control Assoc CA. 1973;41:1–4. [Google Scholar]
- Isoe J, Hagedorn HH. Mosquito vitellogenin genes: Comparative sequence analysis, gene duplication, and the role of rare synonymous codon usage in regulating expression. J Insect Sci. 2007;7:1–49. doi: 10.1673/031.007.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos EH. The effect of larval nutritional level on development of autogeny in colony Culex tarsalis Coq. Proc Mosq Vec Control Assoc CA. 1959;27:71–72. [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch Insect Biochem. 1997;35:491–512. [Google Scholar]
- Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274:47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- Li C, Marrelli MT, Yan G, Jacobs-Lorena M. Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J Hered. 2008;99:275–282. doi: 10.1093/jhered/esn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-H, Yang J, Gu X. Expression divergence between duplicate genes. Trends in Genetics. 2005;21:602–607. doi: 10.1016/j.tig.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mori A, Romero-Severson J, Black WC, Severson DW. Quantitative trait loci determining autogeny and body size in the Asian tiger mosquito (Aedes albopictus) Heredity. 2008;101:75–82. doi: 10.1038/hdy.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Ihle KE, Fondrk MK, P RE, JR, Amdam GV. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biology. 2007;5:0673–0677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RL, Milby MM. Autogeny and blood-feeding by Culex tarsalis (Diptera: Culicidae) and the interval between oviposition and feeding. The Canadian Entomologist. 1982;114:515–521. [Google Scholar]
- Nirmala X, Marinotti O, Sandoval JM, Phin S, Gakhar S, Jasinskiene N, James AA. Functional characterization of the promoter of the vitellogenin gene, AsVg1, of the malaria vector, Anopheles stephensi. Insect Biochem Mol Biol. 2006;36:694–700. doi: 10.1016/j.ibmb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Ohno S. Evolution by Gene Duplication. Springer-Verlag; 1970. [Google Scholar]
- Raikhel AS. The Cell Biology of Mosquito Vitellogenesis. Mem Inst Oswaldo Cruz. 1987;82:93–101. doi: 10.1590/s0074-02761987000700019. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Kokoza VA, Zhu J, Martin D, Wang SF, Li C, Sun G, Ahmed A, Dittmer N, Attardo G. Molecular biology of mosquito vitellogenesis: from basic studies to genetic engineering of antipathogen immunity. Insect Biochem Mol Biol. 2002;32:1275–1286. doi: 10.1016/s0965-1748(02)00090-5. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Previtellogenic development and vitellogenin synthesis in the fat body of a mosquito: an ultrastructural and immunocytochemical study. Tissue and Cell. 1983;15:281–299. doi: 10.1016/0040-8166(83)90023-x. [DOI] [PubMed] [Google Scholar]
- Reeves WC. Epidemiology and control of mosquito-borne arboviruses in California, 1943-1987. Sacramento, California: California Mosquito and Vector Control Association, Inc; 1990. [Google Scholar]
- Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, Woods L, Husted S, Kramer V, Edman J. West Nile virus in California. Emerging Infect Dis. 2004;10:1369–1378. doi: 10.3201/eid1008.040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W, Milby M, Reeves W, Meyer R, Bock M. Population ecology of Culex tarsalis (Diptera: Culicidae) in a foothill environment of Kern County, California: temporal changes in female relative abundance, reproductive status, and survivorship. Ann Entomol Soc Amer. 1983;76:800–808. [Google Scholar]
- Reisen WK, Milby M, Bock M. The effects of immature stress on selected events in the life history of Culex tarsalis. Mosquito News. 1984;44:385–395. [Google Scholar]
- Reisen WK, Milby MM. Studies on autogeny in Culex tarsalis: 3. Life table attributes of autogenous and anautogenous strains under laboratory conditions. J Am Mosq Control Assoc. 1987;3:619–625. [PubMed] [Google Scholar]
- Romans P, Tu Z, Ke Z, Hagedorn HH. Analysis of a vitellogenin gene of the mosquito, Aedes aegypti and comparisons to vitellogenins from other organisms. Insect Biochem Mol Biol. 1995;25:939–958. doi: 10.1016/0965-1748(95)00037-v. [DOI] [PubMed] [Google Scholar]
- Roubaud E. Cycle autogene d’attente et generations hivrenales suractives inaparentes chez le moustique commun Culex pipiens. Les Comptes Rendus de l’Academie des Sciences. 1929;180:735–738. [Google Scholar]
- Sappington TW, Raikhel AS. Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem Mol Biol. 1998;28:277–300. doi: 10.1016/s0965-1748(97)00110-0. [DOI] [PubMed] [Google Scholar]
- Seehuus SC, Norberg K, Gimsa U, Krekling T, Amdam GV. Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci USA. 2006;103:962–967. doi: 10.1073/pnas.0502681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Brust RA. Photoperiodic control of the maintenance and termination of larval diapause in Wyeomyia smithii (Coq.) (Diptera: Culicidae) with notes on oogenesis in the adult female. Canadian J Zool. 1971;49:1065–1073. doi: 10.1139/z71-165. [DOI] [PubMed] [Google Scholar]
- Spadoni RD, Nelson RL, Reeves WC. Seasonal occurrence, egg production, and blood-feeding activity of autogenous Culex tarsalis. Ann Entomol Soc Amer. 1974;67:895–902. [Google Scholar]
- Spielman A. The inheritance of autogeny in the Culex pipiens complex of mosquitoes. Am J Trop Med Hyg. 1957;65:404–425. doi: 10.1093/oxfordjournals.aje.a119878. [DOI] [PubMed] [Google Scholar]
- Su T, Mulla MS. Selection-dependent trends of autogeny and blood feeding in an autogenous strain of Culex tarsalis (Diptera: Culicidae) J Am Mosq Control Assoc. 1997;13:145–149. [PubMed] [Google Scholar]
- Sugino RP, Innan H. Selection for more of the same product as a force to enhance concerted evolution of duplicated genes. Trends Genet. 2006;22:642–644. doi: 10.1016/j.tig.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Trenczek T, Engels W. Occurrence of vitellogenin in drone honeybees (Apis mellifera) Int J Invert Repr Devel. 1986;10:307–311. [Google Scholar]
- Tufail M, Takeda M. Molecular characteristics of insect vitellogenins. J Insect Physiol. 2008;54:1447–1458. doi: 10.1016/j.jinsphys.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’guinn ML, Dohm DJ, Webb JP, Sardelis MR. Vector competence of Culex tarsalis from Orange County, California, for West Nile virus. Vector-borne Zoo Dis. 2003;2:193–196. doi: 10.1089/15303660260613756. [DOI] [PubMed] [Google Scholar]
- Venkatesan M, Westbrook CJ, Hauer MC, Rasgon JL. Evidence for a population expansion in the West Nile virus vector Culex tarsalis. Mol Biol Evol. 2007;24:1208–1218. doi: 10.1093/molbev/msm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts RB, Smith SM. Oogenesis in Toxorhynchites rutilus (Diptera: Culicidae) Canadian J Zool. 1978;56:136–139. [Google Scholar]
- Wheeler DE, Buck NA. A role for storage proteins in autogenous reproduction in Aedes atropalpus. J Insect Physiol. 1996;42:961–966. [Google Scholar]
- Zakharkin SO, Headley VV, Kumar NK, Buck NA, Wheeler DE, Benes H. Female-specific expression of a hexamerin gene in larvae of an autogenous mosquito. Eur J Biochem. 2001;268:5713–5722. doi: 10.1046/j.0014-2956.2001.02514.x. [DOI] [PubMed] [Google Scholar]


