Adiponectin is an adipocytokine that was recently shown to be anti-fibrogenic in hepatic fibrosis. Leptin, on the other hand, promotes hepatic fibrosis. The purpose of the present study was to elucidate a mechanism (or mechanisms) whereby adiponectin dampens leptin signaling in activated hepatic stellate cells (HSC), and prevents excess extracellular matrix production. Activated HSCs, between passage 2 and 5, were cultured and exposed to recombinant human adiponectin and recombinant leptin. Immunoblot analysis for SOCS-3, TIMP-1, and the phosphorylated species of Stat3 and AMPK were conducted. We also examined MMP-1 activity by immunosorbant fluorimetric analysis. In HSCs, adiponectin induced phosphorylation of AMPK, and subsequently suppressed leptin-mediated Stat3 phosphorylation and SOCS-3 induction. Adiponectin also blocked leptin-stimulated secretion of TIMP-1, and significantly increased MMP-1 activity, in vitro. To extend this study, we treated Adiponectin knock-out mice (Ad−/−) daily with 5 mg/kg recombinant leptin and/or carbon tetrachloride (2 ml/kg) for six weeks. Post-necropsy analysis was performed to examine for inflammation, and histological changes in the Ad −/− and wild-type mice. There was no significant difference in inflammation, or aminotransferases, between mice receiving carbon tetrachloride and leptin vs. carbon tetrachloride alone. As anticipated, the combination of leptin and CCl4 enhanced hepatic fibrosis in both wild-type and Ad −/− mice, as estimated by amount of collagen in injured livers, but wild-type mice had significantly higher levels of SOCS-3 and significantly lower levels of TIMP-1 mRNA and protein than did adiponectin KO mice exposed to both CCl4 and leptin. We therefore conclude that the protective effects of adiponectin against liver fibrosis require AMPK activation, and may occur through inhibition of the Jak-Stat signal transduction pathway.
Adiponectin or ACRP is an adipocytokine synthesized and secreted principally by white adipose tissue (WAT). Discovered in 1995, adiponectin is also expressed by bone-forming cells, and is present in other tissues [Berner et al., 2004; Katsiougiannis et al., 2006; Martin et al., 2006]. Full-length adiponectin has biological activity, can be glycosylated, and forms multimeric species of medium and high molecular weight [Garaulet et al., 2007]. The different molecular species have varying degrees of activity and bind two adiponectin receptors: adiponectin receptor I (AdipoR1), found primarily in muscle; and adiponectin receptor II (AdipoR2), found primarily in liver [Yamauchi et al., 2003]. Activation of either of these receptors activates adenosine monophosphate-activated protein kinase (AMPK), although another mechanism involving peroxisome proliferator-activated receptor-alpha (PPARα) has also been described [Kadowaki and Yamauchi, 2005]. AMPK serves as the principal kinase in managing cellular energy stores [Marshall, 2006; Schimmack et al., 2006].
Adiponectin has pleiotropic effects, including the ability to impede formation of cardiovascular plaques [Kadowaki and Yamauchi, 2005], to improve insulin sensitivity and glucose control [Yamauchi et al., 2001]. Adiponectin also protects against certain cancers [Barb et al., 2007]. To date, several groups have reported a protective role for adiponectin in hepatic fibrosis. Kamada and colleagues [Kamada et al., 2003] showed that Adiponectin knockout (Ad −/−) mice are significantly more susceptible than wild-type mice to carbon tetrachloride (CCl4)-induced hepatic fibrosis. Furthermore, co-administration of adiponectin and CCl4 significantly reduced the fibrosis produced by CCl4 alone. Earlier work in our laboratory showed that adiponectin decreased the fibrosis-promoting effects of leptin in activated hepatic stellate cells (HSCs) [Ding et al., 2005]; adiponectin reduced HSC proliferation and rendered HSCs sensitive to apoptosis. Finally Xu, and colleagues [Xu et al., 2003] demonstrated that adiponectin has a protective effect in ob/ob mice and improved hepatic histology as well as other features of the metabolic syndrome.
The molecular mechanisms underlying the anti-fibrotic properties of adiponectin are not well understood, but two recent reports indicate that high molecular weight adiponectin (HMW) or multimeric adiponectin activate AMPK in both human and rodent HSCs [Adachi and Brenner, 2008; Caligiuri et al., 2008; Saxena et al., 2002]. The purpose of this study was to investigate how the adiponectin-AMPK axis could serve as a molecular checkpoint against the leptin signaling axis. The leptin cascade is conducted principally through the long form of the leptin receptor (OB-Rb), and activates Janus kinase 2 (Jak-2) and signal transduction and activator of transcription 3 (Stat3) [Banks et al., 2000; Fruhbeck, 2006]. Jak2/Stat3 signals are regulated in negative feedback fashion by the Suppressors of Cytokine Signaling (SOCS) proteins [Howard and Flier, 2006]. SOCS-3 acts by inhibiting the kinase activity of Jak2, or by targeting the activated receptor complex for degradation [Kubo et al., 2003]. We report here that, in rat HSCs, activation of AMPK by adiponectin disrupts the pro-fibrogenic effects of leptin. This dysregulation follows impairment of leptin-mediated Stat3 phosphorylation, and attenuates the fibrogenic potential of leptin. These data are corroborated by data collected in studies with Ad −/− mice, which further demonstrate the enhanced vulnerability to fibrosis caused by leptin in the CCl4 model.
The role of leptin, a 16 kDa protein hormone, in liver disease may be distinctly important as human obesity and diabetes mellitus type II become more prevalent [Alberti and Zimmet, 1998]. Non-alcoholic steatohepatitis (NASH) is characterized by fatty change of the liver with various degrees of inflammation and fibrosis [Contos and Sanyal, 2002; Diehl, 1999]. The exact mechanism by which leptin promotes liver fibrosis is unknown. Recently, a role for leptin in the molecular pathogenesis of mammalian fibrosis has been substantiated in glomerulosclerosis [Wolf et al., 2002] as well as in liver fibrosis [Honda et al., 2002; Ikejima et al., 2002; Leclercq et al., 2002; Saxena et al., 2002]. In peripheral tissues, leptin exerts control on body weight homeostasis via inhibitory actions on glucose metabolism and insulin secretion [Tartaglia et al., 1995].
Ob-Rb is the functional form of the membrane-associated leptin receptor. It is related to the class II cytokine group receptors binding interleukin-2, interferon, and growth hormone, and is closely related to the gp130 signal-transducing component of the interleukin-6 and the G-CSF receptor [Baumann et al., 1996; Friedman and Halaas, 1998]. The long form of the leptin receptor, Ob-Rb, is present in various peripheral organs, including inflammatory blood cells, lung, kidney, liver, and intestine [Baumann et al., 1996; Friedman and Halaas, 1998; Gainsford et al., 1996; Morton et al., 1998]. Upon leptin interaction with Ob-Rb, activation of Jak-2 occurs via trans-phosphorylation of the receptor as well as subsequent phosphorylation of the Stat proteins. A short, but crucial, amino acid motif is required for Jak-2 activation, which is absent, in short form sequences of Ob-R [Ghilardi et al., 1996].
Two Ob-Rb tyrosine residues have been shown to undergo phosphorylation during receptor activation mediating distinct signal transduction pathways including the mitogen-activated protein kinase (MAPK) pathways [Takahashi et al., 1997]. Leptin has been shown to increase cell proliferation in hematopoietic and embryonic stem cells and CD4+ human T lymphocytes [Bennett et al., 1996; Gainsford et al., 1996; Ghilardi and Skoda, 1997; Lord et al., 1998]. The serine/threonine kinase Akt (protein kinase B or Rac kinase), is known to enhance cell survival [Deregibus et al., 2003; Tang et al., 2000], is another property associated with leptin signal transduction [Kim et al., 2000; Szanto and Kahn, 2000; Vecchione et al., 2002; Zhao et al., 2000].
Materials and Methods
Materials
Recombinant human adiponectin was purchased from Biovendor (Candler, NC). Dulbecco's modified Eagle's medium (DMEM), trypsin-EDTA, and penicillin-streptomycin were all purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from (HyClone, Logan, UT). Recombinant human leptin, Ponceau S solution, and antibodies against β-actin were purchased from Sigma Chemical Co. (St. Louis, MO). SOCS-3 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for AMPKa, phosphorylated AMPKa, phosphorylated Stat3, and Stat3, were purchased from Cell Signaling Technology (Danvers, MA); antibodies against rat TIMP-1 and mouse TIMP-1 were purchased from R&D Systems (Minneapolis, MN). Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from GE Healthcare (Piscataway, NJ). The Sensolyte-Plus™ MMP-1 Assay Kit was purchased from AnaSpec (San Jose, CA), and the adiponectin expression vector, pcDNA-Ad-f was a kind gift from the laboratory of Dr. Amin Xu at the University of Hong Kong, China.
Isolation of hepatic stellate cells
Quiescent HSCs were isolated as described previously [Friedman and Roll, 1987; Saxena et al., 2002]. Sprague–Dawley rats were purchased from Charles River (Boston, MA). All rats received humane care, and the HSC isolation protocol was approved by the Emory University Institutional Animal Care and Use Committee. In brief, in situ perfusion of the liver with 20 mg/dl of Pronase (Boehringer Mannheim, Indianapolis, IN) was followed by collagenase (Crescent Chemical, Hauppauge, NY). Dispersed cell suspensions were layered on a discontinuous density gradient of 8.2% and 15.6% Accudenz (Accurate Chemical and Scientific, Westbury, NY). The resulting upper layer consisted of more than 95% HSCs. Cells were cultured in Medium 199 containing 20% (v/v) FBS (Flow Laboratories, Naperville, IL). Purity of HSCs was assessed by immunolocalization of α-SMA in the monolayer as well as by intrinsic auto fluorescence. Cell viability was verified by propidium iodide exclusion and cell viability for experimental cultures was deemed to be 95%. Sub-confluent activated HSCs in culture, 10 d after isolation and initial plating, were washed twice with PBS and were cultured in DMEM. Growth media was replaced every other day, and activated HSCs were split 1:3 every 7 d, by trypsinization. Only cells between passage 2 and 5 were used in experiments.
Cell lysate production and immunoblotting
Culture-activated HSCs were treated with adiponectin (10 μg/mL), leptin (100 ng/mL), or a combination of leptin and adiponectin after 16 h serum-starvation with 0.1% FBS, 1% penicillin-streptomycin (v/v) in DMEM. Treatment times are indicated in the appropriate figure legends. At the end of the treatments, HSCs were washed in PBS and suspended in ice-cold RIPA buffer (10 μM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% Nonidet P-40 (v/v), 0.5% sodium deoxycholate, 0.1% SDS containing 20 μL/mL protease inhibitor cocktail (Research Products, International, Prospect, IL) for 30 min on ice. Lysates were centrifuged at 12,000 × g for 30 min at 4°C. Supernatant was collected and protein concentrations were determined using the Bradford reagent (Sigma)[Bradford, 1976].
Equal amounts of proteins were resolved on 10% SDS-PAGE [Laemmli, 1970] and transferred onto PVDF membranes by wet transfer. Membranes were stained with Ponceau S (0.1%) to verify equal loading and transfer of proteins. After blocking for 30 min in 5% (w/v) nonfat dry milk in TBS-Tween 20 (20 mM Tris-Cl [pH 7.5]), 137 mM NaCl, 0.05% [w/v] Tween-20), the membranes were exposed overnight, at 4°C, to primary antibody diluted 1:1000, then for 2 h, at room temperature, to the corresponding HRP-conjugated secondary antibody, diluted 1:5000. Equal protein loading was controlled by immunoblot of ß-actin (dilution, 1:2000). Immunoreactive proteins were visualized using HyGlo Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, Metuchen, NJ), and exposure to X-Ray film (Kodak, Rochester, NY). Band density was analyzed using AlphaEase FC Software, version 4.0.1 (Alpha Innotech Corp., San Leandro, CA).
Transfection of HSCs
The adiponectin gene was subcloned into the expression vector pcDNA 3.1+ (Invitrogen) by the Xu laboratory [Wang et al., 2002]. We transfected HSCs with a adiponectin expression vector, pcDNA-Ad-f, using Lipofectamine 2000™ (Invitrogen) per manufacturer's instructions. Two to ten μg of pcDNA-Ad-f were added to 80% confluent HSCs on 100 mm3 tissue culture dishes. After 4 h incubation, the cells were washed twice with PBS and shocked with 10% DMSO (v/v) in DMEM for 3 min. The media were removed, and the cells were provided with fresh DMEM containing 10% FBS for 16 h.
MMP-1 Activity Assay
Microplate strips coated with monoclonal anti-human MMP-1 antibody and all assay reagents were purchased from AnaSpec (San Jose, CA). Primary rat HSCs were treated as described in the appropriate figure legend. Conditioned media was collected from treated HSC cultures, and analysis for MMP-1 activity was performed according to the manufacturer's protocol. To pull down MMP-1, 100 μL of conditioned media was added per well to the microplate. A fluorogenic MMP-1 substrate was added, and MMP-1 activity was analyzed by measuring fluorescence intensity at Excitation/Emission = 490 nm/520 nm. Data were plotted as RFU vs. MMP concentration.
Ad −/− mice care/in vivo studies with carbon tetrachloride and recombinant leptin
Adiponectin knock-out (Ad −/−) mice, the production of which has been previously described [Maeda et al., 2002], were a generous gift from the laboratory of Dr. K. Walsh (Boston University School of Medicine, Boston, MA). The animals were cared for in accordance with protocols approved by the Animal Care and Use Committee of Emory University. Animals were housed in a temperature-controlled environment (20 to 22°C) with a 12:12 h light:dark cycle, and fed ad libitum with Purina Laboratory Chow (Ralston Purina, St. Louis, MO) and water.
Six-week old male littermates from the F2 generation of Ad −/− and wild type mice from the same background were administered CCl4 (2 mL/kg) with olive oil (1:1 ratio) twice weekly by gavage. Control mice were administered sterile saline. All animals were genotyped to confirm wild-type or Ad −/− status. Leptin was administered concomitantly by intraperitoneal (IP) injection every 36 hours for 6 weeks at a dosage of 5 mg/kg. All mice were sacrificed for histologic analysis. Liver tissues were fixed with 10% buffered formalin and embedded with paraffin. Picrosirius red stain was used to detect collagen fibrils as described elsewhere [Junqueira et al., 1979]. Liver tissue samples were also collected to extract mRNA. Before necroscopy, we also collected serum to measure alanine aminotransferase (ALT) levels.
Quantitative RT-PCR
RNA was extracted from mouse liver tissue using RNeasy® (Qiagen). Primers for AMPK, SOCS-3 and TIMP-1 using NIH Primer-BLAST, such that PCR products were 100–200 bp in length and bridged two separate exons. Primer sequence, Accession Number, and Tm (melting temperature) are listed in Table 1. cDNA synthesis was conducted using Bio-Rad's iScript™ cDNA Synthesis kit, according to the manufacturer's recommended parameters. First-strand cDNA synthesis was carried out in 20 μL reaction volumes containing 1 μg of total RNA, 4μL 5X iScript reaction mix, 1 μL iScript reverse transcriptase, and nuclease-free water. Real-time quantitative PCR for SOCS-3 and TIMP-1 were conducted using IQ™ SYBR® Green Supermix (Bio-Rad). PCR reactions were performed according to the manufacturer's protocol, in 25 μL reaction volumes containing nuclease-free water, 1 μL aliquots of cDNA and gene-specific primer pairs, and 12.5 μL SYBR Green Supermix in a MyIQ™ One Color Real Time PCR Detection System. The PCR cycle parameters were set at 95 °C for 20 s, 55 °C for 45 s and 72 °C for 30 s, for a total of 40 cycles. Relative amounts of the target cDNA were estimated by the Ct (threshold cycle) number, and compared with a control gene: GAPDH (glyceraldehyde-3-phosphate dehydrogenase). Three independent samples were analyzed for each condition.
| Gene | Accession No. | Sequence | TM (°C) |
|---|---|---|---|
| mSOCS-3 | NM_007707 | (f) TTCGCTTCGGGACTAGC (r) CTGGTACTCGCTTTTGGA |
60.4 58.5 |
| rSOCS-3 | AJ249240.1 | (f)AAGGCCGGAGACTTTGCTTCGG (r)GCGGGAAACTTGCTGTCTCCCTGA |
59.2 59.9 |
| mTIMP-1 | NM_011593 | (f) CAACTCGGACCTGGTCATAAG (r) CATTTCCCACAGCCTTGAAT |
59.6 59.9 |
Immunoprecipitation
One hundred mg of frozen liver tissue was cut into 0.5 cm × 0.5 cm pieces and allowed to thaw at 4°C in 3 mL lysis buffer [RIPA buffer containing 20 μL/mL protease inhibitor cocktail (Research Products, International, Prospect, IL)] per gram of tissue. While maintaining temperature at 4°C, the tissue was further disrupted by sonication, and incubated on ice for 30 min. The mixtures were transferred to microcentrifuge tubes and centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was transferred to a new tube and centrifuged a second time for 10 min at 10,000 × g; the resulting supernatant constitutes the lysates used for immunoprecipitation.
Equal amounts of protein were incubated with 20 μL of primary antibody for 2 h at 4°C. Twenty μL of protein A/G Plus-Agarose (Santa Cruz Biotechnology) was added to the mixture, and the suspension was incubated at 4°C on a rocking platform overnight. The immunoprecipitates were collected by centrifugation at 1000 × g for 30 s at 4°C, and washed twice in PBS, centrifuging after each wash. The supernatant was discarded, and the pellet resuspended in electrophoresis sample buffer. Immunodetection was conducted as described for immunoblotting.
Statistical Analysis
Animal experiments were performed with 5 to 10 animals in each treatment and control group. Immunoblot analysis was performed with separate samples in triplicate. The data are presented as the mean ± SE. Statistical analysis was performed using Graphpad Prism 4 software (www.graphpad.com). Groups were compared using parametric tests (paired Student's T test or one-way ANOVA with posttest following statistical standards). In all analysis, only p values of less than 0.05 were considered statistically significant.
RESULTS
Adiponectin increases AMPK phosphorylation in HSCs
Multiple effects of adiponectin, and specifically those in the liver, are known to be derived from activation of AMPK [Kadowaki et al., 2006; Yamauchi et al., 2002]. One recent study demonstrated that high molecular weight (HMW) adiponectin inhibits HSC proliferation through multiple effectors, acting downstream of AMPK activation [Adachi and Brenner, 2008]. To determine whether adiponectin signaling in rat HSCs is mediated via activation of AMPK, we treated culture-activated HSCs with recombinant human adiponectin (10 μg/mL) for 5 min, 30 min, and 60 min. and harvested lysates for immunoblotting.
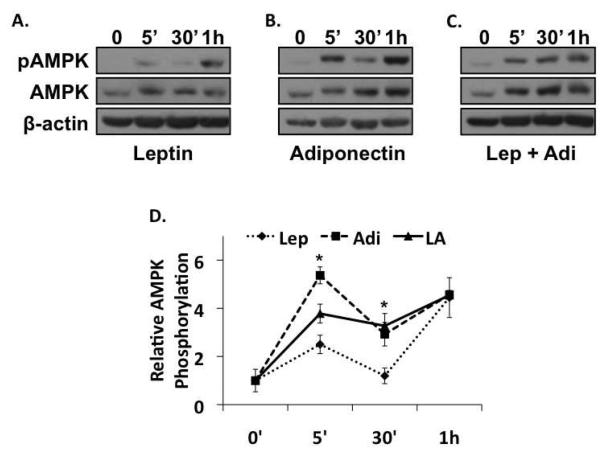
Adiponectin, whether introduced via addition of exogenous recombinant protein (Figure 1B), or by transfection with pcDNA-Ad-F (Figure 2A), increased AMPK phosphorylation. The effect was immediate as evidenced by more than a five-fold increase over baseline (t=0) at 5 min, and receded to a three-fold increase over baseline at 30 min. AMPK phosphorylation, in the presence of leptin, displayed similar kinetics, but was considerably weaker (Figure 1A). In the presence of leptin, AMPK phosphorylation increased only 2.5 fold over baseline at 5 min, before decreasing to basal levels following 30 min exposure to leptin. This finding is consistent with reports that leptin activates AMPK in both liver and muscle [Andreelli et al., 2006; Janovsk· et al., 2008]. In comparison, AMPK phosphorylation in the presence of both adiponectin and leptin was increased 3.8 fold over baseline at 5 min, followed by less robust AMPK phosphorylation at 30 min to 3.2 times the levels observed at baseline (Figure 1C). At 1h, all treatments showed similar increases that were maintained until at least 24h (data not shown).
Figure 1. Adiponectin activates AMPKα2 in activated HSCs.
A). Western blot of the total and active phosphorylated forms of AMPK, and β-actin in lysates prepared from leptin-treated rat HSCs. Leptin treatment alone caused weak increases in AMPK phosphorylation at all time points examined. B). Western blot of the total and active phosphorylated forms of AMPK in lysates prepared from adiponectin-treated rat HSCs demonstrating that adiponectin increased AMPK phosphorylation ~5 fold by 5 min and 3.2-fold at 30 min (*p < 0.05 vs. leptin). C). Co-administration of leptin and adiponectin resulted in a 3.8-fold increase in AMPK phosphorylation at 5min (*p < 0.05 vs. leptin or adiponectin alone), and 3.2-fold at 30 min, (*p < 0.05 vs. leptin). The blots are representative of three independent experiments performed in triplicate.
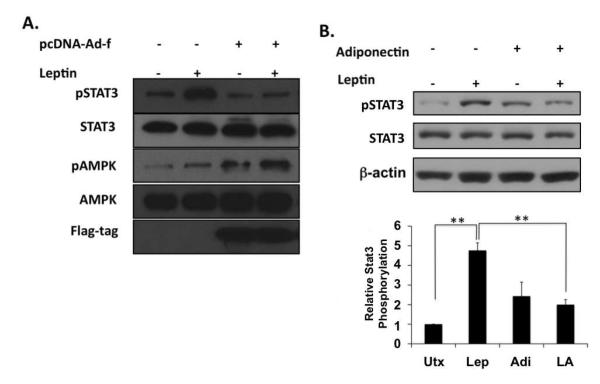
Figure 2. Adiponectin inhibits Stat3 phosphorylation in the presence of leptin.
A) Western blot of total and active phosphorylated forms of Stat3 and AMPK shows that adiponectin over-expression suppresses leptin activation of Stat3. Non-transfected rat HSCs or HSCs transfected with a Flag-tagged adiponectin expression vector (pcDNA-Ad-f) were cultured for 1h in the presence or absence of leptin, and probed with antibodies against Stat3, phosphorylated Stat3 (pStat3), AMPK, or phosphorylated AMPK (pAMPK). Antibodies against the Flag-tag were used to confirm vector expression. B) Western blot of the total and active phosphorylated forms of Stat3 in lysates prepared from HSCs following 1h leptin or adiponectin exposure. β-actin was used as a protein loading control. The blots are representative of three independent experiments performed in triplicate (**p < 0.01).
Adiponectin inhibits leptin-mediated Stat3 phosphorylation in HSCs
The fibrogenic properties of leptin in HSCs are well known [Cao et al., 2006b], and there is a growing body of evidence supporting a role for Jak-2-Stat3 signaling in mediating these effects [Saxena et al., 2002; Saxena et al., 2003; Saxena et al., 2004]. We examined whether the apparent anti-fibrogenic actions of adiponectin could be caused by antagonizing leptin-mediated Jak2-Stat3 signaling. Culture-activated HSCs were treated with recombinant human leptin, recombinant human adiponectin, or both cytokines for 20 min and cellular lysates were subsequently harvested for immunoblotting. As anticipated, leptin increased Stat3 phosphorylation, (Figure 2B). In the presence of both cytokines, however, Stat3 phosphorylation was significantly reduced when compared with that observed in the presence of leptin alone, supporting the hypothesis that adiponectin can down regulate leptin-mediated Jak-Stat signaling. This effect was also evident when adiponectin was expressed via transfection in HSCs (Figure 2A).
Adiponectin promotes suppressors of cytokine signaling 3 (SOCS-3) protein expression
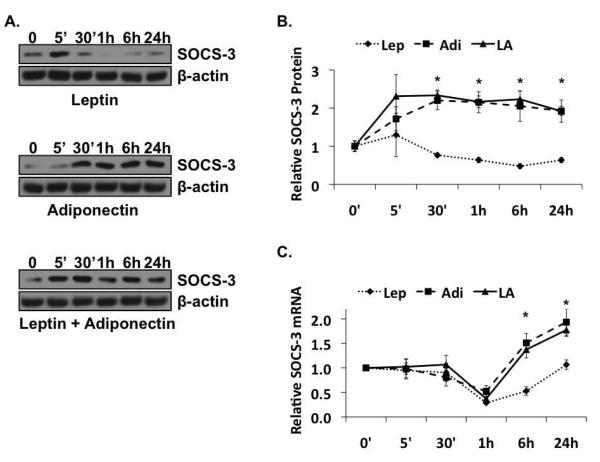
One potential mechanism to explain the apparent antagonistic effect of adiponectin on leptin signal transduction is differential activation of the SOCS-3 negative feedback loop. SOCS proteins are cell-specific transcriptional targets of the Jak/Stat pathway, inhibiting signaling by either, acting as a pseudosubstrate for Jak [Kubo et al., 2003], or targeting the activated receptor complex for ubiquitin-mediated degradation. To investigate differential activation of this loop, we analyzed SOCS-3 protein and mRNA expression in lysates from HSCs treated over a 24 h time period with adiponectin and/or leptin.
The kinetics of change in SOCS-3 expression was similar in the presence of each adipokine alone, as well as in the presence of both. SOCS-3 mRNA was stable for the first 30 min of treatment, followed by a decline at 1h, and recovery, beginning at 6h and maximal at 24h (Figure 3C). There was, however, a significant difference between leptin and adiponectin in their effect on the magnitude of the recovery at the later time points. In the presence of leptin, the increase in SOCS-3 mRNA, beginning at 6h, was relatively small. The increase essentially doubled the amount that was present after 1h (0.286 vs. 0.529), but was still only about half of what was present at t=0 (0.529 vs. 1). Under the same conditions at 24h, SOCS-3 mRNA was statistically equal to what was present at t=0 (1 vs. 1.07). This is very different from what we observed in the presence of adiponectin alone, or the presence of both adipokines. After 6h of exposure to adiponectin, and after a decline at 1h that is comparable to what we saw in the presence of leptin alone, SOCS-3 mRNA levels increased relatively robustly, to 50% higher than baseline (1.51 vs. 1), and almost three times what we observed at the same time point in the presence of leptin alone (1.51 vs. 0.529). After 24h in the presence of adiponectin, the amount of SOCS-3 mRNA was nearly twice that detected at t=0 (1.93 vs. 1), or the in the presence of leptin alone (1.93 vs. 1.07). Finally, in the presence of both leptin and adiponectin, SOCS-3 mRNA was present at levels more similar to adiponectin at both 6h (1.37 vs. 1.51) and 24h (1.73 vs. 1.93), than to leptin [(1.37 vs. 0.529, 6h) and (1.73 vs. 1.07, 24h)].
Figure 3. Adiponectin increases Suppressors of Cytokine Signaling-3 (SOCS-3).
(A) Results of immunoblot analysis of SOCS-3 protein expression in leptin (top panel), adiponectin (middle panel), or co-treatment (bottom panel) of rat HSCs. (B) Quantitative representation of the relative SOCS-3 protein levels during time course treatment of rat HSCs with leptin (Lep), Adiponectin (Adi), or leptin and adiponectin (LA) (C) Results of Real Time PCR analysis of relative SOCS-3 mRNA expression in the presence of leptin (Lep), adiponectin (Adi), or leptin and adiponectin (LA). All blots and qRT-PCR data are representative of three independent experiments performed in triplicate (*p < 0.05 vs. leptin).
The regulation of SOCS-3 protein is apparently more complex. Of the three treatment regimens, only leptin alone (Figure 3A, top panel; Figure 3B, dotted line) generated similar kinetics of expression in both SOCS-3 protein and SOCS-3 mRNA. In the presence of leptin, after a spike at 5 min, the relative amount SOCS-3 protein decreased approximately 50% over the first 6h (from 1.00 at t=0 to 0.48 at 6h) before starting to increase again at 24h. In contrast, in the presence of adiponectin alone (Figure 3A, middle panel; Figure 3B, dashed line), or leptin and adiponectin (Figure 3A, bottom panel; Figure 3B, solid line), SOCS-3 protein also increased (with some variability) as early as 5 min, but remained high throughout the course of the treatment. While interesting, this is not wholly surprising; leptin is well established to regulate SOCS-3 protein expression via transcriptional mechanisms [Bjorbaek et al., 1999; Bjorbaek et al., 1998]. But, while Libby and colleagues describe transcriptional activation of SOCS-3 by adiponectin in macrophages [Folco et al., 2009], and others describe adiponectin as transcriptionally repressive of SOCS-3 in mouse liver during regeneration after partial hepatectomy [Shu et al., 2009], our in vitro data suggest that, in HSCs, adiponectin may regulate SOCS-3 at both the transcriptional and post-translational levels, stabilizing SOCS-3 protein before potentiating SOCS-3 transcription. These responses translate into maintenance of SOCS-3 protein in the presence of adiponectin, or adiponectin and leptin, at levels that are, on average, more than three times what is present in the presence of leptin alone.
Adiponectin stimulates matrix metalloproteinase (MMP-1) activity and blocks leptin-induced tissue inhibitor of metalloproteinase (TIMP)-1 expression
Leptin promotes fibrosis through various mechanisms, the sum of which is to shift the balance of extracellular matrix (ECM) homeostasis to enhanced ECM production and reduced ECM degradation [Cao et al., 2006b; Choudhury et al., 2006; Niu et al., 2007]. Matrix metalloproteinases (MMPs) are enzymes that act to degrade various ECM components. Both MMP-1 and MMP-13 are produced by active HSCs, catalyzing proteolysis of the fibrillar collagens, excess deposition of which underlies the fibrotic phenotype [Benyon and Arthur, 2001; Guo and Friedman, 2007]. TIMPs regulate ECM homeostasis through binding a specific MMP partner and blocking the proteolytic breakdown of its ECM component substrate [Denhardt et al., 1993; Thomas et al., 1999]. This TIMP/MMP regulatory paradigm is critical to maintaining normal extracellular matrix homeostasis.
In vitro, leptin promotes fibrosis by regulating MMP and TIMP expression and activity. Specifically, leptin inhibits MMP-1 expression in both rat HSCs and the human HSC line, LX-2, via Jak/Stat signaling [Cao et al., 2007; Lin et al., 2006]. Leptin also induces TIMP-1 expression in rat HSCs and LX-2 cells [Cao et al., 2006a; Lin et al., 2006], enhancing collagen deposition. Adiponectin suppresses Il-1β–induced MMP 13 expression and [Chen et al., 2006; Lee et al., 2008], and regulates TIMP-1 expression in macrophages [Kumada et al., 2004], but these effects were observed in the context of inflammatory responses. Since the influence of adiponectin on the expression and activity of MMPs and TIMPs is not well understood, we examined the effect of adiponectin on leptin-regulated MMP-1 activity and TIMP-1 expression in rat HSCs, in vitro.
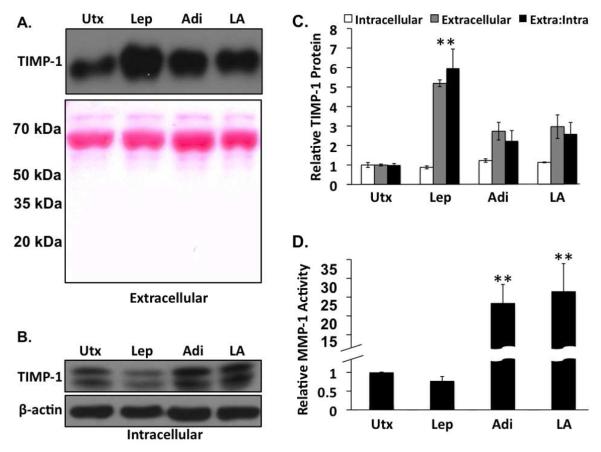
We investigated adiponectin modulation of leptin-regulated TIMP-1 protein expression in lysates from HSCs treated for 24h with leptin, and/or adiponectin. We also examined TIMP-1 protein levels in conditioned media from these cells to investigate TIMP-1 secretion by activated HSCs in the presence of leptin and/or adiponectin. There was a general trend towards reduction (~20%) of intracellular TIMP-1 protein in the presence of leptin, and increase of intracellular TIMP-1 in the presence of adiponectin (22%), or leptin and adiponectin (13%) (Figure 4B; Figure 4C white bars), but none of these differences were statistically significant. We believe the relative differences in intracellular TIMP-1 protein do not reflect actual changes in TIMP-1 production, but relative differences in TIMP-1 secretion instead. As such, less intracellular TIMP-1 is interpreted as increased TIMP-1 secretion. As it relates to ECM remodeling, only extracellular TIMP-1 is relevant. The data collected from conditioned media support this paradigm. When compared with untreated samples, leptin-treated samples showed a more than five-fold increase in extracellular TIMP-1 (Figure 4A; Figure 4C, gray bars). Alternatively, extracellular TIMP-1 increased only 2.7-fold in the presence of adiponectin alone, and 2.9-fold in the presence of adiponectin and leptin. To compare the relative fibrotic potential of the various conditions, we calculated the ratio of extracellular to intracellular TIMP-1, based on these data. The data (Figure 4C, black bars) indicate a five-fold increase in this ratio in the presence of leptin, but only a 2.2 and 2.6-fold increase in this ratio in the presence of adiponectin, or adiponectin and leptin, respectively.
Figure 4. Adiponectin decreases tissue inhibitor of metalloproteinase (TIMP)-1 secretion in the presence of leptin and increases MMP-1 activity.
A) Western analysis of extracellular TIMP-1 from leptin (Lep), adiponectin (Adi), and co-treated (LA) rat HSCs. Ponceau S staining of the membranes was used as a protein loading control. B) Western analysis of intracellular TIMP-1 from leptin (Lep), adiponectin (Adi), and co-treated (LA) rat HSCs. β-actin was used as a protein loading control. (C) Quantitative representation of the relative intracellular (white bars), extracellular (gray bars) TIMP-1 protein levels, and the ratio of extracellular to intracellular (extra:intra, black bars) TIMP-1 protein from leptin (Lep), Adiponectin (Adi), or leptin and adiponectin (LA)-treated rat HSCs, D) Relative MMP-1 activity in rat HSCs was assessed by fluorimetric immunoassay. Adiponectin alone (Adi) or the co-administration of adiponectin and leptin (LA) resulted in a significant increase in MMP-1 activity compared to untreated samples (Utx) or leptin treatment alone (Lep) (**p < 0.01 vs. untreated).
To further relate these modulations in TIMP-1 expression and localization to ECM modulation, we also examined MMP-1 activity in conditioned media collected during these experiments (Figure 4D). Leptin reduced MMP-1 activity 25% compared with untreated samples, while MMP-1 activity increased more than 25-fold over untreated samples in the presence of adiponectin alone. Co-treatment with leptin and adiponectin also increased MMP-1 activity when compared with untreated or leptin-only treated samples, restoring MMP-1 activity to levels similar to that observed in the presence of adiponectin alone. Taken together, these findings further support the paradigm of increased fibrosis in the presence of leptin, and reduced fibrosis in the presence of adiponectin alone, or in the presence of adiponectin and leptin.
Ads −/− mice are more sensitive to CCl4 and leptin-induced fibrosis
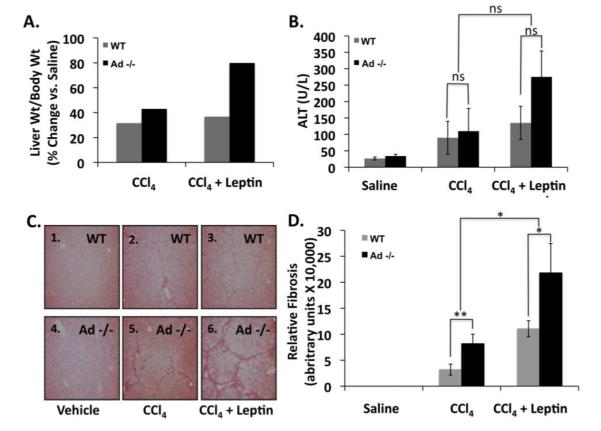
We compared serum ALT, and liver weight-to-body weight ratio (LW/BW) of saline-treated, CCl4-treated, or CCl4 and leptin co-treated wild-type and Ad −/− mice. The increase in LW/BW for Ad −/− mice and wild-type mice was similar after administration of CCl4 alone (~43% vs. 32%, respectively). After CCl4 and leptin co-administration, however the increase in LW/BW was much greater for Ad−/− mice (~80%) than wild-type mice (~37%) (Figure 5A), suggesting that leptin enhances the vulnerability of Ad −/− mice to CCl4-induced hepatic fibrosis. Serum ALT, an indicator of hepatic inflammation, was similar for saline or CCl4-treated Ad −/− and wild-type mice. ALT levels were greater in CCl4/leptin treated Ad −/− mice, compared with their wild-type counterparts, but the difference, however, were not statistically significant. (p > 0.5, n = 8) [Figure 5B]. We also conducted microscopic comparisons of liver sections from the same mice. Figure 5C illustrates that liver sections from CCl4—and particularly in the CCl4/leptin treated Ad −/− mice—are more fibrotic than those from wild-type mice. Control (wild-type) mice (panels 1 and 4), regardless of genotype, displayed normal histological presentation, with little to no fibrosis apparent. Wild-type mice (panels 2 and 3) and Ad −/− mice (panels 5 and 6) develop hepatic fibrosis after CCl4 or CCl4 and leptin co-treatment, as illustrated by increased collagen bridging and hepatocyte dropout. The fibrosis, however, was more severe in livers from Ad−/− mice than wild-type mice, whether exposed to CCl4 alone (Figure 5C, panel 2 vs. panel 5) or CCl4 and leptin (Figure 5C, panel 3 vs. panel 6). Finally, it is evident that leptin enhances the fibrogenic effect in this model (Figure 5C, panel 2 vs. panel 3; panel 5 vs. panel 6), and that Ad −/− mice are more the most vulnerable to leptin-induced fibrosis than wild-type mice (Figure 5C, panel 6 and Figure 5D).
Figure 5. The absence of adiponectin sensitizes mice to CCl4 administration when leptin is co-administered.
A) Percent change in liver weight: body weight for wild-type (WT) and adiponectin knockout (Ad −/−) mice treated with CCl4 alone or CCl4 and leptin. B) Alanine aminotransferase (ALT) in serum collected from WT and Ad −/− mice treated with CCl4 alone or CCl4 and leptin. C) Photomicrographs of Sirius red stained liver sections from WT and Ad −/− animals exposed to CCl4, or CCl4 and leptin. Ad −/− animals treated with CCl4 + leptin had bridging fibrosis and significantly more hepatocyte dropout when compared to Ad −/− mice treated with CCl4 alone, or their wild type counterparts. Quantitative data showing Ad−/− mice are significantly more vulnerable to CCl4 and CCl4/leptin-induced fibrosis. (**p < 0.01; *p < 0.05; NS: p > 0.05)
Ad −/− mouse regulation of SOCS-3 and TIMP-1 expression in response to CCl4 and leptin is impaired, linking the anti-fibrotic properties of adiponectin to SOCS-3
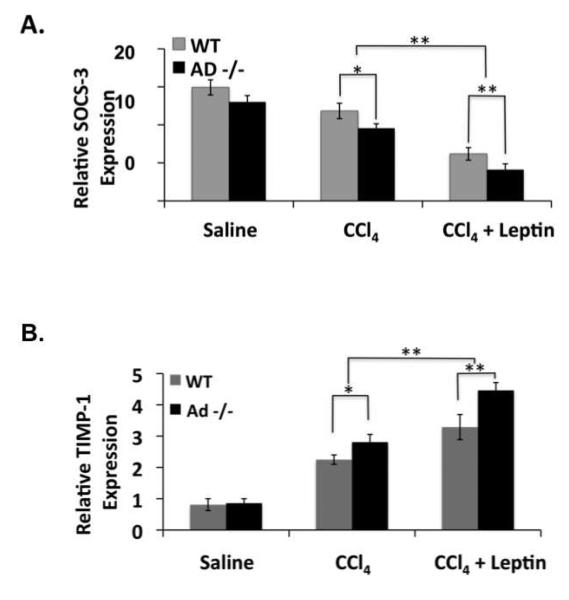
We performed qRT-PCR on RNA extracted from the livers of mice treated with saline, CCl4, or CCl4 and leptin to measure SOCS-3 and TIMP-1 expression. SOCS-3 mRNA expression was similar in saline-treated wild-type and Ad −/− mice (Figure 6A). SOCS-3 expression was reduced in all CCl4-treated mice, but particularly in CCl4/leptin treated Ad −/− mice, which contained significantly less SOCS-3 mRNA than wild-type mice (p < 0.01) (Figure 6A). TIMP-1 mRNA expression was also similar between saline-treated Ad −/− and wild-type mice. However, consistent with the SOCS-3 findings, TIMP-1 mRNA expression was significantly greater (p < 0.01) in CCl4/leptin treated Ad −/− mice, than in any other animal group (Figure 6B).
Figure 6. Ad −/− mice have less SOCS3 mRNA expression and enhanced TIMP-1 mRNA expression in the presence of CCl4 and leptin then wild-type mice do.
Data from quantitative RT-PCR (A) for SOCS-3 and (B) TIMP-1 mRNA expression in livers from wild-type (WT) and adiponectin knockout (Ad −/−) mice exposed to CCl4 alone or CCl4 and leptin. The data are representative of three independent experiments performed in triplicate. (*p < 0.05; **p < 0.01)
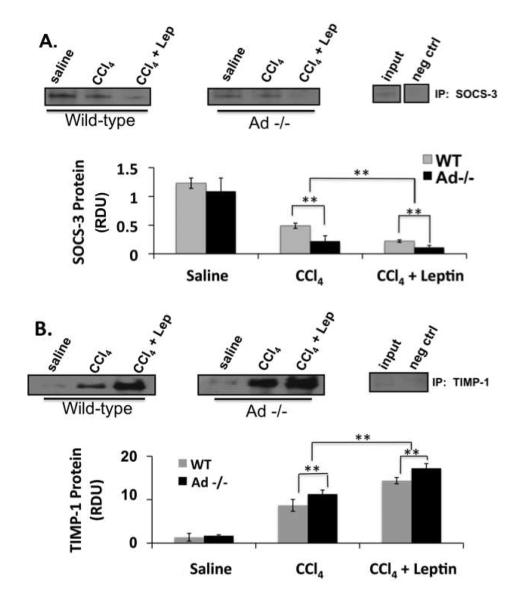
To corroborate the qRT-PCR findings, immunoprecipitation analysis on livers from the respective mice was also performed. These data demonstrate reduced SOCS-3 (Figure 7A) production and enhanced TIMP-1 (Figure 7B) protein expression in vivo. The reduction of SOCS-3 protein and increase of TIMP-1 protein (Fig. 7) was greater in the leptin-treated Ad−/−than in the wild-type mice, consistent with increased vulnerability of Ad−/− mice to fibrogenesis, as shown in Figure 5.
Figure 7. Ad −/− mice have diminished SOCS-3 protein expression and increased TIMP-1 protein expression in the presence of CCl4 and leptin then wild-type mice.
Data from immunoprecipitation for SOCS-3 (A) and TIMP-1 (B) show TIMP-1 expression in livers from wild-type (WT) and adiponectin knockout (Ad −/−) mice exposed to CCl4 alone or CCl4 and leptin. The blots are representative of three independent experiments performed in triplicate. (*p < 0.05; **p < 0.01)
DISCUSSION
A paradigm whereby adipokines play a dynamic role in hepatic fibrogenesis has begun to emerge. Leptin is a profibrogenic adipokine, while recent evidence suggests adiponectin is anti-fibrogenic. While the primary functions of these adipokines--rooted in production from white adipose tissue (WAT)—are related to metabolism and satiety, several key studies, as previously discussed, elaborate the roles of adiponectin and leptin in extracellular matrix homeostasis and other important cellular processes. Recent data also have defined a mechanistic link between adiponectin and AMPK kinase activation in HSCs. These data indicate that adiponectin activation of AMPK slows the activity of HSCs by inhibiting platelet-derived growth factor (PDGF) induced mitogenesis and migration, in addition to down-regulating monocyte chemoattractant protein-1 (MCP-1) protein secretion [Caligiuri et al., 2008]. Furthermore, adiponectin down regulates Akt activity and increases the activity of the cyclin D inhibitors p27 and p21 [Adachi and Brenner, 2008]. Here we provide a plausible mechanism whereby adiponectin can antagonize the leptin-associated HSC signal transduction responsible for its profibrogenic properties.
We have already shown that adiponectin reduces HSC proliferation and sensitizes activated HSCs to apoptosis [Ding et al. 2006]. Here we demonstrate that these biological properties— normally enhanced by leptin—can be antagonized by adiponectin through inhibition of Stat3 phosphorylation. We ascribe this down regulation by adiponectin activation of AMPK and adiponectin blockade of leptin-signal transduction—known to be strongly profibrogenic by our previous work—by modulating SOCS-3 expression.
To dissect the signaling pathways that are responsible for the beneficial effects of adiponectin, we exposed HSCs to either adiponectin, leptin, or both. This series of in vitro studies enabled us to demonstrate several phenomena that provide a molecular basis for the anti-fibrogenic potential of adiponectin. First, adiponectin inhibits leptin-induced Stat3 phosphorylation. Second, adiponectin provides a sustained production of SOCS-3 expression, normally increased by leptin, in vitro, but in a transient fashion. Finally, these effects appear to be mediated through activation of AMPK, which, as described by others, is the principal downstream effector of adiponectin signal transduction in HSCs [Adachi and Brenner, 2008; Caligiuri et al., 2008].
The current understanding of SOCS-3 regulation of leptin-induced Jak2/Stat3 signal transduction describes a negative feedback loop: Leptin-induced Jak2/Stat3 signaling activates SOCS-3 transcription, consequently increasing SOCS-3 protein. To inhibit Jak2/Stat3 signaling, SOCS-3 blocks Stat3 phosphorylation by either targeting the activated receptor complex for degradation, or acting as a pseudosubstrate for Jak2 [Kubo et al., 2003; Yoshimura et al., 2007]. The kinetics of SOCS-3 expression in the present study suggest that the negative feedback loop is turned “off” in rat HSCs as an initial response to leptin signaling, and later, activated again to end the signaling response. Our in vitro studies also suggest that adiponectin uncouples SOCS-3 expression from regulation by leptin, thereby overriding its influence. The consequence of this effect is to effectively leave the negative feedback loop active, preventing a robust response to leptin. These data provide a plausible molecular mechanism for how adiponectin acts as an anti-fibrogenic antagonist of the profibrogenic cytokine leptin. We observe AMPK activation, along with maintenance of relatively high (and constant) SOCS-3 levels, as well as weakened Stat3 activation, as demonstrated by the reduction in leptin-mediated Stat3 phosphorylation. Whether SOCS-3 has any beneficial effect on increasing or stabilizing AMPK phosphorylation is as yet, unknown.
There are numerous reports however; demonstrating that leptin also stimulates AMPK. Therefore, it is plausible that AMPK activation may be necessary, but not sufficient to generate anti-fibrogenic properties of adiponectin. Elements further upstream in the adiponectin signaling pathway, including a specific receptor isoforms (R1 or R2) or LKB1 may be also be required, or sufficient to induce the molecular changes we have described in this report, and in our prior work.
The data also suggest that the kinetics of AMPK activation in liver may be relevant for the development of hepatic fibrosis. Our in vitro data demonstrate prolonged activation of AMPK in the presence of adiponectin, or adiponectin and leptin, when compared with the activation observed in the presence of leptin alone. This difference in temporal activation of AMPK by the two adipokines correlates with the observed protective role for adiponectin against hepatic fibrosis: prolonged activation of AMPK accompanies decreased fibrosis, in addition to decreased expression or activity of the signaling pathways and factors associated with fibrosis, even in the presence of the profibrogenic influence of leptin. Furthermore, the data collected from the in vivo experiments support this assertion. Mice lacking adiponectin, and presumably lacking sustained activation of AMPK, display greater sensitivity to leptin-induced fibrosis, and fibrosis in general. Future studies will investigate this possibility directly.
Of physiologic relevance, we have demonstrated that adiponectin can counter the fibrogenic activity of leptin in HSCs, in vitro. While leptin alone potently stimulated HSC secretion of TIMP-1 into the culture media, adiponectin was able to limit that secretion. In the presence of leptin, the increased secretion of TIMP-1 was associated with reduced MMP-1 activity, suggesting increased fibrotic potential. And, while the amount of TIMP-1 detected in conditioned media increased in the presence of adiponectin, or leptin and adiponectin, in both cases the increase was a fraction of the secretion in the presence of leptin alone. Furthermore, MMP-1 activity increased significantly in the presence of adiponectin, or leptin and adiponectin, This result suggests 1) that the increased secretion of TIMP-1 protein in these conditions was insufficient to block MMP-1 activity, and 2) that other events or changes in expression or function of other factors (perhaps MMP-1, itself) are involved in this anti-fibrogenic mechanism. Although adiponectin has been reported to regulate TIMP-1 expression and secretion [Kumada et al., 2004], whether the observed increases of intracellular TIMP-1 in the presence of adiponectin are caused by transcriptional mechanisms or cellular sequestration are not known. Further studies at the transcriptional level are necessary to ascertain how the interplay of these two cytokines regulates TIMP-1 expression. Finally, TIMP-1/MMP-1 binding, which is critical to controlling matrix degradation, may also be regulated by adiponectin. Taken together, these data offer further evidence that adiponectin plays a direct role in molecular ECM modulation, in vitro, by increasing dense ECM turnover. These properties are in addition to those we have already described, including reduction in HSC proliferation and sensitization of HSCs to apoptosis.
The data from the in vivo experiments using Ad −/− mice are also compelling. There is no question that the Ad −/− mice receiving leptin and CCl4 was the cohort with the most significant fibrosis and the highest liver weight/body weight ratios. These data suggest that not only does the absence of adiponectin render the mice more vulnerable to CCl4 injury, but the presence of leptin also appears to further enhance their vulnerability to the development of liver fibrosis. Although the alanine aminotransferases (ALT) measurements suggest that leptin may increase inflammation, none of those data were statistically significant. While we concede, as other have argued, that several adipokines may contribute to modulating inflammation (e.g., MCP-1); we propose that such inflammatory reactions may not be required for fibrogenic effects in vivo.
Perhaps what is most convincing is the consistency between our observations from the in vitro studies, and the in vivo experiments using Ad −/− mice. Physiologically, the in vivo data further underscore the fibrosis-opposing effects of adiponectin, and support the mechanism suggested by the in vitro experiments. It is clear that Ad −/− mice were more vulnerable to CCl4 and leptin-induced hepatic fibrosis. We also observed elevated TIMP-1 RNA and protein expression in Ad −/− mice, suggesting that adiponectin protects against leptin-induced fibrosis by negatively regulating TIMP-1 expression. That the livers of Ad −/− mice receiving CCl4 and leptin had less SOCS-3 mRNA than wild type mice is also consistent with the in vitro data, strengthening our argument that adiponectin regulates SOCS-3 expression, and that this regulation is important for protecting against leptin-induced fibrosis. The immunoprecipitation data from the in vivo samples also corroborate these findings. While the differences in SOCS-3 and TIMP-1 expression between the wild-type and Ad −/− mice are relatively small, we should note that fibrogenesis is a cumulative process. Therefore, as such small differences persist over extended periods of time (the mice in this study were treated for only six weeks), the disease state becomes progressively worse, and the difference in the severity of the condition between wild-type and knockout animals becomes greater.
At this time we cannot comment on whether SOCS-3 is the only pathway involved in adiponectin down regulation of leptin signaling. LKB1 and/or PTP1B, both components of the adiponectin signaling pathway, could inhibit Jak2 phosphorylation on the long form of the leptin receptor (Ob-Rb), or LKB1 may inhibit the internalization of Ob-Rb. Additional phosphorylation sites on the leptin receptor could also be affected to provide protection by adiponectin against the deleterious effects of leptin in enhanced hepatic ECM production. Discerning these mechanisms may hold promise in preventing or reversing hepatic fibrosis in humans, regardless of the nature of injury.
Acknowledgments
Supported by NIH Public Health Service Grants: DK064399, DK062092, DK075397 and DK076742.
REFERENCES
- Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677–85. doi: 10.1002/hep.21991. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Andreelli F, Foretz M, Knauf C, Cani PD, Perrin C, Iglesias MA, Pillot B, Bado A, Tronche F, Mithieux G, Vaulont S, Burcelin R, Viollet B. Liver Adenosine Monophosphate-Activated Kinase-{alpha}2 Catalytic Subunit Is a Key Target for the Control of Hepatic Glucose Production by Adiponectin and Leptin But Not Insulin. Endocrinology. 2006;147:2432–2441. doi: 10.1210/en.2005-0898. [DOI] [PubMed] [Google Scholar]
- Banks AS, Davis SM, Bates SH, Myers MG., Jr. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:s858–66. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr Biol. 1996;6:1170–80. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–84. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, El-Haschimi K, Frantz JD, Flier JS. The role of SOCS-3 in leptin signaling and leptin resistance. J Biol Chem. 1999;274:30059–65. doi: 10.1074/jbc.274.42.30059. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–25. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trappoliere M, Vizzutti F, Gelmini S, Laffi G, Pinzani M, Marra F. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–76. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. DLPC and SAMe combined prevent leptin-stimulated TIMP-1 production in LX-2 human hepatic stellate cells by inhibiting HO-mediated signal transduction. Liver Int. 2006a;26:221–31. doi: 10.1111/j.1478-3231.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin enhances alpha1(I) collagen gene expression in LX-2 human hepatic stellate cells through JAK-mediated H2O2-dependent MAPK pathways. J Cell Biochem. 2006b;97:188–97. doi: 10.1002/jcb.20622. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol. 2007;46:124–33. doi: 10.1016/j.jhep.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762:711–8. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Choudhury J, Mirshahi F, Murthy KS, Yager DR, Sanyal AJ. Physiologic concentrations of leptin increase collagen production by non-immortalized human hepatic stellate cells. Metabolism. 2006;55:1317–22. doi: 10.1016/j.metabol.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Contos MJ, Sanyal AJ. The clinicopathologic spectrum and management of nonalcoholic fatty liver disease. Adv Anat Pathol. 2002;9:37–51. doi: 10.1097/00125480-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Feng B, Edwards DR, Cocuzzi ET, Malyankar UM. Tissue inhibitor of metalloproteinases (TIMP, aka EPA): structure, control of expression and biological functions. Pharmacol Ther. 1993;59:329–41. doi: 10.1016/0163-7258(93)90074-n. [DOI] [PubMed] [Google Scholar]
- Deregibus MC, Buttiglieri S, Russo S, Bussolati B, Camussi G. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003;278:18008–14. doi: 10.1074/jbc.M300711200. [DOI] [PubMed] [Google Scholar]
- Diehl AM. Nonalcoholic steatohepatitis. Semin Liver Dis. 1999;19:221–9. doi: 10.1055/s-2007-1007111. [DOI] [PubMed] [Google Scholar]
- Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–69. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EJ, Rocha VZ, Lopez-Ilasaca M, Libby P. Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. J Biol Chem. 2009;284:25569–75. doi: 10.1074/jbc.M109.019786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal Biochem. 1987;161:207–18. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–8. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Hernandez-Morante JJ, de Heredia FP, Tebar FJ. Adiponectin, the controversial hormone. Public Health Nutr. 2007;10:1145–50. doi: 10.1017/S1368980007000638. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Skoda RC. The leptin receptor activates janus kinase 2 and signals for proliferation in a factor-dependent cell line. Mol Endocrinol. 1997;11:393–9. doi: 10.1210/mend.11.4.9907. [DOI] [PubMed] [Google Scholar]
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci U S A. 1996;93:6231–5. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27:413–26. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- Honda H, Ikejima K, Hirose M, Yoshikawa M, Lang T, Enomoto N, Kitamura T, Takei Y, Sato N. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36:12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol Metab. 2006;17:365–71. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Ikejima K, Takei Y, Honda H, Hirose M, Yoshikawa M, Zhang YJ, Lang T, Fukuda T, Yamashina S, Kitamura T, Sato N. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- Janovsk· A, Hatzinikolas G, Staikopoulos V, McInerney J, Mano M, Wittert GA. AMPK and ACC phosphorylation: Effect of leptin, muscle fibre type and obesity. Molecular and Cellular Endocrinology. 2008;284:1–10. doi: 10.1016/j.mce.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–92. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H, Okamoto Y, Kihara S, Miyagawa J, Shinomura Y, Funahashi T, Matsuzawa Y. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796–807. doi: 10.1053/j.gastro.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54:2295–9. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- Kim YB, Uotani S, Pierroz DD, Flier JS, Kahn BB. In vivo administration of leptin activates signal transduction directly in insulin-sensitive tissues: overlapping but distinct pathways from insulin. Endocrinology. 2000;141:2328–39. doi: 10.1210/endo.141.7.7536. [DOI] [PubMed] [Google Scholar]
- Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat Immunol. 2003;4:1169–76. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–13. doi: 10.1016/s0168-8278(02)00102-2. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim JH, Park MC, Park YB, Lee SK. Adiponectin mitigates the severity of arthritis in mice with collagen-induced arthritis. Scand J Rheumatol. 2008;37:260–8. doi: 10.1080/03009740801910346. [DOI] [PubMed] [Google Scholar]
- Lin S, Saxena NK, Ding X, Stein LL, Anania FA. Leptin increases tissue inhibitor of metalloproteinase I (TIMP-1) gene expression by a specificity protein 1/signal transducer and activator of transcription 3 mechanism. Mol Endocrinol. 2006;20:3376–88. doi: 10.1210/me.2006-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Marshall S. Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Sci STKE. 2006:re7. doi: 10.1126/stke.3462006re7. 2006. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Woo JG, Geraghty SR, Altaye M, Davidson BS, Banach W, Dolan LM, Ruiz-Palacios GM, Morrow AL. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006;83:1106–11. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J Biol Chem. 1998;273:26194–201. doi: 10.1074/jbc.273.40.26194. [DOI] [PubMed] [Google Scholar]
- Niu L, Wang X, Li J, Huang Y, Yang Z, Chen F, Ni H, Jin Y, Lu X, Cao Q. Leptin stimulates alpha1(I) collagen expression in human hepatic stellate cells via the phosphatidylinositol 3-kinase/Akt signalling pathway. Liver Int. 2007;27:1265–72. doi: 10.1111/j.1478-3231.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35:762–71. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Saliba G, Floyd JJ, Anania FA. Leptin induces increased alpha2(I) collagen gene expression in cultured rat hepatic stellate cells. J Cell Biochem. 2003;89:311–20. doi: 10.1002/jcb.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. Faseb J. 2004;18:1612–4. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack G, Defronzo RA, Musi N. AMP-activated protein kinase: Role in metabolism and therapeutic implications. Diabetes Obes Metab. 2006;8:591–602. doi: 10.1111/j.1463-1326.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- Shu RZ, Zhang F, Wang F, Feng DC, Li XH, Ren WH, Wu XL, Yang X, Liao XD, Huang L, Wang ZG. Adiponectin deficiency impairs liver regeneration through attenuating STAT3 phosphorylation in mice. Lab Invest. 2009;89:1043–52. doi: 10.1038/labinvest.2009.63. [DOI] [PubMed] [Google Scholar]
- Szanto I, Kahn CR. Selective interaction between leptin and insulin signaling pathways in a hepatic cell line. Proc Natl Acad Sci U S A. 2000;97:2355–60. doi: 10.1073/pnas.050580497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Okimura Y, Mizuno I, Iida K, Takahashi T, Kaji H, Abe H, Chihara K. Leptin induces mitogen-activated protein kinase-dependent proliferation of C3H10T1/2 cells. J Biol Chem. 1997;272:12897–900. doi: 10.1074/jbc.272.20.12897. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem. 2000;275:9106–9. doi: 10.1074/jbc.275.13.9106. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Thomas GT, Lewis MP, Speight PM. Matrix metalloproteinases and oral cancer. Oral Oncology. 1999;35:227–233. doi: 10.1016/s1368-8375(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–73. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–9. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- Wolf G, Chen S, Han DC, Ziyadeh FN. Leptin and renal disease. Am J Kidney Dis. 2002;39:1–11. doi: 10.1053/ajkd.2002.29865. [DOI] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- Zhao AZ, Shinohara MM, Huang D, Shimizu M, Eldar-Finkelman H, Krebs EG, Beavo JA, Bornfeldt KE. Leptin induces insulin-like signaling that antagonizes cAMP elevation by glucagon in hepatocytes. J Biol Chem. 2000;275:11348–54. doi: 10.1074/jbc.275.15.11348. [DOI] [PubMed] [Google Scholar]