Abstract
Exposure to air pollutants increases the incidence of cardiovascular disease. Recent toxicity studies revealed that ultra fine particles (UFP, dp<100–200 nm), the major portion of particulate matter (PM) by numbers in the atmosphere, induced atherosclerosis. In this study, we posited that variations in chemical composition in diesel exhausted particles (DEP) regulated endothelial cell permeability to a different extent. Human aortic endothelial cells (HAEC) were exposed to well-characterized DEP (dp<100 nm) emitted from a diesel engine in either idling mode (DEP1) or in urban dynamometer driving schedule (UDDS) (DEP2). Horse Radish Peroxidase-Streptavidin activity assay showed that DEP2 increased endothelial permeability to a greater extent than DEP1 (Control=0.077± 0.005, DEP1=0.175±0.003, DEP2=0.265±0.006, n=3, p<0.01). DEP2 also down-regulated tight junction protein, Zonular Occludin-1 (ZO-1), to a greater extent compared to DEP1. LDH and caspase-3 activities revealed that DEP-mediated increase in permeability was not due to direct cytotoxicity, and DEP-mediated ZO-1 down-regulation was not due to a decrease in ZO-1 mRNA. Hence, our findings suggest that DEP1 versus DEP2 differentially influenced the extent of endothelial permeability at the post-translational level. This increase in endothelium permeability is implicated in inflammatory cell transmigration into subendothelial layers with relevance to the initiation of atherosclerosis.
Keywords: DEP, UFP, permeability, endothelial cell, ZO-1, air pollution
Introduction
Mounting epidemiological evidence demonstrates that exposure to particulate matter (PM) in the atmosphere increases morbidity and mortality (Dockery et al., 1993; Pope et al., 2004; Pope et al., 1995). Ambient PM level was statistically linked to an increased incidence of cardiovascular diseases (Brook et al., 2004), and the concentration of PM was intimately related to acute coronary syndromes (Dockery et al., 1993; Pope et al., 2004; Pope et al., 1995). PM also mediated vascular oxidative stress, favoring early atherosclerosis in ApoE-null mice (Araujo et al., 2008). However, the mechanisms whereby PM air pollutants increase the incidence of cardiovascular diseases remain largely unknown.
By virtue of their high surface to volume ratios per unit mass, ultrafine particles (UFP, dp < 100–200 nm) display unique biochemical characteristics in favor of adsorption or absorption of organic molecules and potential distribution to pulmonary and cardiovascular systems (Frampton, 2001; Nemmar et al., 2002; Oberdorster, 1996). In a recent animal toxicity study, diesel exhaust particles (DEP, dp < 180 nm) induced an increase in atherosclerotic lesions to a greater extent compared to fine particles (PM2.5, dp < 2.5µm) in ApoE knockout mice (Araujo et al., 2008). Approximately 1 % of inhaled nano-sized particles transmigrate across human pulmonary epithelium into systemic arterial circulation (Nemmar et al., 2004; Takenaka et al., 2006; Takenaka et al., 2004). When ultra fine particles accumulate to a high concentration in the so called “hot spot” (Li et al., 2003), cytotoxicity develops. Studies from our laboratory and others demonstrated that vascular endothelial cells exposed to DEP developed oxidative stress and inflammatory responses (Karoly et al., 2007; Li et al., 2009). However, it remained unknown whether DEP affected endothelial permeability.
Endothelial cells line the inner lumen of vasculature forming a layer of physical barrier between circulating blood and underlying tissues. Tight junctions between adjacent endothelial cells modulate vascular homeostasis (Bazzoni and Dejana, 2004). The permeability of endothelial junctions is maintained by junction proteins, including Zonular Oclludin-1 (ZO-1) and Occludin (OCLN) that cross-link to cytoskeleton. Disruption of the tight junctions affects endothelial permeability, promoting leukocyte and lipoprotein transmigration into the subendothelial layers, thereby initiating atherogenesis (Aghajanian et al., 2008).
In this study, we examined whether DEP emitted from a diesel engine operating in either idle mode (DEP1) or in the urban dynamometer driving schedule (UDDS) (DEP2) have differential influences on the endothelial permeability. We observed that DEP-2 increased permeability of human aortic endothelial cells (HAEC) to a greater extent compared to DEP1. This effect was not caused by the direct cytotoxic effects on HAEC, rather, by a reduction in ZO-1 protein at the post-translational level.
Materials and Methods
Materials and Reagents
Endothelial cell culture media and reagents were obtained from Cell Application Inc. Fetal bovine serum (FBS), M199 media, anti-Occludin antibody and anti-ZO-1 antibody were purchased from Invitrogen Inc. Protease inhibitor (PI) and phosphotase inhibitor cocktail were purchased from Sigma Inc. Anti-β-Tubulin was obtained from Millipore. Real time PCR reagents were from Applied Biological Materials Inc.
Preparation of Ultra fine Particles
DEP used in the present study were collected from a 1998 Kenworth truck operating without any emission control technology, as part of a separate project to investigate the effect of emission control technologies, designed to meet the 2007 and 2010 emission standards for heavy-duty diesel vehicles (HDDV) (Biswas, 2008) on the physic-chemical and toxicological characteristics of the emitted PM. Experiments were carried out at the California Air Resource Board (CARB) heavy duty diesel emission testing laboratory (HDETL) in downtown Los Angeles. Holmen and Ayala (Holmen and Ayala, 2002) described the dynamometer specifications in details and the schematic particle collection set up was described by Biswas et al (Biswas, 2008).
A micro-orifice uniform deposited impactor (MOUDI) upstream of a NanoMOUDI (MOUDI-Nano MOUDI, MSP Corp., MN) operating at a nominal flow rate of 10 liters per minute (lpm) was used to collect size-resolved PM samples (10–18nm, 18–32nm, 32– 56nm, 56–100nm, 100–180nm, 180nm-2.5<mu>m). Each stage was loaded with pre-cleaned aluminum foil substrates, which were pre- and post-weighted to determine their gravimetric PM mass. A high volume sampler (Misra et al., 2002) operating at 450 lpm was deployed to collect PM mass on Teflon coated glass fiber filters (20 × 25 cm) (Pallflex Fiberfilm T60A20-8×10, Pall Corp., East Hills, NY). 47 mm Teflon filters (PTFE membrane filter, 2 µm, Pall Life Sciences, Ann Arbor, MI, USA) were deployed to collect particles denuded of volatile species downstream of the dilution channel at 50 lpm. These substrates were subsequently used for the analysis of water-soluble metals and trace elements.
The PM mass of the high volume samples were determined by normalizing the gravimetric PM mass of MOUDI samples based on the flow ratio of the two samplers. The Teflon coated glass fiber filters were then analyzed by Shimadzu TOC-5000A liquid analyzer (Decesari et al., 2001) for water soluble organic carbon (WSOC) and by ion chromatography (IC) technique for the chemical species. A portion of these high volume samples was analyzed by gas chromatography-mass spectrometry (GC/MS) for organic compounds(Schauer et al., 2002). Water-soluble metals and elements were determined by means of Inductively Coupled Plasma - Mass Spectroscopy (ICP-MS) on 47 mm Teflon filters (Lough et al., 2005).
The remaining portion of the high volume samples was used to prepare the DEP suspension for the cell exposure tests. The filters were first soaked in the 10 ml of ultra-pure water (USP grade) for 30 minutes in endotoxin-free glass vial, followed by sonication for 30 minutes. After the particle suspension was transferred to endotoxin-free tube, another 10 ml of ultra-pure water was used to repeat the aforementioned process. Our control suspension was made extracting a blank filter, identical to the ones used for PM collection, with USP grade water, using the exact same procedures described above.
Cell Culture
Human aortic endothelial cells (HAEC) (Cell Application) were cultured with endothelial cell growth media (Cell Application). The cells were used between passages 5 and 11. For DEP treatment, HAEC were incubated with 50 µg/ml of DEP in M199/0.1%FBS for specified time.
Permeability assay
HAEC were seeded into clear transwell ( from Corning Inc, 6.5mm diameter, 0.4µm pore size)at 1×105 per well in 24-well plate. Cells were grown to complete confluence in 5–7 days with media change every 2 days. Cells were then rinsed with treatment media (M199/0.1%FBS) and treated with or without DEP in treatment media in the presence of 1µg/ml of Horse Radish Peroxidase-Streptavidin (HRP-Streptavidin, from Pierce Inc) in the top transwell. One ml of treatment media was added to the bottom well. Changes in endothelial permeability were analyzed by the HRP activities of media in the bottom well.
For the HRP activity assay, five µl of media from bottom well were taken and mixed with 100 µl of TMB solution (HRP substrate, from Pierce Inc) in wells of 96-well plate. After incubation for 5–30 minutes at a room temperature, one hundred µl of 2M sulfuric acid was added to stop the reaction. The absorbance at 450 nm (OD 450) was read as relative to HRP activities.
LDH activity assay
Potential cytotoxicity of DEP on endothelial cells was analyzed by measuring LDH activities. The assay was done with the LDH activity assay kit from BioVision Inc. HAEC cells were seeded in 12 well plates and grown to confluence. Cells were then treated with or without DEP in M199/0.1% FBS for 4 hours. Ten µl of conditioned media was used to measure LDH activities released. For total LDH activity, the cells were lysed by adding 1/5 volume of passive lysis buffer (PLB, Promega Inc) into the wells for 5 min. The lysate were collected after centrifugation. Ten µl of clear cell lysate was used for total LDH activity assay. LDH activities were measured following manufacturer’s instructions. The relative cytotoxicity was expressed as LDH activities released as percentage of total LDH activities.
Caspase-3 Activity Assay
The apoptosis of HAEC was examined by measuring Caspase-3 activities. HAEC were treated with or without DEP or with 10 uM of Camptothecin (CPT, positive control) for 4 hours. The cells were then lysed with passive lysis buffer (PLB). After spin, the clear lysate were used for Caspase-3 activity assay using Caspase-3 colorimetric assay kit from Genscript. The protein concentration of the lysate was measured and the relative Caspase-3 activities were normalized to protein concentration.
Western Blot
HAEC were grown to confluence and treated with or without DEP in M199/0.1%FBS for 4–6 hours. Cells were washed twice with cold PBS and lysed in proper volume of RIPA buffer (50 mM Tris-HCl, pH 8.0, 150mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate supplemented with protease inhibitor (PI) cocktail) and phosphotase inhibitor cocktail at 4 °C for 30 min. After centrifugation for 5 min at 4 °C, supernatant was collected as whole cell lysates. Protein concentration was determined with DCP protein assay kit from Bio-Rad Inc. Samples with equal protein amount of lysates were run on a 4–20% gradient SDS-PAGE gel. The proteins were then transferred to PVDF membrane and blotted with indicated primary and secondary antibodies. Signal was developed with Supersignal Western Pico (Pierce) and recorded with FluorChem FC2 (Alpha Inotech Inc). Densitometry scans of western blots were performed with the software bundled with the FlorChem FC2 machine.
Measurement of ZO-1 expression by Quantitative RT-PCR
Total RNA was isolated using the Bio-Rad kit following manufacturer’s instruction. Potential genomic DNA contamination was removed with on-column DNase I digestion. 0.5–1 µg of total RNA was reverse transcribed with Bio-Rad’s iScript cDNA synthesis kit. The expression of ZO-1 was analyzed at the mRNA levels using quantitative RT-PCR as previously described (Li et al., 2009). The expression of ZO-1 mRNA was normalized to GAPDH. Primers used for ZO-1 were as follows: forward: CGCCAAGAGCACAGCAATGGAGGAA, reverse: CCCACTCTGAAAATGAGGATT-ATCTCGTCC.
Statistical Analysis
All experiments were performed for three or more trials. Data were expressed as mean ± standard deviation (SD). For comparisons between two groups, student t-test was used for significance analysis. For comparisons among multiple values one-way analysis of variance (ANOVA) was used. A P value less than 0.05 was considered statistically significant.
Results
DEP chemical composition
The detailed chemical composition of the DEP used in the present study has been described previously (Li et al., 2010). A list of the mass ratios of the water soluble organic carbon, inorganic ions, organic compounds and trace metals in DEP1 and DEP2 has been provided in the supplemental table.
DEP increased HAEC permeability
DEP exposure promotes atherogenesis in ApoE-null mouse model (Araujo et al., 2008). We and others have shown that DEP induced endothelial cell dysfunctions including the induction of vascular oxidative stress via JNK activation and pro-inflammatory responses by up-regulating inflammatory cytokines such as MCP-1, IL-8 and VCAM (Karoly et al., 2007; Li et al., 2009). Both oxidative stress and inflammatory cytokines such as MCP-1 and IL-8 affected cellular permeability (DeMaio et al., 2006; Gavard et al., 2009). Hence, we assessed whether DEP1 and DEP2 modulated vascular endothelial permeability with differential effects.
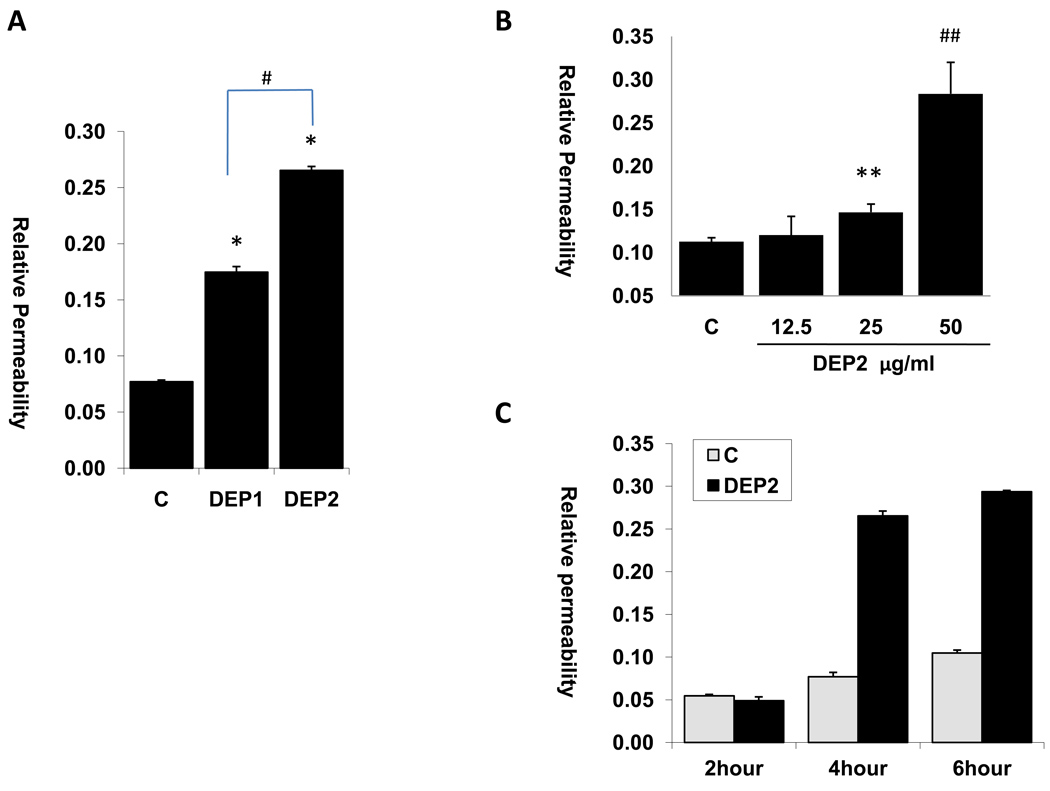
HAEC cells were seeded in transwells to form endothelial monolayers. Streptavidin-HRP migration from the upper chamber through the endothelial monolayer to the lower chamber was used as a marker of endothelium permeability. HRP activities were measured as an index for HAEC permeability. Endothelial cells permeability significantly increased after four hours of exposure to 50 µg/ml of DEP1 or DEP2 (Figure 1A: C = 0.077± 0.005, DEP1 = 0.175±0.003, DEP2 = 0.265±0.006; n=3; versus control, *p < 0.01). DEP2 (emitted from truck engine running under UDDS mode) increased HAEC permeability to a greater extent compared to DEP1 (from truck engine running under idle mode). A lower dose of DEP at 25 µg/ml also significantly increased endothelial permeability, but there seemed to be a significant increase in endothelial permeability at 50 µg/ml DEP (Fig. 1B, C = 0.113± 0.004, 12.5µg/ml = 0.1203±0.02, 25µg/ml = 0.147±0.01, 50µg/ml = 0.284±0.036 ; n=3; versus control, *p < 0.05, # p<0.01). While the base levels of endothelial cell permeability increased with time, DEP-induced increase in permeability occurred mostly after 4 hours of exposure (Fig. 1C).
Fig.1. DEP increased the permeability of endothelial cells.
(A) HAEC cells were grown to confluence in transwell to form an endothelial monolayer. The cells were then treated with or without 50ug/ml DEP1 or DEP2 for 4 hours in the presence of 1ug/ml of Streptavidin-HRP. HRP activity from the bottom well was measured as the permeability of endothelial cells. (* vs. control, n=3, p<0.01; # DEP2 vs. DEP1, n=3, p<0.01). (B) Dose effect of DEP2 on HAEC permeability: HAEC were treated with indicated dose of DEP2 for 4 hours and HRP activity was measured as the permeability of endothelial cells. (n=3, ** vs. control, p<0.05; # # vs. control, p<0.01). (C) Time effect of DEP2 on HAEC permeability: HAEC were treated with 50ug/ml dose of DEP2 for 2, 4 and 6 hours, and HRP activity was measured as the permeability of endothelial cells. (C =Control)
UF-mediated cellular permeability was not caused by direct cytotoxic effect
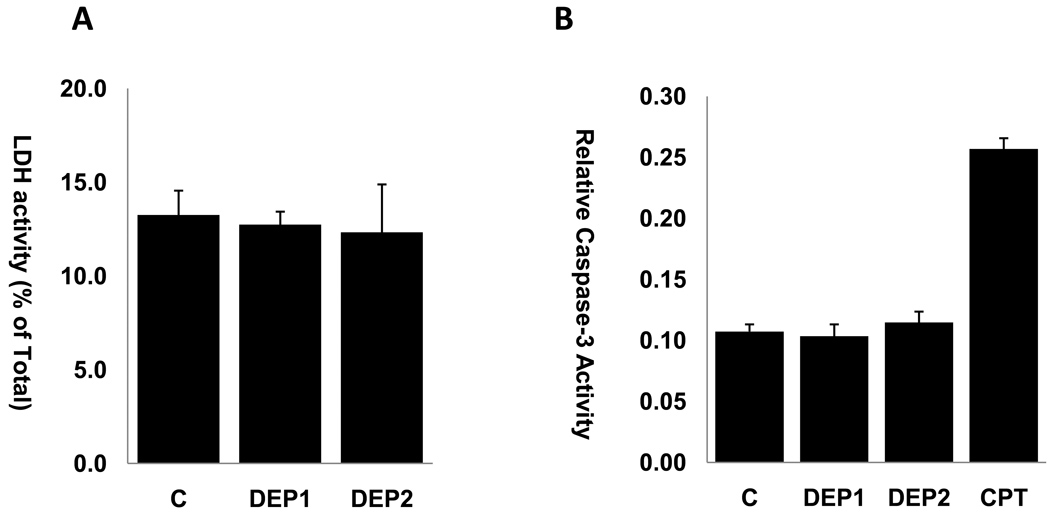
To assess whether DEP-induced increase in cell permeability was due to direct cytotoxicity or cell apoptosis, we measured LDH activities released from HAEC and caspase-3 activities in HAEC exposed to DEP1 and DEP2. Exposure of HAEC to DEP1 or DEP2 at 50 µg/ml for 4 hours did not significantly affect the percentage of LDH released (Fig.2A). Similarly, Caspase-3 activities in HAEC were not significantly changed after exposure to DEP1 or DEP2 at 50 µg/ml for 4 hours (Fig. 2B). Thus, DEP did not cause direct cytotoxicity and apoptosis at the concentration levels and exposure durations used in our studies.
Fig.2. DEP did not cause direct toxicity to HAEC.
(A) HAEC cells grown to confluence were treated with or without 50ug/ml DEP1 or DEP2 for 4 hours. The supernatant was used to measure the activity LDH as indication of cytotoxicity. DEP did not increase the LDH levels released by HAEC. (B) HAEC cells grown to confluence were treated with or without 50ug/ml DEP1 or DEP2 for 4 hours, HAEC apoptosis was examined by measuring Caspase-3 activity. DEP did not cause significant apoptosis of HAEC after 4 hours of treatment. Camptothecin (CPT) at 10uM was used as positive control of apoptosis. (C= control)
Exposure to DEP reduced the levels of tight junction protein ZO-1
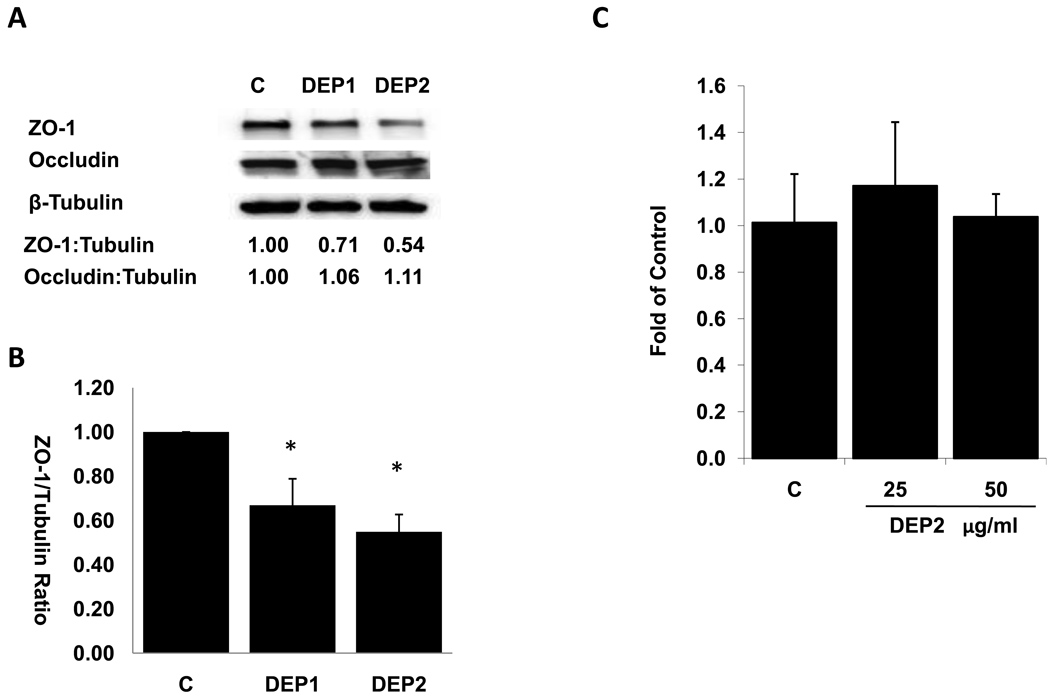
Tight junctions (TJ) are the apical intercellular junction that maintains the physiologic barrier among adjacent endothelial cells. TJ is composed of peripheral membrane proteins such as ZO-1 and transmembrane proteins such as occludin (Aghajanian et al., 2008). ZO-1 plays an important role in assembling the transmembrane protein to the junction sites (Mitic and Anderson, 1998) via linking transmembrane protein such as occludin to actin cytoskeleton (Aghajanian et al., 2008). Thus, ZO-1 is critical in scaffolding the integrity of cellular tight junction. We assessed whether DEP-induced permeability change was associated with junction protein expression. At 50 µg/ml, both DEP1 and DEP2 significantly reduced the protein levels of ZO-1(Fig. 3A, Fig. 3B). We consistently observed that DEP2 reduced ZO-1 levels to a great extent compared to DEP1, although the difference was statistically insignificant (Fig. 3B). The levels of junction protein, Occludin, were not significantly influenced by either DEP1 or DEP2 exposure (Fig 3A).
Fig.3. DEP down-regulated the protein levels of ZO-1.
(A) HAEC cells were grown to confluence in 6-well plate. The cells were then treated with or without 50ug/ml of DEP1 and DEP2 for 4 hours. Cell lysate was prepared and ZO-1 and Occludin protein levels were assessed by western blotting. β-Tubulin blot was performed as loading control and density scan data were used to calculate the relative ratios of ZO-1/Tubulin and Occludin/tubulin. Shown is a representative blot of three independent experiments. (B) The ratio of ZO-1/ β-Tubulin from three independent experiments were calculated based on density scan data and plotted (* vs. control, n=3, p<0.01). (C) HAEC cells were treated with or without DEP2 at 25ug/ml or 50ug/ml for 4 hours. RNA’s were isolated and ZO-1 mRNA expression was measured by quantitative RT-PCR. The level was normalized to GAPDH. DEP did not significantly change the ZO-1 mRNA levels. (C= Control)
Exposure to DEP did not reduce ZO-1 expression at the mRNA level
To examine the underlying mechanism of reduced ZO-1 protein levels in endothelial cells by DEP, we measured ZO-1 mRNA expression by quantitative RT-PCR. DEP2 did not affect ZO-1 mRNA levels (Fig. 3C). This result suggest that down-regulation of ZO-1 protein by DEP is a post-transcriptional event.
Discussion
The novelty of our study is that diesel exhausted particles (DEP) increased endothelial cell permeability in association with a down-regulation of junction protein, ZO-1. Specifically, DEP2 (emitted from diesel engine running under UDDS mode) increased HAEC permeability to a greater extent compared to DEP1 (from engine running under idle mode).
The increase in DEP-mediated endothelial permeability is in agreement with previous observations reported in epithelial cells, in which DEP reduced the transepithelial electric resistance (Lehmann et al., 2009). DEP exposure also enhanced histamine-induced vascular and nasal mucosal permeability in Guinea pigs in a dose-dependent fashion (Hiruma et al., 1999). DEP further enhanced lipopolysaacharide-induced pulmonary permeability (Inoue et al., 2006). In this context, the changes in permeability suggest a common mechanism for DEP-induced pathological effects.
HAEC exposed to DEP as low as 12.5 µg/ml resulted in trend towards an increase in permeability while DEP at 25 and 50 µg/ml induced a statistically significant effect. Despite a low concentration, chronic exposure to DEP in vivo may generate a similar pathological effect as observed at high dose in response to an acute exposure. Hence, the use of relatively high concentration of DEP in our in vitro studies was deemed necessary to assess responses from the cultured endothelial cells (Li et al., 2003).
Our data indicated that DEP had no direct effects on cytotoxicity and apoptosis in endothelial cells in response to the specific levels and durations of DEP exposure in this study. Our findings were similar to the reports by Baulig et al. and Lemann et al. in which epithelial cells exposed to DEP for 24 hours did not cause significant cytotoxicity and apoptosis at concentrations up to 125 µg/ml (Baulig et al., 2004; Lehmann et al., 2009). However, Boland et al reported that cytotoxicity developed when epithelial cells were exposed to DEP for 48 hours (Boland et al., 1999). Hence, acute versus chronic exposures to DEP may implicate a distinct cellular response in terms of cytotoxicity.
Loss of tight junction protein expression can negatively impact on barrier function of endothelial cells (Aghajanian et al., 2008). For example, angiotensin receptor-1 blocker, Telmisartan, increased endothelial permeability via down-regulation of ZO-1 (Bian et al., 2009). Also notable was exposure to histamine, a vasoactive compound, that altered endothelial permeability via reduction of ZO-1 expression (Gardner, 1995). Oxidized phospholipids and VEGF down-regulated junction protein expression such as occludin, leading to an increased in endothelial permeability (DeMaio et al., 2006; Wang et al., 2001). We hereby demonstrate that DEP increased endothelial cell permeability and decreased ZO-1 protein in HAEC without change ZO-1 mRNA levels, suggesting that DEP increased endothelial permeability via ZO-1 at the post-translational level.
The levels of junction protein, Occludin, were not altered by DEP in HAEC. Thus, DEP could reduce the ZO-1 to occludin association ratio, leading to a dissociation of occludin from cytoskeleton and a consequent disruption of tight junction (TJ). Interestingly, epithelial cell exposed to cigarette smoke increased permeability via a decrease in ZO-1/occludin association ratio (Olivera et al., 2009). High concentration of DEP was reported to increase the permeability of 16HBE14o epithelial cells, which may be due to the change in occludin expression (Lehmann et al., 2009). The discrepancy of diesel particle effects on occludin between our observation and that of Lehmann et al. could stem from possible differences in cell types, particle concentrations, and chemical compositions.
DEP was observed to down-regulate ZO-1 expression at the post-translational level in this study. One of the possible mechanisms is that DEP promote ZO-1 protein degradation. DEP activates JNK (Li et al., 2009), and phosphorylation of ZO-1 has been shown to be associated with cell permeability (Aghajanian et al., 2008; Gonzalez-Mariscal et al., 2008). We therefore examined whether inhibition of JNK by JNK inhibitor, SP600125, affected endothelial permeability. Although SP600125 partially inhibited DEP induced permeability, it also reduced the background levels of permeability (Supplemental Fig. 1). We further examined if ZO-1 phosphorylation and ZO-1 ubiquitination were increased by DEP. However, the difference between control and DEP-exposed cells was insignificant (Supplemental Fig. 2). Other proteases may be implicated in the degradation of ZO-1. For example, diabetes-associated blood-brain-barrier permeability increase is associated with increased metalloproteinase activities (Hawkins et al., 2007). Matrix-metalloproteinase-13 has been shown to degrade ZO-1 and is associated with an increase in permeability in brain endothelial cells (Lu et al., 2009). Calpain was also reportedly to degrade proteins in the PDZ family, to which ZO-1 belongs (Jourdi et al., 2005). ZO-1 also undergoes endocytosis with junction protein, Cx43, suggesting possible degradation via lysosome (Fiorini et al., 2008). However, the precise mechanism(s) by which DEP down-regulated ZO-1 protein remains to be defined.
In summary, we demonstrate that both DEP1 and DEP2 increased HAEC permeability while DEP2, containing a higher level of redox active organic compounds and metals on a per PM mass basis, induced a more potent effect. This increase in permeability was associated with the down-regulation of junction protein ZO-1. Hence, our findings provide a new insight into the mechanism whereby DEP promoted the initiation of atherosclerosis by altering vascular endothelial permeability..
Supplementary Material
Acknowledgements
This project was supported by NHLB 083015 (TKH) and NHLBI 068689 (TKH). Additional support was provided by the California Air Resources Board, the South Coast Air Quality Management District (Grant 05-308), and the Southern California Particle Center, funded by the EPA under the STAR program through Grant RD-8324-1301-0 to the University of Southern California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. J Thromb Haemost. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulig A, Poirault JJ, Ausset P, Schins R, Shi T, Baralle D, Dorlhene P, Meyer M, Lefevre R, Baeza-Squiban A, Marano F. Physicochemical characteristics and biological activities of seasonal atmospheric particulate matter sampling in two locations of Paris. Environ Sci Technol. 2004;38:5985–5992. doi: 10.1021/es049476z. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Bian C, Wu Y, Chen P. Telmisartan increases the permeability of endothelial cells through zonula occludens-1. Biol Pharm Bull. 2009;32:416–420. doi: 10.1248/bpb.32.416. [DOI] [PubMed] [Google Scholar]
- Biswas S, Hu S, Verma V, Herner J, Robertson WJ, Ayala A, Sioutas C. Physical Properties of particulate matter (PM) from late model heavy duty diesel vehicles operating with advanced emission control technologies. Atomspheric Environment. 2008;vol. 42 [Google Scholar]
- Boland S, Baeza-Squiban A, Fournier T, Houcine O, Gendron MC, Chevrier M, Jouvenot G, Coste A, Aubier M, Marano F. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am J Physiol. 1999;276:L604–L613. doi: 10.1152/ajplung.1999.276.4.L604. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC, Jr, Tager I. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Decesari S, Facchini MC, Matta E, Lettini F, Mircea M, Fuzzi S, Tagliavini E, Putaud JP. Chemical features and seasonal variation of fine aerosol water-soluble organic compounds in the Po Valley, Italy. Atmospheric Environment. 2001;35:3691–3699. [Google Scholar]
- DeMaio L, Rouhanizadeh M, Reddy S, Sevanian A, Hwang J, Hsiai TK. Oxidized phospholipids mediate occludin expression and phosphorylation in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2006;290:H674–H683. doi: 10.1152/ajpheart.00554.2005. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Fiorini C, Gilleron J, Carette D, Valette A, Tilloy A, Chevalier S, Segretain D, Pointis G. Accelerated internalization of junctional membrane proteins (connexin 43, N-cadherin and ZO-1) within endocytic vacuoles: an early event of DDT carcinogenicity. Biochim Biophys Acta. 2008;1778:56–67. doi: 10.1016/j.bbamem.2007.08.032. [DOI] [PubMed] [Google Scholar]
- Frampton MW. Systemic and cardiovascular effects of airway injury and inflammation: Ultrafine particle exposure in humans. Environmental Health Perspectives. 2001;109:529–532. doi: 10.1289/ehp.01109s4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TW. Histamine, ZO-1 and increased blood-retinal barrier permeability in diabetic retinopathy. Trans Am Ophthalmol Soc. 1995;93:583–621. [PMC free article] [PubMed] [Google Scholar]
- Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29:2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia. 2007;50:202–211. doi: 10.1007/s00125-006-0485-z. [DOI] [PubMed] [Google Scholar]
- Hiruma K, Terada N, Hanazawa T, Nomoto M, Maesako K, Konno A, Kobayashi T. Effect of diesel exhaust on guinea pig nasal mucosa. Ann Otol Rhinol Laryngol. 1999;108:582–588. doi: 10.1177/000348949910800610. [DOI] [PubMed] [Google Scholar]
- Holmen BA, Ayala A. Ultrafine PM emissions from natural gas, oxidation-catalyst diesel, and particle-trap diesel heavy-duty transit buses. Environ Sci Technol. 2002;36:5041–5050. doi: 10.1021/es015884g. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Sakurai M, Oda T, Tamura H, Yanagisawa R, Shimada A, Yoshikawa T. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp Biol Med (Maywood) 2006;231:1626–1632. doi: 10.1177/153537020623101007. [DOI] [PubMed] [Google Scholar]
- Jourdi H, Lu X, Yanagihara T, Lauterborn JC, Bi X, Gall CM, Baudry M. Prolonged positive modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors induces calpain-mediated PSD-95/Dlg/ZO-1 protein degradation and AMPA receptor down-regulation in cultured hippocampal slices. J Pharmacol Exp Ther. 2005;314:16–26. doi: 10.1124/jpet.105.083873. [DOI] [PubMed] [Google Scholar]
- Karoly ED, Li Z, Dailey LA, Hyseni X, Huang YC. Up-regulation of tissue factor in human pulmonary artery endothelial cells after ultrafine particle exposure. Environ Health Perspect. 2007;115:535–540. doi: 10.1289/ehp.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann AD, Blank F, Baum O, Gehr P, Rothen-Rutishauser BM. Diesel exhaust particles modulate the tight junction protein occludin in lung cells in vitro. Part Fibre Toxicol. 2009;6:26. doi: 10.1186/1743-8977-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Hao M, Phalen RF, Hinds WC, Nel AE. Particulate air pollutants and asthma. A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radic Biol Med. 2009;46:775–782. doi: 10.1016/j.freeradbiomed.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Ning Z, Majumdar R, Cui J, Takabe W, Jen N, Sioutas C, Hsiai T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-kappaB signaling. Part Fibre Toxicol. 7:6. doi: 10.1186/1743-8977-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough GC, Schauer JJ, Park JS, Shafer MM, Deminter JT, Weinstein JP. Emissions of metals associated with motor vehicle roadways. Environmental Science & Technology. 2005;39:826–836. doi: 10.1021/es048715f. [DOI] [PubMed] [Google Scholar]
- Lu DY, Yu WH, Yeh WL, Tang CH, Leung YM, Wong KL, Chen YF, Lai CH, Fu WM. Hypoxia-induced matrix metalloproteinase-13 expression in astrocytes enhances permeability of brain endothelial cells. J Cell Physiol. 2009;220:163–173. doi: 10.1002/jcp.21746. [DOI] [PubMed] [Google Scholar]
- Misra C, Kim S, Shen S, Sioutas C. A high flow rate, very low pressure drop impactor for inertial separation of ultrafine from accumulation mode particles. J Aerosol Sci. 2002;33:735–752. [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoet PHM, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- Nemmar A, Hoylaerts MF, Hoet PH, Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol Lett. 2004;149:243–253. doi: 10.1016/j.toxlet.2003.12.061. [DOI] [PubMed] [Google Scholar]
- Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Particulate Science and Technology. 1996;14:135–151. [PubMed] [Google Scholar]
- Olivera D, Knall C, Boggs S, Seagrave J. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke-induced permeability of airway epithelium. Exp Toxicol Pathol. 2009 doi: 10.1016/j.etp.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Schauer JJ, Fraser MP, Cass GR, Simoneit BR. Source reconciliation of atmospheric gas-phase and particle-phase pollutants during a severe photochemical smog episode. Environ Sci Technol. 2002;36:3806–3814. doi: 10.1021/es011458j. [DOI] [PubMed] [Google Scholar]
- Takenaka S, Karg E, Kreyling WG, Lentner B, Moller W, Behnke-Semmler M, Jennen L, Walch A, Michalke B, Schramel P, Heyder J, Schulz H. Distribution pattern of inhaled ultrafine gold particles in the rat lung. Inhal Toxicol. 2006;18:733–740. doi: 10.1080/08958370600748281. [DOI] [PubMed] [Google Scholar]
- Takenaka S, Karg E, Kreyling WG, Lentner B, Schulz H, Ziesenis A, Schramel P, Heyder J. Fate and toxic effects of inhaled ultrafine cadmium oxide particles in the rat lung. Inhal Toxicol. 2004;16 Suppl 1:83–92. doi: 10.1080/08958370490443141. [DOI] [PubMed] [Google Scholar]
- Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol. 2001;280:H434–H440. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.