Abstract
Background
Rats with extended daily cocaine access show escalating cocaine self-administration and behavioral signs of dependence. Regulation of glutamatergic transmission by metabotropic glutamate receptors (mGluR) has emerged as a mechanism in the addictive actions of drugs of abuse. We examined here whether neuroadaptive dysregulation of mGluR function is a factor in escalating cocaine self-administration.
Methods
Rats with 1 h daily cocaine access (short access, ShA) vs. 6 h access (long access, LgA) were tested for differences in the effects of the mGluR2/3 agonist LY379268 and the mGluR5 antagonist MTEP on cocaine-reinforced progressive-ratio (PR) responding and differences in expression levels and functional activity of mGluR2/3 and mGluR5.
Results
The LgA groups showed higher PR breakpoints than ShA groups. LY379268 (0-3 mg/kg, s.c.) dose-dependently lowered breakpoints in the LgA group but reduced breakpoints only at 3 mg/kg in the ShA group. Consistent with this behavioral effect, functional mGluR2/3 activity was significantly elevated following LgA cocaine exposure. MTEP (0-3 mg/kg, i.p.) reduced breakpoints in the ShA group only. LgA cocaine exposure was associated with decreased mGluR5 expression, accompanied by reduced functional mGluR5 activity in the nucleus accumbens. A downward trend developed in mGluR5 protein expression in the medial prefrontal cortex and hippocampus.
Conclusion
Functional upregulation of mGluR2/3 and downregulation of mGluR5 are likely factors in the transition to cocaine dependence. The differential behavioral effects of LY379268 and MTEP in rats with a history of long access to cocaine have implications for the treatment target potential of mGluR2/3 and mGluR5.
Keywords: cocaine, self-administration, compulsive drug taking, metabotropic glutamate receptor, neuroadaptation
Introduction
Drug addiction is a chronically relapsing disorder characterized by compulsive drug use and loss of control over intake (1). Although the neural substrates mediating cocaine reinforcement are well understood, information on the neural mechanisms responsible for the transition from controlled to compulsive cocaine use is limited. Extended daily access to cocaine results in escalation of cocaine intake (2, 3) and provides an effective model for studying the neurobiological processes underlying the transition to compulsive drug use (4).
Regulation of glutamatergic transmission by metabotropic glutamate receptors (mGluR) has received growing attention with respect to a role in drug addiction (5-11). Evidence for a role of mGluR in the pharmacological and behavioral effects of cocaine exists, particularly for the mGlu2/3 and mGlu5 subtypes (12). mGluR2/3 is located at the pre- and perisynaptic level and negatively modulates glutamate transmission by reducing glutamate release or synaptic availability (13-15). mGluR2/3 agonists reverse neurobehavioral consequences of acute and chronic cocaine administration (12) and attenuate cue-induced reinstatement of cocaine seeking (10, 16, 17). mGluR5 is located postsynaptically and positively modulates glutamate transmission by increasing neural excitability (13). Genetic disruption or pharmacological inhibition of mGluR5 attenuates the reinforcing effects of cocaine (5, 8, 9, 18).
Regulation of neural excitability by mGluR2/3 and mGluR5 is susceptible to neuroadaptive disruption, which may be a critical factor in the transition to dependence. Supporting this hypothesis, mGluR2/3 signaling is blunted during withdrawal from repeated noncontingent cocaine administration, reflected by a decrease in G-protein coupling and a decrease in the capacity to regulate glutamate transmission (19, 20). Noncontingent repeated cocaine treatment also attenuates Group I (i.e., mGluR1/mGluR5) mGluR-mediated glutamate release in the nucleus accumbens (NAc) and behavioral activation (21). In rats with a history of escalated cocaine self-administration, increased anxiety-like behavior and increased sensitivity to the anxiolytic-like effects of LY379268, a potent mGlu2/3 agonist, have been reported (22). To test the hypothesis that escalated cocaine self-administration is associated with altered mGluR function, the consequences of cocaine exposure contingencies leading to escalated vs. non-escalated cocaine intake were examined for (i) differential changes in the effects of pharmacological agents that “dampen” glutamatergic transmission via an agonist action at mGluR2/3 (LY379268) or an antagonist action at mGluR5 (MTEP) on the reinforcing magnitude of cocaine, and (ii) differential changes in mGluR2/3 and mGluR5 expression levels or functional activity determined by immunoblotting and measures of G-protein coupling.
Methods and Materials
Animals
Male Wistar rats (200-250 g, Charles River, Wilmington, MA) were maintained on a 12 h/12 h light/dark cycle (lights off at 18:00). Food and water were available ad libitum. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Cocaine self-administration training
Rats (n = 55) were surgically prepared with indwelling silastic catheters. Cocaine self-administration training began 7-10 days after surgery in daily 1 h sessions. Sessions were initiated by extension of both the active and inactive levers. Responses at the active lever were reinforced on a fixed-ratio 1 (FR1) timeout (TO) 20 s schedule by a cocaine injection (0.25 mg/injection for 4 s). After reaching the training criterion (±10% over three consecutive sessions), rats were divided into cocaine short access (ShA, 1 h daily session) and long access (LgA, 6 h daily session) groups. Self-administration during this “escalation phase” was conducted 5-6 days/week until completion of 22 sessions (2).
Behavioral and drug testing procedures
The effects of LY379268 (0-3 mg/kg, s.c., Lilly Research Laboratories, Indianapolis, IN) or MTEP (0-3 mg/kg, i.p., Merck Research Laboratories, Rahway, NJ) were examined on breakpoints for cocaine self-administration on a progressive-ratio (PR) schedule of reinforcement. Doses of LY379268 and MTEP were chosen based on the range of doses previously shown not to interfere with motor performance or operant responding (10, 17). The PR schedule response requirement began with an FR1 and increased according to the following equation: responses/injection = [5 × e (injection number × 0.2)] − 5 (23). The session ended when an animal failed to complete the response requirement within 1 h. The breakpoint was defined as the final ratio completed before the 1 h period during which no infusion was earned. Twenty-three rats (LgA, n = 12; ShA, n = 11) were tested with LY379268, and 18 rats (LgA, n = 9; ShA, n = 9) were tested with MTEP. PR tests were conducted such that 3 days of regular LgA and ShA sessions separated each test. Doses of each drug were tested in a counterbalanced order across rats. The effect of vehicle administration on PR performance was measured twice in each rat, once before and once interspersed with drug testing. Drugs were administered 30 min (LY379268) or 60 min (MTEP) before the test sessions.
Brain dissection
Seventeen hours (LgA) or 22 h (ShA) after the last cocaine training session, rats that were randomly selected from the ShA and LgA groups (n = 6/group) were deeply anesthetized and decapitated, and their brains were rapidly removed and dissected into coronal sections. A group of cocaine-naive rats, matched for age and housing conditions to ShA and LgA animals, were also included. Brain regions of interest—sampled using a 15-gauge tissue punch—included the medial prefrontal cortex (mPFC, 3.20 to 1.80 mm relative to bregma), NAc (1.70 to 0.70 mm), bed nucleus of the stria terminalis (BNST, 0.12 to −0.96 mm), central nucleus of the amygdala (CeA, −1.44 to −2.70 mm), hippocampus (HIP, −1.90 to −3.12 mm), and ventral tegmental area (VTA, −5.20 to −6.30 mm). Brain punches were immediately frozen on dry ice and stored at −80°C.
Protein preparation and mGluR immunoblotting
Brain tissue punches were homogenized and centrifuged in lysis buffer (140 mM NaCl, 50 mM Tris-HCl, 0.5% Triton-100, pH 7.6) to extract the membrane protein. The preparations were saved and stored at −80°C. Samples (30 μg) were loaded onto Tris-Glycin Gel and run for 30 min at 350 V. Protein was transferred onto a polyvinylidene fluoride membrane (0.45 μm) using a semi-dry transfer system. Membranes were then blocked for 30 min at room temperature followed by 30 min incubation with anti-mGlu2/3 (1:5000; Upstate Biotechnology, Waltham, MA), anti-mGlu5 (1:5000; Upstate Biotechnology, Waltham, MA), and β-actin (1:3000; Sigma, St. Louis, MO). Immunoblots were then incubated for 30 min at room temperature with horseradish peroxidase-linked secondary antibody (1:5000 or 1:10000, Jackson Immuno Research, West Grove, PA). Levels of actin were detected using mouse anti-rabbit polyclonal IgG (1:3000, goat anti-rabbit HRP-conjugated, Pierce, Rockford, IL). Immunoblots were developed by chemiluminescent substrate and quantified using Quantity One software (Bio-Rad, Hercules, CA).
Membrane preparation and [35S]GTPγS binding assay
Brain punches were homogenized and centrifuged in 10 volumes (w/v) of 10% sucrose. The pellet was then resuspended in 10 volumes of H2O solution and incubated for 15 min at 37°C. The homogenate was centrifuged at 14800 rpm for 20 min at 4°C. The resulting pellet was resuspended in 10 volumes of HEPES solution and stored at −80°C until the assay was performed. Assay compounds were added along with 10 μM guanosine diphosphate, 10 μg membrane protein, 0.5 U of adenosine deaminase (type X, Sigma, St. Louis, MO), various concentrations of the mGluR2/3 agonist LY379268 (0.01-100 μM) or glutamate (100 μM) combined with various concentrations of the mGluR5 antagonist MTEP (0.01-100 μM), and 0.05 nM [35S]GTPγS (250 μCi, Perkin-Elmer, Waltham, MA) to achieve a total volume of 200 μl in HEPES assay buffer (20 mM HEPES, 10 mM MgCI2, 100 mM NaCl, 0.25 mM EGTA, pH 7.4). Basal binding was measured in the absence of agonist or antagonist, and nonspecific binding was measured in the presence of 10 μM unlabeled GTPγS. The compound was then incubated at 37°C for 1 h, and the reaction was terminated by centrifuge (14500 rpm) at 4°C for 20 min. The supernatant was removed, and the pellet was carefully washed and re-centrifuged twice at 4°C for 20 min. The resulting pellet was dissolved in 100 μl of 2 M NaOH and transferred to a scintillation vial containing 2 ml scintillation cocktail. Radioactivity was measured by liquid scintillation spectrophotometry.
Statistical analysis
Behavioral, Western blot, and [35S]GTPγS binding data were analyzed by analysis of variance (ANOVA) using one-way or mixed factorial designs as appropriate, followed by Fisher’s Least Significant Difference (LSD) tests or simple-effects ANOVA. Differences in self-administration between the first and last session of the escalation phase and differences in baseline PR performance between the cocaine ShA and LgA groups were analyzed by Student’s t-test.
Results
Escalation of cocaine self-administration
Cocaine self-administration progressively increased in the LgA group. Escalation of cocaine intake was observed beginning with session 7 in terms of responding over the entire 6 h session (p < 0.01 vs. session 1 following one-way ANOVA, F21,546 = 8.43, p < 0.001, Fig. 1A left panel) and session 5 in terms of first-hour responding (p < 0.01 vs. session 1 following one-way ANOVA, F21,546 = 7.40, p < 0.001, Fig. 1A right panel) until session 22. In contrast, cocaine self-administration in the ShA rats remained stable for 22 sessions. Escalation of cocaine intake in the LgA group compared with the ShA group was confirmed when comparing the first hour of cocaine self-administration (i.e., LgA 1 h vs. ShA, Fig. 1A right panel) over the course of the escalation period. This was reflected by a significant difference between the LgA and ShA groups (F1,53 = 11.94, p < 0.01) and a significant time (session number) × group interaction (F21,1113 = 4.36, p < 0.001, Fig. 1A right panel). Compared with the ShA group, the increase in cocaine intake by the LgA group was observed from session 5 to 7 and session 9 to 22 (simple effects analysis, p < 0.01, Fig. 1A right panel). Statistical comparison of cocaine intake during the first session vs. final session of the escalation period separately for the behavioral and immunoblotting/GTPγS binding experiments confirmed increased cocaine intake in the LgA group only (behavioral experiment: t20 = 2.62, p < 0.05 for the first hour, t20 = 2.58 for 6 h, Fig. 1B left panel; immunoblotting/GTPγS binding experiments: t5 = 2.32, p < 0.05 for the first hour, t5 = 2.30, p < 0.05 for 6 h, Fig. 1B right panel).
Figure 1.
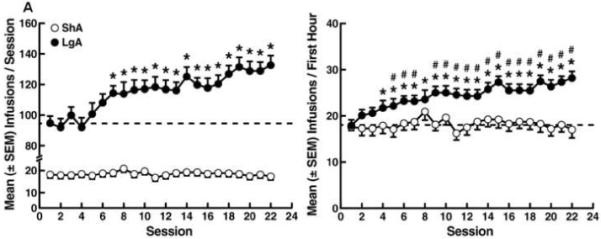
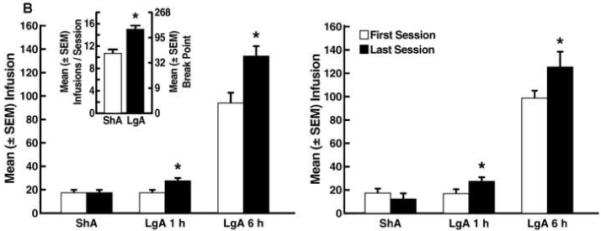
Cocaine self-administration on a fixed-ratio schedule of reinforcement during the escalation phase under short-access (ShA) and long-access (LgA) conditions. (A) Time-course of cocaine escalation during the entire 6 h session (left panel) and the first hour of the sessions (right panel). Data are expressed as mean ± SEM of the number of infusions from the entire 6 h sessions and the first hour of the sessions. *p < 0.05, compared with session 1 (one-way repeated-measures AVOVA). #p < 0.05, compared with ShA animals (mixed-factorial two-way repeated-measures ANOVA followed by Fisher’s LSD and simple-effects post hoc tests). (B) Number of infusions during the first vs. last self-administration session for the behavioral (left panel) and immunoblotting/GTPγS binding experiments (right panel). (Inset) Cocaine self-administration on a progressive-ratio schedule of reinforcement in rats with a history of short access and long access to cocaine self-administration. Data are expressed as the mean ± SEM of the number of infusions during the first hour of the sessions and the entire 6 h sessions. *p < 0.05, compared with session 1 (paired Student’s t-test).
Effects of LY379268 and MTEP on cocaine reinforcement: progressive-ratio tests
Following escalation, rats trained under LgA conditions showed significantly increased breakpoints compared with the ShA group in the PR tests (unpaired t-test: t41 = 4.16, p < 0.05, Fig. 1B inset). In LgA rats, LY379268 dose-dependently lowered the breakpoint for cocaine (Fisher’s LSD post hoc test: p < 0.05 vs. 0 mg/kg; ANOVA: F3,46 = 8.14, p < 0.05, Fig. 2A left panel). In the ShA group, however, only the 3.0 mg/kg dose lowered breakpoints (p < 0.05, Fisher’s LSD following ANOVA: F3,46 = 8.14, p < 0.05, Fig. 2A left panel). In contrast to LY379268, MTEP failed to modify PR performance in LgA rats (Fig. 2A right panel). In ShA rats, however, MTEP dose-dependently lowered breakpoints (F3,34 = 3.40, p < 0.05) at doses of 1.0 and 3.0 mg/kg (p < 0.001, Fisher’s LSD test, Fig. 2A right panel). Additionally, representative responding records also revealed that LgA rats had higher breakpoints compared with ShA rats, with no distinct differences in the response pattern between LgA and ShA rats (Fig. 2B).
Figure 2.
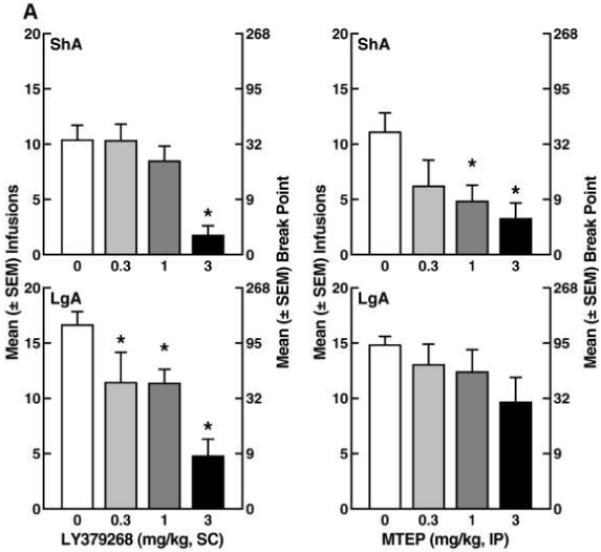

(A) Effects of mGlu2/3 agonist LY379268 and mGlu5 antagonist MTEP on cocaine self-administration on a progressive-ratio schedule of reinforcement by rats under short-access and long-access conditions. The left y-axis indicates the number of infusions per session, and the right y-axis indicates the corresponding breakpoint. Rats were pretreated with LY379268 (0, 0.3, 1.0, and 3.0 mg/kg, s.c., 30 min before the test) or MTEP (0, 0.3, 1.0, and 3.0 mg/kg, i.p., 60 min before the test) in a counterbalanced design. Data are expressed as the mean ± SEM of the number of infusions/session. *p < 0.05, compared with vehicle within each group (one-way ANOVA followed by Fisher’s LSD post hoc test). (B) Representative records of LgA and ShA rats illustrating the pattern of responding during the progressive-ratio tests.
Effects of LgA vs. ShA cocaine self-administration on mGluR protein levels
Following escalation (for behavioral data, see Fig. 1B right panel), LgA rats showed a significant reduction (p < 0.05) in mGluR5 protein levels in the NAc (Fisher’s LSD test following ANOVA: F2,17 = 3.868, p < 0.05, Fig. 4). Although apparent changes in mGluR5 protein were observed in the mPFC (F2,17 = 3.327, 0.05 < p < 0.1) and HIP (F2,17 = 3.560, 0.05 < p < 0.1), with an upward trend in the ShA group and a downward trend in the LgA group, the NAc was the only brain region where a significant decrease in mGluR5 levels was observed compared with naive rats. No significant changes in mGluR2/3 protein levels were observed in any of the sampled brain regions. Only dimer data were used for statistical analysis because dimeric receptor proteins represent functional mGluR2/3 and mGlu5R (24).
Figure 4.
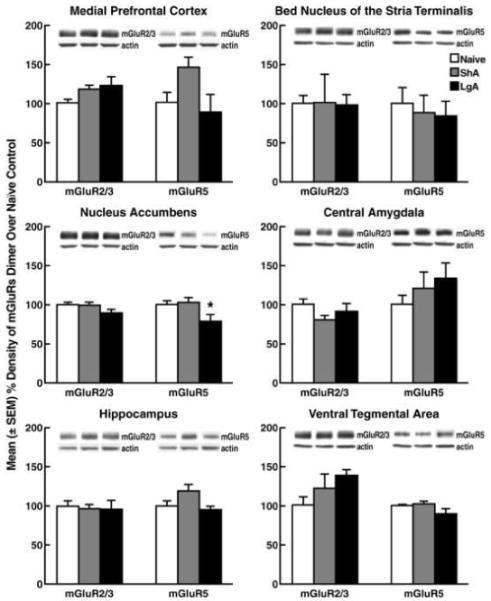
Protein levels of mGluR2/3 and mGluR5 in rats under short-access and long-access cocaine self-administration conditions assessed by Western blot analysis. The bar graph shows the mean ± SEM percent change from cocaine-naive rats for the dimers of mGluR2/3 and mGluR5. Representative Western blots of mGluR2/3, mGluR5 dimer, and the loading control (β-actin) are presented for each group corresponding to the lower bar graphs. *p < 0.05, compared with cocaine-naive rats (one-way ANOVA followed by Fisher’s LSD post hoc test).
Effects of LgA vs. ShA cocaine self-administration on GTPγS binding
LY379268 concentration-dependently stimulated [35S]GTPγS binding in all brain regions tested (Fig. 5). Significant increases in the ability of LY379268 to increase [35S]GTPγS binding were found in the mPFC of LgA rats compared with both ShA and cocaine-naive rats (p < 0.001, Fisher’s LSD test following ANOVA: F2,108 = 20.13, p < 0.001), BNST (p < 0.001, Fisher’s LSD test following ANOVA: F2,108 = 33.26, p < 0.001), CeA (p < 0.001, Fisher’s LSD test following ANOVA: F2,108 = 52.43, p < 0.001), and HIP (p < 0.001, Fisher’s LSD test following ANOVA: F2,108 = 38.59, p < 0.001). Increases in [35S]GTPγS binding rate stimulated by higher concentrations of LY379268 were also found in the VTA of LgA rats compared with ShA rats (10-100 μM, p < 0.01) and cocaine-naive rats (100 μM, p < 0.01; simple effects analysis following overall ANOVA: F2,108 = 6.43, p < 0.01). Only scattered and small changes in [35S]GTPγS binding rate were detected in ShA rats compared with naive rats, with increases at 1 μM LY379268 in the HIP (p < 0.01, simple effects analysis) and decreases at 10-100 μM LY379268 in the CeA (p < 0.01, simple effects analysis).
Figure 5.
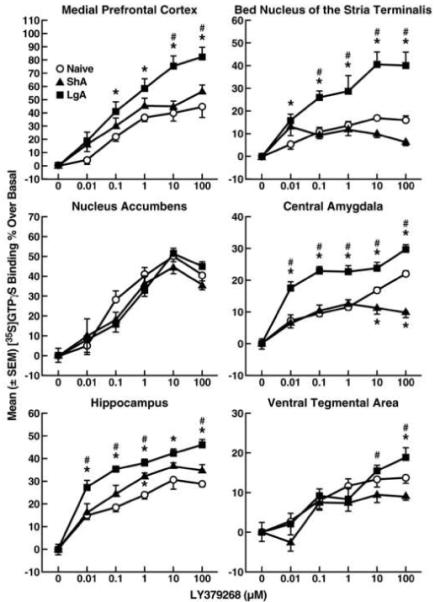
Functional coupling of mGluR2/3 to G-protein in rats under short-access and long-access cocaine self-administration conditions. The mGluR2/3 agonist LY379268 concentration-dependently stimulated [35S]GTPγS binding. Data are expressed as the mean ± SEM of at least three experiments that were each performed in duplicate. *p < 0.05, compared with cocaine-naive rats. #p < 0.05, compared with cocaine ShA rats (two-way ANOVA and simple-effects ANOVA at each dose).
MTEP alone did not modify [35S]GTPγS binding (−7.3 ± 2.9% for 100 μM MTEP). However, MTEP concentration-dependently decreased glutamate-induced [35S]GTPγS binding in all brain regions tested (Fig. 6). Inhibition of [35S]GTPγS binding profiles remained essentially the same in the three cocaine history groups in all brain regions, with the exception of small differences in the mPFC (F2,108 = 3.52, p < 0.05) and NAc (F2,108 = 5.86, p < 0.05).
Figure 6.
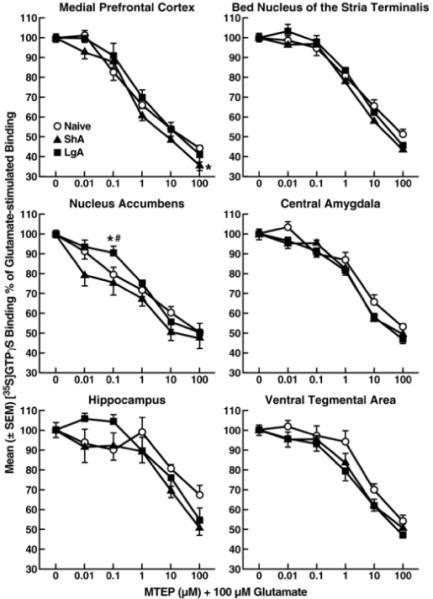
Functional coupling of mGluR5 to G-proteins in rats under short-access and long-access cocaine self-administration conditions. The mGluR5 antagonist MTEP concentration-dependently attenuated 100 μM glutamate-stimulated [35S]GTPγS binding. Data are expressed as the mean ± SEM of at least three experiments that were each performed in duplicate. *p < 0.05, compared with cocaine-naive rats. #p < 0.05, compared with ShA rats (two-way ANOVA and simple-effects ANOVA at each dose).
Discussion
In the present study, two agents known to “dampen” excitatory glutamatergic transmission by activating pre- and perisynaptic autoregulatory mGluR2/3 (LY379268) or by antagonizing excitatory postsynaptic mGluR5 (MTEP) attenuated the reinforcing effects of cocaine on a PR schedule. In rats showing escalated cocaine intake, the ability of the mGluR2/3 agonist LY379268 to diminish cocaine-maintained PR performance increased, whereas the effect of the mGluR5 antagonist MTEP was blunted. This differential shift in the profile of actions of LY379268 and MTEP after cocaine escalation was associated with distinct changes in mGluR2/3 and mGluR5, reflected by enhanced functional mGluR2/3 activity and decreased mGluR5 expression, with overall substantially more pronounced alterations in mGluR2/3 function.
Prolonged daily access to cocaine produced marked escalation of cocaine self-administration associated with increased motivation to “work” for cocaine as previously reported (2, 25, 26). Pharmacological manipulation of either mGluR2/3 or mGluR5 lowered the breakpoints on a PR schedule, indicating the reduced reinforcing value of cocaine. Extending previous findings showing that mGluR2/3 and mGluR5 manipulations interfere with cocaine self-administration on FR schedules of reinforcement (10, 27, 28), this observation strengthens the hypothesis that both mGluR2/3 and mGluR5 participate in the mediation of the reinforcing effects of cocaine. Importantly, however, neuroadaptation occurred as a result of cocaine self-administration under long-access conditions, leading to an increased ability of LY379268 and a decreased ability of MTEP to modify PR responding. These findings implicate neuroplasticity in mGluR2/3 and mGluR5 function in the transition to dependence.
The differential shift in efficacy of LY379268 and MTEP associated with a history of escalated cocaine intake provides evidence for dysregulation of both mGluR2/3 and mGluR5 function that may reflect neuroplasticity in glutamatergic transmission relevant for the development of compulsive cocaine use. Consistent with earlier reports showing increased sensitivity to the anxiolytic-like actions of LY379268 in cocaine-escalated rats (22) and enhanced effects of mGluR2/3 activation on inhibition of excitatory glutamate currents in VTA and NAc neurons following chronic morphine (29, 30), the present results revealed significantly increased sensitivity to LY379268 following cocaine self-administration under long-access conditions. A corresponding significant increase in G-protein binding rate was observed in cocaine-escalated rats (vs. naive and ShA rats) in the mPFC, BNST, CeA, and HIP that likely contributes to the enhanced efficacy of LY379268 in these animals. The mechanism leading to the increased functional activity of mGluR2/3 remain unclear, but the following sequence of events may be proposed. mGlu2/3R negatively modulates presynaptic and glial glutamate release (20) and inhibits the cystine-glutamate exchanger (31), thereby decreasing extracellular glutamate. Glutamate levels increase during cocaine self-administration (32), presumably leading to autoregulatory activation of mGluR2/3. Considering the stable pattern of daily drug intake observed in ShA rats, this increase in mGluR2/3 activity is likely transient, returning to baseline before the onset of the next session, suggested by the absence of increases in either mGluR2/3 function or expression levels in ShA rats. In contrast, upregulated mGluR2/3 activity in response to prolonged high glutamate levels associated with long-access cocaine self-administration presumably fails to “re-adapt” and may ultimately persist (22), resulting in substantially reduced extracellular glutamate levels. Two sets of findings support this possibility. First, extracellular glutamate was recently shown to decrease more profoundly after long-access than short-access cocaine self-administration (Sidhpura, Kerr, Martin-Fardon, Weiss, unpublished observations). Second, administration of N-acetylcysteine, a cysteine prodrug that activates cysteine-glutamate exchange and restores extracellular glutamate levels, prevented long-access-induced escalation in cocaine intake but had no effect on cocaine intake under short-access conditions. Furthermore, N-acetylcysteine only altered cocaine intake once escalation was evident in rats pretreated with saline, suggesting that increased sensitivity of mGluR2/3 develops over repeated cocaine sessions and thus exerts a progressively more profound suppressant effect on extracellular glutamate in LgA rats (33).
Functional mGluR2/3 activity was most profoundly upregulated in the BNST, CeA, and HIP, suggesting that the increased efficacy of LY379268 in cocaine-escalated rats is linked to upregulation of mGluR2/3 function in these brain regions and, more generally, implicating these brain areas as sites of action for the attenuation of cocaine reinforcement by mGluR2/3 activation. These brain regions are components of the circuitry regulating drug reinforcement and incentive motivation (34), but they also mediate anxiety-like behavior and behavioral responses to stress. Thus, the dysregulation of mGluR2/3 function in these brain regions may play a role in hedonic allostasis (35) and in the emergence of negative affect, an integral part of dependence and withdrawal states (36). Cocaine-escalated rats show long-lasting, mGluR2/3 agonist-reversible increases in anxiety-like behavior (22), effects that are likely to be mediated by the CeA and dorsal HIP. Microinjection of mGluR2/3 agonists into these sites exerted potent anxiolytic-like effects (37).
In contrast to the present findings, repeated noncontingent cocaine treatment abolished the inhibitory effects of group II mGluRs on synaptic transmission of CeA neurons in brain slices (38) and decreased mGluR2/3 signaling by reducing binding of the receptor to the G-protein (20). This discrepancy is likely attributable to the divergent effects of response-contingent vs. noncontingent cocaine administration procedures. The behavioral and neurochemical effects of voluntary vs. involuntary cocaine administration are well known to differ (39, 40). Repeated intermittent cocaine treatment typically produces behavioral and neural sensitization (40). Rats with a history of long access to cocaine exhibited decreased sensitization (41) or showed no more sensitization to the psychomotor effects of cocaine than ShA rats (42). These findings suggest that cocaine-induced decreases in mGluR2/3 function may be linked to the development of behavioral sensitization, whereas increases in mGluR2/3 function may be linked to the development of addictive behavior characterized by dose escalation and “loss of control.”
The mGluR5 antagonist MTEP dose-dependently reversed cocaine self-administration on a PR schedule in ShA rats but lost efficacy in LgA rats. Thus, cocaine self-administration under conditions that lead to escalation produces neuroadaptation not only in mGluR2/3 but also mGluR5, albeit with different behavioral consequences. Functional changes in mGluR5 that may account for the diminished behavioral efficacy of MTEP in LgA rats were evident but scattered and much less profound than the functional changes in mGluR2/3. In the NAc, however, LgA rat showed reductions in both mGluR5 expression levels and GTPγS binding. Both effects were small, but the concomitant nature of these changes may have behavioral significance. mGluR5 is highly expressed in the NAc (43), and mGluR5 blockade in the NAc attenuates priming-induced reinstatement of cocaine-seeking (44) and decreases ethanol self-administration (45), findings that implicate NAc mGluR5 in regulating drug-directed behavior. Consistent with such a role, mGluR5 protein levels were decreased exclusively in the NAc after cocaine escalation and, combined with reduced NAc functional mGluR5 activity, may account at least partially for the loss of MTEP’s ability to modify cocaine reinforcement. Recently, Ben-Shahar et al. (46) using a similar cocaine escalation procedure failed to detected changes in mGluR5 expression levels within the NAc 24 h after the last cocaine self-administration session in both ShA and LgA rats. This discrepancy is likely attributable to procedural differences such as in the present study, the animals were subjected to an extended period of cocaine self-administration training that possibly produced stronger and detectable changes in mGluR5 expression level.
The downregulation of mGluR5 function in the NAc of cocaine-escalated rats may be explained by findings showing that mGluR5 undergoes internalization in response to high-dose agonist stimulation (47-50). One may speculate that prolonged stimulation of mGlu5 receptors associated with long-access cocaine self-administration accelerates mGluR5 internalization and, in turn, decomposition within lysosomes (51), leading to downregulation of these receptors. Conversely, glutamate release associated with short-access cocaine exposure may not be sufficient to initiate this cascade of events, leaving mGluR5 function intact. Supporting this possibility, mGluR5 protein levels decrease selectively in the NAc after repeated high-dose cocaine administration (21) and also following withdrawal from 14 days of LgA cocaine exposure (52). MTEP showed a small but significantly decreased ability to antagonize GTPγS binding in the NAc of LgA rats compared to ShA rats. This effect may represent an epiphenomenon of the decreased receptor protein levels, and downstream effectors of the mGluR5 signaling cascade may play a role in regulating functional mGluR5 activity responsible for the change in MTEP’s behavioral efficacy. This may include changes in proteins important for trafficking glutamate receptors and creating signaling microdomains at postsynaptic sites (53), such as Homer, PSD-95, and filamentous (F)-actin, which are known to be altered by chronic cocaine exposure (21, 54, 55).
A trend toward increased mGluR5 protein levels in ShA rats was detected in the mPFC and hippocampus. Correspondingly, GTPγS binding rates, which provide overall information on receptor expression levels and functional activity (56), revealed small enhancements in mGluR5 activity within the mPFC and HIP of ShA animals. Thus, in these brain regions, mGluR5 number or activity increased as a result of ShA cocaine self-administration but returned to control levels when cocaine was available under long-access conditions. This is not an isolated finding because similar patterns of changes induced by cocaine under ShA vs. LgA contingencies have been reported in NAc anandamide levels (57) and c-fos expression (41), although the mechanisms responsible for the reversal to control values of these measures in rats with a history of long access to cocaine remain to be elucidated.
In summary, the results implicate mGluR2/3 and mGluR5-mediated glutamate transmission in regulating cocaine reinforcement and, more importantly, reveal functional upregulation of mGluR2/3 and, possibly, downregulation of mGluR5 as factors in the transition from controlled to compulsive cocaine use. Increased efficacy of the mGluR2/3 agonist LY379268 to interfere with cocaine reinforcement in rats with a history of escalated cocaine self-administration identifies mGluR2/3 as a promising treatment target for severely addicted individuals. However, targeting mGluR2/3 may also produce nonspecific effects (58), although nonspecific effects have been ruled out in numerous studies (10, 16, 17). In contrast, the loss of efficacy of the mGluR5 antagonist MTEP to interfere with cocaine self-administration in cocaine-escalated rats suggests that downregulation of these receptors (or alterations in downstream signaling systems) in rats with extended cocaine exposure may represent an adaptation that further enhances cocaine-seeking behavior. Clearly, however, these findings reveal that the treatment target potential of mGluR5 may be limited to the early stages of the addiction cycle.
Figure 3.
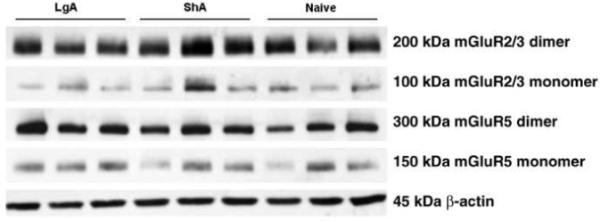
Representative Western blots for mGluR2/3, mGluR5, and the loading control (β-actin). Western blots for both mGluR2/3 and mGluR5 proteins yielded two distinct bands for each subtype (mGlu5: ~150 and ~300 kDa; mGlu2/3: ~100 and ~200 kDa), reflecting monomer and dimer of mGluR2/3 and mGluR5.
Acknowledgements
This work was supported by NIH/NIDA grants DA017097 and DA07348 (FW). We thank Drs. David McKinzie and James Monn at Eli Lilly and Company (Indianapolis, IN) for providing LY379268 and Merck & Co. Inc. (Rahway, NJ) for providing MTEP. We also thank Dr. Wolfram Ruf and Pablito Tejada for providing facilities and expert technical assistance with Western blotting and Michael Arends for editorial assistance. This is publication number 20287 from The Scripps Research Institute.
Abbreviations
- LY379268
(−)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Leshner AI. Drug abuse and addiction treatment research. The next generation. Arch Gen Psychiatry. 1997;54:691–694. doi: 10.1001/archpsyc.1997.01830200015002. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 6.Rasmussen K, Martin H, Berger JE, Seager MA. The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats. Neuropharmacology. 2005;48:173–180. doi: 10.1016/j.neuropharm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- 8.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the Metabotropic Glutamate Receptor 5 Antagonist 2-Methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 10.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 14.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 16.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology (Berl) 2009;203:501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- 20.Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 21.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- 23.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 24.Parnot C, Kobilka B. Toward understanding GPCR dimers. Nat Struct Mol Biol. 2004;11:691–692. doi: 10.1038/nsmb0804-691. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl) 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 26.Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- 27.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 28.Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP on conditioned reinstatement and reinforcement: Comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009 doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999;19:6629–6636. doi: 10.1523/JNEUROSCI.19-15-06629.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin G, Przewlocki R, Siggins GR. Chronic morphine treatment selectively augments metabotropic glutamate receptor-induced inhibition of N-methyl-D-aspartate receptor-mediated neurotransmission in nucleus accumbens. J Pharmacol Exp Ther. 1999;288:30–35. [PubMed] [Google Scholar]
- 31.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 32.Miguens M, Del Olmo N, Higuera-Matas A, Torres I, Garcia-Lecumberri C, Ambrosio E. Glutamate and aspartate levels in the nucleus accumbens during cocaine self-administration and extinction: a time course microdialysis study. Psychopharmacology (Berl) 2008;196:303–313. doi: 10.1007/s00213-007-0958-x. [DOI] [PubMed] [Google Scholar]
- 33.Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, et al. Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci. 2007;27:13968–13976. doi: 10.1523/JNEUROSCI.2808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 36.Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 38.Neugebauer V, Zinebi F, Russell R, Gallagher JP, Shinnick-Gallagher P. Cocaine and kindling alter the sensitivity of group II and III metabotropic glutamate receptors in the central amygdala. J Neurophysiol. 2000;84:759–770. doi: 10.1152/jn.2000.84.2.759. [DOI] [PubMed] [Google Scholar]
- 39.Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. doi: 10.1038/sj.npp.1300834. [DOI] [PubMed] [Google Scholar]
- 43.Tallaksen-Greene SJ, Kaatz KW, Romano C, Albin RL. Localization of mGluR1a-like immunoreactivity and mGluR5-like immunoreactivity in identified populations of striatal neurons. Brain Res. 1998;780:210–217. doi: 10.1016/s0006-8993(97)01141-4. [DOI] [PubMed] [Google Scholar]
- 44.Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gass JT, Olive MF. Role of protein kinase C epsilon (PKCvarepsilon) in the reduction of ethanol reinforcement due to mGluR5 antagonism in the nucleus accumbens shell. Psychopharmacology (Berl) 2009;204:587–597. doi: 10.1007/s00213-009-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, et al. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63:598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dale LB, Bhattacharya M, Seachrist JL, Anborgh PH, Ferguson SS. Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol Pharmacol. 2001;60:1243–1253. doi: 10.1124/mol.60.6.1243. [DOI] [PubMed] [Google Scholar]
- 48.Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 49.Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuroscience. 2008;154:653–666. doi: 10.1016/j.neuroscience.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA, Smith Y. Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J Neurosci. 2007;27:6249–6260. doi: 10.1523/JNEUROSCI.3819-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown MS, Anderson RG, Goldstein JL. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- 52.Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neurosci Lett. 2009;452:167–171. doi: 10.1016/j.neulet.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandler KE, Princivalle AP, Fabian-Fine R, Bowery NG, Kullmann DM, Walker MC. Plasticity of GABA(B) receptor-mediated heterosynaptic interactions at mossy fibers after status epilepticus. J Neurosci. 2003;23:11382–11391. doi: 10.1523/JNEUROSCI.23-36-11382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, et al. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- 55.Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Sovago J, Dupuis DS, Gulyas B, Hall H. An overview on functional receptor autoradiography using [35S]GTPgammaS. Brain Res Brain Res Rev. 2001;38:149–164. doi: 10.1016/s0165-0173(01)00106-0. [DOI] [PubMed] [Google Scholar]
- 57.Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]


