Abstract
According to the ‘Kennard Principle’, there is a negative linear relation between age at brain injury and functional outcome. Other things being equal, the younger the lesioned organism, the better the outcome. But the ‘Kennard Principle’ is neither Kennard’s nor a principle. In her work, Kennard sought to explain the factors that predicted functional outcome (age, to be sure, but also staging, laterality, location, and number of brain lesions, and outcome domain) and the neural mechanisms that altered the lesioned brain’s functionality. This paper discusses Kennard’s life and years at Yale (1931–1943); considers the genesis and scope of her work on early-onset brain lesions, which represents an empirical and theoretical foundation for current developmental neuropsychology; offers an historical explanation of why the ‘Kennard Principle’ emerged in the context of early 1970s work on brain plasticity; shows why uncritical belief in the ‘Kennard Principle’ continues to shape current research and practice; and reviews the continuing importance of her work.
Keywords: Brain Plasticity, Margaret Kennard, Kennard Principle, Hans-Lukas Teuber, Frontal Lobe Function
1. Introduction
A simple statement to explain the supposed age-based differences in maturational brain plasticity has been termed the ‘Kennard Principle’, according to which there is a negative linear relation between age at brain injury and functional outcome. Other things being equal, the younger the lesioned organism, the better the outcome.
The ‘Kennard Principle’ is neither Kennard’s nor a principle. In her work, Kennard sought to explain the factors that predicted functional outcome (age, to be sure, but also staging, laterality, location, and number of brain lesions, and outcome domain) and the neural mechanisms that altered the lesioned brain’s functionality. This paper discusses Kennard’s life and years at Yale (1931–1943); considers the genesis and scope of her work on early-onset brain lesions, which represents an empirical and theoretical foundation for current developmental neuropsychology; offers an historical explanation of why the ‘Kennard Principle’ emerged in the early 1970s; shows why uncritical belief in the ‘Kennard Principle’ continues to shape current research and practice; and reviews the continuing importance of her work.
2. Life
Margaret Alice Kennard (Figs. 1 and 2) was born in Brookline, Massachusetts on 25 September 1899. She graduated from Bryn Mawr in 1922 and registered at Cornell University Medical School on 27 September 1926, graduating 12th in her class in 1930. She held an internship in Medicine at Strong Hospital in Rochester NY from 1930–31. She joined the Laboratory of Physiology at Yale,i as an Honorary Research Fellow (without stipend) from 1931–32, becoming a Research Assistant with Instructor’s rank (with stipend) from 1932–33. She became a Research Assistant with the rank of Assistant Professor (later changed by the Provost’s office to Assistant Professor of Physiology) with stipend of $1200 from the Seessel Fund, 1933–34. From 1934–37, she was an Assistant Professor of Physiology with a stipend of $3000.
Figure 1.

Headshot of Margaret Kennard extracted from her medical school graduating class photograph (1930). Courtesy of Medical Center Archives of New York-Presbyterian/Weill Cornell.
Figure 2.
Photograph of Margaret Kennard with two unidentified colleagues (undated). Courtesy of Cushing/Whitney Medical Historical Library, Yale University.
Kennard travelled to Europe for two years (1934–36) on a Rockefeller Traveling Fellowship. She worked in laboratories in Amsterdam and Breslau, and did clinical work at the National Hospital, Queen’s Square, London and the London Hospital. She spent the last months of her fellowship, studying children with spasticity with Dr. Bronson Crothers at the Children’s Hospital in Boston. During her “crazy and marvelous”ii summer of 1935, she attended the Second International Neurological Congress in London and the XVth International Physiological Congress in Leningrad. In November 1935, she was elected to the Royal Society in London.
In 1942 Kennard passed her specialty boards in Neurology and Psychiatry; in the following five years, she became Associate Professor of Psychiatry at the New York University Medical School and Attending Physician at Bellevue Hospital, New York City. In 1948, she was appointed Associate Professor of Physiology in the University of British Columbia Medical School. In 1956, she became Director of the Washington State Mental Health Research Institute. She was Vice President of the American Neurological Society from 1958–1959, and President of the Society of Biological Psychiatry from 1956–1957. After her active research work ended, Kennard moved to New Hampshire, where she served as psychiatrist at the Elliott Hospital and organized a community guidance center. She died on 12 December 1975iii (not 1976 as indicated in Himwich’s 1977 obituary) of amyotrophic lateral sclerosis.
3. The Yale Years (1931–1943)
In 1931, the Yale Department of Physiology was newly organized into three independent divisions (Fulton, 1932): A Laboratory of Neurophysiology, directed by J. G. Dusser de Barenne; a Laboratory of Comparative Psychobiology, directed by Robert Yerkes, and a Laboratory of Physiology, directed by John F. Fulton. The excitement and synergy in Fulton’s laboratory at this time must have been considerable: He fostered experimental animal work with clinical implications (Davey, 1998), and his revival of primate neurophysiology and his use of techniques of human brain surgery applied to physiological surgery on primates occurred at the same time as his colleagues van Wagenen and Yerkes were advancing primate endocrinology and psychology, respectively (Hoff, 1962).
On 6 March 1931, Kennard wrote to Fulton asking to do research work in his laboratory.iv Her application was accompanied by reference letters from Lusk (with whom she had worked at Cornell), McCann at Strong Memorial Hospital in Rochester (who planned to take her back after her studies with Fulton), and Stanley Cobb at Harvard Medical School (who described her as “... an unusually able girl. She is tall, of a wiry New England type, and has a lot of energy. I would call her attractive without being a Venus.”v) At this time, professional positions for women were made by patronage (Ogilvie and Harvey, 2000).
Fulton wrote back to Kennard immediately,vi agreeing to have her in the lab as a research student. He expressed concern about the lack of space and money, but said her clinical experience appealed to him. He explained that he was working on the physiological basis of the more important neurological signs and thought that the primates in his laboratory were under-used. Fulton transferred funds earmarked for cage cleaning to provide Kennard with limited salary support and then energetically solicited money for her from a variety of sources, including the Rockefeller Foundation and the Eli Lilly drug company. Kennard visited the Yale laboratory in mid-July 1931 and began work in September.
The structure and function of the frontal lobes had been targeted as one of the Fulton laboratory’s interests (Pressman, 1998), so an incoming research student might have been expected to study frontal lobe function. Dusser de Barenne had staked out the sensory functions of the frontal cortex, so motor rather than sensory function might have seemed an appropriate research direction for Kennard, who later said that motor function was a good thing to study because “it has more obvious symptoms than many other syndromes that are the result of injury elsewhere” (Ward and Kennard, 1942, p 189). Kennard’s early publications in Fulton’s laboratory concerned motor functions of the adult frontal cortex: the effects of adult frontal, premotor, and motor lesions on posture, grasping, and perseveration (Fulton et al., 1932); neurological signs after lesions in area 4 and the premotor area 6 (Kennard and Fulton, 1933); motor representation of the autonomic system in premotor area (Fulton et al., 1934); and the premotor syndrome in adult clinical cases (Kennard et al., 1934).
3.1 Mentors and colleagues
On 26 March 1956, Kennard read a paper to the Neuropsychiatric Division of the Vancouver Medical Society entitled Neurophysiology, 1931–56, In Transit in which she reflected on her career and the individuals who had influenced it.vii
In FULTON’S laboratory, for 12 years, I really learned the neurophysiology of the forebrain, by teaching, by experiment, and, I now realize, by contact with some of the best of specialists in that field who came there to work during the 1930s from all the world. Across the court, in psychology, and up above in “Yerkes Lab”, were all the young psychologists. We rubbed together, talked and worked.
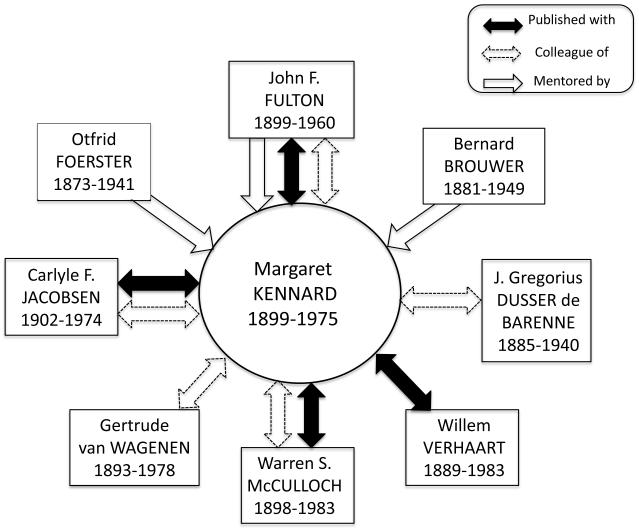
Figure 3 identifies eight of Kennard’s professional associations during her years in Fulton’s laboratory at Yale. The intellectual lineage of her mentors and colleagues is deeply rooted the history of neurology, neurosurgery, and neuroscience. Foerster was taught by Déjerine, Marie, Wernicke, and Babinski; Brouwer by von Monakow; Dusser de Barenne and Fulton by Sherrington; and Jacobsen by Lashley.
Figure 3.
Kennard’s professional associations during her years at Yale (1931–1943). The nature of the associations is discussed in the text. Figure 3 shows Kennard’s connections with the individuals mentioned, but not their connections with each other, which were considerable: For instance, Fulton had long-term professional relations with Foerster, Brouwer, and Verhaart (Koehler, 2003; 2006) and McCulloch published extensively with Dusser de Barenne. Figure 3 does not include a group of people at Queen’s Square and the London Hospital with whom Kennard obtained clinical neurology training during her Rockefeller Travelling Fellowship.
The physiologist and neurosurgeon John F. Fulton obtained a D. Phil. from Oxford in 1925 and a medical degree from Harvard in 1926. He moved to Yale in 1930 as a Sterling Professor and Chair of the Department of Physiology. With Dusser de Barenne in 1938, he founded the Journal of Neurophysiology. Kennard and Fulton maintained common research interests in a variety of topics (forced grasping, the nature of spasticity, autonomic and vasomotor representation in the cerebral cortex, caudate-frontal and cerebellar-frontal connections), but he always recognized the infant monkey work as her own. The two corresponded regularly (and frequently: during some periods of their association, twice per day) on a range of personal and professional topics from 1931 to shortly before his death in 1960.
The neurophysiologist Joannes Gregorius Dusser de Barenne finished medical school and began research at the University of Amsterdam in 1909, continuing his studies after World War I in the Departments of Pharmacology and Physiology at University of Utrecht. In 1930 he took up an appointment as Yale as a Sterling Professor to build the Laboratory of Neurophysiology, where he pioneered studies of physiologically controlled chemistry of the brain in situ and made contributions to the functional organization of the primate sensory cortex (Fulton, 1940; McCulloch, 1940). Although Dusser de Barenne and Kennard did not publish together, each had a paper in the first issue of the Journal of Neurophysiology in 1938, and her Yale work with McCulloch on post-lesion cortical excitability reflects his influence.
The clinical neurologist and neuroanatomist Bernard Brouwer was the first ordinary professor of neurology in the Netherlands, becoming Superintendent of the Neurological Institute in Amsterdam (Koehler and Bruyn, 2003). He lectured in the US for two months in 1926 and again in 1933, when he stayed at Yale for 8 days and met with Fulton, Dusser de Barenne, Yerkes, and Kennard.
Kennard worked in Brouwer’s laboratory from 10 September 1934 to 4 February 1935.viii She studied cerebellar connections, which was important for later plans in Fulton’s lab (Fulton wrote to Brouwer that Kennard was eager to work on the neuroanatomy of the fronto-pontine cerebellar connections; Koehler, 2003). Brouwer’s interest in the subcortical and subtentorial connections of the frontal lobe, as well in the anatomical basis of encephalization, became themes developed in Kennard’s work on frontal lobe motor functions. As part of her work in Brouwer’s laboratory, she published a neuroanatomical paper on primary cortical degeneration of the cerebellum in the Proceedings of the Amsterdam Academy of Sciences.ix
The neurologist and pioneer epilepsy surgeon Otfrid Foerster established the first independent German Department of Neurology in Breslau in 1911 (Piotrowska and Winkler, 2007; Sarikcioglu, 2007; Tan, 2003; Tan and Black, 2001). On his first visit to the USA in 1899 (the others were in 1912, 1914, and 1930; Kennard et al., 1942), he had met John D. Rockefeller, and the Rockefeller Foundation supported the building of his Institute of Neurology in Breslau in 1934. Foerster was known for his use of cortical stimulation in preparation for resecting epileptogenic foci, and, more generally, for his blend of hypothesis testing using human clinical cases and animal models (Tan, 2003; Tan and Black, 2001).
Kennard worked in Foerster’s laboratory from 5 February 1935 to 2 August 1935,x which proved to be a source of enormous excitement for her (on 16 February 1935xi she wrote to Fulton, “I’ve never been in a place where so many ideas are floating around loose.”) She enjoyed the clinical cases and flow of ideas in Foerster’s laboratory, including the emphasis on the temporal evolution of motor symptoms after frontal lesions; the idea that extrapyramidal areas can mutually compensate when any one is lesioned; the view that the ipsilateral cortex is important in recovery after precentral lesions; and Foerster’s embrace of Hughlings Jackson’s idea that cerebral localization involves dynamic, not static plasticity.
Foerster published one of Kennard’s papers on vasomotor changes with cortical lesions in his Zeitschrift and invited her to contribute a chapter to his Handbuch de Neurologie on the cortical influence on the autonomic nervous system.8 She organized the list of his publications to make them accessible to English-speaking audiences. When Foerster died of tuberculosis in 1941, Kennard was senior author of his professional obituary, talking about his wife’s burial, citing a letter received from his sister about the details of the funeral, and noting, “Dr. Foerster had written to us during the preceding winter” (Kennard et al., 1942, p. 1).
The animal psychologist Carlyle Jacobsen obtained his Ph.D. from University of Minnesota in 1928 with a doctoral dissertation on experimental studies of frontal lobe lesions, learning and retention in monkeys (Jacobsen, 1931). He began at Yale as one of Yerkes’s staff of experimental animal psychologists, joining Fulton’s laboratory in 1931 (through whose efforts he obtained Rockefeller Foundation money for advanced primate studies, Getz, 2009). Jacobsen conducted selective behavioral studies of frontal-lesioned monkeys, with behavioral equipment and methodological sophistication provided by Yerkes and surgical facilities by Fulton (Pressman, 1998).
Early on, Jacobsen collaborated with Kennard on studies of motor function after adult frontal lesions (Fulton et al., 1932) and he was actively involved in their joint infant monkey research. Jacobsen cited Kennard’s infant monkey work in formulating key ideas about recovery of function: that the association cortex was less functionally plastic than the motor cortex; and that restitution of function after cortical injury variously involved adjustment without the missing function, vicarious assumption of the missing function by part of the nervous system not previously involved in it, and dynamic reorganization within a partially damaged neural system (Jacobsen et al., 1936). Reciprocally, Kennard’s research investigations reveal the influence of Jacobsen’s methodology in carefully specifying behavioral task demands in studies of functional deficits and sparing of function (he had found that, after bilateral frontal ablations, chimpanzees and baboons could use tools in a multi-stages process to obtain food rewards, but were impaired on tasks with a time-binding component, essentially discovering working memory; Jacobsen, 1931).
The neuroanatomist and neuropsychiatrist Willem Verhaart received a medical degree from the University of Utrecht in 1922 and, in 1930, became superintendant of the clinic at the psychiatric-neurological ward of the school of medicine in Batavia, where he founded a laboratory for brain research, publishing papers between 1931–1936 on neuroanatomical pathways. Verhaart worked at Fulton’s Yale laboratory from 1938–39, collaborating with Kennard, Dusser de Barenne, & McCulloch (Koehler, 2006). Kennard and Verhaart investigated the connections of motor and suppressor areas of the cerebral cortex and the subcortical destinations of fiber tracts in selected precentral motor areas (Verhaart and Kennard, 1940).
The polymath Warren S. McCulloch was a psychologist and neurophysiologist best known as one of the founders of cybernetics (Abraham, 2002, 2003; Andrew, 2004; Christen, 2008). He moved to Yale in the fall of 1930 as an honorary research fellow (a Sterling Fellow from 1935–36) in the Laboratory for Neurophysiology of his mentor and colleague, Dusser de Barenne, with whom he published some 20 papers on the organization of the primate cortex, and whose obituary he authored (McCulloch, 1940). He remained at Yale as an Instructor and Assistant Professor until 1944, during which time he and Kennard published a set of research paper together on the excitability of the cortex and the functional organization of the frontal polar cortex. They corresponded from 1941 until (at least) 1954 on topics both personal (she provided a character reference for his son’s college application to Tufts) and professional (they discussed measurement of post-lesion thresholds for cortical excitability).xii
Gertrude van Wagenen was a pioneer in reproductive endocrinology. In 1931, on Fulton’s invitation, she became an Assistant Professor at Yale University of Medicine (Department of Obstetrics and Gynecology) and established a colony of monkeys for research purposes. van Wageren, whose experimental obstetrics laboratory was down the hall from Fulton’s lab, shared her newborn monkeys with her colleagues (Finger and Almli, 1988), although not without some delicate negotiations. On 15 August 1936, Kennard wrote to Fulton,xiii
Van and I have done a deal whereby she produces monkeys, and I get boy babies, she the girls. We worked out the finances yesterday. I hope you will approve. Score now stand M.A.K.,2 Van,O.
3.2 The genesis of Kennard’s work on motor functions of the immature frontal lobe
Kennard’s research on frontal lesions in infant monkeys, the work for which she is best known, constituted only 10% of her published papers (Finger and Almli, 1988), which has lead these authors to suggest that this research was serendipitous, and that Kennard’s interest in lesions of the immature frontal cortex did not represent a newfound interest in child neurology or developmental neuroscience. I believe, instead, that Kennard’s engagement with early brain lesions emerged at the beginning of her career and was sustained throughout her professional life. Her interest in the developmental course of early lesion effects was fully consistent with the mandate of the Fulton laboratory; she began to collect data on the outcome of infant primate lesions soon after she settled into the Fulton laboratory; her two important European mentors were interested in early lesion effects and she exchanged ideas about early lesions with them; and, as early as 1934, she articulated a plan to spend the rest of her professional life studying early lesions and children’s neurological diseases.
Several of Kennard’s colleagues in the Fulton laboratory were interested in changes in frontal lobe structure and function over phylogeny and over time since lesion in adult primates. The availability of van Wagenen’s infant monkeys provided the Fulton laboratory with material for a new, but compatible, avenue of research into ontogenetic changes in frontal lobe motor function.
Shortly after she begin research in Fulton’s laboratory (while working part time in a clinical service), Kennard wrote to Fultonxiv about the death of a baby orang from bronchopneumonia, the fact that that Jacobsen had taken two baby primates to his house to nurse them back to health, and the effects of forced grasping of the motor and premotor lesions she and Jacobsen had made. Fulton replied from Copenhagen 10 days laterxv that the death of the baby orang was disappointing but that their work with primates had been fruitful beyond any reasonable expectation. From 16 July 1934, when she first mentioned trying to write a paper on the infant monkeys, through the paper’s rejection by Brain, to 19 February 1936, when he told her that the page proofs of the infant monkey paper had arrived and been returned to the printer, Kennard and Fulton were in constant correspondence about the infant monkey paper, and, more generally, about practical and theoretical issues about their joint research on infant primatesxvi
Brouwer had an early pediatric interest: his doctoral thesis in medical school had concerned the acoustic tracts in deaf mutism (Koehler and Bruyn, 2003). On 26 October 1934,xvii Kennard wrote to Fulton from Brouwer’s laboratory:
My paper is not yet typed... I’ve discussed it with both Professor Brouwer and Prof. de Langexviii the pediatrician... Professor de Lange made the statement that, with children before myelinization of the pyramidal tracts, lesions give spasticity s’. after myelinization paresis is greater, spasticity is less...
In 1909, Foerster had been the first to describe a congenital form of cerebral palsy with hypotonia and seizures, diplegia, cerebral ataxia, and motor and mental delay (Pryse-Phillips, 2005). During her stay in Breslau, Kennard reviewed clinical pediatric neurology cases with Foerster. Several of Kennard’s letters to Fulton written from Foerster’s laboratory describe discussions about pediatric issues and her paper on the infant monkeys. On 23 March 1935,xix she wrote to Fulton in some excitement about a discussion with Foerster.
The last evening I spent with Prof. Foerster was most exciting I had given him the baby monkey paper to read, + he was really interested - said that in man, all those things were true, but that no one had ever done any systematic research on them.
On 31 July 1935 in London, Foerster gave the ninth Hughlings Jackson memorial lecture (Foerster, 1936), which included extensive discussion of three pediatric cases, as well as speculation about compensatory mechanisms and reasons for possibly greater recovery of function in immature brains (Foerster, 1936, p. 158). Kennard read Foerster’s Hughlings Jackson lecture before he presented it and corrected his English expressionxx, after which she helped him travel to London.
Energized by her Amsterdam meeting with Foerster, her work with Brouwer, and her progress on the infant monkey lesion paper, and while awaiting the results of the Saar plebiscite to decide whether she would be able to visit Foerster in Germany, Kennard wrote to Fulton from Amsterdam onxxi about her life plan, which, as it happens, she carried out.
Now, for a more important discussion to which I would like your reactions. Don’t think, when you read this that I’m trying to make any sudden + irrevocable decision. I’m not. + my ideas, which have been long simmering may change frequently. But now seems to be a good time for me to think about my past and future professional progress.
The idea has been in my brain for a long time that I should go into neurology for children. What do you think? Do you think that there is a need for children’s neurologists in America, in New Haven, Boston etc? I don’t think that enough is known about the subject. + the children fall between the adult neurologists and the pediatricians. As you may remember, I took all the child patients I could get in Fox’s clinic. I’ve talked to everyone I could find on the subject and everyone agrees that there’s possible need for such specialists. As far as I know Crothers’ and Bailey’s clinics are the only ones with children’s neurologists but I’m sure there are more.
Reasons why I would like to do it are many: the neurology of children’s diseases is more neurophysiology than anything else; I’m very interested in spasticity, paresis + developmental conditions; I’m not really a physiologist.
3.3 Kennard’s 1936 paper on motor cortex lesions in infant monkeys
Kennard’s first paper on motor cortex lesions in infant monkeys was published in the March issue of the American Journal of Physiology. It describes in detail the surgical extirpation of the motor cortex of two monkeys, lesioned at 10 and 40 days of life, respectively, who showed a pattern of motor recovery and motor deficit.
In the first monkey, the entire left hemisphere was removed at the age of 40 days. On recovery from anesthesia, it showed the same deficits as an adult animal with the same operation: hemianopsis and loss of sensibility and motor paresis on the contralateral side. The age-related difference was in the rate of recovery, which was more rapid in the infant than in the adult, although, four months after post-surgery, the infant-lesioned animal still had a slight exaggeration and awkwardness of the movements of the right side.
In the second monkey, removal of the left motor and premotor areas at 10 days of age was followed by a recovery that was actually far from complete. The animal walked about within 24 hours, but with a slight lag in movements of the right side. The animal could produce purposeful movements, although for 10 days the right fingers and toes were used less frequently and a little more awkwardly than the left. Kennard then removed the motor and premotor areas of the second hemisphere at 5 months of age. The monkey showed an “extraordinarily rapid and complete” (p. 143) recovery of voluntary power, righting responses, reaching, and voluntary grasp.
The lesions in this infant monkey were serial rather than successive, the two lesions being separated by a five-month interval. Even with infant monkeys, Kennard reported recovery to be better with serial rather than simultaneous lesions. In Table 1 of the 1936 paper, Kennard presents motor outcome data in young or mature animals according to interval between operations, but it is impossible to determine whether the animals with the longer intervals and better outcomes are also the younger ones. More important, Kennard’s descriptions of the bilaterally lesioned infant monkey revealed diverse long-term motor deficits, which finding seems to contradict her statement about complete recovery.
Table 1.
A sampler of human and animal research published between 1970–75 on the topic of age and neural and functional plasticity
| Annett (1973) | Children with right hemiplegia have more speech deficits than a matched group with left hemiplegia |
| Butters, Rosen, and Stein (1974) | Adult serial ablations of dorsolateral frontal cortex (but not orbitofrontal cortex) in monkeys preserve function as well as early-onset lesions to the same region |
| Denckla (1973) | The association between dexterity and handedness is the same in children and adults, implying early left hemisphere specialization for skilled movements |
| Dennis and Kohn (1975) | Early left lesions and left hemispherectomy produce deficits in the syntactic function of language that early right lesions and right hemispherectomy do not exhibit |
| Douglas (1975) | Unilateral destruction of the hippocampus in infancy produces behavioral effects found only after bilateral lesions in the adult |
| Glassman (1973) | Early (2–14 days) or late (5 months) destruction of sensorimotor cortex in cats affects placing and hopping independent of lesion age |
| Goldman (1971) | Orbitofrontal lesions in the infant monkey produce similar functional effects to later lesions |
| Goldman (1974a) | The strongest evidence for collateral sprouting after early-onset lesions in monkeys is in those subcortical structures and fiber tracts with the least behavioral sparing |
| Goldman (1974b) | Differential development of frontal regions in monkeys accounts for the 1) rate of appearance of deficits after early dorsolateral lesions; 2) disappearance of deficits after early orbital lesions |
| Goldman and Rosvold (1972) | 1) Deficits after anterodorsal caudate lesions in infant monkeys are as severe as those from adult lesions by one year of age; 2) Lesion extent relates to outcome similarly in infants and juveniles |
| Gott (1973) | Hemispherectomy for childhood-onset disease causes more global lowering of function than adult onset hemispherectomy |
| Hicks and D’Amato (1970) | 1) Hemispherectomy in rats produces contralateral loss of tactile placing response in both infants and adults; 2) deficits after infant lesions emerge only on the 7th postnatal day |
| Isaacson (1975) | Damage to the infant brain produces greater anomalies of structure and behavior than are found after brain damage in juvenile or mature animals |
| Johnson (1972) | Septal lesions at 7 days of age produce the same learning and social changes as lesions in the adult rat. |
| Kohn and Dennis (1974) | Early right lesions and right hemispherectomy produce deficits in visuo-spatial function compared to early left lesions and left hemispherectomy |
| Lawrence and Hopkins (1970) | Disruption of medullary pyramids in infancy produces the same deficits in finger dexterity found after similar damage in adult monkeys |
| Milner (1974) | 1) Apparent sparing of language after early-onset lesions comes at the cost of right hemisphere functions; 2) Early-onset lesions in either hemisphere lower IQ |
| Molfese, Freeman, and Palermo (1975) | Newborns and infants show EEG cerebral asymmetry for speech and music sounds |
| Murphy and Stewart (1974) | Striate cortex lesions in the rabbit disrupt visual discrimination whether in infancy or in adulthood |
| Nash (1971) | Neonatal irradiation affect mouse growth more than does irradiation later in life |
| Nonneman and Isaacson (1973) | Neonatal hippocampal destruction produces extreme starting latencies in a passive avoidance task |
| Rudel and Teuber (1971) | Brain-injured children are more impaired than brain-injured adults in egocentric route-finding |
| Rudel, Teuber and Twitchell (1974) | 1) Early brain damage impairs oculomotor function, which is correlated with spatial deficits; 2) Lateralization of physical signs after early brain damage correlates with lateralized cognitive symptoms |
| Schneider (1974) | Anomalous connections after early-onset lesions in hamsters may prevent sparing of function |
| Schneider and Jhaveri (1974) | Neonatal visual cortex lesions retard learning in the hamster but adult lesions spare the same function |
| Stein (1974) | Recovery of function occurs in mature rats, not only in rats with neonatal lesions |
| Teuber (1971) | Children with brain injury show 1) increased starting position deficits compared to controls at all ages; 2) deficits righting from a tilted position younger than age 11 but not thereafter |
| Thompson, Harlow, Blomquist and Schiltz (1971) | Lesions of the monkey dorsolateral prefrontal cortex at 5 months of age (when lesions do not influence delayed response) produce the same effects on oddity learning as do lesions later in life |
| Twitchell (1974) | 1) Cerebral palsy delays sensory-motor maturation and produces hypertrophy of infantile reflexes; 2) Congenital encephalopathies suppress the development of fine finger movements |
| Wada, Clarke, and Hamm (1975) | Asymmetry in the left hemisphere planum temporale exists in fetuses |
| Witelson and Pallie (1973) | Neuroanatomical asymmetry exists in the left hemisphere language areas in newborns |
| Woods and Teuber (1973) | Language is spared after early left hemisphere lesions but at the price of right hemisphere functions |
At the end of the first week after operation it could walk and climb and feed itself by approximating its mouth to the food rather than by using its hands. It climbed rapidly and fairly accurately, sometimes slipping on flat surfaces, and always progressing on a broad base; there was hypermetria and movements were less well performed on the left. At the end of one month... it was sometimes unable to detach itself from the bars of the cage and would cling for hours if not removed. During the next four months...a definite motor deficit was still present, and movements were slower than normal; when placed in a cage with two slightly smaller animals it was unable to hold its own with more agile cage-mates. The walking movements were still hypermetric and the animal developed a gallop, which was like the hopping of a rabbit. The animal clung for hours to the breast of a larger monkey in the manner of the new-born infant. ...It climbed awkwardly and in jumping from chair to desk, a distance of perhaps 5 feet, it frequently missed its aim and fell to the floor...The animal is still alive, 18 months after its last operation, and its neurological condition is unchanged...(Kennard, 1936, pp. 143–144).
Although twice in her paper Kennard makes the general point that infant monkeys recover better from lesions of the motor cortex than do adult monkeys, she also notes that her newborn monkeys had many of the same motor deficits as adult macaques after similar motor cortex lesions.
Infant monkeys at this age show many of the reactions seen in older animals after ablation of the excitable areas. Motor progression is awkward and forced grasping is very pronounced. They exhibit changes in intensity of the reflex grasp on changing position, similar to those seen in the older animals after bilateral extirpation of the motor areas... (Kennard, 1936, p. 142).
Later, in the discussion, Kennard presents a highly nuanced account of the relation between age and recovery of function, the gist of which is certainly not that early lesions produce complete recovery, and the content of which constitutes a significant revision of the general statements about recovery in the introduction of the same paper. She pointed out that adult human hemispherectomy need not prevent contralateral limb movement, which means that even adult organisms have the potential for recovery of motor function after massive unilateral lesions. She explained how the presence of synkinesis implicated the fibres ipsilateral to the lesion in post-injury motor function. She hypothesized that the striate bodies and the cerebellum might influence motor recovery in infant monkeys because their pyramidal tracts are not yet developed and their simple and uncoordinated movements might be integrated at a subcortical level. Importantly, she contrasted the motor recovery she had observed with the more limited recovery of the cognitive function of delayed recall, which was impaired regardless of the age of the animal.
...the ability of an animal to make a correct choice after a given time interval which is normally present in the monkey, is, after extirpation of all of both frontal lobes, entirely and permanently lost, both in infants and in adult animals. (Kennard, 1936, p. 144, italics Kennard’s)
In short, Kennard had identified a number of factors that shaped the degree of motor lesion-induced motor deficit: the size of the lesion within the motor and premotor areas (smaller lesions produced less deficit); the status of the ipsilateral hemisphere (after a recovery from a unilateral lesion, a second lesion in the ipsilateral hemisphere will reinstate the original motor deficits hemisphere and add new, ipsilateral deficits); time interval between the stages in two-stage, non-simultaneous lesions (in adolescent and young adult monkeys a slight degree of motor power returns even in adult animals with a 3–4 week interval between the two lesions); and the age of the animal. In succeeding papers, Kennard went on to explore a number of issues about early lesions that, collectively, form a significant contribution to developmental neuropsychology.
4. Kennard’s Contributions to Developmental Neuropsychology
4.1 Early and adult lesions may produce equivalent effects
In her 1936 paper, Kennard had hypothesized that the attenuation of deficits following immature motor cortex lesions was related to the fact that these areas were incompletely developed in infant monkeys. An entailment of this hypothesis was that infants and adults might show equivalent deficits following lesions in a brain region that developed earlier in ontogeny.
There are in contrast two functions of the cortex which we know to be totally destroyed by ablation both in the infant and in the adult. Vision is as completely and permanently altered by bilateral ablation of the occipital lobes of the infant as of the adult; complete removal of areas 9–12 also produces in both infant and adult, an animal incapable of immediate memory...It may be assumed in these instance that all of the systems necessary for these functions have been destroyed and that there is no vicarious assumption of function. (Kennard and Fulton, 1942, p. 604)
In another paper (Kennard, 1938), Kennard found empirical support for the hypothesis that, if a brain region is functionally developed in infancy, it will produce the same effects when lesioned in infants or adults. In the adult, lesions in area 8 produce paralysis of conjugate deviation of the eyes. Paresis of conjugate deviation of the eyes following lesions of area 8 is more severe and enduring in the infant than is the paresis produced by other cortical ablations, more nearly resembling the paresis in an adult with a similar lesion.
This line of thought allowed her to make some general statements about brain maturation.
The basal ganglia function at birth and continue to show similar function throughout life. (Kennard and Fulton, 1942, p. 605)
4.2 Growing into a deficit
Kennard made longitudinal observations on her young lesioned monkeys. While many of these observations were written up as incidental to the main immediate and short-term effects of motor system lesions, they helped shape her conclusions about early lesions. She demonstrated that there is a complex evolution of functional deficits after some early brain lesions, depending on the development of functional maturity.
Kennard studied the evolution of motor deficits following lesions in the infant. In the adult, lesions of the motor cortex (area 4) produce paresis, while lesions in the premotor area (area 6) produce spasticity and problems in skilled movements. Before complex skilled motor activity has developed, removal of cortical motor areas is not accompanied by any marked and noticeable motor deficit in the infant. Paresis is first seen after ablation of motor cortical areas in the infant at a time when normally skilled coordinated movements should appear. Spasticity (increased resistance to passive manipulation) begins to appear much later than paresis.
After the first six months of life, monkeys thus operated upon in infancy [bilateral ablation of cortical areas 4 and 6] begin to develop spasticity and lose some of the skilled motor performance. (Kennard, 1944, p. 289)
One difference between early and late lesions was the emergence of delayed effects after early lesions. Kennard articulated the idea of delayed cognitive effects following early brain lesions, demonstrating that some infant monkeys had no deficits immediately after the time of their lesions, but gradually appeared to be impaired in the relevant motor abilities as they matured. She discussed the idea that dynamic changes could take place over time and that an initial impression of sparing of function could later be evident as behavioural dysfunction.
Kennard (1940) specifically implicated the sequence of myelination in the temporal emergence of motor deficits after early lesions in the primate brain.
Myelination is generally supposed to be correlated with function, and, since it is known that in neither monkey nor man, ...are corticosubcortical tracts completely myelinated at birth, it is not surprising that discrete “voluntary” motor acts are absent in the newborn infants. (Kennard, 1940, p. 396)
Myelination of the corticospinal tract arising from area 4 occurs before that of other cortical efferent pathways. Functionally, this may be correlated in the monkey with the appearance of “voluntary” prehension during the ninth week of life, in the chimpanzee with the use of the hands and with walking and crawling after the fifth or sixth month, and in the human infant with balancing, walking, and the like, after the ninth month. If, for any reason, the pyramidal tract is unable to function at this time, skilled and coordinate motor performance will not develop completely and impairment appears as failure to learn the skilled acts of which the normal motor system is capable. In the monkey and chimpanzee at least, and probably in man also, there is in such cases no spasticity at this stage of development.
Full functional coordination does not appear in the normal infant macaque until the second half of the first year of life, in the chimpanzee until the third year and in man until several years later. The time of myelination of the extrapyramidal cortical systems of area 6 and of the frontal association areas is not definitely known... except that it occurs later than that of area 4. At any rate, it is probably during this period of myelination that spasticity develops if the cortical extrapyramidal areas have been previously removed. These considerations make it possible to postulate that spasticity is a “release phenomenon.” In the adult, removal of cortical areas (area 6 or 4-s), which normally integrate skilled coordinate movements, results in “release” of other pathways (cortical or subcortical), thus causing spasticity. Removal of these same areas from the infant cortex has no such effect until other reflex circuits (cortical or subcortical) become functionally active, presumably by their myelination. Then, in the absence of the “higher” controlling mechanism, spasticity appears. (Kennard, 1940, p. 396–397)
4.3 Development and regional cortical maturation inferred from age-related lesion effects
Kennard emphasized qualitative differences in motor function between early- and late-lesioned primates.
...removal of a known cortical area from the infant causes a paralysis of a different quality from that which occurs after a similar cortical ablation in an adult of the same species (Kennard, 1940, p. 377).
Kennard suggested that early lesion functional effects might be considered as regression to previous stages of development. After making lesions at various times in the monkey’s “childhood,” she compared the lesion-induced regression to earlier stages of normative motor development, which allowed her to quantify the extent of impairment with reference to normal development of motor function. She showed that the time when motor deficits appeared after perinatal motor cortex lesions could be predicted by knowing the normal developmental sequence of the motor skills.
Kennard’s goal in studying cortical lesions was not only to describe age-related outcomes, but also to plot the time course of regional cortical maturation. Her idea was that systematic comparisons of lesions sustained at different ages could be a useful tool for plotting out cortical maturation.
Area 8 must function before other cortical motor areas since its removal from the infant cortex produces marked deviation of the head and eyes at a time when other motor performance is only slightly affected by cortical ablations elsewhere in the motor areas. (Kennard and Fulton, 1942, p. 602)
The function of the infant basal ganglia must be much like that of the adult. Large bilateral or unilateral lesions of caudate or putamen or both have no immediate visible effect on the motor status of the infant. Excision of the basal ganglia of the adult may result in slight tremor, but only when there is a large bilateral lesion. Bilaterality and lack of focal representation of function in these nuclei have been shown by excision in both age groups. (Kennard and Fulton, 1942, p. 602–603)
Kennard articulated a view of development in which various regions of the cortex initially nonspecific, gradually become committed to a particular function, after which their ability to participate in other forms of functional integration is diminished.
A relative functional non-specificity of cortical areas is partially responsible for this greater compensatory power in infancy... (Kennard, 1940, p. 395)
The process of maturing of the central nervous system is thus shown to be one in which the adaptability of the cortex becomes less and certain areas of cortex become “set” to integrate certain functions which cannot then be integrated through other channels. (Kennard and Fulton, 1942, p. 603)
4.4 Bilateral frontal lesions may produce long-term alterations in global behavior
Kennard observed that bilateral (but not unilateral) frontal lesions produced marked behavioral hyperactivity (Kennard et al., 1941).
Destruction of rostral portions of areas 6 or 8 produced hypermotility. In monkeys the hyperactivity consisted of continual, restless, pacing back and forth; in chimpanzees restless and distractible behavior similar to that observed in man after bilateral frontal lobectomy was seen.” (Kennard et al., 1941, p. 512)
Further, she interpreted the hyperactivity as a loss of inhibitory control and related it to similar behavior in what was then termed ‘problem children.’
the phenomenon of hyperactivity is interpreted as “release of function,” and its possible relationship to restless behavior in problem children.. is stressed. (Kennard et al., 1941, p. 512)
Kennard noted that the hyperactivity was immediate in adult monkeys but emerged with age in monkeys with lesions in infancy.
Removal of these areas in infancy causes no immediate visible change in the elementary motor performance, but with age a compulsive motor hyperactivity appears which cannot be distinguished from that of animals which have had the same areas removed when adolescent or mature. (Kennard and Fulton, 1942, p. 600)
4.5 Serial lesion effects, age and recovery interval
Kennard showed that recovery was better with serial than with simultaneous lesions.
When only a fraction of the sensorimotor cortex is removed at operation, the length of the interval before a second ablation has direct influence on final recovery of function. Thus simultaneous bilateral ablations always result in greater eventual deficit than seriatim ablations and longer intervals between operations are followed by greater recovery of function. (Kennard and Fulton, 1942, p. 598)
She showed, further, that serial lesions had more influence on recovery in younger-than in older-lesioned monkeys.
One other factor intimately affects motor function following cortical ablation in the young monkey to an even greater degree than in the old. (Kennard and Fulton, 1942, p. 598)
4.6 How and where in the brain does recovery take place?
While Kennard observed better recovery after early lesions in a number of circumstances, her real interest was not so much in the fact of recovery as in its mechanism (how? where in the brain? and for how long?). She explored these issues in a series of imaginative studies, beginning with a study in adult monkeys, in which she looked at ipsilateral and contralateral motor function after section of the corpus callosum occurring either before or after ablation of the motor and premotor cortex (Kennard and Watts, 1934).
Kennard then turned her attention to age-related changes in brain organization following frontal lesions. Her procedure was to make motor cortex lesions, wait until motor function had either developed or was once again functional, and then make a second series of lesions in areas that she thought might have been involved in restoring or allowing the development of motor function in the absence of the motor cortex.
Kennard showed that paresis could be increased by removal of non-motor areas. More important, she developed a viable methodology for studying which parts of the brain mediate motor function when the motor cortex is damaged.
If, for instance, the frontal association areas are removed unilaterally or bilaterally from an adult either before, after or simultaneously with areas 4, 6 and 8, no added motor deficit can be detected as the result of removal of these association areas...
However, if, following bilateral removal of areas 4 and 6 in infancy, a young animal is allowed to grow until improvement in motor performance has ceased and then a frontal association area or postcentral gyrus is removed, a markedly increased deficit in motor performance appears. This paresis which is the result of removal of a non-motor area is found, furthermore, both in the contrataleral and ipsilateral extremities. The indication therefore is that, when the motor areas are removed in infancy, there is a reorganization of the remaining non-motor cortex which then integrates both contralateral and ipsilateral motor performance to a degree which is far greater than in the normal cortex. (Kennard and Fulton, 1942, p. 601)
Kennard compared combined cortical and subcortical lesions with those arising from cortical lesions alone. In infants as well as in adults, combined cortical-subcortical resections produced dramatic effects on motor function. She concluded from this that the basal ganglia are functional at birth and continue to show similar function throughout life.
The basal ganglia of both adult and infant affect movement more severely, however, if area 6 of the cortex is removed together with either caudate or putamen. There is then an immediate change in posture and movement. In fact, the only infants with immediate and severe effects as the result of injury to cerebrum are those with this combined cortico-subcortical ablation. Paresis, epilepsy and dysrhythmias have all followed such lesions. (Kennard and Fulton, 1942, p. 603)
Kennard proposed that brain lesions in infant monkeys made the brain more excitable than did similar lesions in adult animals. Comparing pre- and post-operative EEG records of monkeys with lesions in the cortex and basal ganglia, she found that monkeys operated in infancy had the more striking changes.
For it is well known that infants are particularly susceptible to epilepsy under almost any condition than can bring on seizures, whether fever, non-specific trauma or focal cerebral injury. In all infants there must then be some condition of the cerebral cortex or basal ganglia which permits synchronization of cortical potentials into patterns characteristic of epilepsy more readily than in the adult. (Kennard and Nims, 1942, p. 347)
Using an innovative methodology, Kennard and McCulloch (1943) stimulated the ipsilateral cortices of 5 monkeys following motor ablations in infancy (N=4) or in near adult life (N=1), finding that the motor cortex surrounding areas 4 and 6 was more excitable in animals lesioned in infancy than in controls or in the animal with later ablation, and that movements in the infant-lesioned group were more diffuse and required higher thresholds for stimulation. The data show that cortical response after early lesions is abnormal, that early lesions are followed by reorganization - but not normalization - of cortical function, and that functional reorganization occurs within a partially destroyed ipsilateral motor system.
4.7 Human and life-span developmental neuropsychology
Kennard (1940) studied 233 cases of cerebral palsy at the neurologic clinic of the Children’s Hospital of Boston. Comparing children with lesions before or at birth with those whose lesions were acquired later (after 9 months of age), she found that both groups had equivalent arm and leg paresis, but that the later-lesion group showed more spasticity. In those children with bilateral (possibly subcortical) lesions from birth, however, rigidity was evident from the time of birth. In these children, motor deficits became evident when the children began to walk, suggesting that, like infant monkeys, they had grown into their deficit.
Kennard had a strong interest in life-span issues. She analyzed more than 1000 EEG protocols of psychiatric patients aged from 5 to 55 years to explore whether functional brain changes were related to the presence of psychiatric disorders over the life span (her methodology included excluding patients with IQ scores below 80 and using a sibling control group for behavior-disordered children).
Kennard concluded that delayed brain maturation might underlie abnormalities of behaviour but that organic disease was probably involved in schizophrenia.
The incidence of abnormal EEGs in disorders of behavior is directly related to the age of the subjects.
In childhood and adolescence there is a high incidence of abnormal EEGs (above 50 per cent) in both schizophrenics and the behavior disorders which have been variously designated as neurotic, psychopathic, delinquent, or simply behavior problems.
In contrast, the incidence of EEG abnormality in schizophrenia remains high throughout all the ages and, as has been shown previously, is related to the progressive severity of this disorder which ultimately profoundly affects performance of all organic systems including that of the cerebral cortex. (Levy and Kennard 1953, p. 427)
5. How, When, and Why Did Kennard’s Body of Work Become Reduced to the ‘Kennard Principle?’
5.1 Early studies of early-onset lesions
In their review of “The Kennard Principle before Kennard”, Finger and Wolf (1988) point out that Kennard’s contributions may have been more evolutionary than revolutionary, and they show that other investigators had described age-related lesion effects some 70 years earlier. For example, four years after his classical studies on motor speech, Broca (1865) described a woman with childhood brain disease whose language function was spared because, he argued, language laterality had shifted from left to right.
A l’autopsie d’une malade de quarante-sept ans, épileptique depuis sa plus tendre enfance, on constata que la troisième circonvolution frontale gauche faisait défaut, ainsi que la circonvolution pariétale inférieure et la circonvolution temporo-sphénoïdale supérieure. En d’autres termes, on constata l’absence de toute la partie de l’hémisphère gauche qui borde la scissure de Sylvius. [At the autopsy of a sick 47 year-old, epileptic from early childhood, it was recorded that the third left frontal convolution was defective, as well as the inferior parietal and the superior temporal-sphenoidal convolution. In other words, the absence of the entire part of the left hemisphere that borders the Sylvian fissure...] (Broca, 1865, p. 92)
Il suit de ce qui précède qu’un sujet chez lequel la troisième circonvolution frontale gauche, siège ordinaire du langage articulé, serait atrophiée depuis la naissance, apprendrait à parler et parlerait avec la troisième circonvolution frontale droite...[It follows from the preceding that a person in whom the third left frontal convolution, the center of articulate language, is atrophied from birth will learn to talk and speak with the third frontal convolution on the right...]. (Broca, 1865, p. 92)
Throughout the last quarter of the 19th century, clinicians and experimentalists described age-related differences in response to brain injury (Barlow, 1877; Finger et al., 2000). A number of studies addressed specific aspects of early brain injury, such as the equipotentiality of the two cerebral hemispheres for language (Dennis and Whitaker, 1977), although the interpretation of some clinical cases has proved to be equivocal in terms of mechanisms of functional recovery (Finger et al., 2003; Hellal and Lorch, 2007).
5.2 Teuber and the ‘Kennard Principle’
The early 1970s saw an explosion of empirical data, the beginnings of an integration of human and animal work, and a burgeoning body of conceptual discussions of the nature of brain and behavioral plasticity. In this New Look, theoretical formulations pointed out a central anomaly of thinking that the immature brain had boundless plasticity, and empirical research showed that plasticity was not solely a function of a young age. Talking of the ‘myth’ of recovery from early brain damage, Isaacson noted,
If the developing brain were completely “plastic” (a most unfortunate word) and any part capable of doing the work of any other, how are we to explain the tragedies of mental retardation resulting from biological problems occurring before birth? (Isaacson, 1975, p. 1).
The body of new research (Table 1 provides a sampler of human and animal work published between the years 1970–1975) showed that early brain lesions had diverse functional effects, some as or more debilitating than those produced by adult lesions; that several congenital or early-onset brain injury conditions were not as deficit-free as was once supposed; that, like the adult brain, the immature brain showed both structural and functional brain asymmetries; that structural reorganization and functional recovery occurred after both early- and adult-onset lesions; that the apparent sparing of function after early lesions could be mimicked by multiple-stage lesions in the adult; that collateral sprouting after brain lesions was not necessarily associated with recovery of function; and that age interacted with lesion location and timing to produce varied functional outcomes. At this time, key symposia (e.g., the Neurosciences Research Program Symposia) included both human and animal research, as well as both experimental and clinical data; in addition, the community of plasticity researchers was relatively small and shared a common intellectual landscape.
Hans-Lukas Teuber’s (1916–1977) contributions to the emergence of neuropsychology as a discipline were broad, deep, and visionary (Bigler, 2009; Held, 1979; Hécaen, 1979; Pribram, 1977; Richards, 1978). In the final third of his career, Teuber became interested in fundamental problems of perception, including issues of innate schemata for perceptual skills like face recognition (Hécaen, 1979, pp. 121–122). Not coincidentally, perhaps, the last 15 years of Teuber’s life saw him involved in empirical research seeking to identify the effects of early human and animal brain lesions.
Teuber was chief dramaturge of the New Look in brain plasticity. He hired the actors (MIT staff and graduate students); developed the play season (seminars, workshops, Neurosciences Research Program Work Sessions); edited new productions (he was a co-founder of Neuropsychologia); created educational services (his fabled Course Nine Hundred, Lackner, 2009); and interpreted history for old and new players.
Part of Teuber’s presentation of history was his popularization of a ‘Kennard Principle’: “namely, that the time to have one’s cortical lesion, if one can arrange it, should be early because early lesions seemed less disabling than those acquired later in life” (Teuber, 1974, p. 202). Teuber had enormous charisma and a great sense of drama (his associates recalled “the magic with which he outlined the riddles and paradoxes of the brain” [Richards, 1978, p. 357]). With provocative rhetoric and his fabled “pretty taste for paradox” (Gilbert and Sullivan, 1941, p. 133), he regularly unveiled the ‘Kennard Principle’ at the beginning of his discussions of early brain damage (Schneider, 1979). Noting that the Kennard ‘Principle’ is eminently teachable, Teuber would ask: “...but is it true?” (Teuber, 1971, p. 7) and then startle his audiences with the New Look evidence.
Sometimes, a body of research work lies dormant for a generation. Barbara McClintock’s work on ‘jumping genes’ in the 1940s was relatively ignored until the emergence of molecular genetics gave it context (Keller, 1984). One of the reasons why Kennard’s 1930–1940s work became reduced to the eponymous ‘Principle’ in the early 1970s, I believe, was that a superficial reading of her findings appeared to provide a dramatic contrast to the burgeoning body of New Look evidence showing that it was not consistently better to schedule your brain damage earlier rather than later in life. This fact had been known earlier, but never in such volume, or from such diverse sources. Kennard became the straight (wo)man for Teuber’s take-home message, which was that the supposed plasticity of the young brain was ‘likely overrated’ (Teuber, 1971, p. 16); that early brain lesions had diverse effects on function depending on when and how outcome was measured; that age was only one of the factors that shaped recovery and outcome; and that some aspects of developmental brain plasticity were, in fact, maladaptive. Much of this, as we have seen, Kennard had said many years earlier.
6. The ‘Principle’ that Became a Belief
As Sellar & Yeatman [1930/1990] remind us, “History is not what you thought. It is what you can remember.” [p. xxxiii]. Beginning as provocative rhetoric, the exceptionally memorable ‘Kennard Principle’ became the neuropsychology equivalent of Whig history and eventually morphed into a belief. The ‘Kennard Principle’ still matters because it shapes practice and theory: as a belief, it continues to be the prism through which clinicians project rosy expectations about the plasticity of the immature brain; and it is still cited as the extreme plasticity position in theoretical discussions..
6.1 Beliefs dictate practice
Hart and Faust (1988) identified 120 practicing clinicians who had indicated neuropsychology as a primary field or area of specialization in either or both the Directory of the American Psychological Association (1984 Edition) and the National Register of Health Services Providers in Psychology (1983 Edition). These clinicians, who had a mean lifetime clinical experience in neuropsychology of some 5,000 hours, were asked to predict the most likely cognitive outcome (no, mild, moderate, or severe neuropsychological impairment) for two fictitious traumatic brain injury (TBI) case histories, one involving a child and the other an adolescent, but with identical descriptions of a bicycle accident in which the patient hit his/her head, experienced brief loss of consciousness and post-traumatic confusion, and suffered chronic-stage personality change and forgetfulness. The results showed a significant relationship between level of impairment and age (χ2 (1, 72) = 16.0, p < .01). 97% of the clinicians judged the child case to have no or mild impairment, and 3% judged the child case to have moderate or severe impairment; 61% of the clinicians judged the adolescent case to have no or mild impairment, and 39% % judged the adolescent case to have moderate or severe impairment.
In the same vein, Webb et al. (1996) drafted four fictitious clinically based case histories of patients with TBI in which the patient’s age was varied (3, 7, 48, or 55 years). Study participants were 158 professionals in the British National Health Service specializing in neurological problems: neurosurgeons, neurologists, neuropsychologists, general practitioners, nurses, physiotherapists, occupational therapists, and speech therapists. In rating the degree of expected recovery from ‘minimal chance of further recovery’ to ‘anticipate complete recovery with no residual impairment’ the professionals estimated greater recovery for child than for adult cases (F (1,148) = 77. 87, p <.001). Strikingly, time since professional credentialing did not affect estimates of recovery, suggesting that belief in the ‘Kennard Principle’ was not attenuated by access to more information about the vulnerability of the young brain.
Although the ‘Kennard Principle’ suffers from oversimplicity and obsolescence, it remains alive and well in everyday practice. Belief systems dictate practice, and the idea that a young age immunizes children from neurocognitive deficits may well be hazardous to their proper assessment and case management (Hart and Faust, 1988) and to suitable provision for their after-care and rehabilitation (Webb et al., 1996).
7. Kennard’s Place in the History of Developmental Neuropsychology
7.1 Studies following Kennard
Kennard’s imaginative blend of neurosurgery, neurophysiology, developmental observations, and experimental psychology (and early neurochemistry; Ward and Kennard, 1942) makes her an important figure in the history of developmental neuropsychology. She was responsible for methodological innovations, many of which are used in developmental neuropsychology today. She made systematic observations about age effects over a chunk of the lifespan, drafted quasi-formal models of function, set parameters, and analyzed how changing the parameters altered the extent of the lesion effect.
Kennard was perhaps the first to adopt models from infrahuman primates and to relate them explicitly to children with normal and aberrant development. This idea has been central to later investigations of core cognitive functions like inhibitory control (e.g., Diamond, 1990).
Kennard performed exemplary studies of age effects, which she showed to be complex. More recent studies have developed the idea that age effects may be non-linear, that dearly lesions may produce delayed effects (Goldman-Rakic 1980; Pullela et al., 2006), and that there may be multiple developmental periods for good adaptation to brain lesions (Kolb et al., 2000). Kennard advanced the idea that brain pathology alters the developmental sequence of a skill, and that studying these sequences can address questions about the normal time frame for that skill. This is a central tenet of modern developmental neuropsychology models, as is the idea that brain regions become more functionally committed with age. Later work on frontal cortical and caudate lesions in infant monkeys further developed the idea that studying age at lesion effects can provide information, not only about the lesion effects, but also about the ontogenetic maturation of the brain and cortical commitment (Goldman-Rakic, 1980).
Kennard discussed the relative roles of cortical and subcortical brain structures in producing different symptomatology after early brain lesions, an issue of current interest. For example, recent work shows how early lesion produce delayed onset of functional deficits associated with prolonged cortical and subcortical pathogenesis (Pullela et al., 2006). She studied the interaction of age and serial lesions in producing better outcome, a theme developed by later researchers (Butters et al., 1974). She investigated whether reorganization of motor function occurs in the ipsilateral or contralateral hemisphere after early brain lesions, an issue later explored in cases of callosal agenesis (Dennis, 1976). She hypothesized neural and functional mechanisms based on then-current knowledge about Betz cells and the sequence of regional brain myelination (e.g., Kennard, 1940), paving the way for later studies on the morphological correlates of sparing of function (e.g., Kolb and Wishaw, 1989) and current studies on the synaptic mechanisms of plasticity in the neocortex (Feldman, 2009). Later research extended Kennard’s speculations about mechanisms of plasticity to show that, while rearrangement of brain connectivity is one adaptive mechanism underlying recovery of function, anomalous synaptic circuitry is often structurally disastrous (Isaacson, 1975) and may also be responsible for permanent functional deficits (Giza and Prins, 2006; Goldman-Rakic, 1980; Schneider and Jhaveri, 1974).
In childhood, permanent deficits may be indexed by widespread changes in cognition and behavior (see also Hebb, 1942; Isaacson, 1975). In proposing that immature organisms grow into hyperactivity if the development of anterior brain systems is disrupted, Kennard implied that early lesions might produce generalized effects on cognitive development. This idea has been extended in later studies of the effects of radiation on the infant and toddler brain, which is devastating and avoided wherever possible because of its global effects on neurobehavioral development (Cohen et al., 1993).
7.2 Kennard’s relevance for current views of recovery of function and functional plasticity
Kennard’s overarching interest was in recovery of function and how functionality was effected in the lesioned brain. Even had she sought to identify a single principle for recovery of function - and I believe she did not - age at lesion would not have been that principle. For Kennard, early brain damage did not consistently spare function or optimize functional outcome, but could be more, less, or equally disabling than later-onset injury depending on the features of the injury, post-injury neuroanatomical reorganization, the staging of the lesion, and how and when outcome was assessed.
In historical perspective, Kennard’s framing of recovery of function appears more similar to how modern researchers think about cognitive reserve (e.g., Dennis et al., 2006; Stern, 2006) than it is to presentations of a ‘plasticity vs. specialization’ polarity (e.g., Anderson et al., 2009). Kennard argued that the lesion burden, of itself, was a poor predictor of outcome, so investigators needed to identify the mediators and moderators that enhance or impair functional outcome.
Yet it is a remarkable fact that in man during the early acute stages of this paresis the rate and extent of recovery often cannot be predicted and that relatively little is known of the factors other than size and site which affect it. (Ward and Kennard, 1942, p. 189)
As early as 1936, Kennard and her colleagues were clear that age at lesion operated in interaction with both lesion location and behavioral task. They demonstrated that age was more important for recovery of motor function than for association cortex functions like delayed recall:
But in contrast to the considerable motor recovery in infant monkeys, there is no recovery after lesions of the association areas. Impairment of “recall” [delayed response] is as severe as in adult subjects. It seems probably that this difference in recovery after motor and frontal area lesions arises from partial destruction of a dynamic system in the former instance, and complete removal in the latter. Thus the cortical motor area is only one component of the postural-locomotor system, and, under certain conditions, the remaining parts can carry on. Recovery is more complete when injury occurs before this cortical component has been functionally integrated with subcortical mechanisms. On the other hand, no other part of the nervous system can mediate the functions of the frontal association areas. If this region is removed completely, no recovery follows, and it is of little consequence whether such injury occur in infancy or adult life (Jacobsen et al., 1936)
The historical diminution of Kennard’s work to a simple ‘Principle’ of plasticity, which began in the early 1970s and continues to the present time, has fostered an exaggerated polarity between plasticity and specialization, one effect of which has been to obscure factors that age may mask or with which age may interact (Lidzba et al., 2009).
8. Conclusion
Margaret Kennard has enjoyed a strange press. On the one hand, she has been associated with an eponymous principle that does little justice to the breadth and depth of her research. On the other hand, she has been given little credit for tackling, head on and before the age of neuroimaging and neurotransmitters, many of the methodological and conceptual issues about recovery of function with which current developmental neuropsychology still struggles. While she did not have access to later discoveries about the complex series of molecular, cellular, and physiological events that orchestrate optimal function after brain lesions (Giza and Prins, 2006), her vision of the complex relations among the factors that guide recovery of function after brain lesions was consistently clear and prescient. Kennard’s work continues to resonate in a serious contemporary discussion of recovery of function and brain plasticity; the ‘Kennard Principle,’ however, does not. To honor Kennard’s achievements as a founding mother of developmental neuropsychology, we might relegate her eponym to the shadows and bring her real achievements into the light.
ACKNOWLEDGEMENT
Preparation of this paper was supported in part by National Institute of Child Health and Human Development Grants P01 HD35946 & P01 HD35946-06, “Spina Bifida: Cognitive and Neurobiological Variability” and by National Institutes of Health Grant 1RO1 HD04946, “Social Outcomes in Pediatric Traumatic Brain Injury”. Some information in this paper was presented at the Jean Pierre Marie Flourens (1794–1867) Bicentennial History Conference at Université de Québec à Montréal, Quebec, Canada, June 1–2 1994. I thank Rebekah Nelson for assistance with the Broca translation, Arianna Stefanatos for manuscript preparation, Sabine Doebel with help with tracking historical correspondence, and Marcia Barnes, Kim Edelstein, Jack Fletcher, Russell Schachar, Brenda Spiegler, and Keith Yeates for comments on the manuscript. I am most grateful for the assistance of Toby Appel and Florence Gilich, Library Assistant, at the Yale University Cushing/Whitney Medical Library; Diane E. Kaplan, Head of Public Services Manuscripts and Archives at the Yale University Library; and Elizabeth M. Shepard, Assistant Archivist at the Medical Center Archives of New York-Presbyterian/Weill Cornell.
Footnotes
Historical Register of Yale University, 1937–1951 (New Haven: Yale University, 1952, p. 196).
John F. Fulton Papers, MS 1236, Box 96, Folder 1347. (Kennard to Fulton, 23 August 1935).
Dr. Margaret Kennard, Psychiatrist, 76, Dies. New Haven Sunday News 14 December 1975.
John F. Fulton Papers, MS 1236, Box 96, Folder 1344.
John F. Fulton Papers, MS 1236, Box 96, Folder 1344. (Cobb to Fulton, 13 March 1931).
John F. Fulton Papers, MS 1236, Box 96, Folder 1344. (Fulton to Kennard, 9 March 1931).
John F. Fulton Papers, MS 1236, Box 96, Folder 1352.
John F. Fulton Papers, MS 1236, Box 96, Folder 1345–1346.
John F. Fulton Papers, MS 1236, Box 96, Folder 1348. (Fulton to Bayne-Jones, Dean of the School of Medicine at Yale, 23 March 1937).
John F. Fulton Papers, MS 1236, Box 96, Folder 1346.
John F. Fulton Papers, MS 1236, Box 96, Folder 1348.
American Philosophical Society: Warren S. McCulloch papers (Series I-Correspondence, 1931–1968; Kennard, Margaret A. 1947–1954).
John F. Fulton Papers, MS 1236, Box 96, Folder 1346.
John F. Fulton Papers, MS 1236, Box 96, Folder 1345. (Kennard to Fulton, 11 August 1932)
John F. Fulton Papers, MS 1236, Box 96, Folder 1345. (Fulton to Kennard, 23 August 1932).
John F. Fulton Papers, MS 1236, Box 96, Folders 1345–48.
John F. Fulton Papers, MS 1236, Box 96, Folder 1345.
In 1933 Cornelia de Lange described a clinical condition (now termed Brachmann-de Lange syndrome) with feeding difficulties, small head circumference, and upper limb abnormalities.
John F. Fulton Papers, MS 1236, Box 96, Folder 1346.
John F. Fulton Papers, MS 1236, Box 96, Folder 1347 (Kennard to Fulton, 4 July 1935).
John F. Fulton Papers, MS 1236, Box 96, Folder 1345 (Kennard to Fulton, 18 November 1934).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham TH. Integrating mind and brain: Warren S. McCulloch, cerebral localization, and experimental epistemology. Endeavour. 2003;27:32–36. doi: 10.1016/s0160-9327(03)00017-6. [DOI] [PubMed] [Google Scholar]
- Abraham TH. Physiological circuits: The intellectual origins of the McCulloch-Pitts neural networks. Journal of the History of the Behavioral Sciences. 2002;38:3–25. doi: 10.1002/jhbs.1094. [DOI] [PubMed] [Google Scholar]
- Anderson V, Spencer-Smith M, Leventer R, Coleman L, Anderson P, Williams J, Greenham M, Jacobs R. Childhood brain insult: can age at insult help us predict outcome? Brain. 2009;132:45–56. doi: 10.1093/brain/awn293. [DOI] [PubMed] [Google Scholar]
- Andrew AM. A stalled publication - work of Warren McCulloch. Kybernetes. 2004;33:141–146. [Google Scholar]
- Annett M. Laterality of childhood hemiplegia and the growth of speech and intelligence. Cortex. 1973;9:4–33. doi: 10.1016/s0010-9452(73)80014-0. [DOI] [PubMed] [Google Scholar]
- Barlow T. On a case of double hemiplegia, with cerebral symmetrical lesions. The British Medical Journal. 1877;2:103–10. doi: 10.1136/bmj.2.865.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED. Hans-Lukas Teuber and ‘The riddle of frontal lobe function in man’ as published in The frontal granular cortex and behavior (1964) Neuropsychology Review. 2009;19:9–24. doi: 10.1007/s11065-009-9086-1. [DOI] [PubMed] [Google Scholar]
- Broca P. Faculté du langage articulé. Bulletins de la Société d’Anthropologie. 1865;6:377–393. [Google Scholar]
- Butters N, Rosen J, Stein D. Recovery of behavioral functions after sequential ablation of the frontal lobes of monkeys. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. Academic Press; New York: 1974. pp. 429–466. [Google Scholar]
- Christen M. Varieties of publication patterns in neuroscience at the cognitive turn. Journal of the History of the Neurosciences. 2008;17:207–225. doi: 10.1080/09647040601150564. [DOI] [PubMed] [Google Scholar]
- Cohen BH, Packer RJ, Siegel KR, Rorke LB, D’Angio G, Sutton LN, Bruce DA, Schut L. Brain tumors in children under 2 years: Treatment, survival and long-term prognosis. Pediatric Neurosurgery. 1993;19:171–179. doi: 10.1159/000120727. [DOI] [PubMed] [Google Scholar]
- Davey LM. John Farquhar Fulton. Neurosurgery Online. 1998;43:185–187. doi: 10.1097/00006123-199807000-00130. [DOI] [PubMed] [Google Scholar]
- Denckla MB. Development of speech in repetitive and successive finger-movements in normal children. Developmental Medicine and Child Neurology. 1973;15:653–645. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- Dennis M. Impaired sensory and motor differentiation with corpus callosum agenesis: A lack of callosal inhibition during ontogeny? Neuropsychologia. 1976;14:455–469. doi: 10.1016/0028-3932(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Dennis M, Kohn B. Comprehension of syntax in infantile hemiplegics after cerebral hemidecortication: Left-hemisphere superiority. Brain and Language. 1975;2:472–482. doi: 10.1016/s0093-934x(75)80084-8. 1975. [DOI] [PubMed] [Google Scholar]
- Dennis M, Whitaker HA. Hemispheric equipotentiality and language acquisition. In: Segalowitz S, Gruber F, editors. Language development and neurological theory. Academic Press; New York: 1977. pp. 93–106. 1977. [Google Scholar]
- Dennis M, Yeates KO, Taylor HG, Fletcher JM. Brain reserve capacity, cognitive reserve capacity, and age-based functional plasticity after congenital and acquired brain injury in children. In: Stern Y, editor. Cognitive reserve. Psychology Press; New York: 2006. pp. 53–83. 2006. [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys, and the neural bases of inhibitory control in reaching. Annals of the New York Academy of Sciences. 1990;608:267–317. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- Douglas RJ. The development of hippocampal function: Implications for theory and for therapy. In: Isaacson RL, Pribram KJ, editors. The hippocampus: A comprehensive treatise. Plenum; New York: 1975. 1975. [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annual Review of Neuroscience. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger S. Margaret Kennard on sparing and recovery of function: A tribute on the 100th anniversary of her birth. Journal of the History of the Neurosciences. 1999;8:269–285. doi: 10.1076/jhin.8.3.269.1824. [DOI] [PubMed] [Google Scholar]
- Finger S, Almli CR. Margaret Kennard and her “principle” in historical perspective. In: Finger S, LeVere T, Almli CR, Stein DG, editors. Brain injury and recovery: Theoretical and controversial issues. Plenum Press; New York: 1988. pp. 117–132. [Google Scholar]
- Finger S, Beyer T, Koehler PJ. Dr. Otto Soltmann (1876) on development of the motor cortex and recovery after its removal in infancy. Brain Research Bulletin. 2000;53:133–140. doi: 10.1016/s0361-9230(00)00318-x. [DOI] [PubMed] [Google Scholar]
- Finger S, Buckner RL, Buckingham H. Does the right hemisphere take over after damage to Broca’s area? The Barlow case of 1877 and its history. Brain and Language. 2003;85:385–395. doi: 10.1016/s0093-934x(03)00060-9. 2003. [DOI] [PubMed] [Google Scholar]
- Finger S, Wolf C. The ‘Kennard’ effect before Kennard: The early history of age and brain lesions. Archives of Neurology. 1988;45:1136–1142. doi: 10.1001/archneur.1988.00520340090018. [DOI] [PubMed] [Google Scholar]
- Foerster O. The motor cortex in man in the light of Hughlings Jackson’s doctrines. Brain. 1936;59:135–159. [Google Scholar]
- Fulton JF. J.G. Dusser de Barenne 1885–1940. Journal of Neurophysiology. 1940;3:283–292. [Google Scholar]
- Fulton JF, Yale University School of Medicine Department of Physiology Methods and Problems of Medical Education. 1932;20:1–6. [Google Scholar]
- Fulton JF, Jacobsen CF, Kennard MA. A note concerning the relation of the frontal lobes to posture and forced grasping in monkeys. Brain. 1932;55:524–536. [Google Scholar]
- Fulton JF, Kennard MA, Watts JW. Autonomic representation in the cerebral cortex. The American Journal of Physiology. 1934;109:37. Proceedings of the American Physiological Society Forty-Sixth Annual Meeting. [Google Scholar]
- Getz MJ. The ice pick of oblivion: Moniz, Freeman and the development of psychosurgery. Trames. 2009;13:129–152. [Google Scholar]
- Gilbert WS, Sullivan AS. The complete plays of Gilbert and Sullivan. Norton; New York: 1941. [Google Scholar]
- Giza CC, Prins ML. Is being plastic fantastic? Mechanisms of altered plasticity after developmental acquired traumatic brain injury. Developmental Neuroscience. 2006;28:364–379. doi: 10.1159/000094163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman RB. Similar effects of infant and adult sensorimotor cortical lesions on cats’ posture. Brain Research. 1973;63:103–110. doi: 10.1016/0006-8993(73)90079-6. [DOI] [PubMed] [Google Scholar]
- Goldman PS. Functional development of the prefrontal cortex in early life and the problem of neuronal plasticity. Experimental Neurology. 1971;32:366–387. doi: 10.1016/0014-4886(71)90005-7. [DOI] [PubMed] [Google Scholar]
- Goldman PS. An alternative to developmental plasticity: Heterology of CNS structures in infants and adults. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. Academic Press; New York: 1974a. pp. 149–174. [Google Scholar]
- Goldman PS. Recovery of function after CNS lesions in infant monkeys. Neurosciences Research Program Bulletin. 1974b;12:217–222. [PubMed] [Google Scholar]
- Goldman PS, Rosvold HE. The effects of selective caudate lesions in infant and juvenile rhesus monkeys. Brain Research. 1972;43:53–66. doi: 10.1016/0006-8993(72)90274-0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Morphological consequences of prenatal injury to the primate brain. Adaptive capabilities of the nervous system. Progress in brain research. 1980;53:3–19. [PubMed] [Google Scholar]
- Gott PS. Cognitive abilities following right and left hemispherectomy. Cortex. 1973;9:266–274. doi: 10.1016/s0010-9452(73)80004-8. [DOI] [PubMed] [Google Scholar]
- Hart K, Faust D. Prediction of the effects of mild head injury: A message about the Kennard principle. Journal of Clinical Psychology. 1988;44:780–782. doi: 10.1002/1097-4679(198809)44:5<780::aid-jclp2270440519>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The effect of early and late brain injury upon test scores, and the nature of normal adult intelligence. Proceedings of the American Philosophical Society. 1942;85:275–292. [Google Scholar]
- Hécaen H. H. L. Teuber et la fondation de la neuropsychologie experimentale. Neuropsychologia. 1979;17:199–124. doi: 10.1016/0028-3932(79)90003-4. [DOI] [PubMed] [Google Scholar]
- Held R. Hans-Lukas Teuber. Neuropsychologia. 1979;17:117–118. doi: 10.1016/0028-3932(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Hellal P, Lorch MP. The validity of Barlow’s 1877 case of acquired childhood aphasia: Case notes versus published reports. Journal of the History of the Neurosciences. 2007;16:378–394. doi: 10.1080/09647040600653931. [DOI] [PubMed] [Google Scholar]
- Hicks SP, D’Amato CJ. Motor-sensory behavior after hemispherectomy in newborn and mature rats. Experimental Neurology. 1970;29:416–438. doi: 10.1016/0014-4886(70)90069-5. [DOI] [PubMed] [Google Scholar]
- Himwich WA. In memoriam: Margaret Kennard 1899–1976. Biological Psychiatry. 1977;12:603–605. [PubMed] [Google Scholar]
- Hoff HE. John Fulton’s contribution to neurophysiology. Journal of the History of Medicine and Allied Sciences. 1962;XVII:16–37. doi: 10.1093/jhmas/xvii.1.16. [DOI] [PubMed] [Google Scholar]
- Isaacson RL. The myth of recovery from early brain damage. In: Ellis NR, editor. Aberrant development in infancy: Human and animal studies. Lawrence Erlbaum Associates; Hillsdale, NJ: 1975. pp. 1–25. [Google Scholar]
- Jacobsen CF. A study of cerebral function in learning. The frontal lobes. Journal of Comparative Neurology. 1931;52:271–340. [Google Scholar]
- Jacobsen CF, Taylor FV, Haslerud GM. Restitution of function after cortical injury in monkeys. The American Journal of Physiology. 1936;116:85–86. Proceedings of the American Physiological Society Forty-Eighth Annual Meeting. [Google Scholar]
- Johnson DA. Developmental aspects of recovery of function following septal lesions in the infant rat. Journal of Comparative and Physiological Psychology. 1972;78:331–348. doi: 10.1037/h0032174. [DOI] [PubMed] [Google Scholar]
- Keller EF. A feeling for the organism: The life and work of Barbara McClintock. 1984. [Google Scholar]
- Kennard MA. Age and other factors in motor recovery from precentral lesions in monkeys. American Journal of Physiology. 1936;115:138–146. [Google Scholar]
- Kennard MA. Reorganization of motor function in the cerebral cortex of monkeys deprived of motor and premotor areas in infancy. Journal of Neurophysiology. 1938;1:477–496. [Google Scholar]
- Kennard MA. Relation of age to motor impairment in man and in subhuman primates. Archives of Neurology and Psychiatry. 1940;44:377–397. [Google Scholar]
- Kennard MA. Reactions of monkeys of various ages to partial and complete decortication. Journal of Neurophysiology and Experimental Neurology. 1944;3:289–310. [Google Scholar]
- Kennard MA, Fulton JF. Age and reorganization of central nervous system. Journal of the Mount Sinai Hospital. 1942;9:594–606. [Google Scholar]
- Kennard MA, Fulton JF. The localizing significance of spasticity, reflex grasping, and the signs of Babinski and Rossolimo. Brain. 1933;56:213–225. [Google Scholar]
- Kennard MA, Fulton JF, Gutiérrez-Mahoney CG. Otfrid Foerster 1873–1941 An appreciation. Journal of Neurophysiology. 1942;5:1–17. [Google Scholar]
- Kennard MA, McCulloch WS. Motor response to stimulation of cerebral cortex in absence of areas 4 and 6 (macaca mulatta) Journal of Neurophysiology. 1943;6:181–189. [Google Scholar]
- Kennard MA, Nims LF. Effect on electroencephalogram of lesions of cerebral cortex and basal ganglia in macaca mulatta. Journal of Neurophysiology. 1942;5:335–348. [Google Scholar]
- Kennard MA, Spencer S, Fountain G. Hyperactivity in monkeys following lesions of the frontal lobes. Journal of Neurophysiology. 1941;4:512–524. [Google Scholar]
- Kennard MA, Viets HR, Fulton JF. The syndrome of the premotor cortex in man: Impairment of skilled movements, forced grasping, spasticity, and vasomotor disturbance. Brain. 1934;57:69–84. [Google Scholar]
- Kennard MA, Watts JW. The effect of section of the corpus callosum on the motor performance of monkeys. Journal of Nervous and Mental Disease. 1934;79:158–169. [Google Scholar]
- Koehler PJ. The correspondence between Bernard Brouwer and John Fulton (1930–1940) Journal of the History of the Neurosciences. 2003;12:56–69. doi: 10.1076/jhin.12.1.56.13781. [DOI] [PubMed] [Google Scholar]
- Koehler PJ. “The orang lives almost next door” The correspondence between John Fulton (New Haven) and Willem Verhaart (Java) Journal of the History of the Neurosciences. 2006;15:5–16. doi: 10.1080/096470490889376. [DOI] [PubMed] [Google Scholar]
- Koehler PJ, Bruyn GW. Bernard Brouwer’s lecture tours in the United States (1926–1933) Archives of Neurology. 2003;60:1475–1481. doi: 10.1001/archneur.60.10.1475. [DOI] [PubMed] [Google Scholar]
- Kohn B, Dennis M. Selective impairments of visuo-spatial abilities in infantile hemiplegics after right cerebral hemidecortication. Neuropsychologia. 1974;12:505–512. doi: 10.1016/0028-3932(74)90080-3. [DOI] [PubMed] [Google Scholar]
- Kolb B, Cioe J, Wishaw IQ. Is there an optimal age for recovery from motor cortex lesions? I. Behavioral and anatomical sequelae of bilateral motor cortex lesions in rats on postnatal days 1, 10, and in adulthood. Brain Research. 2000;882:62–74. doi: 10.1016/s0006-8993(00)02828-6. [DOI] [PubMed] [Google Scholar]
- Kolb B, Wishaw IQ. Plasticity in the neocortex: Mechanisms underlying recovery from early brain damage. Progress in Neurobiology. 1989;32:235–276. doi: 10.1016/0301-0082(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Lackner JR. Hans-Lukas Teuber: A remembrance. Neuropsychological Review. 2009;19:4–7. doi: 10.1007/s11065-009-9084-3. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Hopkins DA. Bilateral pyramidal lesions in infant rhesus monkeys. Brain Research. 1970;24:543–544. doi: 10.1016/0006-8993(70)90198-8. [DOI] [PubMed] [Google Scholar]
- Levy S, Kennard MA. The EEG pattern of patients with psychologic disorders of various ages. Journal of Nervous and Mental Disease. 1953;118:416–428. doi: 10.1097/00005053-195311000-00005. [DOI] [PubMed] [Google Scholar]
- Lidzba K, Wilke M, Staudt M, Krägeloh-Mann I. Early plasticity versus early vulnerability: the problem of heterogeneous lesion types. Brain. 2009 doi: 10.1093/brain/awp197. doi:10.1093/brain/awp197. [DOI] [PubMed] [Google Scholar]
- McCulloch WS. Joannes Gregorius Dusser De Barenne [1885–1940] Yale Journal of Biology and Medicine. 1940;12:742–746. [PMC free article] [PubMed] [Google Scholar]
- Milner B. Sparing of language functions after early unilateral brain damage. Neurosciences Research Program Bulletin. 1974;12:213–217. [PubMed] [Google Scholar]
- Molfese DL, Freeman RB, Palermo DS. The ontogeny of brain lateralization for speech and nonspeech stimuli. Brain & Language. 1975;2:356–368. doi: 10.1016/s0093-934x(75)80076-9. [DOI] [PubMed] [Google Scholar]
- Murphy EH, Stewart DL. Effects of neonatal and adult striate lesions on visual discrimination in the rabbit. Experimental Neurology. 1974;42:89–96. doi: 10.1016/0014-4886(74)90008-9. [DOI] [PubMed] [Google Scholar]
- Nash DJ. Effects of neonatal irradiation and genotype on postnatal growth in mice. Biologia Neonatorum. 1971;18:17–28. doi: 10.1159/000240342. [DOI] [PubMed] [Google Scholar]
- Nonneman AJ, Isaacson RL. Task dependent recovery after early brain damage. Behavioral Biology. 1973;8:143–172. doi: 10.1016/s0091-6773(73)80016-1. [DOI] [PubMed] [Google Scholar]
- Ogilvie M, Harvey J, editors. The biographical dictionary of women in science. Routledge; New York: 2000. [Google Scholar]
- Piotrowska N, Winkler PA. Otfrid Foerster, the great neurologist and neurosurgeon from Breslau (Wroclaw): his influence on early neurosurgeons and legacy to present-day neurosurgery. Journal of Neurosurgery. 2007;107:451–456. doi: 10.3171/JNS-07/08/0451. [DOI] [PubMed] [Google Scholar]
- Pressman JD. Last resort. Psychosurgery and the limits of medicine. Cambridge University Press; Cambridge, UK: 1998. [Google Scholar]
- Pribram KH. Hans-Lukas Teuber (1916–1977) American Journal of Psychology. 1977;90:705–707. [PubMed] [Google Scholar]
- Pryse-Phillips W. Clinical neurology. 2nd edition Oxford University Press; Oxford, UK: 2003. [Google Scholar]
- Pullela P, Raber R, Pfankuch T, Ferriero DH, Claus C, Koh S-E, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyperactivity and delayed cognitive impairments. Developmental Neuroscience. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Richards W. Obituary H.-L. Teuber (1916–1977) Vision Research. 1978;18:357–359. doi: 10.1016/0042-6989(78)90175-x. [DOI] [PubMed] [Google Scholar]
- Rudel RG, Teuber H-L. Spatial orientation in normal children and in children with early brain injury. Neuropsychologia. 1971;9:401–407. doi: 10.1016/0028-3932(71)90004-2. [DOI] [PubMed] [Google Scholar]
- Rudel RG, Teuber H-L, Twitchell TE. Levels of impairment of sensori-motor functions in children with early brain damage. Neuropsychologia. 1974;12:95–108. doi: 10.1016/0028-3932(74)90031-1. [DOI] [PubMed] [Google Scholar]
- Sarikcioglu L. Historical note: Otfrid Foerster (1873–1941): One of the distinguished neuroscientists of his time. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78:650. doi: 10.1136/jnnp.2006.112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE. Anomalous axonal connections implicated in sparing and alteration of function after early lesions. Neurosciences Research Program Bulletin. 1974;12:222–228. [PubMed] [Google Scholar]
- Schneider GE. Is it really better to have your brain lesion early? A revision of the “Kennard principle”. Neuropsychologia. 1979;17:557–583. doi: 10.1016/0028-3932(79)90033-2. [DOI] [PubMed] [Google Scholar]
- Schneider GE, Jhaveri SR. Neuroanatomical correlations of spared or altered function after brain lesions in the newborn hamster. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. Academic Press; New York: 1974. pp. 65–109. [Google Scholar]
- Sellar WC, Yeatman RJ. 1066 and all that: A memorable history of England, comprising all the parts you can remember, including 103 good things, 5 bad things and 2 genuine dates. Methuen; London: 1930. republished by The Folio Society 1990. [Google Scholar]
- Stein DG. Some variables influencing recovery of function after central nervous system lesions in the rat. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. Academic Press; New York: 1974. pp. 373–428. [Google Scholar]
- Stern Y, editor. Cognitive reserve. Psychology Press; New York: 2006. [Google Scholar]
- Tan T-C. Otfrid Foerster (1873–1941) Journal of Neurology. 2003;250:513–514. doi: 10.1007/s00415-003-0945-z. [DOI] [PubMed] [Google Scholar]
- Tan T-C, Black PM. The contributions of Otfrid Foerster (1873–1941) to neurology and neurosurgery. Neurosurgery. 2001;49:1231–1236. doi: 10.1097/00006123-200111000-00038. [DOI] [PubMed] [Google Scholar]
- Teuber H-L, Angle CR. Mental retardation after early trauma to the brain: Some issues in search of facts. In: Bering EA, editor. Physical trauma as an etiological agent in mental retardation. US Government Printing Office; Washington, DC: 1971. pp. 7–27. [Google Scholar]
- Teuber H-L. Recovery of function after lesions of the central nervous system: history and prospects. Neurosciences Research Program Bulletin. 1974;12:197–211. [PubMed] [Google Scholar]
- Thompson CI, Harlow HF, Blomquist AJ, Schiltz KA. Recovery of function following prefrontal lobe damage in rhesus monkeys. Brain Research. 1971;35:37–48. doi: 10.1016/0006-8993(71)90593-2. [DOI] [PubMed] [Google Scholar]
- Twitchell T. Development of motor coordination in the presence of cerebral lesions. Neurosciences Research Program Bulletin. 1974;12:565–569. [Google Scholar]
- Verhaart WJC, Kennard MA. Corticofugal degeneration following thermocoagulation of areas 4, 6 and 4-s in macaca mulatta. Journal of Anatomy. 1940;74:239–254. [PMC free article] [PubMed] [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Archives of Neurology. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Ward AA, Kennard MA. Effect of cholinergic drugs on recovery of function following lesions of the central nervous system in monkeys. Yale Journal of Biology and Medicine. 1942;15:189–229. [PMC free article] [PubMed] [Google Scholar]
- Webb C, Rose FD, Johnson DA, Attree EA. Age and recovery from brain injury: Clinical opinions and experimental evidence. Brain Injury. 1996;10:303–310. doi: 10.1080/026990596124476. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn: Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–647. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Woods BT, Teuber H-L. Early onset of complementary specialization of cerebral hemispheres in man. Transcripts of the American Neurological Association. 1973;98:113–117. [PubMed] [Google Scholar]